Abstract
Aims
Na+/K+-ATPase and Ca2+/Mg2+-ATPase are enzymes known to maintain intracellular gradients of ions that are essential for signal transduction. The aim of this study was to compare the activities of Na+/K+-ATPase and Ca2+/Mg2+-ATPase in post-mortem brain samples from the cerebellum and frontal, temporal, parietal, and occipital cortices from autistic and age-matched control subjects.
Main methods
The frozen postmortem tissues from different brain regions of autistic and control subjects were homogenized. The activities of Na+/K+-ATPase and Ca2+/Mg2+-ATPase were assessed in the brain homogenates by measuring inorganic phosphorus released by the action of Na+/K+- and Ca2+/Mg2+- dependent hydrolysis of ATP.
Key findings
In the cerebellum, the activities of both Na+/K+-ATPase and Ca2+/Mg2+-ATPase were significantly increased in the autistic samples compared with their age-matched controls. The activity of Na+/K+-ATPase but not Ca2+/Mg2+-ATPase was also significantly increased in the frontal cortex of the autistic samples as compared to the age-matched controls. In contrast, in other regions, i.e., the temporal, parietal and occipital cortices, the activities of these enzymes were similar in autism and control groups.
Significance
The results of this study suggest brain-region specific increases in the activities of Na+/K+-ATPase and Ca2+/Mg2+-ATPase in autism. Increased activity of these enzymes in the frontal cortex and cerebellum may be due to compensatory responses to increased intracellular calcium concentration in autism. We suggest that altered activities of these enzymes may contribute to abnormal neuronal circuit functioning in autism.
INTRODUCTION
Autism is a severe neurological disorder that causes impairment in language, cognition and socialization (Lord et al. 2000). It is a heterogeneous disorder, both etiologically and phenotypically. Autism belongs to a group of neurodevelopmental disorders known as autism spectrum disorders (ASD) that includes Pervasive Developmental Disorder- Not Otherwise Specified (PDD-NOS), Asperger disorder, Childhood Disintegrative Disorder (CDD) and Rett syndrome. According to the Centers for Disease Control (CDC), 1 in 150 children is diagnosed with ASD.
Genetic, neurochemical, neuroimaging and behavioral studies suggest that neural properties may be perturbed in autism, giving rise to abnormalities in processing of neuronal information leading to complex behavioral abnormalities (Belmonte et al., 2004). Na+/K+-ATPase and Ca2+/Mg2+-ATPase are known to play important roles in neuronal transmission. A gradient of high K+ and low Na+ intracellular concentration is needed for the optimum neuronal functions (McCormick and Huguenard, 1994, Palladina et al., 2005). Na+/K+-ATPase is a membrane-bound enzyme involved in maintaining the Na+ and K+ gradient across the cell membrane. It is ubiquitously expressed in neurons (Pietrini et al., 1992), and helps maintain normal neuronal function. The Na+/K+-ATPase extrudes three Na+ ions and imports two K+ ions. This activity is important in the regulation of membrane potential. The Na+/K+-ATPase activity contributes to the resting membrane potential in the cell, and returns Na+ and K+ concentrations to their resting transmembrane levels after bursts of stimulatory activity (Blaustein, 1993). Na+/K+-ATPase abnormality has been reported to be involved in several neurological diseases such as seizures (Brines et al., 1995; Fernandes et al., 1996b), bipolar disorder (Amiet et al., 2008; Christo and el Mallakh, 1993), spongiform encephalopathy (Renkawek et al., 1992), and Alzheimer’s disease (Rose and Valdes, 1994). Na+/K+-ATPase may also have implications in behavioral defects. Lingrel et al. (2007) reported that haploinsuffciency of Na+/K+-ATPase α2 and α3 isoforms results in behavioral defects.
Calcium is an important signaling molecule in cells (Berridge, 1992). Many cellular functions are regulated by intracellular free calcium concentrations. In resting cells, a sub-optimum concentration of intracellular calcium is maintained either by storing calcium in intracellular reserves by the action of ATPase (Nori et al., 1996), by extrusion of calcium by plasma membrane-bound calcium ATPase (Carafoli et al., 1996) or by Na+/ Ca2+ exchange (Blaustein and Lederer, 1999). In stimulated cells, a sudden influx of calcium occurs in a receptor-coupled manner where calcium participates in activating several proteins, which perform specific functions. Neurons use intracellular Ca2+ to control various functions. Disturbances in Ca2+ homeostasis can lead to neuronal dysfunction and eventual neuronal death. Several neurological diseases are caused primarily by malfunctioning of Ca2+ channels or Ca2+/Mg2+-ATPase (Cooper and Jan, 1999; Jacobsen et al., 1999). Recently, Gargus reported genetic calcium signaling abnormalities in several neurological conditions, including seizures, migranes and autism (Gargus, 2009). However, it is not known whether Na+/K+-ATPase and Ca2+/Mg2+-ATPase abnormalities are involved in autism. The present study was undertaken to determine whether the activities of Na+/K+-ATPase and Ca2+/Mg2+-ATPase are affected in autism. We found that activities of Na+/K+-ATPase and Ca2+/Mg2+-ATPase are specifically increased in the frontal cortex and cerebellum of brains from autistic subjects, while they were unchanged in the parietal, occipital and temporal cortices.
MATERIALS AND METHODS
Materials
Samples of postmortem frozen brain regions, i.e., the cerebellum, and cortices from the frontal, temporal, parietal and occipital lobes (N= 6–10 for different brain regions) from autistic and age-matched control subjects (N= 8–10) were obtained from the National Institute of Child Health and Human Development (NICHD) Brain and Tissue Bank for Developmental Disorders. The age (Mean ± S.E.) for autistic subjects was 13 ± 3.7 y and for control subjects, 12.5 ± 3.5 y. The mean postmortem interval (PMI) for the autistic samples was 22 ± 4.5 h, and for control samples, 17 ± 1.3 h. Donors with autism fit the diagnostic criteria of the Diagnostic and Statistical Manual-IV, as confirmed by the Autism Diagnostic Interview-Revised (ADI-R). All brain samples were stored at −70 °C. This study was approved by the Institutional Review Board of the New York State Institute for Basic Research.
Preparation of homogenates
The tissues were homogenized (10% w/v) in cold buffer containing 50 mM Tris-HCl (pH 7.4), 8.5% sucrose, 2 mM EDTA, 10 mM β-mercaptoethanol and protease inhibitor cocktail (Sigma-Aldrich) in a Downs homogenizer with 5 strokes at 4 °C. The protein concentration was assayed by the BioRad protein assay kit.
Measurement of Na+/K+-ATPase activity
The reaction mixture containing 25 µl of 2 M NaCl, 25 µl of 25 mM KCl, 25 µl of 60 mM MgCl2, 5 µl of 10 mM EGTA and brain homogenate (0.5 mg) was adjusted to a total volume of 490 µl with 50 mM Tris-HCl, pH 7.5, and incubated at 37 °C for 10 min. The reaction was started by adding 10 µl of 150 mM ATP. After 1 h, the reaction was stopped by adding 1 ml of cold 15% TCA. The samples were kept on ice for 1 h, followed by centrifugation at 1,000 g for 15 min. Inorganic phosphorus in the 500 µl supernatant was measured by the method of Fiske and Subbaraw (1953).
Measurement of Ca2+/Mg2+-ATPase activity
The reaction mixture containing 25 µl of 2 M NaCl, 25 µl of 25 mM KCl, 25 µl of 60 mM MgCl2, 5 µl of 10 mM Quabain, 10 µl of 10 mM CaCl2, and brain homogenate (0.5 mg) was adjusted to a total volume of 490 µl with 50 mM Tris-HCl, pH 7.5, and incubated at 37 °C for 10 min. The reaction was initiated by adding 10 µl of 150 mM ATP. After 1 h, the reaction was stopped by adding 1 ml of cold 15% TCA. The samples were kept on ice for 1 h, followed by centrifugation at 1,000 g. Inorganic phosphorus in the 500 µl supernatant was then measured.
Statistical analysis
The enzyme activities in autism and control groups were analyzed by unpaired student’s t-test.
RESULTS
Na+/K+-ATPase activity in different brain regions from autistic and control subjects
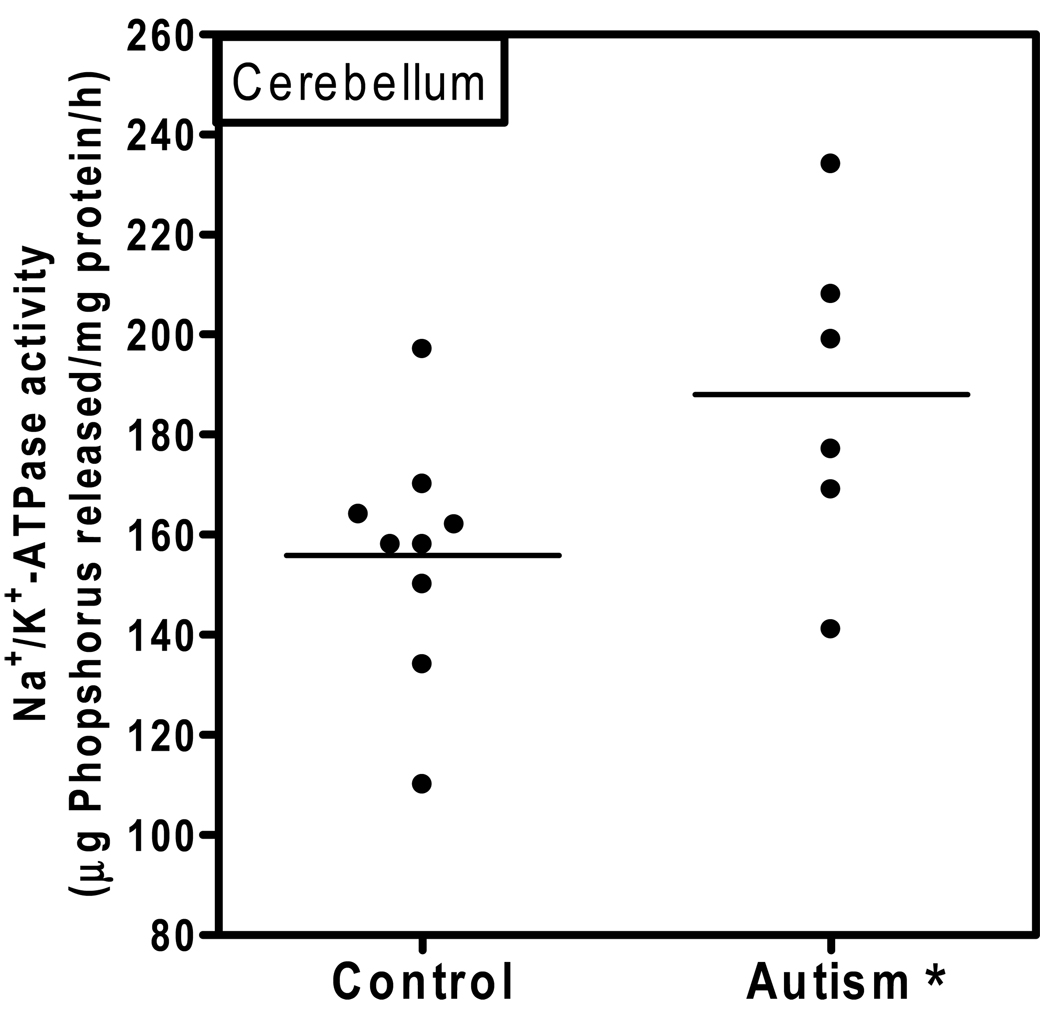
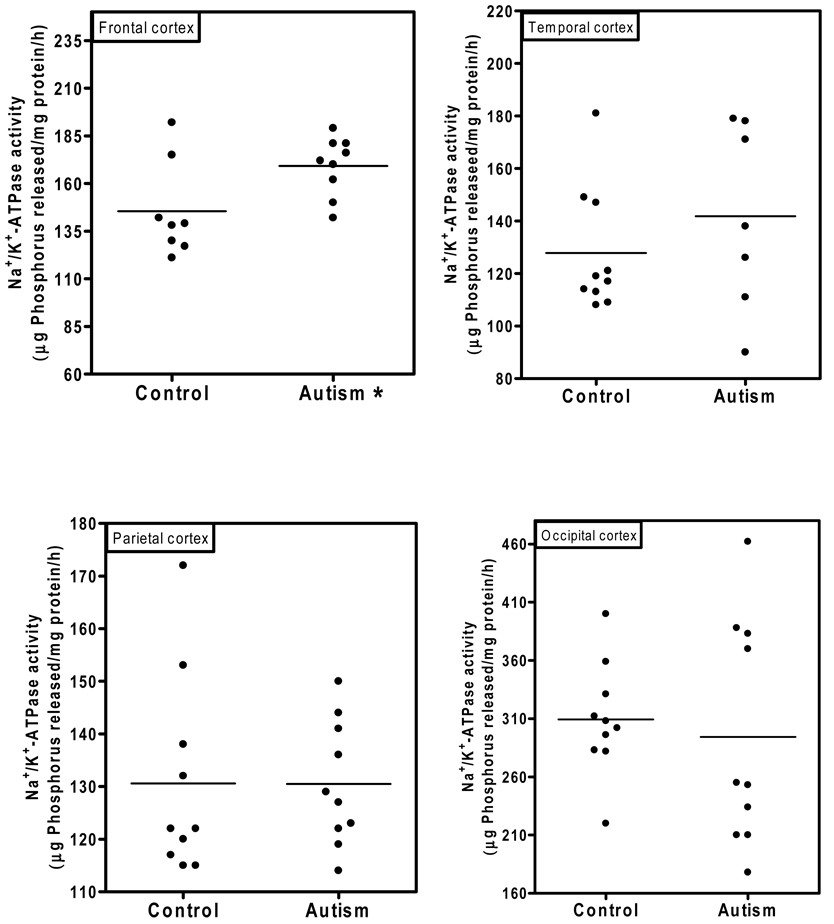
Fig. 1 shows the Na+/K+-ATPase activity in the cerebellum of autistic and age-matched control subjects. The Na+/K+-ATPase activity (µg phosphorus released/mg protein/hr) in the cerebellum of the autistic samples (Mean ± S.E.:188 ±13) was significantly higher (p < 0.03) as compared to control samples (156 ± 8). In Fig. 2, the Na+/K+-ATPase activity in the cortices from frontal, temporal, parietal and occipital regions is shown. The activity of Na+/K+-ATPase in the frontal cortex of the autistic subjects (169 ± 5) was significantly higher (p < 0.03) as compared to the control subjects (145 ± 8.7). However, the activity of the Na+/K+-ATPase was similar between the autistic and control groups in other brain regions, i.e., temporal cortex (autism, 142± 13; and controls, 128 ± 7.5); parietal cortex (autism, 131 ± 4; and controls, 130 ± 6); and occipital cortex (autism, 294 ± 22, and controls, 309 ± 15). No relation was observed between PMI and the activity of this enzyme in the brain.
Fig. 1. Na+/K+ -ATPase activity in the cerebellum of autistic and control subjects.
The enzyme activity was measured in the cerebellum of autistic and control subjects as described in ‘Materials and Methods’. The horizontal line represents average Na+/K+-ATPase activity in each group. * denotes p < 0.05, autism vs. control group.
Fig. 2. Na+/K+ -ATPase activity in the frontal, occipital, parietal and temporal cortices from autistic and control subjects.
The activity of Na+/K+-ATPase was measured in different brain regions from autistic and control subject as described in ‘Materials and Methods’. * denotes p < 0.03, autism vs. control group.
Ca2+/Mg2+-ATPase activity in different brain regions from autistic and control subjects
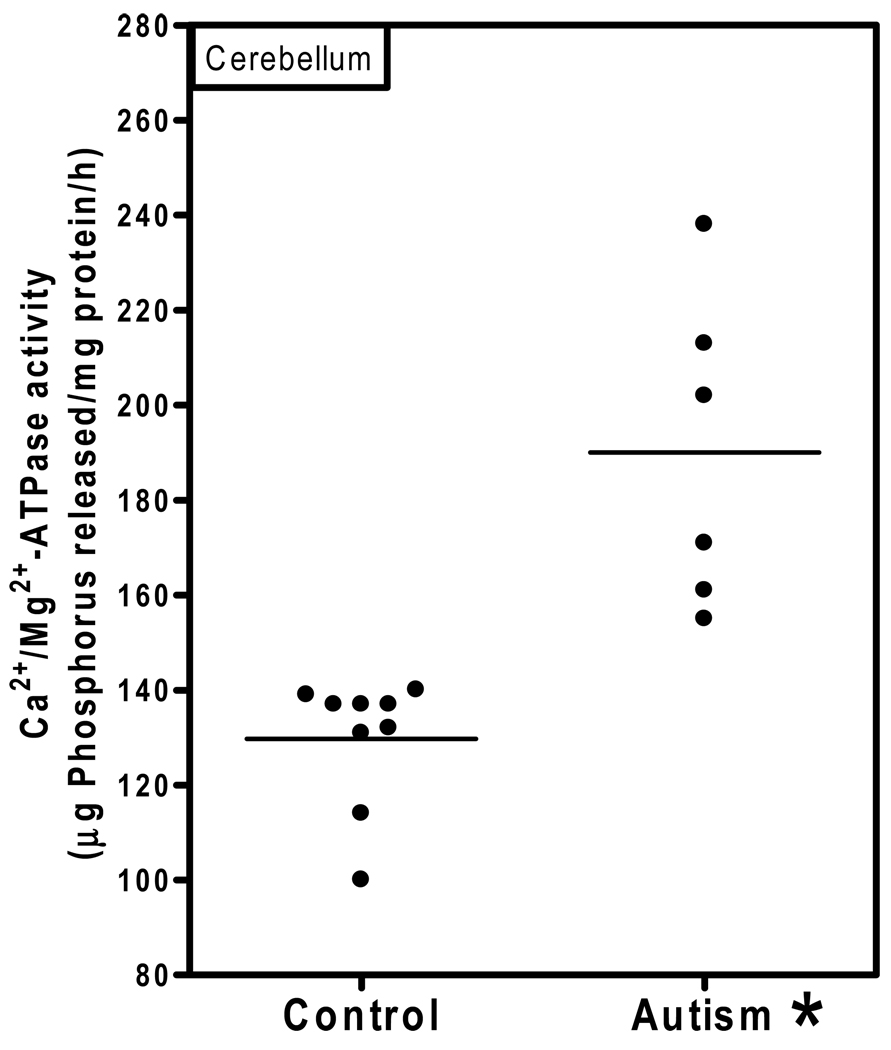
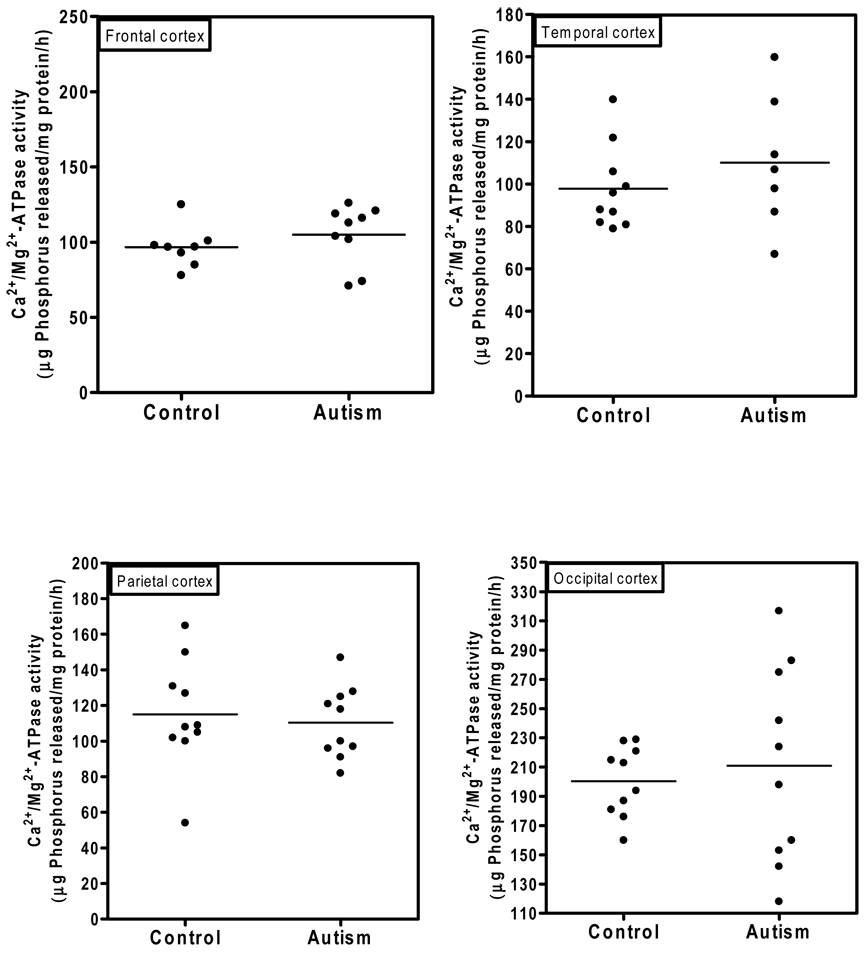
Ca2+/Mg2+-ATPase activity in the cerebellum of autistic and control subjects is shown in Fig. 3. The Ca2+/Mg2+-ATPase activity (µg phosphorus released/mg protein/hr) in the cerebellum of the autistic samples (Mean ± S.E.: 190 ± 13) was significantly higher (p < 0.0005) than in age-matched controls (130 ± 4.5). There was no overlap in the Ca2+/Mg2+-ATPase activity between autistic and control samples. Fig. 4 shows the Ca2+/Mg2+-ATPase activity in the cortices from frontal, temporal, parietal and occipital regions. No significant differences in the activity of Ca2+/Mg2+-ATPase was observed in frontal cortex (autism, 105 ± 6.7; and controls, 96.7 ± 5.6); temporal cortex (autism, 110± 12; and controls, 98 ± 6); parietal cortex (autism, 111 ± 6, and control, 115 ± 10); and occipital cortex (autism, 211 ± 22; and controls, 200 ± 8). No relation was observed between PMI and this activity of the enzyme in the brain.
Fig. 3. Ca2+/Mg2+-ATPase activity in the cerebellum of autistic and control subjects.
The enzyme activity was measured in the cerebellum of autistic and control subjects as described in ‘Materials and Methods’. The horizontal line represents average Ca2+/Mg2+-ATPase activity in each group. * denotes p < 0.0005, autism vs. control group.
Fig. 4. Ca2+/Mg2+-ATPase activity in the frontal, occipital, parietal and temporal cortices of the brain regions from autistic and control subjects.
The activity of Ca2+/Mg2+-ATPase was measured in different brain regions from autistic and control subjects as described in ‘Materials and Methods’.
DISCUSSION
K+, Na+ and Ca2+ play important roles in neuronal signaling due to conduction of electrical activity of neurons (McCormick and Huguenard, 1994). Therefore, control of excitability of neurons is maintained by the ionic environment. Intracellular concentrations of Na+ and Ca2+ are several folds lower and that of potassium are higher as compared to their extracellular concentrations. The net transmembrane potential across the membrane is maintained at −60 mV. If the ionic concentration is perturbed (e.g., levels of intracellular Ca2+ and Na+ or extracellular K+ are altered), this can lead to depolarization and abnormal neuronal activity due to depolarization of neuronal-terminals, neurotransmitters release, depolarization of neurons and discharge of action potential (Somojen, 2002).
Na+/K+-ATPase and Ca2+/Mg2+-ATPase are two ATP-hydrolyzing enzymes which maintain the electrochemical gradient in the cells in an energy-dependent manner. Na+/K+-ATPase extrudes three Na+ molecules in exchange for internalization of two K+ molecules. The Na+/K+-ATPase is composed of multiple isoforms (α1, α2 and α3), and these isoforms differ in their distribution in tissues and during development. Lingrel et al. (2007) reported that haploinsuffciency of its α2 and α3 isoforms results in behavioral defects. In another study, mutations in a C-terminal region of other voltage-gated Na+ channels have been reported to reduce the amount of channel inactivation (Glaaser et al., 2006; Kim et al., 2004). Another report suggests functional deficit of Ca2+-activated K+ channel (BKCa), a synaptic regulator of neuronal excitability with autism (Laumonnier et al., 2006). Disruption of the BKCa gene (KCNMA1) led to haploinsufficiency and reduced BKCa activity in autism. These reports on decrease in BKCa channel activity, and reduced inactivation of voltage-gated Ca2+ channels in individuals with autism, raise the possibility that excessive ion channel activity may lead to ASD.
Since K+, Na+ and Ca2+ play important roles in developing electrochemical gradients and in neuronal signaling, the altered activities of Na+/K+-ATPase and Ca2+/Mg2+-ATPase may have a significant impact on brain function in autistic subjects. Our results show that the activities of both Na+/K+-ATPase and Ca2+/Mg2+-ATPase were significantly increased in the cerebellum in autism as compared with age-matched controls, while the activity of Na+/K+-ATPase was also significantly increased in the frontal cortex in autism. In other regions of cerebrum i.e., occipital, parietal and temporal cortex, the activities of Na+/K+-ATPase and Ca2+/Mg2+-ATPase were similar between autistic and control subjects. Increased activity of Na+/K+-ATPase has been reported in several other pathological conditions such as in experimentally induced epilepsy (Fernandes et al., 1996; Reime et al., 2007), and in Crush syndrome (Desai and Desai, 2007). In chronic fatigue syndrome, the activities of both Na+/K+-ATPase and Ca2+/Mg2+-ATPase are increased in sarcoplasmic reticulum membranes (Fulle et al., 2003). In addition, Takser et al. (2003) reported a correlation of ATPase activities with early psychomotor development in humans. Rapid eye movement sleep deprivation has also been reported to increase Na+/K+-ATPase activity (Mallick et al., 2000). In addition, certain environmental factors such as lead have been reported to increase the activity of Na+/K+-ATPase (Regunathan and Sundaresan, 1985).
After a stimulus, calcium flows rapidly into neurons through various types of membrane channels including voltage-dependent and receptor-coupled channels. Intracellular Ca2+ concentrations are quickly restored to resting levels primarily through Ca2+/Mg2+-ATPase, Na+/Ca+ exchange, and endoplasmic sequestration. Calcium is essential for neurotransmitter release, and Ca2+ influx is essential for neuronal excitability. Improper intracellular regulation of calcium has been linked with several neurological disorders. The receptor-coupled increase in intracellular levels of calcium is important for neuronal survival, differentiation, migration, and synaptogenesis (Aamodt and Constantine-Paton, 1999; Cline, 2001; Komuro and Rakic, 1998; Moody and Bosma, 2005; Represa and Ben Ari, 2005; Spitzer et al., 2004). Defects in these developmental processes can lead to neuroanatomical abnormalities, such as increased cell-packing density, decreased neuron size and arborizations, and alterations in connectivity. Such abnormalities have been associated with ASD patients (Courchesne et al., 2005; DiCicco-Bloom et al., 2006). Plasma membrane calcium-ATPase plays an important role in the translocation of calcium from the cytosol to the extracellular milieu. Our results suggest that Ca2+/Mg2+-ATPase activity is significantly increased in the cerebellum of autistic subjects, but not in other regions of the brain. Although Ca2+/Mg2+-ATPase activity in the frontal cortex of autism subjects was not significantly changed but a trend towards increased Ca2+/Mg2+-ATPase activity was observed as compared to controls. The median Ca2+/Mg2+-ATPase activity in the frontal cortex for autism was 113 µg phosphorus released/mg protein/hr while for control group, it was 99 µg phosphorus released/mg protein/hr. A differential effect of ATPase activity in different regions of brain is not unique. In epilepsy, the intrasynaptosomal Ca2+/Mg2+-ATPase activity was reported to be decreased in the hippocampus, but not in the temporal cortex (Nagy et al., 1990).
Voltage-gated calcium channels mediate calcium influx in response to membrane depolarization and regulate intracellular processes such as contraction, secretion, neurotransmission, and gene expression. Their activity is essential for coupling electrical signals on the cell surface to physiological events in cells. Functional mutations in genes encoding voltage-gated Ca2+ channels have been suggested as a possible cause of ASD (Hemara-Wahanui et al., 2005b; Splawski et al., 2006; Splawski et al., 2004). Point mutations in the gene encoding the L-type voltage-gated Ca2+channel CaV1.2 (CACNA1C) cause Timothy syndrome, a multisystem disorder that includes cardiac abnormalities and autism (Splawski et al., 2005; Splawski et al., 2004). CaV1.2 plays an important role in the activation of transcription factors, such as cAMP response-element-binding protein (CREB) and myocyte enhancer factor 2 (MEF2), involving neuronal survival and dendritic arborization (West et al., 2001). The mutations associated with Timothy syndrome prevent voltage-dependent inactivation of CaV1.2, which causes the channels to remain open longer and allow the influx of more Ca2+ than wild-type channels (Splawski et al., 2005; Splawski et al., 2004) leading to increased intracellular Ca2+. Additional evidence of calcium’s involvement in autism comes from a mutation identified in the CACNA1F gene, which encodes the L-type voltage-gated Ca2+ channel, CaV1.4. This mutation was reported to cause autistic symptoms in a New Zealand family where the affected subjects have stationary night blindness (Hemara-Wahanui et al., 2005a; Hope et al., 2005). ASD-associated mutations have been identified not only in genes encoding Ca2+ channels themselves but also in genes encoding ion channels whose activity is directly modulated by Ca2+ such as Ca2+-dependent Na+ channels. Several point mutations in SCN1A and SCN2A genes, which encode the voltage-activated Na+ channels NaV1.1 and NaV1.2 respectively has been reported (Kamiya et al., 2004; Weiss et al., 2003).
Wingless-type mouse mammary tumor virus (MMTV) integration site member (Wnt) proteins are known to form a family of highly conserved and secreted signaling molecules, which regulate cell-to-cell interactions during embryogenesis. The role of WNT2 has been implicated in ASD. Two families with mutations in WNT2 have been identified, and a polymorphism in an upstream region of WNT2 has been associated with families characterized with severe language abnormalities (Wassink et al., 2001). Increase in Ca2+ concentration has been reported to enhance the synthesis and release of Wnt through the activity of the Ca2+-regulated transcription factor CREB (Wayman et al., 2006). Because of the pivotal role of calcium in cellular signaling, calcium may play an important role in the etiology of ASD.
The increased activity of Ca2+/Mg2+-ATPase in the cerebellum of autistic subjects may be attributable to several factors. Ca2+/Mg2+-ATPase activity may increase due to compensatory mechanisms in response to increased intracellular calcium levels in autism. Heguilen et al. (2009) reported increases in Ca2+/Mg2+-ATPase activity in patients with hypercalciuric nephrolithiasis. In addition, Ca2+/Mg2+-ATPase activity can also be activated by lysophosphatidylcholine, a phospholipase A2 (PLA2)–mediated lipolytic product in the membrane. It has also been reported that the levels of polyunsaturated fatty acids, another lipolytic product of PLA2, are decreased in the erythrocyte membranes of autistic subjects as compared with normal control subjects (Bell et al., 2000). Increased activity of PLA2, an enzyme that removes unsaturated fatty acids from phospholipids, has also been reported in erythrocytes from autistic subjects (Bell et al., 2004). Additionally, increased levels of phospholipase A2 have been observed in the erythrocytes of patients with schizophrenia (Ward, 2000) and dyslexia (MacDonell et al., 2000). Since chromosomal linkage studies in autism point to a locus which includes the PLA2 gene (Lamb et al., 2000), this enzyme may also have an important role in the etiology of autism. In conclusion, Na+/K+-ATPase and Ca2+/Mg2+-ATPase activities in autism may be increased in response to increased intracellular calcium concentration, and may contribute to altered neocotical circuitry in the cerebellum and frontal cortex of individual with autism.
Acknowledgement
This work was supported in part by funds from the New York State Office of Mental Retardation and Developmental Disabilities, NIH Grant No. AG020992 (VC), an Autism Collaboration (autism.org) grant (VC), Department of Defense (AC), Autism Speaks (AC) and Autism Research Institute (AC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aamodt SM, Constantine-Paton M. The role of neural activity in synaptic development and its implications for adult brain function. Adv Neurol. 1999;79:133–144. [PubMed] [Google Scholar]
- Amiet C, Gourfinkel-An I, Bouzamondo A, Tordjman S, Baulac M, Lechat P, Mottron L, Cohen D. Epilepsy in autism is associated with intellectual disability and gender, evidence from a meta-analysis. Biol Psychiatry. 2008;64:577–582. doi: 10.1016/j.biopsych.2008.04.030. [DOI] [PubMed] [Google Scholar]
- Bell JG, MacKinlay EE, Dick JR, MacDonald DJ, Boyle RM, Glen AC. Essential fatty acids and phospholipase A2 in autistic spectrum disorders. Prostaglandins Leukot Essent Fatty Acids. 2004;71:201–204. doi: 10.1016/j.plefa.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Bell JG, Sargent JR, Tocher DR, Dick JR. Red blood cell fatty acid compositions in a patient with autistic spectrum disorder, a characteristic abnormality in neurodevelopmental disorders? Prostaglandins Leukot Essent Fatty Acids. 2000;63:21–25. doi: 10.1054/plef.2000.0186. [DOI] [PubMed] [Google Scholar]
- Belmont MK, Cook EH, Jr, Anderson GM, Rubenstein JL, Greenough WT, Beckel-Mitchener A, Courchesne E, Boulanger LM, Powell SB, Levitt PR, Perry EK, Jiang YH, DeLorey TM, Tierney E. Autism as a disorder of neural information processing, directions for research and targets for therapy. Mol Psychiatry. 2004;9:646–663. doi: 10.1038/sj.mp.4001499. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium oscillations. Adv Second Messenger Phosphoprotein Res. 1992;26:211–223. [PubMed] [Google Scholar]
- Blaustein MP. Physiological effects of endogenous ouabain, control of intracellular Ca2+ stores and cell responsiveness. Am J Physiol. 1993;264:C1367–C1387. doi: 10.1152/ajpcell.1993.264.6.C1367. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Lederer WJ. Sodium/calcium exchange, its physiological implications. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Brines ML, Tabuteau H, Sundaresan S, Kim J, Spencer DD, de Lanerolle N. Regional distributions of hippocampal Na+,K+.-ATPase, cytochrome oxidase, and total protein in temporal lobe epilepsy. Epilepsia. 1995;36:371–383. doi: 10.1111/j.1528-1157.1995.tb01012.x. [DOI] [PubMed] [Google Scholar]
- Carafoli E, Garcia-Martin E, Guerini D. The plasma membrane calcium pump, recent developments and future perspectives. Experientia. 1996;52:1091–1100. doi: 10.1007/BF01952107. [DOI] [PubMed] [Google Scholar]
- Christo PJ, el Mallakh RS. Possible role of endogenous ouabain-like compounds in the pathophysiology of bipolar illness. Med Hypotheses. 1993;41:378–383. doi: 10.1016/0306-9877(93)90089-9. [DOI] [PubMed] [Google Scholar]
- Cline HT. Dendritic arbor development and synaptogenesis. Curr Opin Neurobiol. 2001;11:118–126. doi: 10.1016/s0959-4388(00)00182-3. [DOI] [PubMed] [Google Scholar]
- Cooper EC, Jan LY. Ion channel genes and human neurological disease, recent progress, prospects, and challenges. Proc Natl Acad Sci U.S.A. 1999;96:4759–4766. doi: 10.1073/pnas.96.9.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Redcay E, Morgan JT, Kennedy DP. Autism at the beginning, microstructural and growth abnormalities underlying the cognitive and behavioral phenotype of autism. Dev Psychopathol. 2005;17:577–597. doi: 10.1017/S0954579405050285. [DOI] [PubMed] [Google Scholar]
- Desai SN, Desai PV. The study of Na+, K+.-ATPase activity of rat brain during Crush syndrome. Neurochem Res. 2007;32:1843–1848. doi: 10.1007/s11064-007-9370-5. [DOI] [PubMed] [Google Scholar]
- DiCicco-Bloom E, Lord C, Zwaigenbaum L, Courchesne E, Dager SR, Schmitz C, Schultz RT, Crawley J, Young LJ. The developmental neurobiology of autism spectrum disorder. J Neurosci. 2006;26:6897–6906. doi: 10.1523/JNEUROSCI.1712-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes MJ, Naffah-Mazzacoratti MG, Cavalheiro EA. Na+K+ ATPase activity in the rat hippocampus, a study in the pilocarpine model of epilepsy. Neurochem Int. 1996;28:497–500. doi: 10.1016/0197-0186(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Fiske CH, Subbarow Y. The colorimetric determination of phosphorus. J Biol Chem. 1953;66:375–400. [Google Scholar]
- Fulle S, Belia S, Vecchiet J, Morabito C, Vecchiet L, Fano G. Modification of the functional capacity of sarcoplasmic reticulum membranes in patients suffering from chronic fatigue syndrome. Neuromuscul Disord. 2003;13:479–484. doi: 10.1016/s0960-8966(03)00042-7. [DOI] [PubMed] [Google Scholar]
- Gargus JJ. Genetic calcium signaling abnormalities in the central nervous system, seizures, migraine, and autism. Ann N Y Acad Sci. 2009;1151:133–156. doi: 10.1111/j.1749-6632.2008.03572.x. [DOI] [PubMed] [Google Scholar]
- Glaaser IW, Bankston JR, Liu H, Tateyama M, Kass RS. A carboxyl-terminal hydrophobic interface is critical to sodium channel function. Relevance to inherited disorders. J Biol Chem. 2006;281:24015–24023. doi: 10.1074/jbc.M605473200. [DOI] [PubMed] [Google Scholar]
- Heguilen RM, Gimenez MI, Imperiali N, Bernasconi A, Algranati SL. Ca2+/Mg2+-ATPase activity in erythrocyte membrane in hypercalciuric nephrolithiasic patients. Nepharology. 2009;7:12–17. [Google Scholar]
- Hemara-Wahanui A, Berjukow S, Hope CI, Dearden PK, Wu SB, Wilson-Wheeler J, Sharp DM, Lundon-Treweek P, Clover GM, Hoda JC, Striessnig J, Marksteiner R, Hering S, Maw MA. A CACNA1F mutation identified in an X-linked retinal disorder shifts the voltage dependence of Cav1.4 channel activation. Proc Natl Acad Sci U S A. 2005;102:7553–7558. doi: 10.1073/pnas.0501907102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope CI, Sharp DM, Hemara-Wahanui A, Sissingh JI, Lundon P, Mitchell EA, Maw MA, Clover GM. Clinical manifestations of a unique X-linked retinal disorder in a large New Zealand family with a novel mutation in CACNA1F, the gene responsible for CSNB2. Clin Experiment Ophthalmol. 2005;33:129–136. doi: 10.1111/j.1442-9071.2005.00987.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen NJ, Lyons I, Hoogendoorn B, Burge S, Kwok PY, O'Donovan MC, Craddock N, Owen MJ. ATP2A2 mutations in Darier's disease and their relationship to neuropsychiatric phenotypes. Hum Mol Genet. 1999;8:1631–1636. doi: 10.1093/hmg/8.9.1631. [DOI] [PubMed] [Google Scholar]
- Kamiya K, Kaneda M, Sugawara T, Mazaki E, Okamura N, Montal M, Makita N, Tanaka M, Fukushima K, Fujiwara T, Inoue Y, Yamakawa K. A nonsense mutation of the sodium channel gene SCN2A in a patient with intractable epilepsy and mental decline. J Neurosci. 2004;24:2690–2698. doi: 10.1523/JNEUROSCI.3089-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Ghosh S, Liu H, Tateyama M, Kass RS, Pitt GS. Calmodulin mediates Ca2+ sensitivity of sodium channels. J Biol Chem. 2004;279:45004–45012. doi: 10.1074/jbc.M407286200. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Orchestration of neuronal migration by activity of ion channels, neurotransmitter receptors, and intracellular Ca2+ fluctuations. J Neurobiol. 1998;37:110–130. [PubMed] [Google Scholar]
- Lamb JA, Moore J, Bailey A, Monaco AP. Autism, recent molecular genetic advances. Hum Mol Genet. 2000;9:861–868. doi: 10.1093/hmg/9.6.861. [DOI] [PubMed] [Google Scholar]
- Laumonnier F, Roger S, Guerin P, Molinari F, M'rad R, Cahard D, Belhadj A, Halayem M, Persico AM, Elia M, Romano V, Holbert S, Andres C, Chaabouni H, Colleaux L, Constant J, Le Guennec JY, Briault S. Association of a functional deficit of the BKCa channel, a synaptic regulator of neuronal excitability, with autism and mental retardation. Am J Psychiatry. 2006;163:1622–1629. doi: 10.1176/ajp.2006.163.9.1622. [DOI] [PubMed] [Google Scholar]
- Lingrel JB, Williams MT, Vorhees CV, Moseley AE. Na,K-ATPase and the role of alpha isoforms in behavior. J Bioenerg Biomembr. 2007;39:385–389. doi: 10.1007/s10863-007-9107-9. [DOI] [PubMed] [Google Scholar]
- Lord C, Cook EH, Leventhal BL, Amaral DG. Autism spectrum disorders. Neuron. 2000;28:355–363. doi: 10.1016/s0896-6273(00)00115-x. [DOI] [PubMed] [Google Scholar]
- MacDonell LE, Skinner FK, Ward PE, Glen AI, Glen AC, Macdonald DJ, Boyle RM, Horrobin DF. Increased levels of cytosolic phospholipase A2 in dyslexics. Prostaglandins Leukot Essent Fatty Acids. 2000;63:37–39. doi: 10.1054/plef.2000.0189. [DOI] [PubMed] [Google Scholar]
- Mallick BN, Adya HV, Faisal M. Norepinephrine-stimulated increase in Na+, K+-ATPase activity in the rat brain is mediated through alpha1A-adrenoceptor possibly by dephosphorylation of the enzyme. J Neurochem. 2000;74:1574–1578. doi: 10.1046/j.1471-4159.2000.0741574.x. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Huguenard JR. Electrophysiology of neuron: An interactive tutorial. Oxford: Oxford University Press; 1994. p. 12. [Google Scholar]
- Moody WJ, Bosma MM. Ion channel development, spontaneous activity, and activity-dependent development in nerve and muscle cells. Physiol Rev. 2005;85:883–941. doi: 10.1152/physrev.00017.2004. [DOI] [PubMed] [Google Scholar]
- Nagy AK, Houser CR, Delgado-Escueta AV. Synaptosomal ATPase activities in temporal cortex and hippocampal formation of humans with focal epilepsy. Brain Res. 1990;529:192–201. doi: 10.1016/0006-8993(90)90827-x. [DOI] [PubMed] [Google Scholar]
- Nori A, Fulceri R, Gamberucci A, Benedetti A, Volpe P. Biochemical and functional heterogeneity of rat cerebrum microsomal membranes in relation to SERCA Ca2+.-ATPases and Ca2+ release channels. Cell Calcium. 1996;19:375–381. doi: 10.1016/s0143-4160(96)90110-4. [DOI] [PubMed] [Google Scholar]
- Palladino MJ, Bower JE, Kreber R, Ganetzky B. Neuronal dysfunction and neurodegeneration in Drosophila Na+/K+ ATPase alpha subunit mutants. J. Neurosci. 2003;23:1276–1286. doi: 10.1523/JNEUROSCI.23-04-01276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrini G, Matteoli M, Banker G, Caplan MJ. Isoforms of the Na, K-ATPase are present in both axons and dendrites of hippocampal neurons in culture. Proc Natl Acad Sci U S A. 1992;89:8414–8418. doi: 10.1073/pnas.89.18.8414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regunathan S, Sundaresan R. Effects of organic and inorganic lead on synaptosomal uptake, release, and receptor binding of glutamate in young rats. J Neurochem. 1985;44:1642–1646. doi: 10.1111/j.1471-4159.1985.tb08807.x. [DOI] [PubMed] [Google Scholar]
- Reime KE, Arida RM, Mara dO, Silva Fernandes MJ. The Na+/K+ ATPase activity is increased in the hippocampus after multiple status epilepticus induced by pilocarpine in developing rats. Brain Res. 2007;1138:203–207. doi: 10.1016/j.brainres.2006.12.068. [DOI] [PubMed] [Google Scholar]
- Renkawek K, Renier WO, de Pont JJ, Vogels OJ, Gabreels FJ. Neonatal status convulsivus, spongiform encephalopathy, and low activity of Na+/K+.-ATPase in the brain. Epilepsia. 1992;33:58–64. doi: 10.1111/j.1528-1157.1992.tb02283.x. [DOI] [PubMed] [Google Scholar]
- Represa A, Ben Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005;28:278–283. doi: 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Rose AM, Valdes R., Jr Understanding the sodium pump and its relevance to disease. Clin Chem. 1994;40:1674–1685. [PubMed] [Google Scholar]
- Spitzer NC, Root CM, Borodinsky LN. Orchestrating neuronal differentiation, patterns of Ca2+ spikes specify transmitter choice. Trends Neurosci. 2004;27:415–421. doi: 10.1016/j.tins.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Decher N, Kumar P, Sachse FB, Beggs AH, Sanguinetti MC, Keating MT. Severe arrhythmia disorder caused by cardiac L-type calcium channel mutations. Proc Natl Acad Sci U S A. 2005;102:8089–8096. doi: 10.1073/pnas.0502506102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. CaV.1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Splawski I, Yoo DS, Stotz SC, Cherry A, Clapham DE, Keating MT. CACNA1H mutations in autism spectrum disorders. J Biol Chem. 2006;281:22085–22091. doi: 10.1074/jbc.M603316200. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Ion regulation in the brain: implications for pathophysiology. Neuroscientist. 2002;8:254–267. doi: 10.1177/1073858402008003011. [DOI] [PubMed] [Google Scholar]
- Takser L, Campagna D, Blot P, Huel G. Could monoamine plasma levels and erythrocyte membrane ATPase activities at birth be predictive for future hand performance? Pediatr Res. 2003;54:358–363. doi: 10.1203/01.PDR.0000077484.55921.A0. [DOI] [PubMed] [Google Scholar]
- Ward PE. Potential diagnostic aids for abnormal fatty acid metabolism in a range of neurodevelopmental disorders. Prostaglandins Leukot Essent Fatty Acids. 2000;63:65–68. doi: 10.1054/plef.2000.0193. [DOI] [PubMed] [Google Scholar]
- Wassink TH, Piven J, Vieland VJ, Huang J, Swiderski RE, Pietila J, Braun T, Beck G, Folstein SE, Haines JL, Sheffield VC. Evidence supporting WNT2 as an autism susceptibility gene. Am J Med Genet. 2001;105:406–413. doi: 10.1002/ajmg.1401. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron. 2006;50:897–909. doi: 10.1016/j.neuron.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Escayg A, Kearney JA, Trudeau M, MacDonald BT, Mori M, Reichert J, Buxbaum JD, Meisler MH. Sodium channels SCN1A, SCN2A and SCN3A in familial autism. Mol Psychiatry. 2003;8:186–194. doi: 10.1038/sj.mp.4001241. [DOI] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci U S A. 2001;98:11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]