Abstract
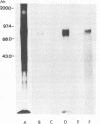
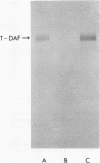
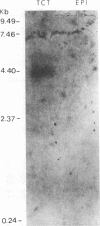
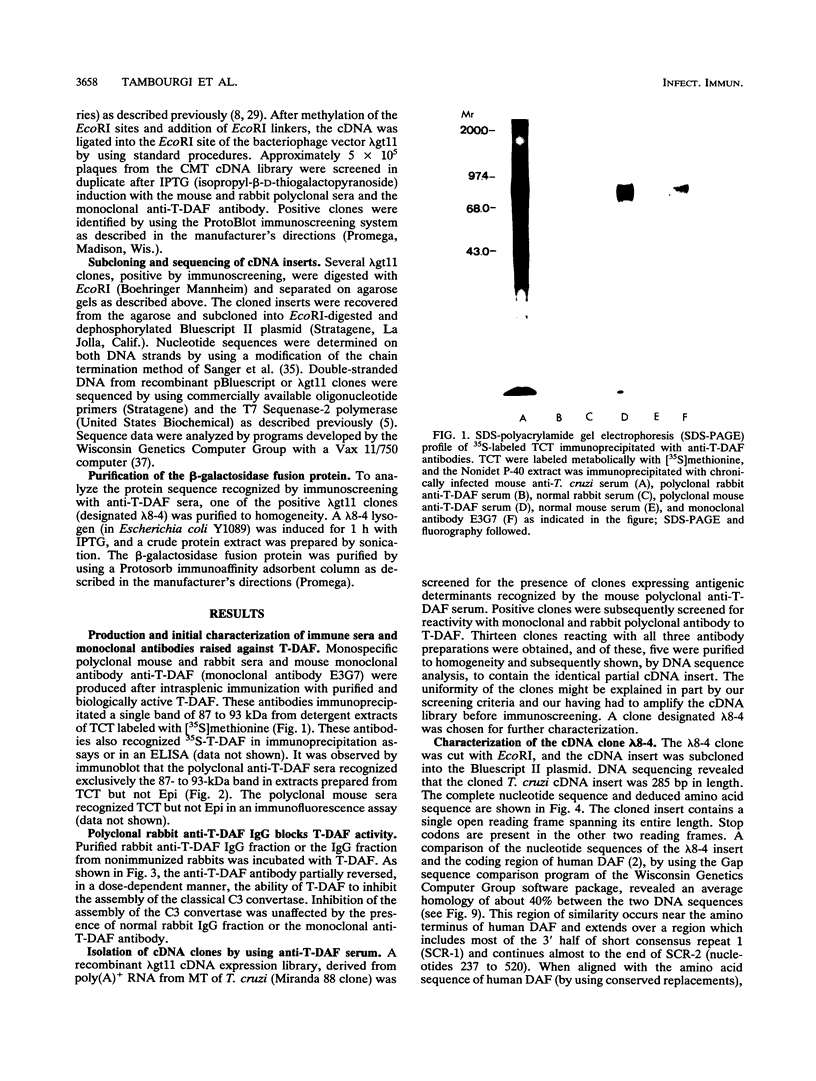
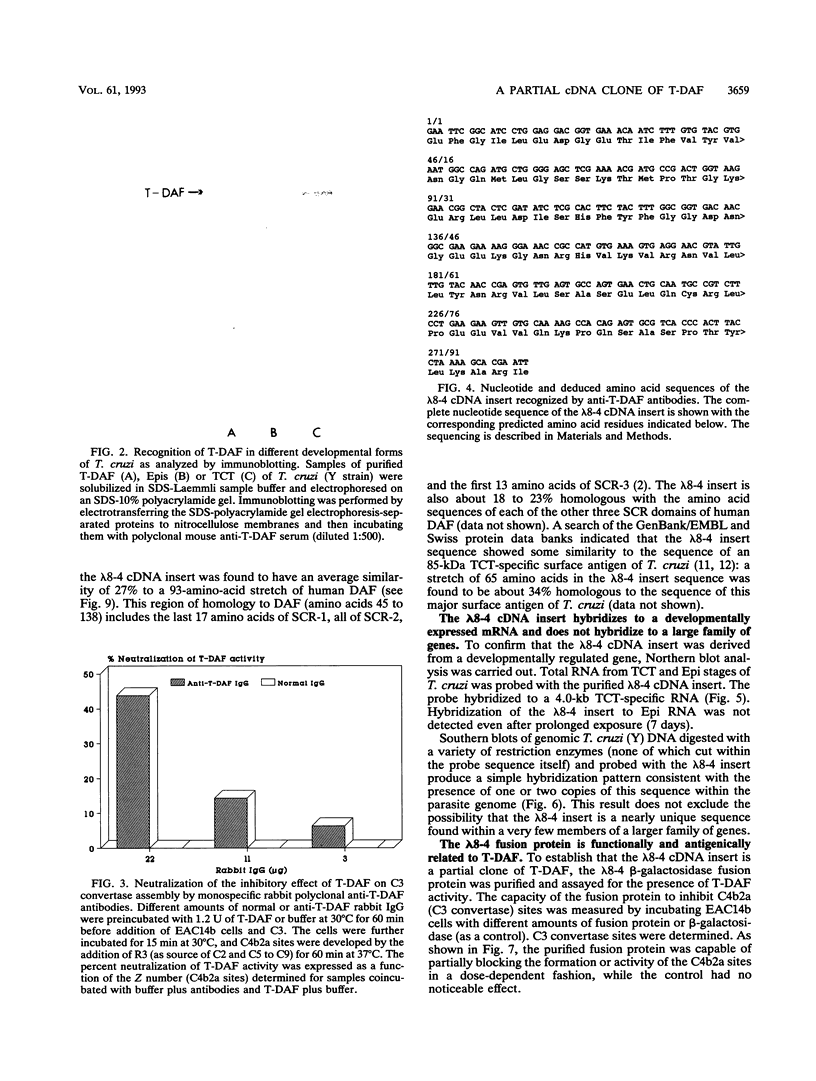
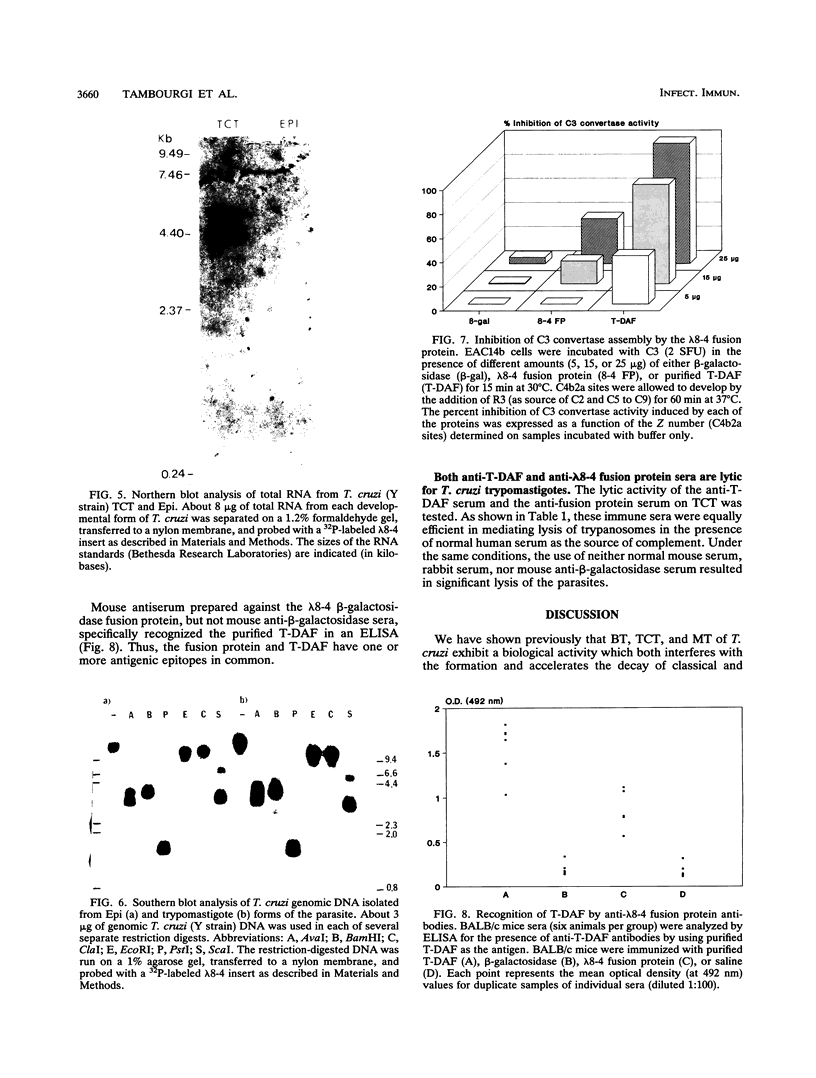
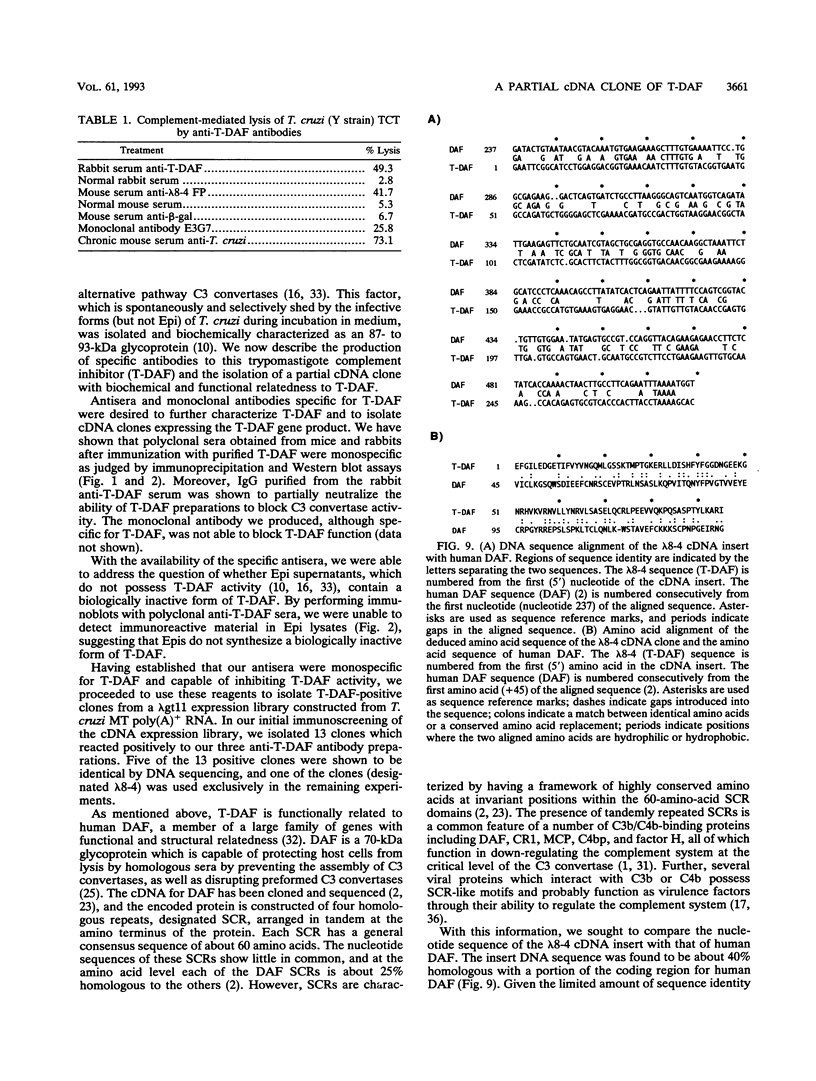
Resistance to complement-mediated lysis in Trypanosoma cruzi is due to the expression of complement-regulatory factors by the virulent developmental forms of this protozoan parasite. An 87- to 93-kDa molecule, which we have termed T-DAF (trypomastigote decay-accelerating factor), is present on the surface of the parasite and inhibits complement activation in a manner functionally similar to the mammalian complement regulatory component, decay-accelerating factor. In this report, we characterized monospecific polyclonal and monoclonal antibodies which were obtained from mice and rabbits immunized with fast protein liquid chromatography-purified T-DAF. These polyclonal antibodies were shown to inhibit T-DAF activity and were capable of inducing lysis of the parasites. Both the polyclonal and monoclonal antibodies were used to screen a cDNA expression library prepared from T. cruzi trypomastigote mRNA. From this library, we obtained a partial lambda gt11 cDNA clone which showed genetic and functional similarity to the human C3 convertase inhibitor DAF (A. Nicholson-Weller, J. Burge, D. T. Fearon, P. F. Weller, and K. F. Austen, J. Immunol. 129:184-189, 1982).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell R. D., Law S. K., Reid K. B., Sim R. B. Structure, organization, and regulation of the complement genes. Annu Rev Immunol. 1988;6:161–195. doi: 10.1146/annurev.iy.06.040188.001113. [DOI] [PubMed] [Google Scholar]
- Caras I. W., Davitz M. A., Rhee L., Weddell G., Martin D. W., Jr, Nussenzweig V. Cloning of decay-accelerating factor suggests novel use of splicing to generate two proteins. Nature. 1987 Feb 5;325(6104):545–549. doi: 10.1038/325545a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Qasba P. K. Alkaline transfer of DNA to plastic membrane. Biochem Biophys Res Commun. 1984 Jul 18;122(1):340–344. doi: 10.1016/0006-291x(84)90480-7. [DOI] [PubMed] [Google Scholar]
- DALMASSO A. P., MUELLER-EBERHARD H. J. INTERACTION OF AUTOLOGOUS COMPLEMENT WITH RED CELLS IN THE ABSENCE OF ANTIBODY. Proc Soc Exp Biol Med. 1964 Dec;117:643–650. doi: 10.3181/00379727-117-29658. [DOI] [PubMed] [Google Scholar]
- Del Sal G., Manfioletti G., Schneider C. The CTAB-DNA precipitation method: a common mini-scale preparation of template DNA from phagemids, phages or plasmids suitable for sequencing. Biotechniques. 1989 May;7(5):514–520. [PubMed] [Google Scholar]
- Evans R., Kamdar S. J. Stability of RNA isolated from macrophages depends on the removal of an RNA-degrading activity early in the extraction procedure. Biotechniques. 1990 Apr;8(4):357–360. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hung S. L., Srinivasan S., Friedman H. M., Eisenberg R. J., Cohen G. H. Structural basis of C3b binding by glycoprotein C of herpes simplex virus. J Virol. 1992 Jul;66(7):4013–4027. doi: 10.1128/jvi.66.7.4013-4027.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A., daSilva W. D., Rimoldi M. T., Hammer C. H., Sher A., Kipnis T. L. Biochemical characterization of a factor produced by trypomastigotes of Trypanosoma cruzi that accelerates the decay of complement C3 convertases. J Biol Chem. 1988 Aug 15;263(23):11327–11335. [PubMed] [Google Scholar]
- Kahn S., Colbert T. G., Wallace J. C., Hoagland N. A., Eisen H. The major 85-kDa surface antigen of the mammalian-stage forms of Trypanosoma cruzi is a family of sialidases. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4481–4485. doi: 10.1073/pnas.88.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn S., Van Voorhis W. C., Eisen H. The major 85-kD surface antigen of the mammalian form of Trypanosoma cruzi is encoded by a large heterogeneous family of simultaneously expressed genes. J Exp Med. 1990 Aug 1;172(2):589–597. doi: 10.1084/jem.172.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierszenbaum F., Lima M. F. Susceptibility of insect-borne, metacyclic forms of Trypanosoma cruzi to antibody-mediated mechanisms of destruction. Am J Trop Med Hyg. 1983 Nov;32(6):1236–1241. doi: 10.4269/ajtmh.1983.32.1236. [DOI] [PubMed] [Google Scholar]
- Kipnis T. L., David J. R., Alper C. A., Sher A., da Silva W. D. Enzymatic treatment transforms trypomastigotes of Trypanosoma cruzi into activators of alternative complement pathway and potentiates their uptake by macrophages. Proc Natl Acad Sci U S A. 1981 Jan;78(1):602–605. doi: 10.1073/pnas.78.1.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis T. L., Krettli A. U., Dias da Silva W. Transformation of trypomastigote forms of Trypanosoma cruzi into activators of alternative complement pathway by immune IgG fragments. Scand J Immunol. 1985 Aug;22(2):217–226. doi: 10.1111/j.1365-3083.1985.tb01874.x. [DOI] [PubMed] [Google Scholar]
- Kipnis T. L., Tambourgi D. V., Sucupira M., Dias-da-Silva W. Effect of Trypanosoma cruzi membrane components on the formation of the classical pathway C3 convertase. Braz J Med Biol Res. 1986;19(2):271–278. [PubMed] [Google Scholar]
- Kotwal G. J., Moss B. Vaccinia virus encodes a secretory polypeptide structurally related to complement control proteins. Nature. 1988 Sep 8;335(6186):176–178. doi: 10.1038/335176a0. [DOI] [PubMed] [Google Scholar]
- Krych M., Hourcade D., Atkinson J. P. Sites within the complement C3b/C4b receptor important for the specificity of ligand binding. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4353–4357. doi: 10.1073/pnas.88.10.4353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lublin D. M., Atkinson J. P. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol. 1989;7:35–58. doi: 10.1146/annurev.iy.07.040189.000343. [DOI] [PubMed] [Google Scholar]
- Medof M. E., Lublin D. M., Holers V. M., Ayers D. J., Getty R. R., Leykam J. F., Atkinson J. P., Tykocinski M. L. Cloning and characterization of cDNAs encoding the complete sequence of decay-accelerating factor of human complement. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2007–2011. doi: 10.1073/pnas.84.7.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Nogueira N., Bianco C., Cohn Z. Studies on the selective lysis and purification of Trypanosoma cruzi. J Exp Med. 1975 Jul 1;142(1):224–229. doi: 10.1084/jem.142.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris K. A., Bradt B., Cooper N. R., So M. Characterization of a Trypanosoma cruzi C3 binding protein with functional and genetic similarities to the human complement regulatory protein, decay-accelerating factor. J Immunol. 1991 Oct 1;147(7):2240–2247. [PubMed] [Google Scholar]
- Norris K. A., Harth G., So M. Purification of a Trypanosoma cruzi membrane glycoprotein which elicits lytic antibodies. Infect Immun. 1989 Aug;57(8):2372–2377. doi: 10.1128/iai.57.8.2372-2377.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. S., Fouts D. L., Manning J. E. The 85-kd surface antigen gene of Trypanosoma cruzi is telomeric and a member of a multigene family. EMBO J. 1989 Dec 1;8(12):3911–3916. doi: 10.1002/j.1460-2075.1989.tb08571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B., Day A. J. Structure-function relationships of the complement components. Immunol Today. 1989 Jun;10(6):177–180. doi: 10.1016/0167-5699(89)90317-4. [DOI] [PubMed] [Google Scholar]
- Rey-Campos J., Rubinstein P., Rodriguez de Cordoba S. Decay-accelerating factor. Genetic polymorphism and linkage to the RCA (regulator of complement activation) gene cluster in humans. J Exp Med. 1987 Jul 1;166(1):246–252. doi: 10.1084/jem.166.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi M. T., Sher A., Heiny S., Lituchy A., Hammer C. H., Joiner K. Developmentally regulated expression by Trypanosoma cruzi of molecules that accelerate the decay of complement C3 convertases. Proc Natl Acad Sci U S A. 1988 Jan;85(1):193–197. doi: 10.1073/pnas.85.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen K. M., Lamperti E. D., Villa-Komaroff L. Optimizing the northern blot procedure. Biotechniques. 1990 Apr;8(4):398–403. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel-Dugan C., Ponce de Leon M., Friedman H. M., Eisenberg R. J., Cohen G. H. Identification of C3b-binding regions on herpes simplex virus type 2 glycoprotein C. J Virol. 1990 May;64(5):1897–1906. doi: 10.1128/jvi.64.5.1897-1906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]
- Takle G. B., Young A., Snary D., Hudson L., Nicholls S. C. Cloning and expression of a trypomastigote-specific 85-kilodalton surface antigen gene from Trypanosoma cruzi. Mol Biochem Parasitol. 1989 Nov;37(1):57–64. doi: 10.1016/0166-6851(89)90102-3. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]