Abstract
Vascular smooth muscle cell hypertrophy, proliferation or migration occurs in hypertension, atherosclerosis and restenosis after angioplasty leading to pathophysiological vascular remodeling. Angiotensin II and platelet-derived growth factor are well known participants of vascular remodeling, and activate a myriad of downstream protein kinases including PAK1. PAK1, an effector kinase of small GTPases, phosphorylates several substrates to regulate cytoskeletal reorganization. However, the exact role of PAK1 activation in vascular remodeling remains to be elucidated. Here, we have hypothesized that PAK1 is a critical target of intervention for prevention of vascular remodeling. Adenoviral expression of dominant-negative PAK1 inhibited both angiotensin II- and platelet-derived growth factor-stimulated vascular smooth muscle cell migration. It also inhibited vascular smooth muscle cell proliferation induced by platelet-derived growth factor. PAK1 was activated in neointima of the carotid artery after balloon injury in rat. Moreover, marked inhibition of the neointima hyperplasia was observed in dominant-negative PAK1 adenovirus treated carotid artery after the balloon injury. Taken together, these results suggest that PAK1 is involved in both angiotensin II and platelet-derived growth factor mediated VSMC remodeling, and inactivation of PAK1 in vivo could be effective in preventing pathophysiological vascular remodeling.
Keywords: angiotensin II, platelet-derived factor, signal transduction, arterial injury, restenosis
Introduction
An increasing body of evidence strongly suggests that activation of a certain set of protein kinases is shared by multiple risk factors implicated in cardiovascular remodeling under hypertension or atherosclerosis 1. PAK1, an effector kinase of small G proteins, could be one of these kinases, thus representing a novel therapeutic target. Except for plants, nearly all eukaryotes encode one or more PAK genes, indicating an important physiological function for this family of kinases 2. PAK has been implicated in cell growth, survival and migration in non-cardiovascular cells 2-5. In cultured vascular smooth muscle cells (VSMCs), PAK1 is the major isoform expressed. Multiple ligands implicated in pathological vascular remodeling such as angiotensin II (AngII) and platelet-derived growth factor BB (PDGF-BB) have been shown to activate PAK1 in VSMCs 6-9. The PAK1 activation mechanism by these ligands in VSMCs has been extensively studied, which appears to include intracellular Ca2+ elevation, reactive oxygen species, protein kinase C-δ, small G protein Rac1, PYK2, c-Src, phosphoinositide-dependent kinase 1, and Nck 8, 10, 11.
In contrast, limited information is available regarding the functional significance of PAK1 activation in VSMCs. Thus far, PAK1 has been shown to be required for VSMC hypertrophy and migration induced by AngII and PDGF-BB, respectively 8, 11. However, the exact role of PAK1 activation in vascular remodeling remains to be elucidated. In the present study, we have hypothesized that PAK1 is a critical target of intervention for prevention of vascular remodeling by inhibiting VSMC proliferation and migration. The hypothesis was tested in vitro with primary culture of arterial VSMCs treated with AngII or PDGF-BB as well as in vivo with carotid artery after balloon angioplasty. Our data support that PAK1 is one of the critical protein kinases involved in pathological vascular remodeling.
Materials and Methods
Reagents
AngII was purchased from Sigma. PDGF-BB was purchased from R & D Systems. Phospho-specific antibodies to detect Ser192/204-phosphorylated PAK1 for immunoblotting and Thr423-phosphorylated PAK1 for immunohistochemistry were purchased from Cell Signaling. Antibody to detect total PAK1 was purchased from Santa Cruz Biotechnology. Antibody against proliferation cell nuclear antigen (PCNA) was purchased from Chemicon.
Cell Culture
Isolation and characterization of rat aortic VSMCs in culture were described previously 12. Cells were subcultured in DMEM containing 10% fetal bovine serum, penicillin and streptomycin as previously described 12. Cells at passage 3-12 at ~80% confluence in culture wells were made quiescent by incubation with serum-free medium for 24 h before the adenovirus infection.
Adenoviral Infection
Generation and characterization of replication-deficient adenovirus encoding kinase-inactive/dominant negative K299R/dnPAK1 was described previously 3. The adenovirus titer was determined by Adeno-X™ Rapid Titer Kit (BD Biosciences). VSMCs were infected with adenovirus for 2 days as previously described 13. The infection efficiency was estimated to be 90-100% as defined by infection with adenovirus (50-100 moi) encoding green fluorescent protein (GFP).
Immunoblotting
Immunoblotting was performed as previously described 14. Cell lysates were subjected to SDS-PAGE gel electrophoresis and electrophoretically transferred to a nitrocellulose membrane. The membranes were then exposed to primary antibodies overnight at 4 °C. After incubation with the peroxidase linked secondary antibody for 1h at room temperature, immunoreactive proteins were visualized by a chemiluminescence reaction kit. The results were quantified by densitometry in the linear range of film exposure using CanoScan N670U (Canon) and Un-Scan-It Gel 5.3 software (Silk Scientific) 15. An example of data supporting the linearity has been demonstrated 16.
Wound Healing Assay
VSMC migration was measured using a monolayer-wounding protocol in which cells migrated from a confluent area into an area that was mechanically denuded of cells. VSMCs infected with adenovirus for 2 days were scraped by a metal dental pick (DenTek) and stimulated by 100 nmol/L AngII for 24 h with 5 mmol/L hydroxyurea to completely block proliferation. VSMC migration was quantified as previously reported 16.
Cell Proliferation
VSMCs infected with adenovirus for 2 days were stimulated by 100 ng/mL PDGF-BB for 72 h, and then, cell numbers were counted by Coulter counter.
Balloon Angioplasty and Gene Transfer
Left common carotid artery balloon angioplasty was performed in male Sprague-Dawley rats (Charles River Breeding Laboratory) that were under pentobarbital sodium anesthesia as previously reported 17. Subsequently, adenovirus encoding dnPAK or control GFP was delivered to the injured artery (1×109 pfu/mL) 18. The vessels were harvested 14 days later, fixed, and histology was determined as described 17. These investigations conform with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and Temple University 18.
Immunohistochemistry and morphometry
Immunohistochemistry was performed with phosho-PAK1 Thr423 antibody and PCNA antibody as described previously 18. For vascular morphometry, digitized images were averaged from at least three representative stained tissue sections using Image Pro Plus (Media Cybernetics). The circumference of the lumen, the area encircled internal elastic lamina, and the external elastic lamina were quantified. The medial and intimal areas were then calculated 18.
Statistical Analysis
Data are presented as mean±SEM. Groups were compared using ANOVA followed by Tukey post hoc test (Figure 2), student t test (Figure 3 and 4), or paired t test (Figure 1). The null hypothesis was rejected when p<0.05.
Figure 2.
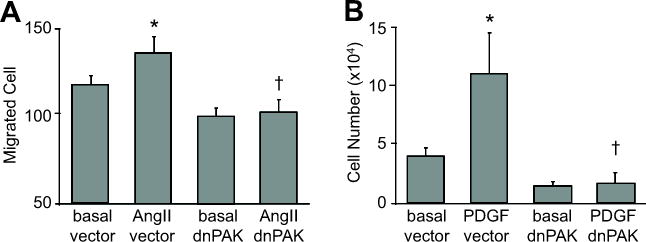
dnPAK1 inhibited migration and proliferation of VSMCs. A, Confluent VSMCs infected with adenovirus (100 moi) encoding dnPAK1 or the control GFP vector were scraped by a metal dental pick and stimulated with AngII for 24 hours in the presence of 5 mmol/L hydroxyurea to block cell proliferation completely. The nucleus was stained with Hoechst 33342 dye, and migrated VSMCs from the wound edge were counted in 4 independent view fields (100X). Data are mean±SEM of 4 experiments. B, VSMCs were infected with adenovirus (100 moi) encoding control GFP vector or dnPAK1 for 48 hours. The cells were then stimulated with 100 ng/mL PDGF-BB for 3 days and the cell number counted. Data are mean±SEM of 3 experiments. *p<0.05 compared to the basal control. †p<0.05 compared to the stimulated control.
Figure 3.
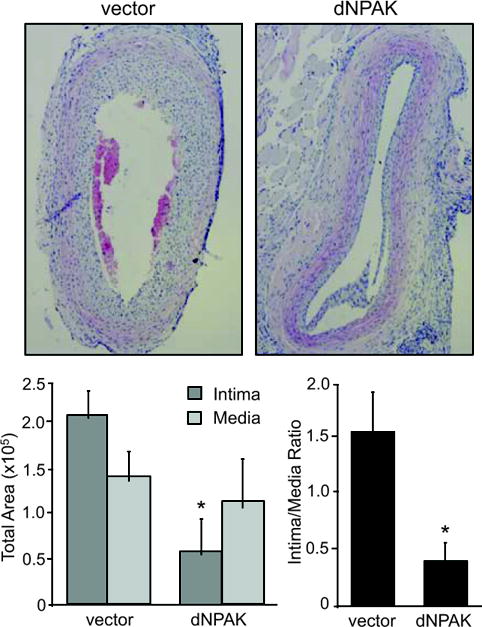
dnPAK1 inhibited arterial neointima formation after balloon injury. Representative sections (x40 magnification) are shown. 14 days after the injury, common carotid artery was stained and the area of neointima and media were quantified. The circumference of the lumen, the area encircled by internal elastic lamina (IEL), and the external elastic lamina (EEL) were measured. The medial area was calculated by subtracting the area defined by the IEL from the area defined by the EEL, and the intimal area calculated as the difference between the area inside the IEL and the luminal area using an automated computer-based image analyzer. Data are mean±SEM of sections from 4 rats. *p<0.05 compared to the GFP adenovirus-infected control.
Figure 4.
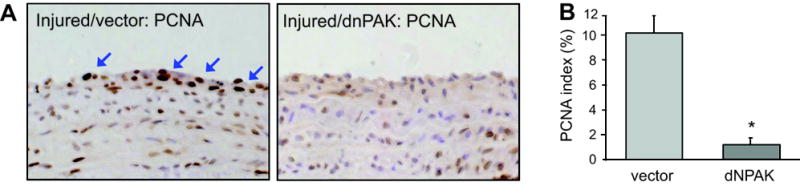
Histological analysis of cell proliferation in arterial cross-sections obtained after balloon injury. Arterial sections obtained on day 14 after injury with infection of adenovirus encoding GFP or dnPAK1 were stained with PCNA antibody (x400 magnification). Representative sections (each from 4 sections, x400 magnification) are shown. A, Cells were considered positive for PCNA expression only in the presence of an intense brown staining of the nucleus. The arrows indicate representative PCNA positive cells in the neointima lesion. B, The number of PCNA-positive cells was expressed as a percentage of the total cell number in the neointima (PCNA index). Data are mean±SEM of 4 sections. *p<0.05 compared to the GFP adenovirus-infected control.
Figure 1.
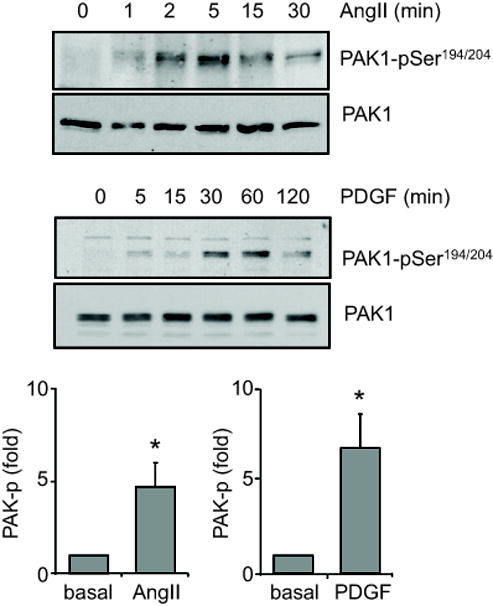
Phosphorylation of PAK1 by AngII and PDGF-BB. VSMCs were stimulated with 100 nmol/L AngII or 100 ng/mL PDGF-BB for the indicated time periods. The cell lysates were immunoblotted with a phospho-selective antibody, which detects PAK1-pSer194/204 phosphorylation, and with anti-PAK1 antibody. The bar graphs show quantification of the PAK1 phosphorylation by densitometry at 5 min and 30 min induced by AngII and PDGF-BB, respectively. Data are mean±SEM of 3 experiments. *p<0.05 compared to the basal control.
Results
In vitro experiments
To test whether AngII and PDGF-BB activate PAK1 in VSMCs, immunoblot analysis was performed with antibody selectively recognizing Ser192/204-phosphorylated PAK1. As shown in Figure 1, both agonists markedly stimulated PAK1 phosphorylation in a time dependent manner. The results are consistent with past publications 8, 11. We have demonstrated that PAK1 activation is required for AngII-induced VSMC hypertrophy 11. Here, we have further questioned whether PAK1 is required for VSMC migration by AngII and proliferation by PDGF-BB in vitro. As shown in Figure 2A, infection of adenovirus encoding dnPAK1 completely inhibited VSMC migration induced by Ang II. Moreover, infection of dnPAK1 adenovirus completely inhibited VSMC proliferation induced by PDGF-BB (Figure 2B).
In vivo experiments
Above in vitro experiments prompted us to further test the effect of dnPAK1 on arterial neointimal hyperplasia after balloon angioplasty since this type of vascular remodeling involves VSMC migration and proliferation. As shown in Figure 3, dnPAK1 gene transfer by adenovirus significantly inhibited neointima formation at the carotid artery 14 days after the injury, whereas it had no effect on the medial area of the injured artery. PCNA positive cells were detected in neointima of the carotid artery 14 days after the injury, especially at the lumen side, and were almost completely attenuated in the neointima treated with dnPAK adenovirus (Figure 4). These data suggest the crucial participation of PAK1 within pathological vascular hyperplasia. To confirm the causal role of PAK1 in vascular remodeling, activation of PAK1 in the artery 14 days after the injury was assessed by immunohistochemistry using a phospho-specific PAK1 antibody. Marked staining was specifically observed in the neointima region of the artery at the cytosol as well as nucleus, which was attenuated in the dnPAK1-treated artery (Figure 5).
Figure 5.
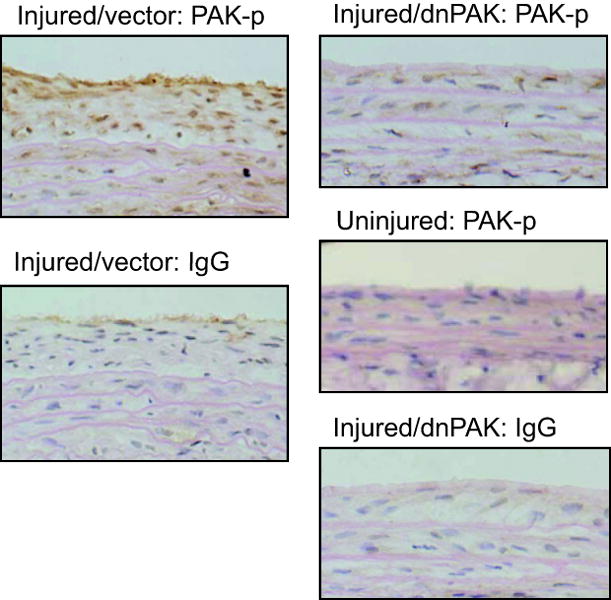
Histological analysis of PAK1 phosphorylation in arterial cross-sections obtained after balloon injury. Arterial sections obtained on day 14 after injury with infection of adenovirus encoding GFP or dnPAK1 were stained with a phospho-selective antibody, which detects PAK1-pThr423 phosphorylation, or with control IgG (x400 magnification). Representative sections (each from 3 to 5 sections, x400 magnification) are shown.
Discussion
PAK1 has been shown to be required for migration of cultured tracheal smooth muscle cells and VSMCs 3, 8. We have previously reported that PAK1 activity is required for enhanced protein synthesis induced by AngII in cultured VSMCs 11. PAK1 was also implicated in regulation of smooth muscle contraction 19, 20 and vascular permeability in atherogenesis 21. However, studies focusing on the causal roles of PAK1 activation in cardiovascular diseases have been limited. The data presented here support our initial hypothesis that PAK1 represents a novel therapeutic target in pathological vascular remodeling. Importantly, we demonstrate for the first time that PAK1 is required for proliferation of VSMCs induced by PDGF in vitro as well as in vivo associated with arterial injury.
By using a Boyden chamber assay, the involvement of PAK1 in mediating VSMC migration stimulated by PDGF-BB and thrombin has been reported 8. Although the Boyden chamber method has an advantage for assessing a directional migration toward a stable gradient of an attractant, the method may not distinguish between treatment effects on cell migration, cell spreading, or cell attachment 22. Thus, our current finding with the wound healing assay complements these past findings and strongly supports the critical role of PAK1 in migration of VSMCs.
Although there are multiple animal models to conduct research on pathological vascular remodeling, both AngII and PDGF are strongly implicated in neointima formation after the balloon arterial injury in rodent 23-26, justifying the model selection in the present study. Several distinct factors involved in vascular remodeling have been shown to activate PAK1 in cultured VSMCs 6-9 as in the present study. Our in vivo data further highlighted PAK1 as a potential therapeutic target toward vascular occlusive diseases such as restenosis and atherosclerosis, which is also supported by a recent report 27. Moreover, our data demonstrated that there was a persistent activation of PAK1 in the vascular neointima region and that dnPAK1 inhibited cell proliferation in the intima. These data suggest that PAK1 activation is not only involved in the early stage of vascular remodeling which is mainly regulated by VSMC migration but also in the chronic stage of the remodeling associated with VSMC proliferation.
The downstream mechanism by which PAK1 participates in neointima formation remains unclear at this point. It should be noted however that some of the known or predicted PAK1 downstreams in VSMCs have been shown to contribute to vascular remodeling in response to arterial injury, which include apoptosis signal-regulating kinase 1, c-Jun NH2-terminal kinase, and c-Jun 28-30. Therefore, it is possible that PAK1 participates in neointimal hyperplasia in part through c-Jun induction via sequential activation of the above mentioned two kinases at the cytosol.
Alternatively, PAK1 may also participate in vascular remodeling via its nuclear signal transduction. In this regard, over expression of PAK1 in cancer cells results in an NFκB-dependent promoter stimulation of cyclin D1, which drives cell cycle progression 31. However, NFκB activation seems to be dispensable for PDGF-BB-induced cyclin D1 expression in VSMCs 32. In line with our novel observation of PAK1 phosphorylation at the cytosol and nucleus in the neointima lesion, PAK1 has been shown to be involved in mitotic regulation at the nucleus 33. Therefore, an important future project will be the identification of the downstream target of PAK1 such as a nuclear substrate in VSMCs by which PAK1 supports neointima formation.
Perspectives
In addition to our present study suggesting the causal role of PAK1 in restenosis and potentially atherosclerosis, PAK1 is also implicated in contractile regulation of VSMCs. Therefore further research on PAK1 in hypertensive animal models should be performed to expand our findings to lead to a better treatment of cardiovascular diseases associated with hypertension.
Acknowledgments
We thank Kyoko Hinoki for her technical assistance.
Sources of Funding This work was supported by National Institute of Health Grants, HL076770 (S.E.), HL076575 (G.F.), HL063810 (M.A.), HL090885 (M.A.), by American Heart Association Established Investigator Award, 0740042N (S.E.), and by W. W. Smith Charitable Trust Grant, H0605 (S.E.). K.I. was supported by a Japan Heart Foundation Bayer Yakuhin Research Grant Abroad.
Footnotes
Conflict(s) of Interest/Disclosure(s) None.
References
- 1.Shirai H, Autieri M, Eguchi S. Small GTP-binding proteins and mitogen-activated protein kinases as promising therapeutic targets of vascular remodeling. Curr Opin Nephrol Hypertens. 2007;16(2):111–115. doi: 10.1097/MNH.0b013e3280148e4f. [DOI] [PubMed] [Google Scholar]
- 2.Hofmann C, Shepelev M, Chernoff J. The genetics of Pak. J Cell Sci. 2004;117(Pt 19):4343–4354. doi: 10.1242/jcs.01392. [DOI] [PubMed] [Google Scholar]
- 3.Dechert MA, Holder JM, Gerthoffer WT. p21-activated kinase 1 participates in tracheal smooth muscle cell migration by signaling to p38 Mapk. Am J Physiol Cell Physiol. 2001;281(1):C123–132. doi: 10.1152/ajpcell.2001.281.1.C123. [DOI] [PubMed] [Google Scholar]
- 4.Bokoch GM. Biology of the p21-activated kinases. Ann Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 5.Arias-Romero LE, Chernoff J. A tale of two Paks. Biol Cell. 2008;100(2):97–108. doi: 10.1042/BC20070109. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz U, Ishida T, Ishida M, Surapisitchat J, Hasham MI, Pelech S, Berk BC. Angiotensin II stimulates p21-activated kinase in vascular smooth muscle cells: role in activation of JNK. Circ Res. 1998;82(12):1272–1278. doi: 10.1161/01.res.82.12.1272. [DOI] [PubMed] [Google Scholar]
- 7.Schmitz U, Thommes K, Beier I, Vetter H. Lysophosphatidic acid stimulates p21-activated kinase in vascular smooth muscle cells. Biochem Biophys Res Commun. 2002;291(3):687–691. doi: 10.1006/bbrc.2002.6493. [DOI] [PubMed] [Google Scholar]
- 8.Weber DS, Taniyama Y, Rocic P, Seshiah PN, Dechert MA, Gerthoffer WT, Griendling KK. Phosphoinositide-dependent kinase 1 and p21-activated protein kinase mediate reactive oxygen species-dependent regulation of platelet-derived growth factor-induced smooth muscle cell migration. Circ Res. 2004;94(9):1219–1226. doi: 10.1161/01.RES.0000126848.54740.4A. [DOI] [PubMed] [Google Scholar]
- 9.Beier I, Dusing R, Vetter H, Schmitz U. Epidermal growth factor stimulates Rac1 and p21-activated kinase in vascular smooth muscle cells. Atherosclerosis. 2008;196(1):92–97. doi: 10.1016/j.atherosclerosis.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz U, Thommes K, Beier I, Wagner W, Sachinidis A, Dusing R, Vetter H. Angiotensin II-induced Stimulation of p21-activated Kinase and c-Jun NH2-terminal Kinase is Mediated by Rac1 and Nck. J Biol Chem. 2001;276:22003–22010. doi: 10.1074/jbc.M102450200. [DOI] [PubMed] [Google Scholar]
- 11.Woolfolk EA, Eguchi S, Ohtsu H, Nakashima H, Ueno H, Gerthoffer WT, Motley ED. Angiotensin II-Induced Activation of P21-Activated Kinase 1 Requires Ca2+ and Protein Kinase C {delta} in Vascular Smooth Muscle Cells. Am J Physiol Cell Physiol. 2005;289:C1286–C1294. doi: 10.1152/ajpcell.00448.2004. [DOI] [PubMed] [Google Scholar]
- 12.Eguchi S, Matsumoto T, Motley ED, Utsunomiya H, Inagami T. Identification of an essential signaling cascade for mitogen-activated protein kinase activation by angiotensin II in cultured rat vascular smooth muscle cells. Possible requirement of Gq-mediated p21ras activation coupled to a Ca2+/calmodulin-sensitive tyrosine kinase. J Biol Chem. 1996;271(24):14169–14175. doi: 10.1074/jbc.271.24.14169. [DOI] [PubMed] [Google Scholar]
- 13.Eguchi S, Iwasaki H, Ueno H, Frank GD, Motley ED, Eguchi K, Marumo F, Hirata Y, Inagami T. Intracellular signaling of angiotensin II-induced p70 S6 kinase phosphorylation at Ser(411) in vascular smooth muscle cells. Possible requirement of epidermal growth factor receptor, Ras, extracellular signal-regulated kinase, and Akt. J Biol Chem. 1999;274(52):36843–36851. doi: 10.1074/jbc.274.52.36843. [DOI] [PubMed] [Google Scholar]
- 14.Eguchi S, Numaguchi K, Iwasaki H, Matsumoto T, Yamakawa T, Utsunomiya H, Motley ED, Kawakatsu H, Owada KM, Hirata Y, Marumo F, Inagami T. Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J Biol Chem. 1998;273(15):8890–8896. doi: 10.1074/jbc.273.15.8890. [DOI] [PubMed] [Google Scholar]
- 15.Ohtsu H, Mifune M, Frank GD, Saito S, Inagami T, Kim-Mitsuyama S, Takuwa Y, Sasaki T, Rothstein JD, Suzuki H, Nakashima H, Woolfolk EA, Motley ED, Eguchi S. Signal-crosstalk between Rho/ROCK and c-Jun NH2-terminal kinase mediates migration of vascular smooth muscle cells stimulated by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25(9):1831–1836. doi: 10.1161/01.ATV.0000175749.41799.9b. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki H, Kimura K, Shirai H, Eguchi K, Higuchi S, Hinoki A, Ishimaru K, Brailoiu E, Dhanasekaran DN, Stemmle LN, Fields TA, Frank GD, Autieri MV, Eguchi S. Endothelial nitric oxide synthase inhibits G12/13 and rho-kinase activated by the angiotensin II type-1 receptor: implication in vascular migration. Arterioscler Thromb Vasc Biol. 2009;29(2):217–224. doi: 10.1161/ATVBAHA.108.181024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Autieri MV, Carbone C, Mu A. Expression of allograft inflammatory factor-1 is a marker of activated human vascular smooth muscle cells and arterial injury. Arterioscler Thromb Vasc Biol. 2000;20(7):1737–1744. doi: 10.1161/01.atv.20.7.1737. [DOI] [PubMed] [Google Scholar]
- 18.Sommerville LJ, Xing C, Kelemen SE, Eguchi S, Autieri MV. Inhibition of allograft inflammatory factor-1 expression reduces development of neointimal hyperplasia and p38 kinase activity. Cardiovasc Res. 2009;81(1):206–215. doi: 10.1093/cvr/cvn242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McFawn PK, Shen L, Vincent SG, Mak A, Van Eyk JE, Fisher JT. Calcium-independent contraction and sensitization of airway smooth muscle by p21-activated protein kinase. American Journal of Physiology - Lung Cellular & Molecular Physiology. 2003;284(5):L863–870. doi: 10.1152/ajplung.00068.2002. [DOI] [PubMed] [Google Scholar]
- 20.Wirth A, Schroeter M, Kock-Hauser C, Manser E, Chalovich JM, De Lanerolle P, Pfitzer G. Inhibition of contraction and myosin light chain phosphorylation in guinea-pig smooth muscle by p21-activated kinase 1. Journal of Physiology. 2003;549(Pt 2):489–500. doi: 10.1113/jphysiol.2002.033167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orr AW, Stockton R, Simmers MB, Sanders JM, Sarembock IJ, Blackman BR, Schwartz MA. Matrix-specific p21-activated kinase activation regulates vascular permeability in atherogenesis. J Cell Biol. 2007;176(5):719–727. doi: 10.1083/jcb.200609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerthoffer WT. Mechanisms of vascular smooth muscle cell migration. Circ Res. 2007;100(5):607–621. doi: 10.1161/01.RES.0000258492.96097.47. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Kawamura M, Wanibuchi H, Ohta K, Hamaguchi A, Omura T, Yukimura T, Miura K, Iwao H. Angiotensin II type 1 receptor blockade inhibits the expression of immediate-early genes and fibronectin in rat injured artery. Circulation. 1995;92(1):88–95. doi: 10.1161/01.cir.92.1.88. [DOI] [PubMed] [Google Scholar]
- 24.Abe J, Deguchi J, Matsumoto T, Takuwa N, Noda M, Ohno M, Makuuchi M, Kurokawa K, Takuwa Y. Stimulated activation of platelet-derived growth factor receptor in vivo in balloon-injured arteries: a link between angiotensin II and intimal thickening. Circulation. 1997;96(6):1906–1913. doi: 10.1161/01.cir.96.6.1906. [DOI] [PubMed] [Google Scholar]
- 25.Ferns GA, Raines EW, Sprugel KH, Motani AS, Reidy MA, Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- 26.Jawien A, Bowen-Pope DF, Lindner V, Schwartz SM, Clowes AW. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest. 1992;89(2):507–511. doi: 10.1172/JCI115613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D, Paria BC, Zhang Q, Karpurapu M, Li Q, Gerthoffer WT, Nakaoka Y, Rao GN. A role for Gab1/SHP2 in thrombin activation of PAK1: gene transfer of kinase-dead PAK1 inhibits injury-induced restenosis. Circ Res. 2009;104(9):1066–1075. doi: 10.1161/CIRCRESAHA.109.196691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izumi Y, Kim S, Namba M, Yasumoto H, Miyazaki H, Hoshiga M, Kaneda Y, Morishita R, Zhan Y, Iwao H. Gene transfer of dominant-negative mutants of extracellular signal-regulated kinase and c-Jun NH2-terminal kinase prevents neointimal formation in balloon-injured rat artery. Circulation Research. 2001;88(11):1120–1126. doi: 10.1161/hh1101.091267. [DOI] [PubMed] [Google Scholar]
- 29.Yasumoto H, Kim S, Zhan Y, Miyazaki H, Hoshiga M, Kaneda Y, Morishita R, Iwao H. Dominant negative c-jun gene transfer inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia in rats. Gene Therapy. 2001;8(22):1682–1689. doi: 10.1038/sj.gt.3301590. [DOI] [PubMed] [Google Scholar]
- 30.Izumi Y, Kim S, Yoshiyama M, Izumiya Y, Yoshida K, Matsuzawa A, Koyama H, Nishizawa Y, Ichijo H, Yoshikawa J, Iwao H. Activation of apoptosis signal-regulating kinase 1 in injured artery and its critical role in neointimal hyperplasia. Circulation. 2003;108(22):2812–2818. doi: 10.1161/01.CIR.0000096486.01652.FC. [DOI] [PubMed] [Google Scholar]
- 31.Balasenthil S, Sahin AA, Barnes CJ, Wang RA, Pestell RG, Vadlamudi RK, Kumar R. p21-activated kinase-1 signaling mediates cyclin D1 expression in mammary epithelial and cancer cells. J Biol Chem. 2004;279(2):1422–1428. doi: 10.1074/jbc.M309937200. [DOI] [PubMed] [Google Scholar]
- 32.Mehrhof FB, Schmidt-Ullrich R, Dietz R, Scheidereit C. Regulation of vascular smooth muscle cell proliferation: role of NF-kappaB revisited. Circulation Research. 2005;96(9):958–964. doi: 10.1161/01.RES.0000166924.31219.49. [DOI] [PubMed] [Google Scholar]
- 33.Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6(6):459–471. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]


