Abstract
Pineal hemorrhage only occurs in rare cases, and this known to have several different causes such as germ cell tumors, pineal cysts and vascular malformations, including the cavernous malformations. Pineal cavernous malformations are extremely rare: to date only fifteen cases have been reported worldwide. Although the diagnosis of pineal cavernous malformation is not easy because of the extreme rareness of this condition, the presence of this lesion can be suspected based on its typical radiological findings. Case 1. A 42-year- old man presented with a limitation in his upward gazing. Radiologic examinations showed acute hemorrhage in the pineal region. He underwent ventriculo-peritoneal (VP) shunting but the patient's condition deteriorated after the shunting surgery. We operated and totally removed the tumor and the hemorrhages via an occipital-transtentorial approach. Case 2. A 37-year-old man presented with diplopia. Radiologic examinations showed acute hemorrhage in the third ventricle. He underwent VP shunting, and after this procedure the diplopia was aggravated. We operated and totally removed the tumor and the hemorrhages via an occipital-transtentorial approach. If there is no doubt about the pineal cavernous malformation on MR imaging, we strongly recommend early surgical intervention without performing a risky biopsy. In this study, we describe our experiences for the diagnosis of cavernous malformations in the pineal region with special emphasis on the radiological aspects and the clinical course of this disease.
Keywords: Cavernous malformation, pineal region
INTRODUCTION
Cavernous malformations were previously thought to be a rather rare pathology occurring predominantly in adults. However, new radiological techniques such as MR imaging have demonstrates that these lesions are more frequent than had been previously thought, and especially in the pediatric patients. Intracranial cavernous malformations accounted for 5 to 16% of all cerebral vascular malformations found on an autopsy series.1,2 Cavernous malformations can occur in all parts of the central nervous system, but they most commonly arise in the cerebral hemispheres, and they may especially be found along the midline in regions such as the basal ganglia and brain stem.3,4 However, the pineal region is one of the most infrequent locations for cavernous malformations to be found, with only fifteen cases being reported so far in the literature.5-7 Since very little has been written about the diagnostic features of these tumors when they occur in the pineal region, they are often clinically and radiologically confused with other tumors of the pineal region, particularly the germ cell tumors.8 When radiotherapy is performed without the benefit of biopsy, cavernous malformations as well as other radioresistant neoplasms are sometimes unnecessarily treated. In the study, we describe our experiences for the diagnosis of cavernous malformations in the pineal region with special emphasis being placed on the radiological aspects and the clinical course of this disease.1-4
CASE REPORT
Case 1
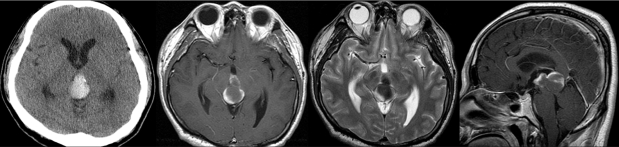
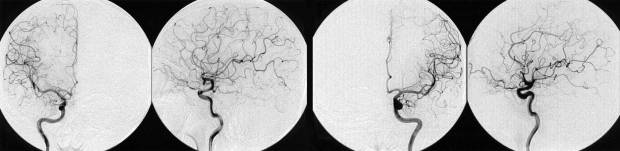
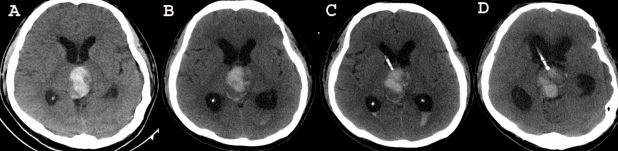
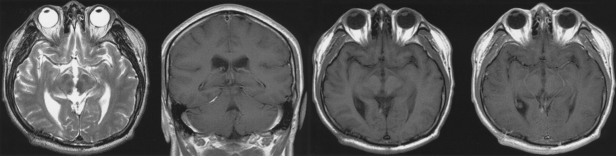
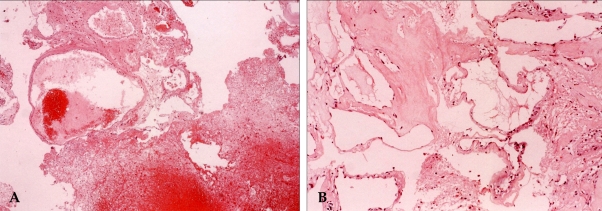
A 42-year-old man was admitted to our hospital because of a suddenly developed diplopia and severe headache. His medical history included no relevant information that would account for the development of these symptoms. The neurological examination on admission revealed a limitation in his upward gazing of both eyeballs without papilledema. His best-corrected visual acuities were 20/30 OD and 20/25 OS. The color vision and pupillary function were both normal. The brain CT and MR imaging showed acute hemorrhage in the pineal region extending to the third ventricle (Fig. 1). The differential diagnosis for this patient included germ cell tumor, pineal parenchymal tumors, glial tumors, and vascular malformations. To rule out the vascular malformations, we performed a 4-vessel angiography procedure, but we could not locate any vascular anomaly (Fig 2). Tumor markers such as β-HCG, α-FP, and PLAP were all within their normal limits. During the period of time that the differential diagnosis was being performed, the patient's condition progressively deteriorated. A follow-up brain CT demonstrated the presence of a newly developed hemorrhage and enlarged ventricles (Fig. 3A and B). We treated him for acute hydrocephalus by means of VP shunting with a Heyer-Schulte highpressure shunt system (Fig. 3C). However, the patient's condition continued to deteriorate and he became stuporous 2 weeks after the shunting. We repeated the brain CT and it revealed the presence of a new, huge hemorrhage in the pineal region that was of a different age than the previous lesion, and the patient's hydrocephalus had worsened (Fig. 3D). We decided to operate via an occipital-transtentorial approach, and we found and totally removed the hemorrhages and also a tumor that was found mixed in the acute hematomas. The tumor was filled with xanthochromic, reddish brown colored old blood. The patient was subsequently cured with some residual limitation of the upward gaze remaining (Fig. 4). The histologic examination of the tumor showed the presence of a cavernous malformation composed of a compact mass of sinusoidal-type vessels that were immediately contiguous with each other and had no intervening parenchyma (Fig. 5 A and B)
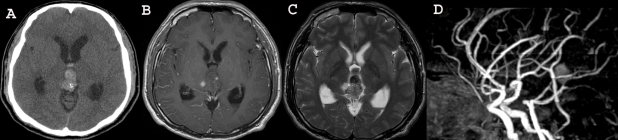
Fig. 1.
Brain CT and MR imaging showing the acute hemorrhage in the pineal region extending to the third ventricle.
Fig. 2.
Conventional 4-angiography revealed no vascular anomaly.
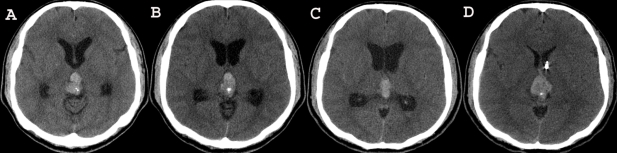
Fig. 3.
Follow-up brain CT demonstrating the repeat hemorrhages in the pineal region and the obstructive hydrocephalus. A follow-up brain CT demonstrates the presence of a newly developed hemorrhage and the enlarged ventricles (A and B). We treated the acute hydrocephalus by shunting (C). Two days after shunting, the patient's condition further deteriorated and he became stuporous. Repeat brain CT reveals the presence of a new, huge hemorrhage of different ages in the pineal region and the aggravated hydrocephalus (D).
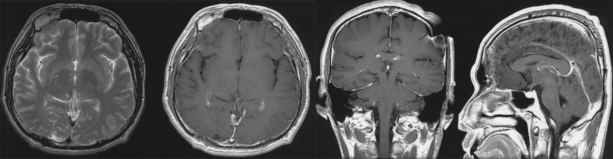
Fig. 4.
Follow-up brain MRI revealed no residual mass, and the postop changes.
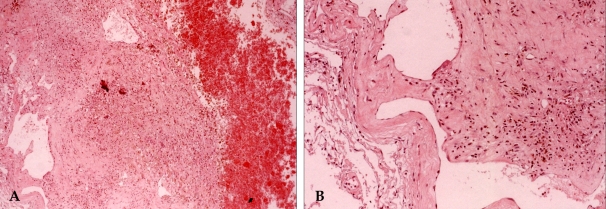
Fig. 5.
Histologic examination showed the presence of a cavernous malformation composed of hematoma (right side), aggregations of hemosiderin laden macrophages (A, ×40) and variable sized hyalinized vascular channels (B, ×100).
Case 2
A 37-year-old man visited a private ophthalmic clinic because of diplopia that was aggravated during his upward gazing. No significant abnormalities were found upon ophthalmologic examination except for a mild conjugation problem occurring during his upward gazing. He was referred to the department of neurosurgery of our institute and he underwent a brain CT. The brain CT showed acute hemorrhage in the third ventricle and there were also spots of calcification (Fig. 6A). This hemorrhage showed mixed signals that indicated different ages of the pathology, but there was no hemosiderin rim on the T1- and T2-weighted MR (Fig. 6B and C). The clinical differential diagnosis was primarily split between intratumoral hemorrhages originating from a pineal germ cell tumor, specifically choriocarcinoma vs. hemorrhage from a pineal cyst. The presence of a cavernous malformation was considered to be a less likely diagnostic possibility. We were easily able to rule out the possibility of vascular malformations because MR angiography revealed no such abnormalities (Fig. 6D). Two weeks later, he experienced an improvement in the diplopia, but the headache that he had been experiencing became progressively more severe. Brain CTs, performed weekly after the first attack of symptoms, revealed a resolving hemorrhage and the progressively enlarging ventricles (Fig. 7A, B, and C). We performed VP shunting using the HAKIM programmable valve without the prechamber Shunt System, and it was adjusted to an opening pressure of 120 mm CSF. Two days after the shunting, he complained of a sudden aggravation of his diplopia. The follow-up brain CT revealed rebleeding from the previous lesion (Fig. 7D). The patient underwent an emergency operation consisting of an occipital-transtentorial approach to the pineal region, and this resulted in the complete excision of a discovered tumor and the removal of the hematoma. The lesion mass was composed of acute and subacute hematomas and it was well encapsulated. These surgical findings corresponded to a cavernous malformation. The mass was easily separated from the surrounding tissues that included the midbrain. Although the mass had some fibrous connection with the internal cerebral vein, it was successfully removed in its entirety without any violation of the deep venous structures. After surgery, his conjugation problem was not completely resolved right away, but he was otherwise in good health (Fig. 8). The pathology of this case was confirmed as being a cavernous malformation (Fig. 9).
Fig. 6.
Brain CT showing the acute hemorrhages in the pineal region extending into the third ventricle and spots of calcification (A). This hemorrhage shows mixed signals with different ages on T1- and T2 weighted MR imaging (B and C). There was no vascular abnormality on the MR angiography (D).
Fig. 7.
Follow-up Brain CT in case 2. Brain CTs, performed weekly after the first attack of symptoms, reveal the resolving hemorrhage and progressively enlarged ventricles (A, B, and C). Rebleeding from the previous lesion is found on the brain CT 2 days after shunting (D).
Fig. 8.
Follow-up brain MRI revealed no residual mass, but there is hemosiderin deposition from old hemorrhagic residue at the posteromedial aspect of the right thalamus and roof of the third ventricle.
Fig. 9.
Histologic examination showed the presence of a cavernous malformation composed of hematoma, variable sized vascular channels (A, ×40) and hyalinized vascular channels without intervening glial tissue (B, ×100).
DISCUSSION
It is very important to perform the differential diagnosis in such cases because pineal hemorrhage is very rare and it may be associated with many different causes. The possible causes of pineal hemorrhages are pineal region tumors,8-14 vascular malformations and pineal cysts,15-17 and tumors are the most common cause of pineal hemorrhage. Various pineal tumors have been reported to cause intra-tumoral hemorrhage including ganglioglioma,18 pineocytoma,19 choriocarcinoma,10,11 meningioma20 and hemangiopericytoma.21 Embryonal carcinoma and metastatic melanoma are also included in the differential diagnosis.9,22 However, there have been no incidences of recurrent massive hemorrhage originating from these tumors except in the case of choriocarcinoma.11 Choriocarcinoma can be discriminated by checking the β-HCG level from the serum or from the cerebrospinal fluid. In our two cases, the tumor markers for the differential diagnosis of germ cell tumors, β-HCG and α-FP, were within the normal limits. Pineal cyst is the next most likely cause of pineal hemorrhage. Pineal cysts are relatively common, and they occurr in as much as 1.4 to 4.3% of the patients that undergo MR imaging. These cysts can become symptomatic, and there have been about 75 verified cases reported in the literature so far.5,15,17,23 However, their hemorrhagic manifestation is extremely rare, with only 8 such cases being found in the literature.5,15-17,23-27 Moreover, there have been no occurrences of repeat intracystic hemorrhage reported. The last possibility is hemorrhage resulting from a vascular malformation in the pineal region, and particularly cavernous malformations; our cases belonged to this category. Cavernous malformations represent one of the four common cerebrovascular malformations. Although the prevalence of cavernous malformations within the general population is unknown, they have been found to affect 0.5% to 0.7% of the general population, and they account for 5% to 16% of all the cerebral vascular malformations found in several autopsy series.1-4 Cavernous malformations most commonly occur in the cerebral hemispheres and especially along the midline in regions such as the basal ganglia and brain stem. However, they have a susceptibility to occur in all parts of the central nervous system, including the pineal region in some rare cases. Only fifteen cases of pineal hemorrhage have so far been reported in the literature. Repeated hemorrhage is a characteristic of these cavernous malformations.5-7 In our present cases, both patients experienced repeated hemorrhage on at least 3 occasions. The MRI characteristics of cavernous malformations are sufficiently unique to allow the diagnosis to be made in the majority of cases.3,7,28 The lesions are best appreciated on the heavily T2-weighted spin-echo images. The classic signature appearance is a mixed signal "variegated" core surrounded by a ring of low-signal intensity. The MRI signal intensity depends on whether there has been recent hemorrhage or thrombosis.3 In the presence of subacute hemorrhage, the central core will be hyperintense. As the hematoma ages, the methemoglobin is rapidly broken down and converted to hemosiderin and ferritin. This breakdown of the blood products initially occurs along the margins of the hematoma, and it results in the gradual deposition of hemosiderin in the cerebral tissue that surrounds the cavernous malformation. MR imaging in our cases of pineal cavernous malformation showed mixed signal intensities with different ages, but there was a lack of any hemosiderin ring. This may have happened on account of the pineal gland being in a special location; it is surrounded by the third ventricle and several cisterns. Since pineal cavernous malformations were difficult to diagnose before the introduction of MRI, some incidences of this disorder were erroneously treated with irradiation before surgical resection was performed.7,29,30 Although endoscopic or stereotactic biopsy for pineal lesions could be useful for the confirmation of the histologic diagnosis,8 such tests run the high risk of repeated, potentially fatal bleeding occurring in the case of pineal cavernous malformations. The issue of surgical intervention in the case of cavernous malformation is controversial. It appears that asymptomatic cavernous malformations do not require prophylactic surgery or radiosurgery. This is because the morbidity and mortality associated with hemorrhage originating from these lesions are relatively low, and the rebleeding rate would also be expected to be low.3,31 However, recent reports have recommended surgical intervention in the case of clinically significant hemorrhage, uncontrollable seizures or progressive neurological deterioration.32-35 Moreover, once hemorrhage had occurred, the rebleeding rate is relatively high.3 As observed in the present cases, cavernous malformations in the pineal region are not only associated with a higher rebleeding rate than cavernous malformations arising in other locations, but these lesions can also induce acute obstructive hydrocephalus. In the present cases, shunting for obstructive hydrocephalus had to be performed. However, shunting before the resection of pineal cavernous malformation would have been very dangerous because it might have caused huge hemorrhages originating from the lesions, and this would have caused a sudden deterioration in the patients' condition. We strongly recommend early surgical intervention if the MR imaging leaves no doubt as to the presence of pineal cavernous malformation. The surgical lesion should be completely resected because a subtotal removal of a cavernous malformation is associated with a high risk of symptomatic recurrence. Moreover, the origin of the lesion may be the tectum, and the clinical course of brain stem cavernous malformation, when treated conservatively, is unfavorable because of progressive brain stem dysfunction.6
In conclusion, pineal hemorrhage does occur in rare cases, and it is known to have several different causes such as germ cell tumors, pineal cysts and vascular malformations, including the cavernous malformations. Although the diagnosis of pineal cavernous malformation is not so easy because of the extreme rareness of this condition, the presence of this lesion can be suspected based on the typical radiological findings. If MR imaging leaves no doubt as to the presence of pineal cavernous malformation, we strongly recommend early surgical intervention without resorting to a risky biopsy.
References
- 1.Al-Shahi R, Bhattacharya JJ, Currie DG, Papanastassiou V, Ritchie V, Roberts RC, et al. Prospective, population-based detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS) Stroke. 2003;34:1163–1169. doi: 10.1161/01.STR.0000069018.90456.C9. [DOI] [PubMed] [Google Scholar]
- 2.Hang Z, Shi Y, Wei Y. A pathological analysis of 180 cases of vascular malformation of brain. Zhonghua Bing Li Xue Za Zhi. 1996;25:135–138. Chinese. [PubMed] [Google Scholar]
- 3.Kim DS, Park YG, Choi JU, Chung SS, Lee KC. An analysis of the natural history of cavernous malformations. Surg Neurol. 1997;48:9–18. doi: 10.1016/s0090-3019(96)00425-9. [DOI] [PubMed] [Google Scholar]
- 4.Mazza C, Scienza R, Beltramello A, Da Pian R. Cerebral cavernous malformations (cavernomas) in the pediatric age-group. Childs Nerv Syst. 1991;7:139–146. doi: 10.1007/BF00776709. [DOI] [PubMed] [Google Scholar]
- 5.McNeely PD, Howes WJ, Mehta V. Pineal apoplexy: is it a facilitator for the development of pineal cysts? Can J Neurol Sci. 2003;30:67–71. doi: 10.1017/s031716710000247x. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi S, Kamagata M, Nakamura M, Nakazato Y, Sasaki T. Pineal apoplexy due to massive hemorrhage associated with cavernous angioma: case report. Surg Neurol. 2001;55:365–371. doi: 10.1016/s0090-3019(01)00461-x. [DOI] [PubMed] [Google Scholar]
- 7.Lombardi D, Scheithauer BW, Villani RM, Giovanelli M, de Tribolet N. Cavernous haemangioma of the pineal region. Acta Neurochir (Wien) 1996;138:678–683. doi: 10.1007/BF01411472. [DOI] [PubMed] [Google Scholar]
- 8.Hung YC, Lee EJ, Wang LC, Chen HH, Yan JJ, Yu CY. Mixed germ cell tumor presenting as intratumoral hemorrhage: report of a case originated from the pineal region. Kaohsiung J Med Sci. 1999;15:498–503. [PubMed] [Google Scholar]
- 9.Maruki C, Takara K, Abe K, Tsunoda A, Ebato M, Ikeya F. Primary pineal embryonal carcinoma occurring in a middle aged man. No Shinkei Geka. 2000;28:909–912. Japanese. [PubMed] [Google Scholar]
- 10.Nogueira K, Liberman B, Pimentel-Filho FR, Goldman J, Silva ME, Vieira JO, et al. hCG-secreting pineal teratoma causing precocious puberty: report of two patients and review of the literature. J Pediatr Endocrinol Metab. 2002;15:1195–1201. doi: 10.1515/jpem.2002.15.8.1195. [DOI] [PubMed] [Google Scholar]
- 11.Sakurada K, Kayama T, Kawakami K, Saino M, Sato S. A successfully operated case of choriocarcinoma with recurrent intratumoral hemorrhage. No Shinkei Geka. 2000;28:67–72. Japanese. [PubMed] [Google Scholar]
- 12.Kida Y, Banno M, Kanzaki M, Kobayashi T, Kageyama N. Pineal choriocarcinoma presenting massive ventricular hemorrhage--a case report. No Shinkei Geka. 1985;13:641–645. Japanese. [PubMed] [Google Scholar]
- 13.Wakai S, Yamakawa K, Manaka S, Takakura K. Spontaneous intracranial hemorrhage caused by brain tumor: its incidence and clinical significance. Neurosurgery. 1982;10:437–444. doi: 10.1227/00006123-198204000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Fujii T, Itakura T, Hayashi S, Komai N, Nakamine H, Saito K. Primary pineal choriocarcinoma with hemorrhage monitored by computerized tomography. Case report. J Neurosurg. 1981;55:484–487. doi: 10.3171/jns.1981.55.3.0484. [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee KK, Banerji D, Sharma R. Pineal cyst presenting with intracystic and subarachnoid haemorrhage: report of a case and review of the literature. Br J Neurosurg. 1999;13:189–192. doi: 10.1080/02688699943970. [DOI] [PubMed] [Google Scholar]
- 16.Mena H, Armonda RA, Ribas JL, Ondra SL, Rushing EJ. Nonneoplastic pineal cysts: a clinicopathologic study of twenty-one cases. Ann Diagn Pathol. 1997;1:11–18. doi: 10.1016/s1092-9134(97)80004-4. [DOI] [PubMed] [Google Scholar]
- 17.Vallee B, Meriot P, Dam Hieu P, Person H. Benign hemorrhagic cyst of the pineal region. Treatment by stereotactic puncture. 4-year follow-up. Neurochirurgie. 1997;43:303–307. French. [PubMed] [Google Scholar]
- 18.Tekkok IH, Ventureyra EC. Spontaneous intracranial hemorrhage of structural origin during the first year of life. Childs Nerv Syst. 1997;13:154–165. doi: 10.1007/s003810050061. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto K, Imaoka T, Tomita S, Ohmoto T. Pineocytoma with massive intratumoral hemorrhage after ventriculoperitoneal shunt- case report. Neurol Med Chir (Tokyo) 1997;37:911–915. doi: 10.2176/nmc.37.911. [DOI] [PubMed] [Google Scholar]
- 20.Ohba S, Kurisu K, Arita K, Sugiyama K, Inoue T, Satoh H, et al. Falcotentorial meningioma accompanied by temporal lobe hematoma. Hiroshima J Med Sci. 2001;50:75–77. [PubMed] [Google Scholar]
- 21.Cervoni L, Artico M, Salvati M, Bristot R, Wierzbicki V, Gagliardi FM. Haemangiopericytoma and meningioma presenting clinically with intracranial haemorrhage: report of three cases and review of the literature. Zentralbl Neurochir. 1993;54:20–23. [PubMed] [Google Scholar]
- 22.Vaquero J, Martinez R, Magallon R, Ramiro J. Intracranial metastases to the pineal region. Report of three cases. J Neurosurg Sci. 1991;35:55–57. [PubMed] [Google Scholar]
- 23.Swaroop GR, Whittle IR. Pineal apoplexy: an occurrence with no diagnostic clinicopathological features. Br J Neurosurg. 1998;12:274–276. doi: 10.1080/02688699845140. [DOI] [PubMed] [Google Scholar]
- 24.Decq P, Le Guerinel C, Sakka L, Roujeau T, Sol J, Palfi S, et al. Endoscopic surgery of third ventricle lesions. Neurochirurgie. 2000;46:286–294. [PubMed] [Google Scholar]
- 25.Koenigsberg RA, Faro S, Marino R, Turz A, Goldman W. Imaging of pineal apoplexy. Clin Imaging. 1996;20:91–94. doi: 10.1016/0899-7071(94)00079-4. [DOI] [PubMed] [Google Scholar]
- 26.Osborn RE, Deen HG, Kerber CW, Glass RF. A case of hemorrhagic pineal cyst: MR/CT correlation. Neuroradiology. 1989;31:187–189. doi: 10.1007/BF00698853. [DOI] [PubMed] [Google Scholar]
- 27.Richardson JK, Hirsch CS. Sudden, unexpected death due to "pineal apoplexy". Am J Forensic Med Pathol. 1986;7:64–68. doi: 10.1097/00000433-198603000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Merhemic Z, Kadenic Z, Sulejmanpasic G. Magnetic resonance and magnetic resonance angiography in the diagnosis of vascular anomalies in childhood. Med Arh. 2000;58:114–116. Bosnian. [PubMed] [Google Scholar]
- 29.Vishteh AG, Nadkarni T, Spetzler RF. Cavernous malformation of the pineal region: short report and review of the literature. Br J Neurosurg. 2000;14:147–151. doi: 10.1080/02688690050004624. [DOI] [PubMed] [Google Scholar]
- 30.Slavin KV, Dujovny M, McDonald LW, Camras LR, Ausman JI. Pineal region: rare location of a cavernous haemangioma. Neurol Res. 1994;16:133–136. doi: 10.1080/01616412.1994.11740211. [DOI] [PubMed] [Google Scholar]
- 31.Porter PJ, Willinsky RA, Harper W, Wallace MC. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg. 1997;87:190–197. doi: 10.3171/jns.1997.87.2.0190. [DOI] [PubMed] [Google Scholar]
- 32.Chang HS, Hongo K, Nakagawa H, Tsuge T. Surgical decision-making on cerebral cavernous malformations. J Clin Neurosci. 2001;8:416–420. doi: 10.1054/jocn.2000.0857. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz-Sandoval JL, Cantu C, Barinagarrementeria F. Intracerebral hemorrhage in young people: analysis of risk factors, location, causes, and prognosis. Stroke. 1999;30:537–541. doi: 10.1161/01.str.30.3.537. [DOI] [PubMed] [Google Scholar]
- 34.Amin-Hanjani S, Ogilvy CS, Ojemann RG, Crowell RM. Risks of surgical management for cavernous malformations of the nervous system. Neurosurgery. 1998;42:1220–1228. doi: 10.1097/00006123-199806000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Braun V, Antoniadis G, Rath S, Richter HP. Cavernoma. Indications for surgical removal and outcome. Nervenarzt. 1996;67:301–305. German. [PubMed] [Google Scholar]