Abstract
The angiotensin-converting enzyme (ACE) gene DD homozygote has been suggested to be a significant risk factor for the progression of diabetic nephropathy. We analyzed clinical parameters and ACE genotype distribution between type 2 diabetic patients at the extremes of renal risk, i.e. an end-stage renal failure (ESRF) group (n = 103, group 1) who were on dialysis therapy due to progression of diabetic nephropathy, and a no progression group (n = 88, group 2) who had maintained normal renal function and normoalbuminuria for more than 15 years. There were no significant differences in age, sex, body mass index, HbA1c level, or lipid profiles between the two groups (p > 0.05). Group 1 had a significantly higher prevalence of hypertension [group 1: 82.5% (85/103) vs. group 2: 50.0% (44/88), p < 0.05] and diabetic retinopathy [group 1: 103/103 (100%) vs. group 2: 28/88 (31.8%), p < 0.05] than group 2. Daily urinary albumin excretion was also higher in group 1 than in group 2 [group 1: 2873 ± 2176 mg/day vs. 12 ± 7 g/day, p < 0.05]. The frequencies of the DD, ID, and II genotypes of the ACE gene in group 1 and group 2 were 26.2%, 47.6%, and 26.2%, and 7.9%, 57.9%, and 34.2%, respectively. The ACE genotype frequencies between the two groups were significantly different according to a chi-square test with Bonferroni's correction (p = 0.004). The presence of the DD genotype increased the risk of ESRF 4.286-fold compared to the II genotype [odds ratio 4.286, 95% CI 1.60-11.42, p = 0.005]. The frequency of the D-allele was higher in both male and female patients in group 1 compared to group 2, but reached statistical significance only in males [male, group 1: 50.8% vs. group 2: 35.0%, p = 0.018, female, group 1: 48.8% vs. group 2: 39.5%, p = 0.231]. This study, although limited by sample size, showed that type 2 diabetic ESRF patients more frequently expressed the DD genotype. These findings may substantiate the previously noted relationship between the ACE DD genotype and the progression of diabetic nephropathy in Korean type 2 diabetic patients.
Keywords: ACE gene polymorphism, end-stage renal failure, type 2 diabetes
INTRODUCTION
Approximately 10 to 40% of diabetic patients develop diabetic nephropathy. The pathogenesis of this drastic complication is not clearly understood, but available data suggest that multiple factors contribute to this complication, such as hemodynamic alterations, metabolic abnormalities, various growth factors, and genetic factors.1 Recent publications suggest that genetic predisposition plays a role in the development of diabetic nephropathy, which clusters within families in both type 1 and type 2 diabetes mellitus.2-5 Furthermore, antihypertensive drugs such as ACE inhibitors or angiotensin II receptor blockers (ARBs) have beneficial effects over other classes of antihypertensive drugs in retarding the development and progression of diabetic nephropathy.6-8 These findings suggest that genes involved in the reninangiotensin system (RAS) are important in the pathogenesis of diabetic nephropathy. Various genes, including the angiotensinogen gene, renin gene, ACE gene, and angiotensin II type 1 receptor gene, are involved in the RAS. The insertion/deletion (I/D) polymorphism of the ACE gene has been the most extensively studied among the candidate genes implicated in the development and progression of diabetic nephropathy, although conflicting results have been reported to date.9-11 Differences in the frequency of genetic polymorphisms of the ACE gene among different ethnic groups have been implicated as an explanation for the observed differences.12 High ACE activity in the DD genotype, compared to the ID and II genotypes, has been demonstrated not only in the plasma but also in several tissues, including the heart and kidneys.13,14 Some studies have demonstrated a more progressive decline in renal function in diabetic patients with the DD genotype.15 We have recently reported the importance of the ACE DD genotype in the progression of diabetic nephropathy on the basis of renal function impairment in Korean patients with type 2 diabetes.16 An excess of DD homozygotes has been noted among Caucasian hemodialysis patients with type 2 diabetes.17 In this context, we conducted this cross-sectional study to reassess the association of the ACE gene polymorphism with ESRF due to diabetic nephropathy by comparing two extreme groups of patients with type 2 diabetes.
MATERIALS AND METHODS
Subjects
Type 2 diabetic patients attending the diabetic and renal clinics at Yongdong Severance Hospital in Korea from 1985 to 2001 were screened. The criteria for recruitment were similar to those described previously.16 The diagnosis of type 2 diabetes was based on clinical characteristics that included: 1) no episodes of ketoacidosis; 2) diagnosis of diabetes after the age of 40 years using American Diabetes Association criteria;18 and 3) treatment by diet alone, or diet combined with oral hypoglycemic agents, or fasting serum C-peptide values greater than 1.0 ng/mL (0.333 nmol/L) in patients treated with insulin. Patients with a diabetes history of more than 15 years, and those who had normal renal function, defined as a serum creatinine level less than 1.2 mg/dL (106 µmol/L), at the time of diagnosis were recruited for the study. A total of 191 patients satisfied the above criteria. The patients were then allocated into two groups. Group 1 (n = 103) included patients who had progressed to end-stage renal failure due to diabetic nephropathy and were on dialysis. Group 2 (n = 88) consisted of patients who had normal renal function (serum creatinine less than 1.2 mg/dL (106 µmol/L), normoalbuminuria, and a duration of diabetes of more than 15 years. The two extreme groups of type 2 diabetic patients were compared based on various clinical parameters and on ACE genotype distributions.
Methods
Measurement of biochemical parameters
After 12 hours of overnight fasting, 5 ml of EDTA-anticoagulated blood samples were drawn from the patients, and the samples were centrifuged within four hours. Buffy coat layers were refrigerated for DNA extraction at -20℃. The BUN, serum creatinine, total cholesterol, triglycerides, and HDL-cholesterol were measured from the separated plasma. Urinary albumin excretion and creatinine clearance were measured by 24-hour urine collection, and urinary albumin excretions were measured by the nephelometric method (Behring Nephelometer 100, Marburg, Germany). Total cholesterol was determined with the cholesterol oxidase method. Triglyceride levels were determined with glycerol 3-phosphate oxidase-peroxidase (without glycerol blanks) on the Synchron CX5 (Beckman Instruments, Brea, CA, USA). LDL cholesterol was calculated by Friedewald and Delong's equation. HbA1c was determined with ion capture.
Determination of ACE genotypes
For the determination of ACE I/D polymorphism, genomic DNA was extracted from peripheral blood leukocytes. A 287-bp I/D polymorphism in intron 16 of the ACE was examined by polymerase chain reaction (PCR). PCR was performed according to the method of Tiret et al.,19 with slight modification. The sequences of the sense and the antisense primers were 5'-CTGGA GAC-CACTCCCATCCTTTCT-3' and 5'-GATGTG GC CATCACAT-TCGTC AGAT-3', respectively. PCR was performed in a final volume of 20 µL that contained 200 ng of genomic DNA, 0.5 µmol of each primer, 0.25 mM dNTP, 1.5 mM/L MgCl2, 50 mM/L KCl, 10 mM/L Tris-HCl (PH 8.4), and 1 U Taq DNA polymerase. Amplification was carried out for 35 cycles with steps of denaturation at 94℃ for 2 min, annealing at 58℃ for 15 sec, and extension at 72℃ for 30 sec. The PCR products were subjected to electrophoresis in 1.5% agarose gels and stained by ethidium bromide for visualization. To avoid mistyping of the ID genotype as DD, we added dimethyl sulfoxide to the PCR reaction mix to enhance the amplification of the I-allele and repeated the genotyping procedure with the DD genotype.
Blood pressure and ophthalmological evaluation
Blood pressures were measured by sphygmomanometer in the supine position after 10 minutes of rest, and the mean of two consecutive blood pressure readings was recorded. Fundoscopic examination and fluoroscein angiography were performed by a retinal specialist.
Statistical analysis
Variables with a normal distribution were tested with Student's t-test. Variables with skewed distributions (e.g., cholesterol, triglyceride, urinary albumin excretion, BUN, and creatinine) were logarithmically transformed and then compared with t-tests. Concomitantly, the non-parametric Mann-Whitney U tests were used to analyze variables with skewed distribution. The ACE genotype distributions in the two groups were analyzed using the Hardy-Weinberg equation, and differences in ACE genotype frequency between the two groups were analyzed using a chi-square test with Bonferroni's correction. The contribution of ACE I/D polymorphism in relation to ESRF was explored with a logistic regression analysis. We calculated the odds ratios of DD and ID, compared to II. For each odds ratio, we calculated a 95% confidence interval and p-value. The level of significance was defined as p<0.05.
RESULTS
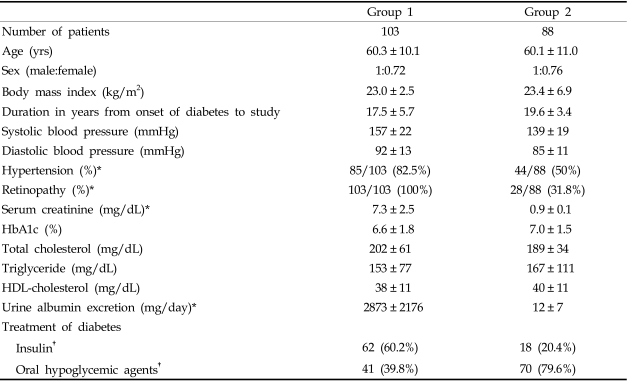
Demographic data are shown in Table 1. All patients were Korean. There were no significant differences in age, sex ratio, BMI, HbA1c levels, or lipid profiles between the two groups (p>0.05). As expected, group 1 (82 patients on hemodialysis and 21 patients on continuous ambulatory peritoneal dialysis) had a significantly higher prevalence of hypertension [group 1: 82.5% (85/103) vs. group 2: 50.0% (44/88), p<0.05] and diabetic retinopathy [group 1: 103/103 (100%) vs. group 2: 28/88 (31.8%), p<0.05] than group 2 (no progression group). Statistically, more patients in group 1 were taking ACE inhibitors or ARBs [group 1: 72.3% (75/103) vs. group 2: 48% (42/88), p<0.05], and daily urinary albumin excretion was also higher in group 1 than in group 2 [group 1: 2873 ± 2176 mg/day vs. 12 ± 7 mg/day, p<0.05]. For glycemic control, group 1 patients were more often treated with insulin, whereas oral hypoglycemic agents were more often used in group 2 patients (Table 1).
Table 1.
Clinical Characteristics of Group 1 (ESRF) and Group 2 (No Progression Group)
Data are mean ± SD.
HDL, high density lipoprotein; ESRF, end stage renal failure.
*p<0.05 by t-test.
†p<0.05 by chi-square test.
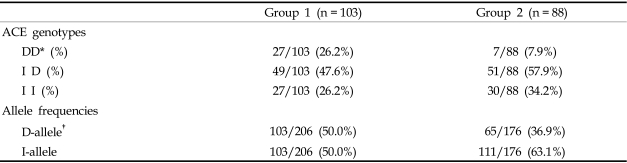
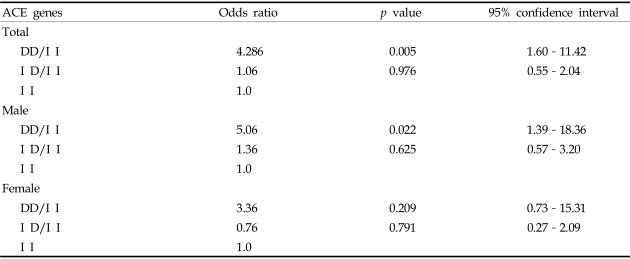
The distribution of ACE genotypes followed the Hardy-Weinberg equilibrium for the whole group (χ2 = 0.7468, p = 0.3875). Both the genotype and allele distributions of the ACE I/D polymorphism of group 1 were significantly different as compared with group 2 (after chi-square test with Bonferroni's correction, p = 0.004; Table 2) or with the background Korean population.20 The DD genotype was over-represented in type 2 diabetic ESRF patients (group 1), as compared with the group 2 patients. The odds ratio for ESRF of the DD genotype compared to the II genotype was 4.286 (95% CI 1.60-11.42, p = 0.005), and the ID genotype compared to the II genotype was 1.06 (95% CI 0.55-2.04, p = 0.976) (Table 3).
Table 2.
ACE Genotype Frequencies between Group 1 (ESRF) and Group 2 (No Progression Group)
D, Deletion; I, Insertion; ESRF, end stage renal failure.
*p = 0.004 by χ2 test with Bonferroni's correction.
†p = 0.01 by t-test.
Table 3.
Odds Ratios of ACE Genes for End Stage Renal Failure in Type 2 Diabetic Patients
D, Deletion; I, Insertion.
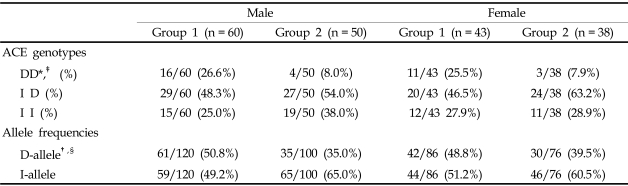
To examine the sex-specific association of the ACE genotype in type 2 diabetic ESRF patients, we compared genotype and allele distributions separately among men and women. Male patients showed a significant difference in ACE genotype frequencies between group 1 and group 2 (chi-square test with Bonferroni's correction, p = 0.032, Table 4). The frequency of D-alleles was also higher in group 1 than in group 2 (group 1: 50.8% vs. group 2: 35.0%, p = 0.018, Table 4). The odds ratio for ESRF in males with the DD genotype compared to II genotype was 5.06 (95% CI 1.39- 18.36, p = 0.022), and was 1.36 (95% CI 0.57-3.20, p = 0.625, Table 3) for the ID compared to the II genotype. However, in females, the ACE genotype frequency between group 1 and group 2 was not significantly different after a chi-square test with Bonferroni's correction (p = 0.096). The frequency of D-alleles showed a tendency to be higher in group 1 than in group 2, but was not statistically significant (group 1: 48.8% vs. group 2: 39.5%, p = 0.231, Table 4). The odds ratio for ESRF in females with the DD genotype compared to II genotype was 3.36 (95% CI 0.73-15.31, p = 0.209), and the odds ratio for the ID genotype compared to the II genotype was 0.76 (95% CI 0.27-2.09, p = 0.791, Table 3).
Table 4.
ACE Genotype Frequencies between Male and Female Patients
D, Deletion; I, Insertion.
*group 1 vs. group 2 in male (p=0.032), ‡group 1 vs. group 2 in female (p=0.096), by χ2 test.
†group 1 vs. group 2 in male (p=0.018), §group 1 vs. group 2 in female (p= 0.231), by t-test.
DISCUSSION
We recently reported that progression of diabetic nephropathy occurs more rapidly in type 2 diabetic patients who are homozygous for the D allele.16 In the present study, we investigated the frequency of I/D polymorphism of the ACE gene in type 2 diabetic ESRF patients by comparing type 2 diabetic patients at the extremes of renal risk. Two major findings, although limited by the small sample size, of the present study are: 1) the DD genotype is over-expressed in type 2 diabetic patients on dialysis (ESRF), and 2) gender-dependent interactions are present between the ACE gene DD genotype and type 2 diabetic ESRF patients.
Many of the previous association studies that focused on the association between ACE gene polymorphism and the development and progression of diabetic nephropathy yielded conflicting results. A meta-analysis performed using data from 18 studies, which included 4,773 diabetic patients, reported that the D-allele was significantly associated with diabetic nephropathy in a dominant manner.21 Two subsequent meta-analyses,22,23 however, found no significant association between D-alleles and diabetic nephropathy among Caucasian patients with type 1 or type 2 diabetes, but did confirm an association between nephropathy and the ID or DD genotype among Japanese type 2 diabetic patients. The results of meta-analyses are frequently confounded by publication bias resulting in the less frequent publication of negative results, as well as differences in study design, patient selection, and case definitions. Typically, these studies defined diabetic renal disease as the presence of micro- or macro-albuminuria, thereby not taking into account that patients with microalbuminuria do not always progress to overt proteinuria and ESRF. Some of the patients might even regress to normoalbuminuria,24 which would lead to perplexing results. Furthermore, the ethnic differences in the frequency of I/D polymorphism may contribute to the discrepant results of the association studies.
The specific issue of an association of the DD genotype with the progression of diabetic nephropathy in an Asian population has been addressed in previous studies.15,16,25 The insertion and deletion allele frequency of the ACE gene in Koreans is very similar to that of Chinese or Japanese subjects.20 The study of Yoshida et al.15 that included 168 Japanese type 2 diabetic patients followed up for 10 years showed that the ACE DD genotype has a high prognostic value for the progressive deterioration of renal function. This study noted a higher frequency of DD homozygotes in Japanese patients with type 2 diabetes who showed evidence of progression. Moreover, their analysis of the time to ESRF indicated that the DD genotype accelerated the progression to chronic renal failure in diabetic nephropathy. Our recent study also reported that DD alleles were associated with an increased risk for the deterioration of renal function in diabetes.16 However, such a relationship has not been shown in the Chinese population. The study of Wong et al.,25 which included substantial pre-dialysis patients with macroalbuminuria in their significant nephropathy group (comparable to group 1 in our present study), reported that ACE I/D or ATG M235T polymorphism does not influence the development and progression of renal disease in type 2 Chinese diabetic patients. Notably, the distribution of ACE I/D polymorphisms was similar between those with or without significant nephropathy, and the prevalence of the DD genotype was lower in the significant diabetic nephropathy group in Wong's study. The authors suggested that genetic factors other than I/D polymorphisms might be involved in the progression of nephropathy. However, Wong's study might have failed to assess the influence of ACE gene polymorphism on the progression of diabetic nephropathy, due to the fact that their case and control selections were not focused on the progression of renal disease.
It has been reported that patients who are homozygous for the D-allele progress more rapidly to ESRF,26 and Caucasian type 2 diabetic patients on dialysis therapy have a greater frequency of the DD genotype.27 The risk of ESRF in Caucasian patients with type 1 diabetes has been shown to be increased two-fold in patients with the DD genotype, as compared to patients with other genotypes. The ACE genotype distribution in the patient group was not in accordance with the Hardy-Weinberg equilibrium, due to a significant over-representation of the DD genotype. This resulted in a significant increase in the D-allele frequency in these cases compared to the controls.28 A recent report has also shown a rapid progression towards ESRF in individuals with the DD genotype, particularly in those who also have the MM genotype for angiotensinogen.29 Yoshida et al.15 reported a higher frequency of DD homozygotes in Japanese patients with type 2 diabetes who showed evidence of progression. In the dialyzed cohort, however, an excess of DD homozygotes was not noted. In the present study, we found a significant difference in the allele distributions between the group of patients on dialysis (group 1) and the group with no progression (group 2). The patients on dialysis showed a high prevalence of the ACE DD genotype and a low prevalence of the ACE II genotype. The risk of ESRF in Korean type 2 diabetic patients was increased 4.286-fold in patients with the ACE DD genotype as compared to the ACE II genotype (p=0.005), a result that closely resembled the data from the previous Japanese study. The risk of ESRF was increased 1.06-fold in the ACE ID genotype compared to the ACE II genotype (p=0.976). Thus, the presence of one D-allele did not increase the risk of ESRF, but the presence of two D-alleles increased the risk of ESRF approximately four-fold, compared to the ACE II genotype. These findings suggest that the D-allele is a genetic risk factor that behaves as a recessive trait, requiring the presence of two alleles to contribute to the progression of ESRF. It is plausible that increased risk for ischemic heart disease30 and the reported adverse effects of the deletion polymorphism of the ACE gene on survival in diabetic patients with ESRF31 may have contributed to decrease prevalence of DD genotype in the Chinese ESRF patients. However, it has previously been proposed that patients who are homozygous for the D-allele progress more rapidly to ESRF. The observation of an accumulation of the DD genotype in the cohort of dialyzed patients would be consistent with the notion that these individuals progress more rapidly to ESRF, and that accumulation outweighs loss secondary to the known increase in mortality.
It has been suggested that association studies determining genotype-phenotype relationships require several criteria to enhance study quality, such as a stringently defined phenotype and a large sample size.12 We attempted to examine a cohort of type 2 diabetic patients at the extremes of renal risk, restricting the study to Korean diabetic patients to eliminate racial- and disease-specific differences on the effects of the ACE genotype.
Gender has been reported to have an impact on the development and progression of renal insufficiency.32 The meta-analysis by Neugarten et al. analyzed the effect of gender on the development and progression of nondiabetic renal diseases, and indicated that men with chronic renal disease of various etiologies show a more rapid decline in renal function with time than do women.33 It has been speculated that direct receptor-mediated effects of sex hormones may determine susceptibility to renal damage.34 The effect of sex on the development and progression of diabetic nephropathy, however, is still controversial. Several prospective studies have suggested that men were more likely to develop albuminuria than women,35,36 but conflicting results indicating that African-American women were more likely to develop diabetic nephropathy as a cause of end-stage renal disease than men,37 were also reported. In the present study, the subgroup analysis of the allele distributions between males and females found gender-dependent interactions between the ACE gene and type 2 diabetic ESRF patients. In males, the ACE genotype frequency between the two groups was significantly different (p=0.032), and the frequency of D-allele was also higher in group 1 than in group 2 [group 1: 50.8% vs. group 2: 35.0%, p=0.018]. However, in females, the ACE genotype frequency between the two groups was not statistically significant (p=0.096), and the frequency of the D-allele showed a higher incidence in group 1 than in group 2, but was not statistically significant [group 1: 48.8% vs. group 2: 39.5%, p=0.231]. Logistic regression analysis also failed to show any significance of the DD genotype for the progression to ESRF in females. These results suggest that the female gender may ameliorate the risk of progression to ESRF in Korean type 2 diabetic patients with the DD genotype. Our observations may have been due to chance, due to the small sample size or to the small size of the female patient group compared to the male group. Furthermore, whether this finding resulted from possible interactions between female sex hormones and the ACE activity of the RAS, which may counteract the negative effects of the DD genotype,34,38 is at present speculative.
In summary, we observed a significant excess of DD homozygotes and possible gender-dependent interactions between the ACE gene in type 2 diabetic ESRF patients. Although our study is limited by sample size, these findings may substantiate the previously observed relationship between the ACE DD genotype and the progression of diabetic nephropathy in Korean type 2 diabetic patients.
References
- 1.Tarnow L. Genetic pattern in diabetic nephropathy. Nephrol Dial Transplant. 1996;11:410–412. [PubMed] [Google Scholar]
- 2.Seaquist ER, Goetz FC, Rich S, Barbosa J. Familial clustering of diabetic kidney disease. Evidence of genetic susceptibility to diabetic nephropathy. N Engl J Med. 1989;320:1161–1165. doi: 10.1056/NEJM198905043201801. [DOI] [PubMed] [Google Scholar]
- 3.Borsch-Johnsen K, Norgaard K, Hommel E, Mathiesen ER, Jensen JS, Deckert T, et al. Is diabetic nephropathy an inherited complication? Kidney Int. 1992;41:719–722. doi: 10.1038/ki.1992.112. [DOI] [PubMed] [Google Scholar]
- 4.Pettitt DJ, Saad MF, Bennett PH, Nelson RG, Knowler WC. Familial predisposition to renal disease in two generations of Pima Indians with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1990;33:438–443. doi: 10.1007/BF00404096. [DOI] [PubMed] [Google Scholar]
- 5.Freedman BI, Tuttle AB, Spray BJ. Familial predisposition to nephropathy in African-Americans with non-insulin dependent diabetes mellitus. Am J Kidney Dis. 1995;25:710–713. doi: 10.1016/0272-6386(95)90546-4. [DOI] [PubMed] [Google Scholar]
- 6.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 7.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 8.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effect of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 9.Taal MW. Angiotensin-converting enzyme gene polymorphisms in renal disease: clinically relevant? Curr Opin Nephrol Hypertens. 2000;9:651–657. doi: 10.1097/00041552-200011000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Marre M, Bernadet P, Gallois Y, Savagner F, Guyene TT, Hallab M, et al. Relationships between angiotensin I converting enzyme gene polymorphism, plasma levels, and diabetic retinal and renal complications. Diabetes. 1994;43:384–388. doi: 10.2337/diab.43.3.384. [DOI] [PubMed] [Google Scholar]
- 11.Kunz R, Bork JP, Fritsche L, Ringel J, Sharma AM. Association between the angiotensin-converting enzyme-insertion/deletion polymorphism and diabetic nephropathy: a methodologic appraisal and systemic review. J Am Soc Nephrol. 1998;9:1653–1663. doi: 10.1681/ASN.V991653. [DOI] [PubMed] [Google Scholar]
- 12.Grimm R, Rettig R. Association studies between the angiotensin-converting enzyme insertion/deletion polymorphism and hypertension: still interesting. J Hypertens. 2002;20:1049–1051. doi: 10.1097/00004872-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danser AH, Schalekamp MA, Bax WA, van den Brink AM, Saxena PR, Riegger GA, et al. Angiotensin-converting enzyme in the human heart. Effect of the deletion/insertion polymorphism. Circulation. 1995;92:1387–1388. doi: 10.1161/01.cir.92.6.1387. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida H, Kuriyama S, Atsumi Y, Tomonari H, Mitarai T, Hamaguchi A, et al. Angiotensin I converting enzyme gene polymorphism in non-insulin dependent diabetes mellitus. Kidney Int. 1996;50:657–664. doi: 10.1038/ki.1996.362. [DOI] [PubMed] [Google Scholar]
- 16.Ha SK, Park HC, Park HS, Kang BS, Lee TH, Hwang HJ, et al. ACE gene polymorphism and progression of diabetic nephropathy in Korean type 2 diabetic patients: effect of ACE gene DD on the progression of diabetic nephropathy. Am J Kidney Dis. 2003;41:943–949. doi: 10.1016/s0272-6386(03)00191-4. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt S, Strojek K, Grzeszczak W, Bergis K, Ritz E. Excess of DD homozygotes in haemodialysed patients with type II diabetes. The Diabetic Nephropathy Study Group. Nephrol Dial Transplant. 1997;12:427–429. doi: 10.1093/ndt/12.3.427. [DOI] [PubMed] [Google Scholar]
- 18.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 19.Tiret L, Rigat B, Visvikis S, Breda C, Corvol P, Cambien F, et al. Evidence, from combined segregation and linkage analysis, that a variant of the angiotensin I-converting enzyme (ACE) gene controls plasma ACE levels. Am J Hum Genet. 1992;51:197–205. [PMC free article] [PubMed] [Google Scholar]
- 20.Ha SK, Seo JK. Insertion/deletion polymorphism in ACE gene as a predictor for progression of diabetic nephropathy. Kidney Int Suppl. 1997;52:S28–S32. [PubMed] [Google Scholar]
- 21.Fujisawa T, Ikegami H, Kawaguchi Y, Hamada Y, Ueda H, Shintani M, et al. Meta-analysis of association of insertion/deletion polymorphism of angiotensin I-converting enzyme gene with diabetic nephropathy and retinopathy. Diabetologia. 1998;41:47–53. doi: 10.1007/s001250050865. [DOI] [PubMed] [Google Scholar]
- 22.Kunz R, Bork JP, Fritsche L, Ringel J, Sharma AM. Association between the angiotensin-converting enzyme insertion/deletion polymorphism and diabetic nephropathy: a methodologic appraisal and systemic review. J Am Soc Nephrol. 1998;9:1653–1663. doi: 10.1681/ASN.V991653. [DOI] [PubMed] [Google Scholar]
- 23.Tarnow L, Gluud C, Parving HH. Diabetic nephropathy and the insertion/deletion polymorphism of the angiotensin-converting enzyme gene. Nephrol Dial Transplant. 1998;13:1125–1130. doi: 10.1093/ndt/13.5.1125. [DOI] [PubMed] [Google Scholar]
- 24.Parving HH, Mauer M, Ritz E. Diabetic nephropathy. In: Brenner BM, editor. Brenner and Rector's The Kidney. 7th ed. Philadelphia, PA: WB Saunders; 2004. pp. 1777–1805. [Google Scholar]
- 25.Wong TY, Chan JC, Poon E, Li PK. Lack of association of angiotensin-converting enzyme (DD/II) and angiotensinogen M235T gene polymorphism with renal function among Chinese patients with type II diabetes. Am J Kidney Dis. 1999;33:1064–1070. doi: 10.1016/S0272-6386(99)70143-5. [DOI] [PubMed] [Google Scholar]
- 26.Bjorck S, Blohme G, Sylven C, Mulec H. Deletion insertion polymorphism of the angiotensin converting enzyme gene and progression of diabetic nephropathy. Nephrol Dial Transplant. 1997;12(Suppl 2):67–70. [PubMed] [Google Scholar]
- 27.Schmidt S, Ritz E. Angiotensin I converting enzyme gene polymorphism and diabetic nephropathy in type II diabetes. Nephrol Dial Transplant. 1997;12:37–41. [PubMed] [Google Scholar]
- 28.Vleming LJ, van der Pijl JW, Lemkes HH, Westendorp RG, Maassen JA, Daha MR, et al. The DD genotype of the ACE gene polymorphism is associated with progression of diabetic nephropathy to end stage renal failure in IDDM. Clin Nephrol. 1999;51:133–140. [PubMed] [Google Scholar]
- 29.Lovati E, Richard R, Frey BM, Frey FJ, Ferrari P. Genetic polymorphisms of the renin-angiotensin-aldosterone system in end-stage renal disease. Kidney Int. 2001;60:46–54. doi: 10.1046/j.1523-1755.2001.00769.x. [DOI] [PubMed] [Google Scholar]
- 30.Tarnow L, Cambien F, Rossing P, Nielsen FS, Hansen BV, Lecerf L, et al. Insertion/deletion polymorphism in the angiotensin-I-converting enzyme gene is associated with coronary heart disease in IDDM patients with diabetic nephropathy. Diabetologia. 1995;38:798–803. doi: 10.1007/s001250050355. [DOI] [PubMed] [Google Scholar]
- 31.Sakka Y, Babazono T, Sato A, Ujihara N, Iwamoto Y. ACE gene polymorphism, left ventricular geometry, and mortality in diabetic patients with end-stage renal disease. Diabetes Res Clin Pract. 2004;64:41–49. doi: 10.1016/j.diabres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Seliger SL, Davis C, Stehman-Breen C. Gender and the progression of renal disease. Curr Opin Nephrol Hypertens. 2001;10:219–225. doi: 10.1097/00041552-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000;11:319–329. doi: 10.1681/ASN.V112319. [DOI] [PubMed] [Google Scholar]
- 34.Silbiger SR, Neugarten J. The impact of gender on the progression of chronic renal disease. Am J Kidney Dis. 1995;25:515–533. doi: 10.1016/0272-6386(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 35.Savage S, Nagel NJ, Estacio RO, Lukken N, Schrier RW. Clinical factors associated with urinary albumin excretion in type II diabetes. Am J Kidney Dis. 1995;25:836–844. doi: 10.1016/0272-6386(95)90565-0. [DOI] [PubMed] [Google Scholar]
- 36.Ravid M, Brosh D, Ravid-Safran D, Levy Z, Rachmani R. Main risk factors for nephropathy in type 2 diabetes mellitus are plasma cholesterol levels, mean blood pressure, and hyperglycemia. Arch Intern Med. 1998;158:998–1004. doi: 10.1001/archinte.158.9.998. [DOI] [PubMed] [Google Scholar]
- 37.Garza R, Medina R, Basu S, Pugh JA. Predictors of the rate of renal function decline in non-insulin-dependent diabetes mellitus. Am J Nephrol. 1997;17:59–67. doi: 10.1159/000169073. [DOI] [PubMed] [Google Scholar]
- 38.Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res. 2002;53:672–677. doi: 10.1016/s0008-6363(01)00479-5. [DOI] [PubMed] [Google Scholar]