Abstract
Human NK cells lyse Ab-coated target cells through the process of Ab-dependent cellular cytotoxicity (ADCC). Improving ADCC responses is desirable because it is thought to be an important antitumor mechanism for some Abs. NK cell inhibitory receptors, such as killer cell Ig-like receptors, engage with MHC class I molecules on self-cells to block NK cell activation. Accordingly, we enhanced ADCC responses by blocking NK cell inhibitory receptors, thus perturbing induction of the self-recognition signal. In a cell line model of anti-lymphoma therapy, the combination of rituximab with an Ab that blocks inhibitory self-recognition yielded increased NK cell-mediated target cell lysis when compared with rituximab alone. To validate this proof-of-concept, we then used a more representative approach in which an individual’s fresh primary NK cells encountered autologous, EBV-transformed B cells. In this system, rituximab and a combination of Abs that block NK cell inhibitory receptors yielded improved NK cell-mediated lysis over rituximab alone. The results show, for the first time, that disruption of inhibitory self-recognition can efficiently promote ADCC in a human model, applying an autologous system in which physiologic checkpoints are in place. This method provides an alternative approach to potentiate the therapeutic benefit of antitumor Abs that mediate ADCC.
The process of Ab-dependent cell-mediated cytotoxicity (ADCC)3 is considered to be a major antitumor mechanism (1–3). This property is dependent upon interactions between the Ab Fc domains and Fc receptors expressed by accessory cells (4). Several families of Fc receptors have been identified, and specific leukocyte populations characteristically express defined Fc receptors (5). In particular, human NK cells express the A (transmembrane) isoform of CD16 (FcγRIIIA) (6). Fc-mediated engagement of CD16A leads to phosphorylation of ITAMs on the receptor-associated FcγRI and TCR-ζ adaptor proteins. The phosphorylated ITAM then serves as a docking site for either the Syk or ZAP70 tyrosine kinases, triggering a downstream cascade of activation events that can lead to NK cell lysis of the Ab-coated target (7). The mechanism of attack is analogous to that of CTLs, involving the release of cytoplasmic granules containing perforin and granzymes (8). Thus, ADCC uses the engagement of an Fc receptor by Abs to direct an Ag-specific attack by NK cells that otherwise lack specificity for a particular Ag. This mechanism of NK cell lysis of Ab-coated cells has been shown to be a major mechanism for direct antitumor effects in some settings, and also for the regulation of innate and adaptive immune responses (1, 9–13). Indeed, several groups have provided a mechanistic basis for the link between NK cells and Ag presentation, showing that NK cells directly interact with and reciprocally activate dendritic cells (14–17).
Rituximab is a chimeric anti-CD20 mAb that was the first un-conjugated therapeutic anticancer mAb to be approved by the FDA, and it is now a component of effective treatment for B cell lymphomas that express CD20 (18–20). Rituximab efficiently initiates ADCC through CD16, thus its function is dependent on the interaction between the Ab Fc domain and the FcγRIIIA (21). Valine (V) or phenylalanine (F) genetic polymorphisms of FcγRIIIA aa 158 alter affinity toward IgG Fc. Low-grade lymphoma patients with the homozygous higher affinity (V/V) polymorphism exhibit improved clinical response rates compared with those possessing the lower affinity (V/F or F/F) polymorphism after treatment with rituximab (1, 22, 23). Strategies to improve mAb therapy are mainly focused on modifying mAb structure to improve the affinity of Abs for FcγRIII and other FcγRs to more efficiently mediate ADCC (24).
NK cells are defined as a unique subset of lymphocytes that do not express rearranged Ag recognition receptors (e.g., TCR or BCR), but rather express numerous Ig-like receptors and C-type lectin receptors that deliver a finely tuned balance of inhibitory and activating signals. These receptors allow the NK cells to discriminate self, healthy cells from transformed or pathogen-infected cells, and regulate their effector function (“the missing self hypothesis”) (25–28). It is now apparent that NK cells recognize and kill target cells as a result of a balance of signaling by both inhibitory and activating NK cell receptors (29, 30). Most inhibitory receptors recognize MHC class I and class I-like molecules, whereas the function and ligand specificity of some activating receptors still remains to be elucidated (11). The two major inhibitory receptors in NK cells are the killer cell Ig-like receptors (KIRs), which recognize HLA-A, HLA-B, or HLA-C and C-type lectin CD94/NKG2A heterodimers, which recognize HLA-E (31–34).
FcγRIIIA engagement, as described, represents only one of numerous mechanisms by which NK cells can be activated. The triggering of natural cytotoxicity receptors (reviewed in Ref. 35), activating CD94/NKG2 receptors, activating forms of KIRs, as well as a lack of self-recognition by inhibitory KIRs or NKG2A/CD94 could provide alternative activation signals (36).
KIRs constitute a polymorphic group of molecules that are encoded by multiple loci on chromosome 19 and vary in certain structural features. Each member interacts with a different group of closely related class I HLA-A, HLA-B, or HLA-C molecules. Members of the KIR family have been shown to be highly polymorphic at the allelic and haplotypic level. To adapt to the rapid evolution and divergence of MHC class I molecules, Khakoo et al. (37) have shown that KIRs have also evolved very rapidly in primates. Significant diversity of KIRs occurs in human populations because a given person inherits a defined repertoire of receptors and these are differentially expressed on the individual NK cells (38). Only the KIR family members with long cytoplasmic domains (KIR2DL and KIR3DL) are associated with inhibitory signaling. The inhibitory signal from the KIR is transduced via the ITIMs located in the cytoplasmic domain of the receptor, whereas the triggering signal from the activating KIRs is transduced via association with DAP12 adaptor proteins bearing an ITAM. Given that inhibitory and activating receptors are being expressed on the same NK cell, a predominance of inhibitory signaling ensures tolerance to self HLA-expressing autologous cells (35). Although the full complement of inhibitory receptors inherited by an individual is not expressed on every NK cell of that individual, most, but not all NK cells have at least one inhibitory receptor for a self MHC class I molecule, which either can be a KIR or CD94/NKG2A receptor (12, 39).
The role for KIR engagement in the process of negative control of NK cell activation was described in several molecular-based studies (40, 41). Also, the loss of MHC class I molecules from the surfaces of virally infected cells or tumor cells was shown to be associated with increased susceptibility to NK cell lysis (42). Basic limitations in the ability to obtain in vivo mechanistic proof-of-concept in rodent models are due to lack of KIR in mice. However, several studies in syngeneic murine models have shown that the manipulation of inhibitory NK cell signaling can prime NK cells to exert in vivo antitumor activity and prevent allograft rejection (43–45). These data also show that this blockade is frequently insufficient to mediate complete tumor eradication because the blockade is not associated with sufficiently potent concomitant activation signals provided by the tumors.
Our approach differs from other approaches for the enhancement of the ADCC properties of antitumor Abs in that we aim to down-regulate the signaling mediated by NK cell inhibitory self-recognition receptors to tip the balance toward ADCC-mediated tumor attack. This approach can thus complement attempts to create more powerful activating Abs that signal through CD16. In this study, we define the potential clinical value of blocking inhibitory self-recognition by human NK cells to promote mAb-mediated ADCC. We show that lowering the threshold for NK cell activation through the interruption of inhibitory self-recognition checkpoints improves NK cell cytotoxicity toward Ab-coated transformed B cells.
Materials and Methods
Cells
NK-92 and NK-92.26.5 cell lines (a subclone generated to express novel genes, notably KIRs, by treatment with 5-aza-2′-deoxycytidine, as described (46) were maintained in α-MEM (Life Technologies) containing 10% FBS (HyClone Laboratories), 10% horse serum, 2 mM L-glutamate, 100 μg/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate (all from Life Technologies), 100 μM 2-ME (Fisher), 2 mM folic acid (Sigma-Aldrich), 20 mM myoinositol (Sigma-Aldrich). Cell lines were supplemented with 2% culture supernatant of J558L cells transfected with the human IL-2 gene provided by A. Lanzavecchia (Institute for Research in Biomedicine, Bellinzona, Switzerland). Cells were passed with fresh IL-2 every 4 days.
Lymphoblast transfectant cell lines 721.221-B*5101 (B51) and 721.221-Cw4 (47) were maintained in RPMI 1640 containing 10% FBS, 2 mM L-glutamate, 100 μg/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, 1 mM HEPES buffer, and 50 μM 2-ME.
SK-OV-3 ovarian carcinoma cells were maintained in DMEM containing 10% FBS, 2 mM L-glutamate, 100 μg/ml penicillin, 100 μg/ml streptomycin, and 50 μM 2-ME. NK cells were purified from donor whole blood using the RossetteSep NK cell enrichment mixture (StemCell Technologies) according to the manufacturer protocol, and under Institutional Review Board approval. EBV transformation of autologous B cells obtained from donors was performed in the Fox Chase Cancer Center Cell Culture Facility using the B95-8 strain of the EBV (48).
Generation of CD16-expressing NK-92 cells
CD16 cDNA (V158 polymorphic variant) provided by Dr. B. Perussia (Thomas Jefferson University, Philadelphia, PA) was ligated into the bi-cistronic retroviral expression vector, pBMN-IRES-EGFP, provided by Dr. G. Nolan (Stanford University, Stanford, CA) using the BamHI/NotI restriction sites (49) to produce recombinant retrovirus for transduction of NK cell lines with stably integrated cDNA. The oligonucleotides 5′-CTT CTG CAG GGG GCT TTT TGG GAG TAA AAA TGT GTC T and 5′-AGA CAC ATT TTT ACT CCC AAA AAG CCC CCT GCA GAA G were used to generate the CD16-F158 variant as well as primers overlapping to the pBMN-IRES-EGFP vector 5′-GCA TCG CAG CTT GGA TAC AC and 5′-GGC GGA ATT TAC GT AGC G and digestion with the BamHI and NotI restriction sites. The integrity of all constructs was confirmed by sequencing in the Fox Chase Cancer Center Automated DNA Sequencing Facility (Applied Biosystems). Transduction of the CD16 construct was as previously described (49). Briefly, the packaging cell line, Phoenix-Amphotropic was transfected with the pBMN-IRES-EGFP vector containing the CD16 gene using Lipofectamine Plus reagent (Life Technologies). Supernatants of these transfected cells grown in serum-free Opti-MEM medium (Life Technologies) for 2 days were cocultured with NK-92 or NK-92.26.5 cell lines for 8 h in the presence of Lipofectamine Plus reagent. Complete α-MEM containing IL-2 was added for 3 days. At that time 5–10% of the infected NK cells efficiently expressing enhanced GFP and CD16 were sorted on a FACSVantage flow cytometer (BD Biosciences) in the Fox Chase Cancer Center Cell Sorting Facility.
Ab agents
KIR and NK cell receptor-directed Abs used in these studies were: DX9 binding KIR3DL1 (mouse IgG1) produced from a hybridoma obtained from Dr. L. Lanier (University of California, San Francisco, CA), HP3E4 binding KIR2DL1, KIR2DS1, and KIR2DS4 (mouse IgM; BD Pharmingen), 143211 binding KIR2DL1 (mouse IgG1; R&D Systems), GL183 binding KIR2DL2, KIR2DL3, and KIR2DS2 (mouse IgG1; Immunotech), 5.133 binding KIR3DL1, KIR3DL2 and KIR2DS4 (mouse IgG1) produced from a hybridoma obtained from Dr. M. Colonna (Washington University, St. Louis, MO), Z199 binding NKG2A (mouse IgG2b; Beckman Coulter), and HP3B1 binding CD94 (mouse IgG2a; Immunotech). B159 binding CD56 (mouse IgG1) produced from a hybridoma obtained from Dr. B. Perussia was used as a control Ab in ADCC assays. CD16 Ab, CLB-Fc obtained from Dr. B. Perussia, and 3G8 (BD Pharmingen) were used to detect CD16 expression of the two polymorphic variants at residue 158 (i.e., valine or phenylalanine). Rituximab (anti-CD20) and trastuzumab (anticerbB2) Abs were used to direct NK cell cytotoxicity (in ADCC assays). CD56 R-PE (NCAM 16.2; BD Biosciences) and CD3-FITC (Leu-4; BD Biosciences) conjugated Abs were used to identify NK cell populations. DX17 is an Ab reactive with all HLA class I (HLA-A, -B, -C, -E, -G, and -F) molecules provided by Dr. L. Lanier.
CD16 polymorphism analysis
Donors’ CD16 polymorphisms were determined in the Fox Chase Cancer Center Cell Cancer Biomarker and Genotyping Facility using Sequence-Specific Primer SSP kits (SSP UniTray; Pel-Freez) from Dynal Biotech.
ADCC assays
ADCC studies were performed as previously described (50). Target cells were labeled with Na251CrO4 (100 μCi/106 targets; PE Life Sciences) for 1 h at 37°C in 500 μl of FBS. The 51Cr-labeled target cells were washed twice and resuspended at the desired concentration in RPMI 1640. Ten thousand cells were added to individual wells of 96-well flat-bottom plates (Costar) containing NK cells (effector cells) at the indicated E:T ratio and/or at indicated concentrations of Abs in supplemented RPMI 1640. Each well contained a total volume of 200 μl, and all assays were performed in triplicate. The plates were centrifuged at 300 × g for 3 min, incubated for 4 h in a 5% (v/v) CO2 incubator at 37°C, and then centrifuged again at 300 × g for 3 min. Supernatant (100 μl) were removed from each well for counting on a Packard Instruments Cobra Quantum, Series 5002 (PE Life Sciences). Cytotoxicity was estimated by measuring the quantity of label released into culture supernatants using the formula: percentage of lysis = 100 × (experimental release (cpm) − spontaneous release (cpm))/(total counts (cpm)/2 − spontaneous release (cpm)), where the experimental release was defined as cpm released by target cells in the presence of effector cells or Ab and the spontaneous release was defined as cpm released by target cells alone.
Flow cytometry
The expression levels of NK cell receptors were determined by flow cytometry with previously described techniques (51). Briefly, 1 × 106 cells were incubated with the relevant Ab for 30 min at 4°C. The cells were washed before the addition of fluorochrome-conjugated goat anti-mouse κ Ab (Southern Biotechnology Associates). The degree of fluorescence was determined using a FACScan flow cytometer (BD Biosciences), and data were analyzed using FlowJo software (Tree Star).
Results
Our goal in this study was to establish NK cell to target cell models to study the impact of blocking self-inhibitory receptor interactions with Abs to increase NK cell-mediated ADCC of Ab-coated lymphoma cells.
KIR and CD16 expression on effector cells
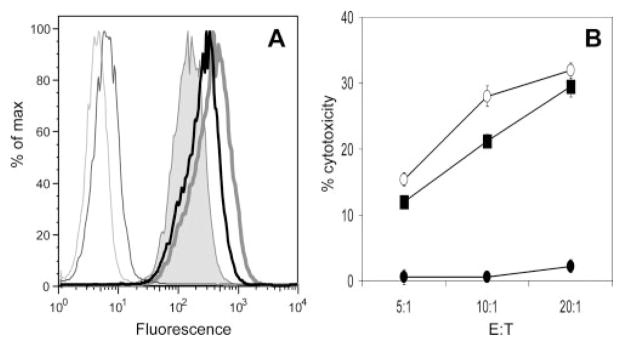
To determine the impact of KIR blockade we first used the NK-92 subclone NK-92.26.5. These cells have been described elsewhere (46) and we confirmed by flow cytometry that KIRs are expressed on the surface (Fig. 1A). Abs that have been reported to functionally block KIR recognition were used for the flow cytometry analysis. These Abs are HP3E4 (52), DX9 (53), GL183 (54), and 5.133 (55). Of these Abs, only DX9 is monospecific for a single inhibitory receptor KIR3DL1. To study the ability of KIR3DL1 on the NK-92.26.5 cells to deliver an inhibitory signal a “reverse ADCC” assay (56, 57), using mouse FcγR-positive P815 target cells, was performed (Fig. 1B). Inhibition of P815 lysis by NK-92.26.5 was observed when using the DX9 Ab, indicating that KIR3DL1 inhibitory signaling was triggered by the engagement with DX9 via Fc receptors on the P815 cells. These results indicated that KIR3DL1 is functional on NK-92.26.5 cells, as it can deliver a dominant negative signal to those cells.
FIGURE 1.
A, KIR expression NK-92.26.5 effector cells. Flow cytometry was used to determine KIR expression on NK-92.26.5 cells. The Abs HP3E4 (thin black histogram) binding KIR2DL1, KIR2DS1 and KIR2DS4; DX9 (gray-shaded histogram) binding KIR3DL1; GL183 (thick gray histogram) binding KIR2DL2, KIR2DL3, and KIR2DS2; and 5.133 (thick black histogram) binding KIR3DL1, KIR3DL2 and KIR2DS4 are used. Thin gray histogram is isotype control stain. B, KIR3DL1 is a functional inhibitory receptor on NK-92.26.5 cell line. NK-92.26.5 cells were incubated with 51Cr-labeled FcγR-expressing P815 cells (redirected cytotoxicity assay) at various E:T ratios with no Ab (○), anti-CD56 (B159) (■), or anti-KIR3DL1 (DX9) (●) Abs at 1 μg/ml each. 51Cr release was measured 4 h later. Results shown are representative of at least three independent experiments.
NK cell-mediated ADCC depends on FcγRIIIA (CD16) interactions (24). However, NK-92.26.5 does not naturally express CD16 (Fig. 2A). Two genetic variants of the CD16 receptor have different affinities for the Fc domain of the Ab and their presence correlates with different clinical response rates when the ADCC-mediating anti-CD20 Ab, rituximab, is used as a therapeutic agent (1). To make NK-92.26.5 cells competent to mediate ADCC, these cells were modified by retroviral transduction of CD16 cDNA to stably express CD16. The two common polymorphic variants of CD16-cDNA were separately transduced into NK-92.26.5 cells. The variants contained the amino acid valine or phenylalanine at position 158 of the protein sequence, with sequence assignment based on numbering of the mature polypeptide (GenBank accession nos. BC017865.1 and NM_000569.6, respectively) (58). To determine CD16 expression levels on the cells, flow cytometry assays were performed using two different anti-CD16 Abs, CLB-Fc and 3G8 (Fig. 2). Using the CLB-Fc Ab we confirmed that CD16 expression was at a comparable level in the two variant cell lines. Fluorescence intensity staining with 3G8 was higher for CD16-V158.NK-92, most likely because of higher affinity binding compared with the CD16-F158.
FIGURE 2.
Flow cytometric analysis of CD16 transduced NK-92.26.5 cells. NK-92.26.5 cells were transduced with CD16 cDNA encoding either F158 or V158 variant as described in Materials and Methods. Cells were stained with anti-CD16 Abs to compare expression. Anti-CD16 Abs are CLB-Fc (gray-filled histogram) and 3G8 (thick black histogram). Reactivity was detected with an R-PE-conjugated anti-mouse κ Ab. Thin black histogram is secondary Ab alone. NK-92.26.5 cells (A), CD16-F158 transduced NK-92.26.5 cells (B), and CD16-V158 transduced NK-92.26.5 cells (C) are shown.
ADCC activity
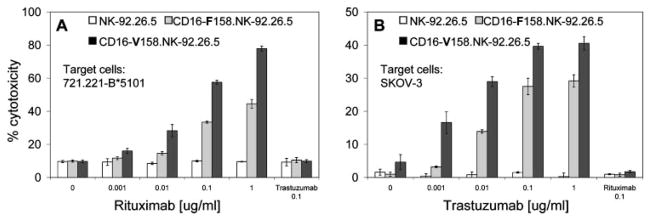
To confirm that the CD16 NK-92.26.5 cells can mediate ADCC, we used CD20+ 721.221-B*5101 (B51) or c-erbB2+ SK-OV-3 target cells, in the presence of serial dilution of the Ag-reactive mAbs rituximab or trastuzumab (59), respectively. ADCC was mediated only toward target cells that expressed the specific Ag (Fig. 3). CD16-V158 NK-92.26.5 cells mediated higher maximal cytotoxicity and were more sensitive to lower concentrations of Ab compared with the corresponding CD16-F158 cells. This result is in accordance with the flow data and previous reports regarding the higher affinity of the CD16-V158 variants to the Fc domain of the Ab (60). ADCC was not mediated by CD16− NK-92.26.5 cells or by Ab that does not bind to the target cells (trastuzumab to B51 or rituximab to SK-OV-3).
FIGURE 3.
ADCC is induced to different levels by CD16-F158 or CD16-V158 polymorphic variants of NK-92.26.5 cell line. NK-92.26.5 (□), CD16-F158.NK-92.26.5 ( ), or CD16-V158.NK-92.26.5 (■) were incubated with 51Cr-labeled 721.221-B*5101 (B51), CD20-expressing B cells (A) or 51Cr-labeled SK-OV-3, c-erbB2-expressing ovarian cancer cells (B) at a 10:1 ratio, in the presence of the indicated concentrations of rituximab or trastuzumab, respectively. 51Cr release was measured 4 h later. Results shown are mean ± SD of one representative experiment of at least three independent experiments.
), or CD16-V158.NK-92.26.5 (■) were incubated with 51Cr-labeled 721.221-B*5101 (B51), CD20-expressing B cells (A) or 51Cr-labeled SK-OV-3, c-erbB2-expressing ovarian cancer cells (B) at a 10:1 ratio, in the presence of the indicated concentrations of rituximab or trastuzumab, respectively. 51Cr release was measured 4 h later. Results shown are mean ± SD of one representative experiment of at least three independent experiments.
Increasing NK cell cytotoxicity by KIR blockade
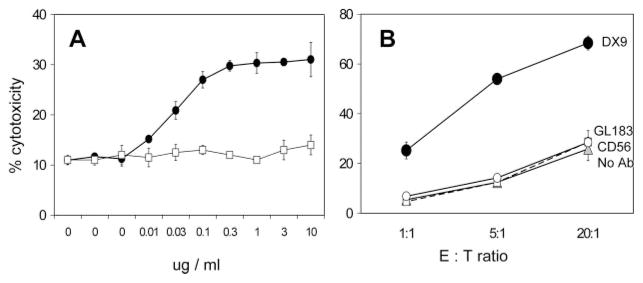
To determine whether cytotoxicity of NK-92.26.5 against relevant target cells could be increased by blocking inhibitory self-recognition we performed cytotoxicity assays using B51 cells as targets. B51 B cells were transfected to solely express HLA-B*5101, containing the HLA-Bw4 epitope (47) for the engagement by KIR3DL1 receptor. In this setting, blockade of the KIR3DL1 interaction by the DX9 Ab promoted cytotoxicity in a concentration-dependent manner (Fig. 4A). Attempted blockade of the other inhibitory receptor KIR2DL2 and KIR2DL3 by GL183 blocking Ab failed to induce cytotoxicity against the B51 target cells (Fig. 4B). Because these mechanisms are CD16-independent, results are shown for the NK-92.26.5 cells but were equivalent to the results with CD16-V158 or CD16-F158 transduced NK-92.26.5 cells (data not shown).
FIGURE 4.
KIR3DL1 blocking Ab induces cytotoxicity against 721.221-B*5101 (B51) cells by NK-92.26.5 cells. A, NK-92.26.5 cells were incubated at an E:T ratio of 5:1 with 51Cr-labeled 721.221-B*5101 (HLA-Bw4 transfected B cells) in the presence of various concentrations of B159 Ab (□) binding CD56 or DX9 Ab (●) binding KIR3DL1. B, Cytotoxicity was measured at different E:T ratios with no Ab (gray-filled triangle), in the presence of B159 (□), DX9 (●), or GL183 binding KIR2DL2, KIR2DL3, and KIR2DS2 (○) all at 1 μg/ml. 51Cr release was measured 4 h later. Results shown are mean ± SD of one representative experiment of at least three independent experiments.
To confirm that in the presence of the intact DX9 Ab CD16.NK-92.26.5 cells are not cross-linked to each other to result in killing of the NK cell population (i.e., by the Fc domain of DX9 that potentially can bind CD16 on NK cells and the binding domain of the DX9 Ab that binds KIR3DL1 on a second NK cell), a cytotoxicity assay was performed with only half of the CD16.NK-92.26.5 cells labeled with 51Cr (target cells), and in the presence of intact DX9 Ab or F(ab′)2 of the DX9 Ab. The results were consistent with no detected cytotoxicity (data not shown).
Blocking inhibitory self-recognition improves ADCC
We then assessed the impact of combining the potentially complementary NK cell-activating mechanisms of CD16 engagement and blockade of inhibitory self-recognition.
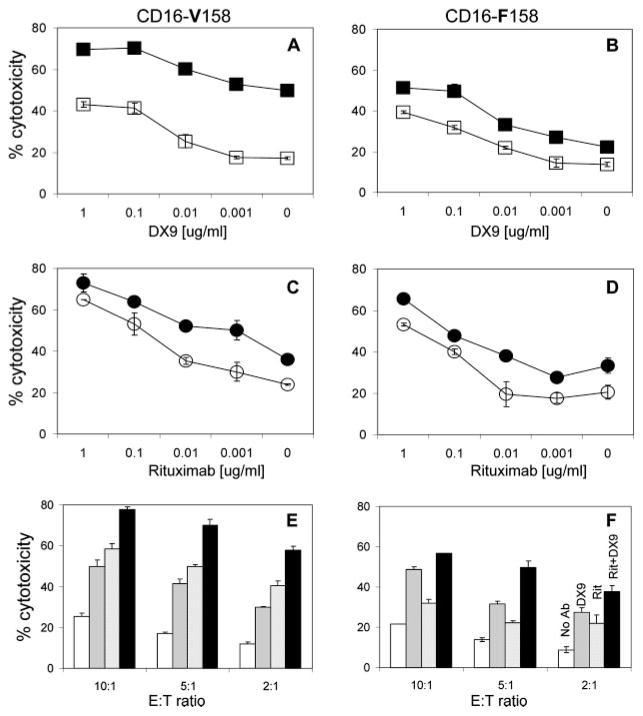
Effector cells (NK-92.26.5 CD16-V158 or CD16-F158) were incubated with B51 targets, at varied E:T ratios (5:1 E:T ratio shown) using different concentrations of DX9 and in the presence or absence of rituximab (10 ng/ml) (Fig. 5, A and B). The results of the cytotoxicity assays for both CD16-V158 and CD16-F158 variants show that at any given DX9 concentration, adding rituximab improved NK cell-mediated target cell lysis. We repeated this experiment with both intact DX9 Ab and F(ab′)2 fragments of DX9 and found consistent results (data not shown). As expected, at this low rituximab concentration, the amplitude of the effect was greater with the higher affinity CD16 variant (V158). We then incubated the effector and target cells in different concentrations of rituximab and in the presence or absence of DX9 (0.1 μg/ml) (Fig. 5, C and D). As shown, combining DX9 with any given rituximab concentration yielded higher NK cell-mediated target cell lysis than with rituximab alone. Cytotoxicity was also compared using the different combinations of blocking inhibitory Abs at varied E:T ratios (Fig. 5, E and F). A combination of rituximab plus DX9 yielded the highest NK cell-mediated target cell lysis, when either CD16-V158.NK-92.26.5 or CD16-F158.NK-92.26.5 was the effector cell (Fig. 5, E and F, respectively). KIR3DL1 blockade alone efficiently promoted B51 cell killing; in this E:T cell combination, KIR3DL1 is the only receptor that contributes to inhibitory self-recognition. In the CD16 high affinity effector cell setting, CD16-V158.NK-92.26.5, rituximab alone mediated a significant level of killing and target cell lysis was only modestly promoted when rituximab and DX9 were used together (Fig. 5E). In the CD16-F158 effector cell setting, the level of cytotoxicity significantly increased when combining DX9 and rituximab compared with rituximab alone (Fig. 5F) and was equivalent to the level of cytotoxicity achieved by the CD16-V158 cells when tested with rituximab alone. This experiment provides a proof-of-concept that blockade of inhibitory self-recognition can be applied to increase the degree of ADCC by human NK cells.
FIGURE 5.
ADCC is augmented by blocking the inhibitory self-recognition receptor KIR3DL1. CD16-V158 (A, C, and E) or CD16-F158 (B, D, and F) variants of the NK-92.26.5 cell line were incubated with 51Cr-labeled 721.221-B*5101 (B51) cells, at a 5:1 E:T ratio and with various concentrations of DX9 (A and B) and with (■) or without (□) 10 ng/ml rituximab, or with various concentrations of rituximab (C and D) and with (●) or without (○) 0.1 μg/ml DX9. E and F, Cells were incubated with no Ab (□), DX9 Ab alone ( ) at 0.1 μg/ml, rituximab (gray-striped box) at 10 ng/ml, or with rituximab plus DX9 Ab (■). 51Cr release was measured 4 h later. Results shown are mean ± SD of one representative experiment of at least three independent experiments.
) at 0.1 μg/ml, rituximab (gray-striped box) at 10 ng/ml, or with rituximab plus DX9 Ab (■). 51Cr release was measured 4 h later. Results shown are mean ± SD of one representative experiment of at least three independent experiments.
A primary NK cell model with autologous target cells
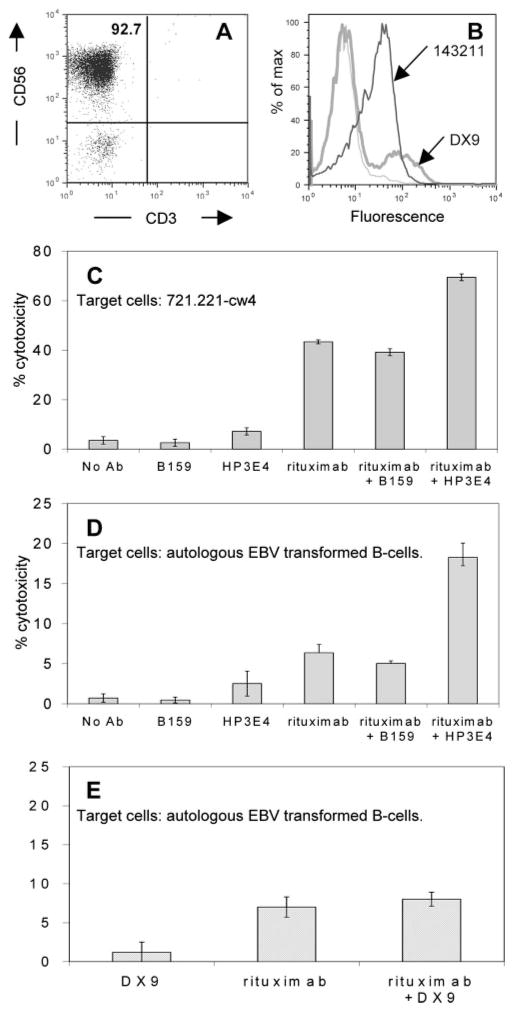
Although our NK-92 cell line model system expressing KIR3DL1 provides a proof-of-concept, endogenous NK cells express multiple inhibitory receptors to sustain MHC class I-mediated self-recognition. The therapeutic potential of KIR blockade in improving antitumor ADCC responses is best modeled using NK cells and autologous targets. In screening a number of volunteer donors, we identified one unique individual who expressed KIR2DL1 on all NK cells. As revealed by HLA phenotyping, this donor also expresses the ligand for KIR2DL1 (HLA-C5, HLA-C6; American Red Cross Laboratories). Flow cytometry analysis performed on purified NK cells showed that an anti-KIR2DL1-specific Ab (143211) stained all of this donor’s NK cells (Fig. 6B). In contrast to a previous publication that described a severe immunodeficiency syndrome in a patient with KIR2DL1 expressed on all NK cells (61) our donor is healthy and exhibits entirely normal immune function. Freshly isolated, highly purified NK cells (92%) (Fig. 6A) were used as effector cells in ADCC experiments against 721.221-Cw4 B cells (expressing HLA-Cw4, a ligand for KIR2DL1) or EBV-transformed B cells from the same individual. Both target B cell populations were positively stained by rituximab (data not shown). As shown in Fig. 6C, 721.221-Cw4 target cells were not effectively killed by purified donor NK cells in the presence of either no Ab, inhibitory receptor blocking Ab HP3E4 (blocking KIR2DL1), or the B159 anti-CD56 Ab. Rituximab or a combination of rituximab plus B159 control Ab achieved 40% target cell lysis, while the combination of HP3E4 KIR2DL1 blocking Ab plus rituximab yielded a further 30% increase in cytotoxicity over rituximab alone. In Fig. 6D, target cells were autologous EBV-transformed B cells. Although the absolute percentage of lysis varied between targets, the pattern was similar. The lower intensity of cytotoxicity against autologous cells is presumably due to additional protective interactions or lack of activating signals (62). Importantly, however, exposure to only the KIR2DL1 blocking Ab (HP3E4) did not increase NK cell cytotoxicity against autologous B cells (Fig. 6D), indicating that blocking KIR alone does not break tolerance. In contrast, the combination of rituximab and the KIR2DL1 blockade significantly improved the ability of the NK cells to lyse these targets. In this donor, ADCC was promoted by blocking the function of an inhibitory self-recognition receptor that is ubiquitously expressed on all NK cells.
FIGURE 6.
ADCC promotion by blocking inhibitory self-recognition receptor on fresh purified NK cells. NK cells were purified from a whole blood sample obtained from a healthy donor as described in Materials and Methods. A, Purity of NK cells was analyzed by flow cytometry staining with CD3-FITC and CD56-PE Abs. B, Flow cytometric analysis was used to determine KIR expression on purified NK cells. Staining with 143211 Ab (thick black histogram) binding KIR2DL1 and DX9 Ab (thick gray histogram) binding KIR3DL1) is shown. Thin gray histogram is secondary Ab alone. NK cells from this donor (expressing KIR2DL1 on all NK cells, but KIR3DL1 on only a subpopulation, see Fig. 5B) were incubated with 51Cr-labeled 721.221-Cw4 cells (C) or with autologous EBV-transformed B cells (D and E) at a 10:1 E:T ratio. Cytotoxicity was calculated using a 4-h 51Cr release assay in the presence of B159 binding CD56 (C and D), HP3E4 binding KIR2DL1, KIR2DS1, and KIR2DS4 (C and D), DX9 binding KIR3DL1 (E), and rituximab (C–E) in different combinations as indicated. All Abs were used at 1 μg/ml concentration. Results shown are mean ± SD of one representative experiment of at least three independent experiments.
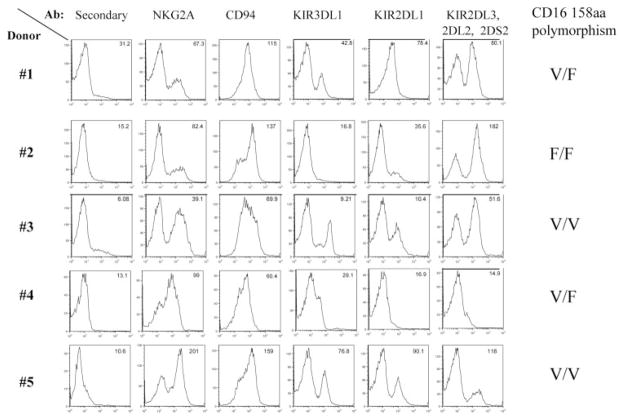
The expression of KIRs and other NK cell receptors was studied in four other donors using specific Abs: Z199 (NKG2A), HP3B1 (CD94), DX9 (KIR3DL1), HP3E4 (KIR2DL1, 2DS1, 2DS4), and GL183, which is an Ab with a broader KIR specificity (KIR2DL3, KIR2DL2, KIR2DS2). Fig. 7 shows the diversity in expression patterns as revealed by flow cytometry analysis. As shown, considerable interdonor variability was observed in the expression of the various NK cell receptors. CD16 polymorphisms were also determined. We then performed ADCC assays using freshly isolated NK cells (effector cells) and autologous EBV-transformed B cells (target cells). Effector and target cells were incubated with NK cell Abs, with or without rituximab. Table I shows the lysis of EBV-transformed B cells when incubated with different Ab combinations. When each of the NK cell receptors Abs was individually used to block inhibitory self-recognition in rituximab-based ADCC assays, we determined that the impact of interference with self-recognition has variable and generally modest effects on ADCC promotion as compared with rituximab alone.
FIGURE 7.
Expression of the diversity of NK cell receptors and CD16 polymorphisms among donors. NK cells were purified from whole blood samples obtained from healthy donors as described in Materials and Methods. Flow cytometry analysis was used to determine NK cell receptor expression on purified NK cells of each individual using available Abs described in Materials and Methods. CD16 polymorphism status was determined by the Fox Chase Cancer Center Biomarker and Genotyping Facility as described in Materials and Methods.
Table I.
Reversal of inhibitory self-recognition (RISER) enhances rituximab-promoted ADCCa
| Percentage of Lysis of EBV-Transformed B Cells by Autologous NK Cells |
|||||
|---|---|---|---|---|---|
| Reagent Donor | 1 | 2 | 3 | 4 | 5 |
| No Ab | 1 ± 0.2 | 0.2 ± 0.1 | 1.2 ± 0.2 | 3.4 ± 1.7 | 3.2 ± 2.2 |
| Pan anti-MHC I Ab (DX17) | 0.4 ± 0.1 | 0.5 ± 0.1 | 10.6 ± 0.1 | 5.9 ± 2.7 | 11.5 ± 0.1 |
| RISER combinationb | 1.3 ± 0.1 | 0 ± 0.1 | 8.2 ± 0.3 | 2.7 ± 0.5 | 13.2 ± 0.8 |
| Rituximab | 6.4 ± 0.1 | 14.9 ± 1.5 | 50.9 ± 3.4 | 25.8 ± 5.8 | 79.1 ± 1.5 |
| Rituximab + CD56 | 4 ± 0.8 | 14.5 ± 0.5 | 43.4 ± 1.4 | 27.5 ± 5.5 | 81.5 ± 1.6 |
| Rituximab + NKG2A (Z199) | 11.6 ± 0.6 | 30.3 ± 0.6 | 58.8 ± 3.9 | 41.6 ± 0.3 | 92.6 ± 1.5 |
| Rituximab + CD94 (HP3B1) | 9.6 ± 0.6 | 20.2 ± 1.3 | 53.3 ± 4.4 | 29.3 ± 0.5 | 88.9 ± 1.8 |
| Rituximab + KIR3DL1 (DX9) | 6 ± 0.85 | 22 ± 0.3 | 55.8 ± 3.0 | 20.1 ± 2.1 | 81.2 ± 4.5 |
| Rituximab + KIR2DL1 (HP3E4) | 21.2 ± 0 | 13.2 ± 1.3 | 51.5 ± 1.1 | 29.6 ± 5.2 | 78.7 ± 7.5 |
| Rituximab + KIR3DL1, 3DL2, 2DS4 (5.133) | 4.4 ± 0.3 | 15.5 ± 0.9 | 47.3 ± 0.8 | 29.2 ± 1.9 | 73.2 ± 0.7 |
| Rituximab + KIR2DL3, 2DL2, 2DS2 (GL183) | 6 ± 0.6 | 13.1 ± 0.6 | 59.8 ± 1.2 | 41.1 ± 0.1 | 81.9 ± 1.8 |
| Rituximab + Pan anti MHC I antibody (DX17) | 24.4 ± 0.8 | 15.3 ± 0.3 | 60.3 ± 2.2 | 25.2 ± 4.4 | 82.2 ± 1.7 |
| Rituximab + RISER combination* | 31.2 ± 0.2 | 43.4 ± 1.4 | 76.3 ± 1.6 | 53.4 ± 3.9 | 96.9 ± 1.4 |
NK cells were freshly purified from healthy donors whole blood samples (effector cells). EBV-transformed B cells were labeled with 51Cr (target cells). Cells were incubated at effector to target cells ratio of 10:1 in the presence of Ab combination as specified. All Abs were used at 1 μg/ml concentration. Cytotoxicity was calculated using a 4 h 51Cr -release assay. Results shown are representative of at least three independent experiments.
RISER combination, a combination of NK cell receptor antibodies (Z199, HP3B1, DX9, HP3E4, 5.133, GL183, 1 μg/ml each).
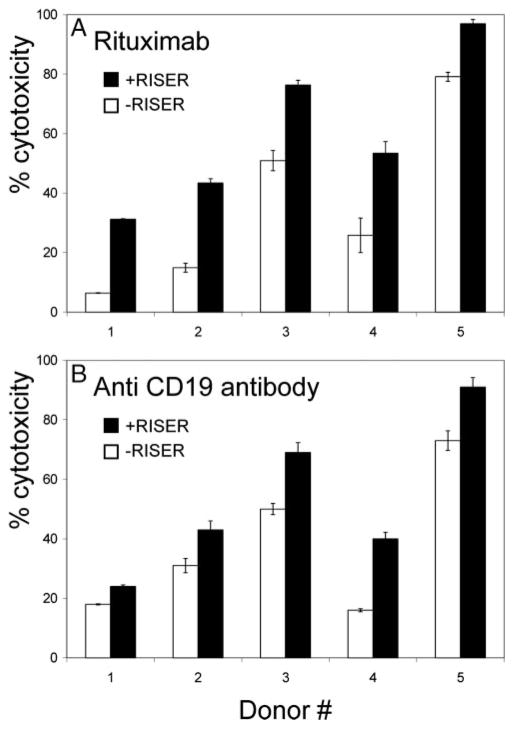
We then tested the hypothesis that interfering with multiple inhibitory receptors would effectively tip the balance in favor of Ab-promoted cytotoxicity. We used a combination of Abs, termed reversal of inhibitory self-recognition (RISER) combination, to block inhibitory self-recognition. The RISER combination consists of the Abs that were used in the experiments described in this study. We found that a mixture of Abs with broad coverage of a large number of NK cell receptors, most of which are inhibitory, had a dramatic influence on the capacity of donor NK cells to lyse autologous B cell targets in the presence of rituximab, but not in its absence (Table I). This effect was equivalent or more pronounced than that obtained when autologous self-recognition was blocked through incubation with DX17, a pan anti-MHC class I Ab (63). As re-demonstrated in Fig. 8A, applying rituximab and the RISER combination of Abs was superior in achieving Ab-mediated NK cell cytotoxicity over rituximab alone. The improvement in cytotoxicity was independent of CD16 polymorphism status.
FIGURE 8.
RISER augments Ab-induced NK cell-mediated ADCC. NK cells from five healthy donors (n = 5) were incubated with 51Cr-labeled autologous EBV-transformed B cells at a 10:1 E:T ratio in the presence of rituximab (A) or mutant anti-CD19 Ab (Xencor) (B) or in the absence (□) or presence (■) of NK cell RISER Abs combination binding NKG2A, CD94, KIR3DL1, KIR2DL1, KIR2DL3, KIR2DL2, and KIR2DS2. Cytotoxicity was calculated using a 4-h 51Cr release assay. All Abs were used at 1 μg/ml concentration. Results shown are mean ± SD of one representative experiment of at least three independent experiments.
We also tested an anti-CD19 Ab (Xencor) in this system (Fig. 8B). The Fc domain of this Ab was manipulated to improve affinity to Fc receptor and thus to more efficiently promote ADCC compared with an Ab of identical specificity and affinity, but with a wild-type IgG1 Fc domain (data not shown). When freshly isolated NK cells and autologous EBV-transformed B cells were incubated with the manipulated CD19 Ab in the absence or presence of the NK cell RISER combination of Abs, NK cell-mediated cytotoxicity was augmented in all donors, suggesting that the blockade of inhibitory self-recognition provides a benefit that is additive to the effects of optimizing Ab structure to promote ADCC.
Discussion
In this study, we show for the first time that ADCC can be promoted by blocking inhibitory self-recognition receptors on human NK cells. This mechanism was shown in an appropriate autologous human system, providing physiologic “checkpoints”. This approach offers a new direction in the development and improvement of new and existing therapeutic Ab treatments of cancer. A broad assortment of NK cell inhibitory and activating receptors create important immune response checkpoints by surveying self-cells that express different ligands (39). These checkpoints are exquisitely regulated in part by the defined inherited repertoire of inhibitory KIRs distributed on different NK cell subpopulations within an individual; this basis provides a mechanism by which NK cells sense changes in self-molecule expression or expression of stress-activating ligands while protecting healthy cells. Based on our results, disrupting interactions that lead to inhibitory self-recognition can efficiently improve ADCC for the elimination of Ab-coated malignant cells.
CD16 is the predominant Fc receptor on NK cells and has been shown to be involved in the capacity of NK cells to mediate ADCC (2). In our cell line model, NK-92.26.5 cells were transduced with one of the two variants of CD16 (determined by the aa 158 polymorphism valine (V) or phenylalanine (F)) (Fig. 2). CD16.NK-92.26.5 effector cells were treated with DX9 to block KIR3DL1 inhibitory receptor and used to mediate ADCC against rituximab-coated 721.221-B*5101 (B51) target cells. Lysis of the target cells was improved when DX9 was used in combination with rituximab, compared with rituximab alone, indicating that our approach can improve ADCC (Fig. 5).
The results show that NK cells that do not express CD16 predictably do not mediate Ab-dependent cytotoxicity. However, CD16-expressing NK cells efficiently mediate ADCC against cells bearing relevant target Ags, CD20+ or c-erbB2+, using rituximab or trastuzumab, respectively (Fig. 3). Moreover, in determining how the impact of CD16 polymorphisms on the degree of response correlates with Fc binding affinity (1), an enhanced cytotoxicity was seen with the V158 variant compared with F158 at any given Ab concentration (Fig. 3).
To validate our hypothesis that RISER will augment Ab-dependent NK cell-mediated cytotoxicity we combined the two complementary NK cell-activating mechanisms of CD16 engagement and blockade of inhibitory self-recognition (Fig. 5). The Ab isotypes of mouse origin that we used in our study show low affinity binding to human CD16 (64). We initially used F(ab′)2 fragments of the DX9 Ab in our cytotoxicity experiments. This process was performed in anticipation of the potential problem of cross-linking two NK cells and a possible competition with the Ab that mediates ADCC. In our CD16.NK-92 cells system, an intact mouse IgG1 Ab (DX9) was used with no such effect, as described in Results. In our model, the blockade of KIR3DL1 on NK-92.26.5 cells was sufficient to significantly activate lysis toward 721.221-B*5101 (B51) target cells (on which the KIR3DL1 cognate ligand HLA-B B*5101 is the only expressed class I MHC molecule). We found that although blocking of KIR3DL1-HLA-Bw4 interaction by the DX9 Ab or mediating ADCC by rituximab were separately effective in triggering NK cell cytotoxicity alone, the combination of these two approaches yielded the most efficient cytotoxicity level. Our results indicate that blocking KIR-HLA interactions could overcome the effects of CD16 Fc receptor low affinity in rituximab-mediated ADCC.
An individual NK cell population contains a diverse repertoire of NK cells, each expressing one of a varied spectrum of inhibitory receptors that recognize class I MHC self-molecules and thereby achieve tolerance. Therefore, it is predictable, that in a biologic setting, the blockade of any single receptor is unlikely to have a substantial overall impact on inhibitory self-recognition. Thus, broad targeting of inhibitory NK cell receptors might be required to effectively block NK cell recognition, allowing a nonrestricted enhanced antitumor response in patients. The NK-92.26.5 studies provided a useful starting point for obtaining proof-of-concept and elucidating key principles that must be fulfilled in order for inhibitory self-recognition to cooperate with CD16 signaling to efficiently promote ADCC. Nonetheless, an autologous setting using primary NK cells and controlled by realistic checkpoints was needed.
A unique donor gave us the opportunity to test how KIR blockade affected NK-mediated lysis in a human autologous in vitro system (Fig. 6). Ab blockage of KIR2DL1, which was expressed on virtually all NK cells from this subject, did not allow cytotoxicity of either 721.221 or autologous target cells that expressed HLA-C ligands for KIR2DL1. However, Ab blockage of KIR2DL1 did augment rituximab-mediated ADCC of these target cells. This property offers potential advantages for clinical translation because cytotoxicity will not be directed to healthy cells, but more specifically toward Ab-coated tumor cells. When we tested DX9 to block KIR3DL1 (expressed on 13% of the donor NK cells) in Fig. 6B, neither basal NK activity nor ADCC were augmented (Fig. 6E), suggesting that the inhibitory receptors on a more substantial proportion of the NK cell population must be blocked to effectively improve NK cell cytotoxicity.
More donors were characterized for varying expression patterns of inhibitory receptors and CD16 polymorphism status (Table I), and these cells were tested for the impact of blocking inhibitory receptors on ADCC. Because the interference with self-recognition through a single inhibitory receptor had variable and generally modest effects on ADCC promotion, we applied a combination of NK cell-binding Abs to interfere with multiple inhibitory receptors. One challenge for this set of experiments was that most anti-KIR Abs bind to polymorphic extracellular domain determinants that are shared among several activating and inhibitory receptors of a given KIR family (e.g., KIR2D). However, we found that a mixture of mAb molecules with broad coverage of a large number of NK cell receptors, most of which are inhibitory, had a dramatic influence on the capacity of donor PBMC or NK cells to lyse autologous B cell targets in the presence of rituximab, but not in its absence (Table I). The results of blocking autologous recognition by pan anti-MHC class I Ab were similar to those obtained when autologous self-inhibition was blocked with the NK cell receptor combination of Abs, suggesting that we successfully achieved maximal inhibition of MHC class I-regulated self-recognition, even though some activating signals were targeted (Table I). Due to the variability among our donors and among NK cell subpopulations within each donor, attempts to identify a critical subset of KIR receptors required to achieve these effects have not yielded consistent results, suggesting that optimal ADCC promotion by interfering with inhibitory self-recognition requires the blocking of multiple inhibitory receptors. A combination of Abs targeting NKG2A/CD94 and multiple KIRs generally yielded the most consistent promotion of cytotoxicity.
Other laboratories have used strategies to modify mAb structure to better interact with the immune system and more efficiently mediate ADCC (65). These approaches have included manipulation of the mAb Fc region. Examples include introducing mutations within the Fc domain of mAb to selectively tune the affinity for FcγRIII and other FcγRs (66) and modifying Fc glycosylation by the Ab-producing cell line (67). Importantly, the improvement in rituximab-mediated ADCC when combined with the RISER combination of Abs (Fig. 8A) was also seen using an anti-CD19 Ab with a mutated Fc domain that mediates enhanced ADCC (CD19 Ab) obtained from Xencor (Fig. 8B). Accordingly, the blockade of inhibitory self-recognition further augments ADCC promoted by optimized CD16 signaling. Thus, if the current generation of high affinity ADCC promoting anti-CD20 Abs possesses improved antitumor activity in lymphoma, additional gains may be anticipated by combining our approach.
Interestingly, in some of our donors, the relatively low in vitro rituximab-promoted cytotoxicity of autologous cells was not consistent with the ubiquitous and rapid systemic B cell depletion that follows rituximab therapy. It must be considered that we have used short-term assay with purified NK cells. However, additional mechanisms such as macrophage- and neutrophil-mediated damage of Ab-coated cells, complement fixation, and signaling perturbation of the B cells probably contributed to rituximab-initiated B cell depletion in vivo. We believe, nonetheless, that more efficient ADCC promotion is still a valuable benefit, particularly because CD16 polymorphism status can dictate clinical response to rituximab therapy in lymphoma. Increased ADCC thus offers the possibility of improving treatment outcomes, particularly in individuals who carry the low response polymorphism forms of CD16 (F/F and V/F). This accomplishment can be furthered by altering target cell sensitivity to effector cell lysis, by modulating the affinity of the Ag-binding domains for the tumor target, and by improving the affinity of the Ab Fc domain for activating cellular Fc receptors (65). Our results suggest that lowering the threshold for NK cell activation through the blockade of inhibitory cellular receptors can complement attempts to create more powerful activating Abs that signal through CD16. This novel approach offers a clear pathway for clinical development of a new way to improve Ab therapy.
Acknowledgments
We thank Christine Quigley and Sharon Howard in the Fox Chase Cancer Center Cell Culture Facility for establishing EBV-transformed cell lines from donor blood samples.
Footnotes
Abbreviations used in this paper: ADCC, Ab-dependent cellular cytotoxicity; KIR, killer cell Ig-like receptor; RISER, reversal of inhibitory self-recognition.
Disclosures
K.S.C. has submitted a patent application for NK-92 cells transduced to express CD16 variants.
This work was supported by Grants CA50633, CA06927, AI050656, AI050656, and CA083859 from the National Institutes of Health, by grants from the Frank Strick Foundation and the Bernard A. and Rebecca S. Bernard Foundation, and by an appropriation from the Commonwealth of Pennsylvania.
References
- 1.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 3.Johnson P, Glennie M. The mechanisms of action of rituximab in the elimination of tumor cells. Semin Oncol. 2003;30:3–8. doi: 10.1053/sonc.2003.50025. [DOI] [PubMed] [Google Scholar]
- 4.Steplewski Z, Lubeck MD, Koprowski H. Human macrophages armed with murine immunoglobulin G2a antibodies to tumors destroy human cancer cells. Science. 1983;221:865–867. doi: 10.1126/science.6879183. [DOI] [PubMed] [Google Scholar]
- 5.Heijnen IA, van de Winkel JG. Human IgG Fc receptors. Int Rev Immunol. 1997;16:29–55. doi: 10.3109/08830189709045702. [DOI] [PubMed] [Google Scholar]
- 6.Sulica A, Morel P, Metes D, Herberman RB. Ig-binding receptors on human NK cells as effector and regulatory surface molecules. Int Rev Immunol. 2001;20:371–414. doi: 10.3109/08830180109054414. [DOI] [PubMed] [Google Scholar]
- 7.Campbell KS, Colonna M. Human natural killer cell receptors and signal transduction. Int Rev Immunol. 2001;20:333–370. doi: 10.3109/08830180109054413. [DOI] [PubMed] [Google Scholar]
- 8.Wallace ME, Smyth MJ. The role of natural killer cells in tumor control-effectors and regulators of adaptive immunity. Springer Semin Immunopathol. 2005;27:49–64. doi: 10.1007/s00281-004-0195-x. [DOI] [PubMed] [Google Scholar]
- 9.Smyth MJ, Thia KY, Cretney E, Kelly JM, Snook MB, Forbes CA, Scalzo AA. Perforin is a major contributor to NK cell control of tumor metastasis. J Immunol. 1999;162:6658–6662. [PubMed] [Google Scholar]
- 10.Kelly JM, Darcy PK, Markby JL, Godfrey DI, Takeda K, Yagita H, Smyth MJ. Induction of tumor-specific T cell memory by NK cell-mediated tumor rejection. Nat Immunol. 2002;3:83–90. doi: 10.1038/ni746. [DOI] [PubMed] [Google Scholar]
- 11.Farag SS, Fehniger TA, Ruggeri L, Velardi A, Caligiuri MA. Natural killer cell receptors: new biology and insights into the graft-versus-leukemia effect. Blood. 2002;100:1935–1947. doi: 10.1182/blood-2002-02-0350. [DOI] [PubMed] [Google Scholar]
- 12.Farag SS, Fehniger TA, Becknell B, Blaser BW, Caligiuri MA. New directions in natural killer cell-based immunotherapy of human cancer. Expert Opin Biol Ther. 2003;3:237–250. doi: 10.1517/14712598.3.2.237. [DOI] [PubMed] [Google Scholar]
- 13.Hallett WH, Murphy WJ. Positive and negative regulation of natural killer cells: therapeutic implications. Semin Cancer Biol. 2006;16:367–382. doi: 10.1016/j.semcancer.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 15.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Münz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiner GJ, Link BK. Monoclonal antibody therapy of B cell lymphoma. Expert Opin Biol Ther. 2004;4:375–385. doi: 10.1517/14712598.4.3.375. [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD. Monoclonal antibody therapy for B-cell malignancies. Semin Oncol. 2006;33:S2–S14. doi: 10.1053/j.seminoncol.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Coiffier B. Standard treatment of advanced-stage diffuse large B-cell lymphoma. Semin Hematol. 2006;43:213–220. doi: 10.1053/j.seminhematol.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Lopes de Menezes DE, Denis-Mize K, Tang Y, Ye H, Kunich JC, Garrett EN, Peng J, Cousens LS, Gelb AB, Heise C, et al. Recombinant interleukin-2 significantly augments activity of rituximab in human tumor xenograft models of B-cell non-Hodgkin lymphoma. J Immunother. 2007;30:64–74. doi: 10.1097/01.cji.0000211315.21116.07. [DOI] [PubMed] [Google Scholar]
- 22.Weng WK, Czerwinski D, Levy R. Humoral immune response and immunoglobulin G Fc receptor genotype are associated with better clinical outcome following idiotype vaccination in follicular lymphoma patients regardless of their response to induction chemotherapy. Blood. 2007;109:951–953. doi: 10.1182/blood-2006-03-013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatjiharissi E, Xu L, Santos DD, Hunter ZR, Ciccarelli BT, Verselis S, Modica M, Cao Y, Manning RJ, Leleu X, et al. Increased natural killer cell expression of CD16, augmented binding and ADCC activity to rituximab among individuals expressing the FcγRIIIa-158 V/V and V/F polymorphism. Blood. 2007;110:2561–2564. doi: 10.1182/blood-2007-01-070656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, Chan C, Chung HS, Eivazi A, Yoder SC, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci USA. 2006;103:4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 26.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–861. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- 27.Long EO. Regulation of immune responses through inhibitory receptors. Annu Rev Immunol. 1999;17:875–904. doi: 10.1146/annurev.immunol.17.1.875. [DOI] [PubMed] [Google Scholar]
- 28.Ruggeri L, Mancusi A, Capanni M, Urbani E, Carotti A, Aloisi T, Stern M, Pende D, Perruccio K, Burchielli E, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110:433–440. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacFarlane AW, IV, Campbell KS. Signal transduction in natural killer cells. Curr Top Microbiol Immunol. 2006;298:23–57. doi: 10.1007/3-540-27743-9_2. [DOI] [PubMed] [Google Scholar]
- 30.Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev. 2006;20:123–137. doi: 10.1016/j.blre.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Borges L, Hsu ML, Fanger N, Kubin M, Cosman D. A family of human lymphoid and myeloid Ig-like receptors, some of which bind to MHC class I molecules. J Immunol. 1997;159:5192–5196. [PubMed] [Google Scholar]
- 32.Lanier LL. Follow the leader: NK cell receptors for classical and non-classical MHC class I. Cell. 1998;92:705–707. doi: 10.1016/s0092-8674(00)81398-7. [DOI] [PubMed] [Google Scholar]
- 33.López-Botet M, Bellón T, Llano M, Navarro F, García P, de Miguel M. Paired inhibitory and triggering NK cell receptors for HLA class I molecules. Hum Immunol. 2000;61:7–17. doi: 10.1016/s0198-8859(99)00161-5. [DOI] [PubMed] [Google Scholar]
- 34.Cooley S, Xiao F, Pitt M, Gleason M, McCullar V, Bergemann T, McQueen KL, Guethlein LA, Parham P, Miller JS. A subpopulation of human peripheral blood NK cells that lacks inhibitory receptors for self MHC is developmentally immature. Blood. 2007;110:578–586. doi: 10.1182/blood-2006-07-036228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bakker AB, Wu J, Phillips JH, Lanier LL. NK cell activation: distinct stimulatory pathways counterbalancing inhibitory signals. Hum Immunol. 2000;61:18–27. doi: 10.1016/s0198-8859(99)00160-3. [DOI] [PubMed] [Google Scholar]
- 36.Valiante NM, Uhrberg M, Shilling HG, Lienert-Weidenbach K, Arnett KL, D’Andrea A, Phillips JH, Lanier LL, Parham P. Functionally and structurally distinct NK cell receptor repertoires in the peripheral blood of two human donors. Immunity. 1997;7:739–751. doi: 10.1016/s1074-7613(00)80393-3. [DOI] [PubMed] [Google Scholar]
- 37.Khakoo SI, Rajalingam R, Shum BP, Weidenbach K, Flodin L, Muir DG, Canavez F, Cooper SL, Valiante NM, Lanier LL, Parham P. Rapid evolution of NK cell receptor systems demonstrated by comparison of chimpanzees and humans. Immunity. 2000;12:687–698. doi: 10.1016/s1074-7613(00)80219-8. [DOI] [PubMed] [Google Scholar]
- 38.Borrego F, Kabat J, Kim DK, Lieto L, Maasho K, Pena J, Solana R, Coligan JE. Structure and function of major histocompatibility complex (MHC) class I specific receptors expressed on human natural killer (NK) cells. Mol Immunol. 2002;38:637–660. doi: 10.1016/s0161-5890(01)00107-9. [DOI] [PubMed] [Google Scholar]
- 39.Miller JS, Cooley S, Parham P, Farag SS, Verneris MR, McQueen KL, Guethlein LA, Trachtenberg EA, Haagenson M, Horowitz MM, et al. Missing KIR-ligands is associated with less relapse and increased graft versus host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109:5058–5061. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blery M, Delon J, Trautmann A, Cambiaggi A, Olcese L, Biassoni R, Moretta L, Chavrier P, Moretta A, Daeron M, Vivier E. Reconstituted killer cell inhibitory receptors for major histocompatibility complex class I molecules control mast cell activation induced via immunoreceptor tyrosine-based activation motifs. J Biol Chem. 1997;272:8989–8996. doi: 10.1074/jbc.272.14.8989. [DOI] [PubMed] [Google Scholar]
- 41.Binstadt BA, Brumbaugh KM, Dick CJ, Scharenberg AM, Williams BL, Colonna M, Lanier LL, Kinet JP, Abraham RT, Leibson PJ. Sequential involvement of Lck and SHP-1 with MHC-recognizing receptors on NK cells inhibits FcR-initiated tyrosine kinase activation. Immunity. 1996;5:629–638. doi: 10.1016/s1074-7613(00)80276-9. [DOI] [PubMed] [Google Scholar]
- 42.Lanier LL. Turning on natural killer cells. J Exp Med. 2000;191:1259–1262. doi: 10.1084/jem.191.8.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koh CY, Blazar BR, George T, Welniak LA, Capitini CM, Raziuddin A, Murphy WJ, Bennett M. Augmentation of antitumor effects by NK cell inhibitory receptor blockade in vitro and in vivo. Blood. 2001;97:3132–3137. doi: 10.1182/blood.v97.10.3132. [DOI] [PubMed] [Google Scholar]
- 44.Koh CY, Ortaldo JR, Blazar BR, Bennett M, Murphy WJ. NK-cell purging of leukemia: superior antitumor effects of NK cells H2 allogeneic to the tumor and augmentation with inhibitory receptor blockade. Blood. 2003;102:4067–4075. doi: 10.1182/blood-2003-04-1367. [DOI] [PubMed] [Google Scholar]
- 45.Raziuddin A, Longo DL, Bennett M, Winkler-Pickett R, Ortaldo JR, Murphy WJ. Increased bone marrow allograft rejection by depletion of NK cells expressing inhibitory Ly49 NK receptors for donor class I antigens. Blood. 2002;100:3026–3033. doi: 10.1182/blood.V100.8.3026. [DOI] [PubMed] [Google Scholar]
- 46.Lutz CT, Kurago ZB. Human leukocyte antigen class I expression on squamous cell carcinoma cells regulates natural killer cell activity. Cancer Res. 1999;59:5793–5799. [PubMed] [Google Scholar]
- 47.Litwin V, Gumperz J, Parham P, Phillips JH, Lanier LL. Specificity of HLA class I antigen recognition by human NK clones: evidence for clonal heterogeneity, protection by self and non-self alleles, and influence of the target cell type. J Exp Med. 1993;178:1321–1336. doi: 10.1084/jem.178.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allday MJ, Crawford DH, Griffin BE. Epstein-Barr virus latent gene expression during the initiation of B cell immortalization. J Gen Virol. 1989;70(Pt 7):1755–1764. doi: 10.1099/0022-1317-70-7-1755. [DOI] [PubMed] [Google Scholar]
- 49.Yusa S, Catina TL, Campbell KS. SHP-1- and phosphotyrosine-independent inhibitory signaling by a killer cell Ig-like receptor cytoplasmic domain in human NK cells. J Immunol. 2002;168:5047–5057. doi: 10.4049/jimmunol.168.10.5047. [DOI] [PubMed] [Google Scholar]
- 50.Weiner LM, Holmes M, Richeson A, Godwin A, Adams GP, Hsieh-Ma ST, Ring DB, Alpaugh RK. Binding and cytotoxicity characteristics of the bispecific murine monoclonal antibody 2B1. J Immunol. 1993;151:2877–2886. [PubMed] [Google Scholar]
- 51.Adams GP, Schier R, McCall AM, Crawford RS, Wolf EJ, Weiner LM, Marks JD. Prolonged in vivo tumour retention of a human diabody targeting the extracellular domain of human HER2/neu. Br J Cancer. 1998;77:1405–1412. doi: 10.1038/bjc.1998.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katz G, Gazit R, Arnon TI, Gonen-Gross T, Tarcic G, Markel G, Gruda R, Achdout H, Drize O, Merims S, Mandelboim O. MHC class I-independent recognition of NK-activating receptor KIR2DS4. J Immunol. 2004;173:1819–1825. doi: 10.4049/jimmunol.173.3.1819. [DOI] [PubMed] [Google Scholar]
- 53.Bakker AB, Phillips JH, Figdor CG, Lanier LL. Killer cell inhibitory receptors for MHC class I molecules regulate lysis of melanoma cells mediated by NK cells, γδT cells, and antigen-specific CTL. J Immunol. 1998;160:5239–5245. [PubMed] [Google Scholar]
- 54.Moretta A, Vitale M, Bottino C, Orengo AM, Morelli L, Augugliaro R, Barbaresi M, Ciccone E, Moretta L. P58 molecules as putative receptors for major histocompatibility complex (MHC) class I molecules in human natural killer (NK) cells. Anti-p58 antibodies reconstitute lysis of MHC class I-protected cells in NK clones displaying different specificities. J Exp Med. 1993;178:597–604. doi: 10.1084/jem.178.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Münz C, Holmes N, King A, Loke YW, Colonna M, Schild H, Rammensee HG. Human histocompatibility leukocyte antigen (HLA)-G molecules inhibit NKAT3 expressing natural killer cells. J Exp Med. 1997;185:385–391. doi: 10.1084/jem.185.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sconocchia G, Titus JA, Mazzoni A, Visintin A, Pericle F, Hicks SW, Malavasi F, Segal DM. CD38 triggers cytotoxic responses in activated human natural killer cells. Blood. 1999;94:3864–3871. [PubMed] [Google Scholar]
- 57.Perussia B, Loza MJ. Assays for antibody-dependent cell-mediated cytotoxicity (ADCC) and reverse ADCC (redirected cytotoxicity) in human natural killer cells. Methods Mol Biol. 2000;121:179–192. doi: 10.1385/1-59259-044-6:179. [DOI] [PubMed] [Google Scholar]
- 58.Leppers-van de Straat FG, van der Pol WL, Jansen MD, Sugita N, Yoshie H, Kobayashi T, van de Winkel JG. A novel PCR-based method for direct Fcγ receptor IIIa (CD16) allotyping. J Immunol Methods. 2000;242:127–132. doi: 10.1016/s0022-1759(00)00240-4. [DOI] [PubMed] [Google Scholar]
- 59.Goldenberg MM. Trastuzumab, a recombinant DNA-derived humanized monoclonal antibody, a novel agent for the treatment of metastatic breast cancer. Clin Ther. 1999;21:309–318. doi: 10.1016/S0149-2918(00)88288-0. [DOI] [PubMed] [Google Scholar]
- 60.Bowles JA, Weiner GJ. CD16 polymorphisms and NK activation induced by monoclonal antibody-coated target cells. J Immunol Methods. 2005;304:88–99. doi: 10.1016/j.jim.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 61.Gazit R, Garty BZ, Monselise Y, Hoffer V, Finkelstein Y, Markel G, Katz G, Hanna J, Achdout H, Gruda R, et al. Expression of KIR2DL1 on the entire NK cell population: a possible novel immunodeficiency syndrome. Blood. 2004;103:1965–1966. doi: 10.1182/blood-2003-11-3796. [DOI] [PubMed] [Google Scholar]
- 62.Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Phillips JH, Chang C, Mattson J, Gumperz JE, Parham P, Lanier LL. CD94 and a novel associated protein (94AP) form a NK cell receptor involved in the recognition of HLA-A, HLA-B, and HLA-C allotypes. Immunity. 1996;5:163–172. doi: 10.1016/s1074-7613(00)80492-6. [DOI] [PubMed] [Google Scholar]
- 64.Hulett MD, Hogarth PM. Molecular basis of Fc receptor function. Adv Immunol. 1994;57:1–127. doi: 10.1016/s0065-2776(08)60671-9. [DOI] [PubMed] [Google Scholar]
- 65.Weiner LM, Carter P. Tunable antibodies. Nat Biotechnol. 2005;23:556–557. doi: 10.1038/nbt0505-556. [DOI] [PubMed] [Google Scholar]
- 66.Hayes RJ, Bentzien J, Ary ML, Hwang MY, Jacinto JM, Vielmetter J, Kundu A, Dahiyat BI. Combining computational and experimental screening for rapid optimization of protein properties. Proc Natl Acad Sci USA. 2002;99:15926–15931. doi: 10.1073/pnas.212627499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Umaña P, Jean-Mairet J, Moudry R, Amstutz H, Bailey JE. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nat Biotechnol. 1999;17:176–180. doi: 10.1038/6179. [DOI] [PubMed] [Google Scholar]