Abstract
Background and Aims of the Study
Histopathological studies of rejected orthotopic heart transplants suggest that cardiac valve endothelium is spared the inflammatory cell infiltration and tissue damage that occurs in the myocardium. To test whether this apparent protection from leukocyte invasion might be an inherent feature of the valve endothelium, we analyzed leukocyte adhesion molecule expression and function in human pulmonary valve endothelial cells (HPVEC). Use of cultured HPVEC allowed us to delineate the potential contribution of functional adhesion molecules from the contribution of hemodynamic forces exerted on the leaflet surface in vivo
Methods and Results
HPVECs express E-selectin, ICAM-1, and VCAM-1 in response to the inflammatory cytokine tumor necrosis factor-∀ (TNF-∀) similarly to other types of cultured human endothelial cells. In a static cell adhesion assay, E-selectin mediated adhesion of HL-60 cells, a human promyelocytic leukemia cell line, and U937 cells, a human monocytic cell line, was determined in cells treated with TNF-∀ for 4 hours. After 24 hours of TNF-∀, adhesion of U937 cells to HPVECs was mediated primarily by VCAM-1, consistent with the high expression of VCAM-1 and diminished expression of E-selectin at 24 hours.
Conclusion
These results demonstrate that HPVECs express functional leukocyte adhesion molecules in vitro and suggest that cardiac valve endothelium is competent to initiate leukocyte adhesion. Thus, other factors, such as the hemodynamic forces exerted on the valve, may contribute to the apparent protection from inflammatory cell infiltration in vivo.
Keywords: Heart valve endothelium, leukocyte adhesion molecules, E-selectin, VCAM-1, inflammation
Introduction
The identification of the endothelial cell surface constituents that orchestrate the adhesion and extravasation of leukocytes into tissue at sites of inflammation has been achieved by the efforts of many investigators (1). A series of endothelial cell surface glycoproteins that interact with counter ligands on leukocytes to mediate critical events in cell adhesion have been characterized using molecular, biochemical and transgenic mouse approaches (2). Two major classes of endothelial adhesion molecules have been described. The first is the selectins: a three-member family of calcium-dependent lectins that share extensive homology at the amino acid sequence level and have similar carbohydrate recognition properties. Endothelial cells express two selectins, E- and P-selectin, both of which can bind to neutrophils, monocytes and some T cells. The second major class is the immunoglobulin superfamily: important members include ICAM-1 (intercellular adhesion molecule-1) and VCAM-1 (vascular cell adhesion molecule-1), both of which mediate adhesion by binding to specific leukocyte integrins. ICAM-1 mediates firm adhesion of neutrophils, monocytes, and lymphocytes while VCAM-1 is primarily responsible for adhesion of lymphocytes and monocytes, but not neutrophils. The temporal expression of these adhesion molecules, in response to inflammatory cytokines, and recognition of cell-specific counter ligands, orchestrates the recruitment and activation of leukocytes into inflamed tissues to fight infections. However, in certain pathological conditions, leukocyte infiltration contributes to tissue damage and organ rejection, such as can occur following allogenic heart transplantation.
In a study of aortic valves from orthotopic heart transplants (n=16), Mitchell and colleagues (3) found that the valve leaflets were spared the inflammatory cell infiltration that occurred elsewhere in the heart, even in cases of fatal cardiac rejection (n=4). Mild T lymphocyte infiltration was observed, but did not differ substantially from what is typically observed in normal, disease-free valves. The authors speculate that the remarkable absence of inflammatory injury in the valves may be due to attenuated expression of leukocyte adhesion molecules in the endothelium overlying the valves, or alternatively, that hemodynamic conditions at the leaflet surface may prevent significant binding of inflammatory cells. Additional evidence for an apparent protection of valve leaflets from leukocyte infiltration was seen when pig hearts were transplanted into baboons (n=3) (4). Hyperacute rejection occurred, with dramatic tissue damage due to IgM, but the aortic and pulmonary valves were spared. Hyperacute rejection occurs by recognition of porcine galactose ∀-1,3-galactose epitopes by circulating anti-∀-galactose antibodies present in primates. Expression of the galactose ∀-1,3-galactose antigen was detected on porcine myocardial microvascular endothelium, but not on aortic and pulmonary valve endothelium, providing evidence for a mechanism for the apparent protection from IgM-mediated injury, and suggesting that phenotypic differences between valve endothelium and microvascular endothelium exist. In summary, clinical and experimental evidence exists to suggest that the endothelium of valve leaflets is protected from inflammatory and immune-mediated injury compared to cardiac microvascular endothelium.
Despite these in vivo observations, few in vitro studies on cardiac valve endothelial cells have been reported. Endothelial cells from human aortic valves have been isolated and partially characterized using immunohistochemical techniques for expression of cell surface antigens (5,6). Recently, we showed that human pulmonary valve endothelial cells (HPVEC) employ a unique NFATc1-dependent pathway when stimulated to proliferate in response to VEGF and that NFATc1 is expressed in subsets of endothelial cells on human pulmonary valve leaflets in vivo (7). NFATc1 is required for formation of the aortic and pulmonary valves during embryonic development (8, 9); our results suggest that NFATc1 plays a role post-natal valves as well. We have also shown that ovine aortic valve endothelial cells can be induced to undergo an endothelial-to-mesenchymal transdifferentiation that is reminiscent of events that occur during valve development (10). The ability of valve endothelial cells to transdifferentiate appears to be unique, in that endothelial cells isolated from large vessels or the microvasculature could not be induced to transdifferentiate. These two studies support the concept that the valvular endothelium has unique properties compared to the endothelium located in other vascular beds. Furthermore, the studies demonstrate that certain aspects of the valvular endothelial phenotype are maintained in vitro. In the present study, we examined a panel of leukocyte adhesion molecules in activated human pulmonary valve endothelial cells to determine if the level of expression of these molecules and ability to support leukocyte adhesion might be attenuated, and thereby offer a potential mechanism that would explain the apparent protection from leukocyte adhesion/infiltration seen in vivo in cardiac transplant rejection.
Materials and Methods
Isolation and culture of HPVECs
HPVECs were isolated from pulmonary valve leaflets obtained from children, ages 5 months to 20 years of age, undergoing open heart surgery at Children’s Hospital Boston. Removal of the pulmonary valve was a planned part of the procedure. Valve tissue was obtained in accordance with a protocol approved by the Committee on Clinical Investigation, Children’s Hospital Boston. HPVEC were isolated using the same procedures as described previously for ovine aortic and pulmonic valve leaflets (7, 10). Briefly, leaflets were incubated in 0.2% collagenase 5 minutes at 37EC. The supernatant containing released cells was sedimented at 200 x g, and resuspended in a growth medium designated EBM-B. EBM-B contains EBM-131 (Clonetics, Inc.), 10% heat-inactivated fetal bovine serum (FBS) (Hyclone), a mixture of 1X glutamine, penicillin, and streptomycin (GPS) (Irvine Scientific), and 2ng/ml recombinant human basic fibroblast growth factor (bFGF) (R&D Systems). Cells were grown on gelatin-coated culture dishes at 37EC in a 5% CO2 incubator. About 1 week later, endothelial cells from primary cultures of human pulmonary valve were isolated using Ulex europaeus-I-coated Dynabeads (Dynal, Inc.) as described (11). Human dermal microvascular endothelial cells (HDMEC) were isolated from newborn foreskin as described and grown in EBM-B (12). Cells were shown to be endothelial by immunostaining with antibodies against von Willebrand factor, CD31, and E-selectin as described (7, 14). Both HPVEC and HDMEC were passaged 1:3 every 4 - 6 days, and used in experiments at passages 4 - 14.
Cells lines
The human promyelocytic leukemia cell line HL-60 (ATCC) and monocytic lymphoma cell line U937 (kindly provided by Dr. Michael Klagsbrun, Children’s Hospital Boston) were grown in RPMI 1640 medium (Biowhittaker) supplemented with 10% FBS, 1x GPS, and maintained at a density of less than 1×106 cells/ml.
Antibodies
Mouse monoclonal antibodies (mAb) against human E-selectin (7A9), P-selectin (HPDG 2/3), and ICAM-1 (Hu 5/3) (13) were kindly provided by Dr. F. William Luscinskas, Vascular Research Division, Brigham and Women’s Hospital, Boston, MA. Anti-human VCAM-1 (1.G11B1) mAb was obtained from Biosource International; mouse IgG1 (DAK-G01) and polyclonal rabbit anti-human von Willebrand Factor (vWF) were obtained from Dako; polyclonal goat anti-human CD31/PECAM-1 IgG was obtained from Santa Cruz Biotechnology; Texas Red anti-rabbit-IgG, Texas Red anti-goat-IgG, and FITC anti-mouse-IgG were obtained from Vector Laboratories.
Inflammatory Cytokines
TNF-∀ and interleukin-1∃ (IL-1∃) were obtained from R&D Systems and were used at 10ng/ml and 10U/ml concentrations, respectively. Lipopolysaccharide (LPS) was obtained from Sigma and used at 1ug/ml.
Flow cytometry
Endothelial cells were cultured in EBM-B with or without TNF-∀ for the indicated times. Cells were washed twice with 10ml Hank’s balanced salt solution, incubated in 4ml dissociation buffer (Gibco) for 45 min at 37EC in a 5% CO2. Repeat pipetting was used to detach cells from plates. Cells were suspended further in 6 ml blocking buffer (RPMI-10%FBS) and centrifuged at 315 x g for 5 min. The cell pellet was resuspended in blocking buffer and cells were aliquoted for incubation with different primary antibodies (10ug/ml) for 45 min at 4EC. After washing twice, cells were incubated with secondary FITC-conjugated anti-mouse IgG at 4EC for 45min. Cells were then washed twice with blocking buffer, once with PBS with Ca++ /Mg++, and resuspended in 1% formaldehyde in PBS. Flow cytometric analyses were performed using a Becton Dickinson FACScan flow cytometer.
Adhesion assay
HPVEC and HDMEC plated on 35-mm dishes were assayed at 80% confluency as described (15). Briefly, cells were fed fresh EBM-B, with or without inflammatory stimuli, for 5h or 24h. For antibody blocking, the monolayers were incubated in 1ml EBM-B with antibody or isotype-control (10ug/ml) for 30 min at room temperature on a rotating platform prior to addition of leukocytes. Medium was removed and cell monolayers were washed once with RPMI with or without 2.5mM EGTA. HL-60 or U937 cells were centrifuged at 200 x g and resuspended in RPMI with or without 2.5mM EGTA at a concentration of 3.33×106 cells/ml. A total of 2×106 cells were added to the monolayers and incubated at 4EC on a rocking platform for 45 min, washed 5 times with 2ml RPMI with or without 2.5mM EGTA, and fixed with 2.5% glutaraldehyde in PBS. Bound leukocytes were counted in 10 randomly selected fields. The number of bound cells is expressed as mean +/− standard error of the mean. Statistical significance was determined using the Student’s t-test. A p value of less than 0.05 was considered statistically significant.
Results
Cytokine-Inducible Cell Adhesion Molecule Expression
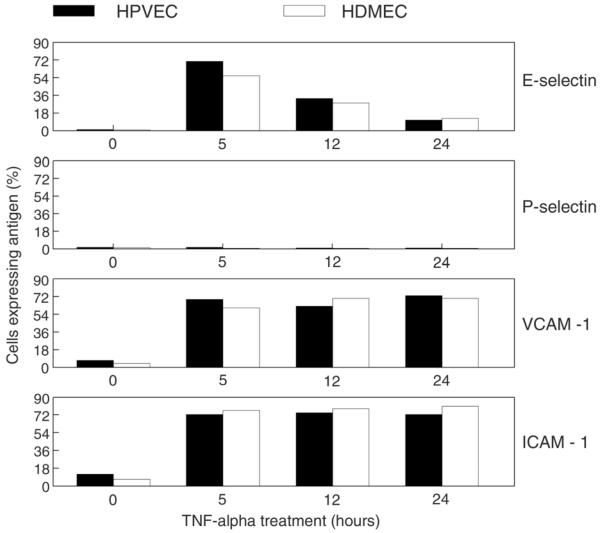
To determine if HPVEC upregulate leukocyte adhesion molecules, as has been described for other types of human endothelial cells, we analyzed the time course of expression of E-selectin, P-selectin, VCAM-1 and ICAM-1 in response to TNF-∀. Cell surface expression of each was detected by flow cytometry using well-characterized mAbs (13,15). As shown in Fig. 1, the time course and levels of expression observed in HPVEC and HDMEC were very similar. Approximately 70% of the cells expressed E-selectin after 5 hours of TNF-∀ treatment; levels were reduced to ~30% by 12 hours and to ~10% by 24 hours. As expected, cell surface P-selectin was not detected at any time point. In human endothelial cells, P-selectin is not induced by TNF-∀ (1). Both VCAM-1 and ICAM-1 were expressed at low basal levels (0 hours), but upregulated such that ~70% of the cells expressed antigen on the cell surface after 5, 12 and 24 hours of TNF-∀ treatment. The prolonged expression of VCAM-1 and ICAM-1 at 24 hours, when E-selectin expression levels returned towards basal levels, is in good agreement with studies on human umbilical vein endothelial cells - the model endothelial culture system in which these adhesion molecules were discovered (1). These results demonstrate that, in vitro, HPVEC upregulate leukocyte adhesion molecules in a manner very similar to what has been reported for large and small vessel-derived human endothelial cells.
Figure 1. Cell surface expression of leukocyte adhesion molecules.
HPVEC (black bars) and HDMEC (open bars) were treated with 10ng/ml TNF-∀ for 0, 5, 12, and 24 hours. Cells expressing E-selectin, P-selectin, VCAM-1, and ICAM-1 on the cell surface were measured by flow cytometry as described in Materials and Methods.
Functional analyses
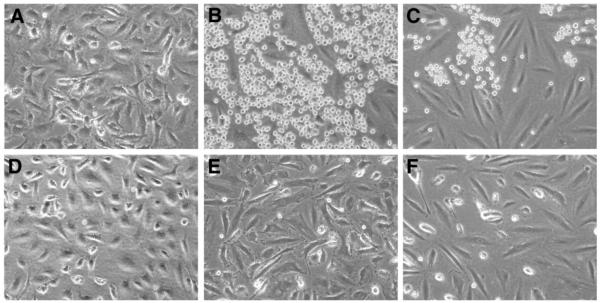
To determine if E-selectin, ICAM-1 and VCAM-1 expressed in HPVEC are functional, we assayed their ability to mediate leukocyte adhesion in a static cell adhesion (15) assay using HL-60 cells, a human promyelocytic leukemia line used as a model for human neutrophils. Adherance of HL-60 cells to TNF-∀-treated HPVEC and HDMEC monolayers is shown in Fig. 2. As expected, HL-60 cells did not adhere to non-stimulated HPVEC (Fig. 2A) because of the low or undectable expression of E-selectin, ICAM-1 or VCAM-1 in HPVEC. Robust binding to HPVEC treated with TNF-∀ was seen (Fig. 2B). Binding to HDMEC treated with TNF-∀ is shown for comparison (Fig. 2C). For each of these combinations, binding was abolished when EGTA was included, indicating calcium/magnesium-dependent binding (Fig. 2D-F).
Figure 2. HL-60 cell adhesion to HPVEC and HDMEC.
Phase contrast micrographs of HL-60 cell adhesion to HPVEC (Panels A, B, D, E) and HDMEC (Panels C and F). Prior to addition of HL-60 cells, endothelial cells were incubated without (Panels A and D) or with (Panels B, C, E, and F) TNF-∀ for 5 hours. Cell adhesion assays were carried out in the absence (Panels A-C) or presence (Panels D-F) of 2.5mM EGTA. Adherent HL-60 cells are the small, round, highly refractible bodies seen only in panels B and C. Magnification in all panels is 20x.
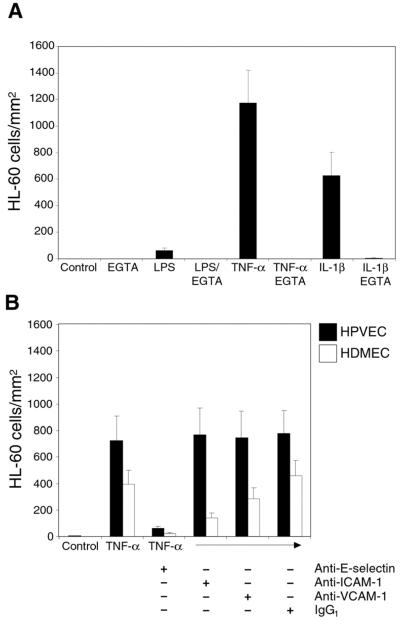
We next compared HL-60 cell adhesion to HPVEC treated with LPS, TNF-∀, or IL-1∃ for 5 hours. IL-1∃ and LPS are known to upregulate adhesion molecules in a manner similar to TNF-∀. Adhesion of HL-60 cells was performed in the absence and presence of EGTA. Quantification of HL-60 cell binding to HPVEC under these conditions is shown in Fig. 3A. As visualized in Fig. 2, HL-60 cells did not bind to control HPVEC monolayers in the absence or presence of EGTA. Robust binding was observed in cells treated with TNF-∀ or IL-1∃. Binding was completely blocked in presence of EGTA. A small amount of binding was observed in LPS-treated cells.
Figure 3. E-selectin-dependent adhesion of HL-60 cells to activated HPVEC and HDMEC.
(A) To determine which cytokine would result in the most robust adhesion of HL-60 cells, HPVEC were incubated with LPS (1ug/ml), TNF-∀ (10ng/ml), or IL-1∃ (10U/ml) for 5 hours. HL-60 cell adhesion was performed in the absence and presence of 2.5mM EGTA. The number of adherent cells in 10 randomly selected fields were counted and expressed as mean number of cells/field +/− standard error of the mean. (B) HPVEC (black bars) and HDMEC (open bars) were treated with 10ng/ml TNF-∀ for 5 hours. Endothelial cells were incubated with designated mAbs for 30 minutes prior to adding HL-60 cells. Mouse IgG1 served as a negative control. Adhesion was quantitated as in (A).
To determine which leukocyte adhesion molecule was responsible for the HL-60 cell binding, we analyzed binding to HPVEC and HDMEC that had been treated with TNF-∀ for 5 hours (Fig. 3B). Prior to addition of HL-60 cells, endothelial monolayers were incubated with antibodies against E-selectin, ICAM-1, or VCAM-1. Each of the antibodies has been shown previously to block cell adhesion of the respective adhesion molecule (13,15). Anti-E-selectin mAb abolished binding of HL-60 cells to both HPVEC (p =0.0032) and HDMEC (p = 0.0031). In contrast, anti-ICAM-1 and anti-VCAM-1 had no effect on HL-60 cell binding to HPVEC. Anti-ICAM-1 inhibited HL-60 cell binding to HDMEC by 65% (p = 0.041). Anti-VCAM-1 antibody had no effect on adhesion to HDMEC. An isotype-matched IgG1 mouse mAb served as a negative control.
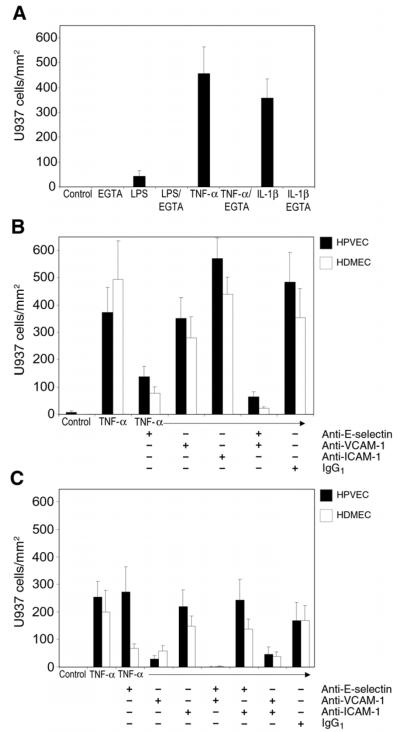
We next evaluated the ability of the monocytic cell line U937 to bind to HPVEC. U937 cells were derived from a histiocytic lymphoma and are used to model monocyte-endothelial adhesion interactions in which VCAM-1 is known to participate. HPVEC were activated as before with LPS, TNF-∀, and IL-1∃ and tested for their ability to support adhesion of U937 cells in the presence and absence of EGTA. Similar to what was observed with HL-60 cells, U937 cells bound to cells treated with TNF-∀ or IL-1∃, but less so to cells treated with LPS (Fig. 4A). Binding was abolished in the presence of EGTA, consistent with the requirement for divalent cations for binding to leukocyte integrins.
Figure 4. E-selectin- and VCAM-1-dependent adhesion of U937 cells to HPVEC and HDMEC.
(A) Inflammatory stimuli LPS (1ug/ml), TNF-∀ (10ng/ml), and IL-1∃ (10U/ml) were used to upregulate adhesion molecules in HPVEC. Adhesion of U937 cells was quantitated in the presence and absence of 2.5mM EGTA. (B and C) Endothelial cells were treated with TNF-∀ (10ng/ml) for 5h (B) or 24h (C) and incubated with mAbs for 30 minutes as designated. U937 cells were added in the presence or absence of 2.5mM EGTA. Adhesion was quantitated as described in Material and Methods.
U937 adhesion to HPVEC, and for comparison, to HDMEC, was measured in the presence or absence of the same function-blocking mAbs used above. Endothelial cells were treated with TNF-∀ for 5 hours to upregulate E-selectin, ICAM-1 and VCAM-1. Anti-E-selectin mAb substantially blocked adhesion of U937 cells to HPVEC (p = 0.014) and HDMEC (p = 0.0042) (Fig. 4B). Anti-VCAM-1 and anti-ICAM-1 antibodies had no significant effect on binding. Addition of both anti-E-selectin and anti-VCAM-1 antibody did not result in significantly greater inhibition compared to anti-E-selectin mAb alone. Thus, E-selectin appeared to mediate most of the U937 cell adhesion in this assay.
The same experiment was carried out on endothelial cells treated for 24 hours with TNF-∀. Under these conditions, only 10% of the cells express E-selectin while over 70% of the cells express VCAM-1 and ICAM-1 (Fig 1). In this experiment, anti-E-selectin mAb had no effect on U937 cell adhesion to HPVEC (Fig. 4C). In contrast, anti-VCAM-1 significantly blocked adhesion to HPVEC (p = 0.001), but did not have a statistically significant effect on adhesion to HDMEC. Anti-ICAM-1 mAb had no effect on U937 adhesion to either endothelial cell type. Combinations of mAb were also analyzed: anti-VCAM-1 and anti-E-selectin resulted in 100% inhibition (p = 0.0002); anti-ICAM-1 and anti-E-selectin had no effect; and anti-ICAM-1 and anti-VCAM-1 produced no greater inhibition than with anti-VCAM-1 alone. The additional inhibition seen with combined anti-E-selectin and anti-VCAM-1 was statistically significant compared to anti-VCAM-1 alone (p = 0.033). These results are consistent with decreased expression of E-selectin, compared to VCAM-1, after 24-hour treatment with TNF-∀. Adhesion of U937 cells to HDMEC was similar, with the exception that the only statistically significant inhibition was in presence of anti-VCAM-1 and anti-E-selectin (p = 0.022). Although the mechanism for this is not known, the results suggests a phenotypic difference in the activity of these adhesion molecules between HPVEC and HDMEC.
Comments
This study demonstrates that endothelial cells from human pulmonary valve, cultured in vitro, express the leukocyte adhesion molecules E-selectin, ICAM-1 and VCAM-1 when stimulated with the inflammatory cytokine TNF-∀. HL-60 and U937 cells, cell lines used to model neutrophilic- and monocytic-endothelial interactions respectively, adhered to HPVEC in a EGTA-sensitive manner. In HPVEC stimulated for 5 hours with TNF-∀, E-selectin, VCAM-1 and ICAM-1 are expressed at high levels; however, most of the leukocytic binding was inhibited by an anti-E-selectin neutralizing mAb. In contrast, in HPVEC stimulated for 24 hours with TNF-∀, in which VCAM-1 and ICAM-1 levels remain high but E-selectin levels are reduced, adhesion of U937 cells is mediated primarily by VCAM-1. These results are consistent with the well-established roles of E-selectin in early inflammatory events associated with acute rejection and ischemia/reperfusion injury, and with role of VCAM-1 in monocyte adhesion in prolonged chronic inflammatory events, such as occur in allograft rejection.
Use of cultured HPVEC allowed us to delineate the potential contribution of functional adhesion molecules from the contribution of hemodynamic forces exerted on the leaflet surface in vivo. In other words, the HPVEC, which we have shown retain a valve-specific phenotype in vitro (8), enabled us to test functionality of adhesion molecules in the absence of hemodynamic forces, which would not be possible in vivo. Cardiac valve endothelial cells, like lymphatic endothelial cells (16) and infantile hemangioma-derived endothelial cells (14,17), are excellent examples of endothelial cells isolated from specific vascular beds and studied in vitro that have enabled investigators to identify unique aspects of the endothelial phenotypes and which have been corroborated by in vivo findings.
Expression of E-selectin, VCAM-1 and ICAM-1 in vivo in heart valve specimens obtained after surgical removal of diseased valves has been reported in two recent studies. In the first study, 34 degenerative aortic valves were analyzed (18). Although affected by fibrosis and calcification, no signs of acute or chronic inflammation were evident in the valves. All three adhesion molecules were detected on many of the valves analyzed: 21/34 were positive for E-selectin; 25/34 positive for VCAM-1 and 15/34 were positive for ICAM-1. In the second study, out of the 22 aortic valves analyzed by immunohistochemistry, over two-thirds were positive for E-selectin, VCAM-1 and ICAM-1 (19). These findings support our in vitro results and indicate that these adhesion molecules can be expressed on valve leaflet surface endothelium in vivo. Thus, the lack of inflammatory cell infiltration into valve leaflets seen in cardiac transplant recipients cannot be explained by the absence or attenuated expression of these adhesion molecules. Understanding the function of these adhesion molecules in vivo will require further investigation.
Several lines of evidence suggest that the valvular endothelium is unique compared to other types of endothelium. These include defects in valvular endothelium and valvulogenesis that have been noted in a number of gene “knockout” experiments in mice. For example, mice in which the transcription factor NFATc1 has been knocked out fail to form aortic and pulmonary valves (8,9), whereas the rest of the vasculature forms normally. We showed recently that NFATc1 is required for maximal VEGF-induced proliferation in HPVEC, suggesting a post-natal role for NFATc1 in cardiac valves (7). Other knockouts with defective valvulogenesis include endoglin, Smad6, and neurofibromin-1 (20-22). Mice mutant for Egfr and Shp2 have also been found to have defects in valvulogenesis (23). Such studies suggest unique pathways exist in endothelium of developing heart valves. Whether such pathways affect the phenotype and function of valvular endothelium in post-natal valve remains to be investigated.
Understanding features that are unique to valve endothelium, as well as common features shared with other types of endothelium will contribute greatly to the many efforts underway to develop improved heart valve substitutes, in particular in the creation of tissue-engineered heart valves using autologous endothelial cells. Our results indicate that it will not be necessary to attenuate or inhibit expression of leukocyte adhesion molecules in autologous cells used to endothelialize tissue-engineered valve replacements in order to mimic the valvular endothelium.
Acknowledgements
The authors would like to thank Bill Luscinskas in the Vascular Research Divsion at Brigham and Women’s Hospital for providing monoclonal antibodies against human endothelial adhesion molecules and for technical advice on measuring adhesion molecules by flow cytometry. We would also like to thank Dr. John E. Mayer, Jr, Cardiovascular Surgery at Children’s Hospital Boston for providing human pulmonary valve leaflet tissue for the isolation of HPVEC. This work was supported by R01 HL60490 from the National Heart Lung and Blood Institute.
References
- 1.Bevilacqua MP. Endothelial-leukocyte adhesion molecules. Ann. Rev. Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 2.Ley K. Molecular mechanisms of leukocyte recruitment in the inflammatory process. Cardiovasc. Res. 1996;32:733–742. [PubMed] [Google Scholar]
- 3.Mitchell RN, Jonas RA, Schoen FJ. Pathology of explanted cryopreserved allograft heart valves: comparison with aortic valves from orthotopic heart transplants. J.Thorac. Cardiovasc. Surg. 1998;115:118–127. doi: 10.1016/s0022-5223(98)70450-7. [DOI] [PubMed] [Google Scholar]
- 4.Chen RH, Kadner A, Mitchell RN, Adams DH. Fresh porcine cardiac valves are not rejected in primates. J. Thorac.Cardiovasc. Surg. 2000;119:1216–1220. doi: 10.1067/mtc.2000.106526. [DOI] [PubMed] [Google Scholar]
- 5.Simon A, Zavazava N, Sievers HH, Muller-Ruchholtz W. In vitro cultivation and immunogenicity of human cardiac valve endothelium. J. Cardiac Surg. 1993;8:656–665. doi: 10.1111/j.1540-8191.1993.tb00426.x. [DOI] [PubMed] [Google Scholar]
- 6.Simon A, Wilhelmi M, Steinhoff G, Harringer W, Brucke P, Haverich A. Cardiac valve endothelial cells: relevance in the long-term function of biologic valve prostheses. J. Thorac. Cardiovasc. Surg. 1998;116:609–616. doi: 10.1016/s0022-5223(98)70167-9. [DOI] [PubMed] [Google Scholar]
- 7.Johnson EN, Lee YM, Sander TL, Rabkin E, Schoen FJ, Kaushal S, Bischoff J. NFATc1 mediates VEGF-induced proliferation in human pulmonary valve endothelial cells. J. Biol. Chem. doi: 10.1074/jbc.M210250200. in press, published on-line 11.09.02 www.jbc.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Pompa JL, Timmerman LA, Takimoto H, Yoshida H, Elia AJ, Samper E, Potter J, Wakeham A, Marengere L, Langille BL, Crabtree GR, Mak TW. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature. 1998;392:182–186. doi: 10.1038/32419. [DOI] [PubMed] [Google Scholar]
- 9.Ranger AM, Grusby MJ, Hodge MR, Gravallese EM, de la Brousse FC, Hoey T, Mickanin C, Baldwin HS, Glimcher LH. The transcription factor NF-ATc is essential for cardiac valve formation. Nature. 1998;392:186–189. doi: 10.1038/32426. [DOI] [PubMed] [Google Scholar]
- 10.Paranya G, Vineberg S, Dvorin E, Kaushal S, Roth SJ, Rabkin E, Schoen FJ, Bischoff J. Aortic valve endothelial cells undergo transforming growth factor-beta-mediated and non-transforming growth factor-b-mediated transdifferentiation in vitro. Am. J. Path. 2001;159:1335–1343. doi: 10.1016/s0002-9440(10)62520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson CJ, Garbett PK, Nissen B, Schrieber L. Binding of human endothelium to Ulex europaeus I-coated Dynabeads: application to the isolation of microvascular endothelium. J. Cell Sci. 1990;96:257–262. doi: 10.1242/jcs.96.2.257. [DOI] [PubMed] [Google Scholar]
- 12.Kraling BM, Bischoff J. A simplified method for growth of human microvascular endothelial cells results in decreased senescence and continued responsiveness to cytokines and growth factors. In Vitro Cell Dev. Biol. 1998;33:308–315. doi: 10.1007/s11626-998-0007-z. [DOI] [PubMed] [Google Scholar]
- 13.Boyce JA, Mellor EA, Perkins B, Lim YC, Luscinskas FW. Human mast cell progenitors use alpha4-integrin, VCAM-1, and PSGL-1 E-selectin for adhesive interactions with human vascular endothelium under flow conditions. Blood. 2002;99:2890–2896. doi: 10.1182/blood.v99.8.2890. [DOI] [PubMed] [Google Scholar]
- 14.Boye E, Yu Y, Paranya G, Mulliken JB, Olsen BR, Bischoff J. Clonality and altered behavior of endothelial cells from hemangioma. J. Clin. Invest. 2001;107:745–752. doi: 10.1172/JCI11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiely JM, Luscinskas FW, Gimbrone MJ. Leukocyte-endothelial monolayer adhesion assay (static conditons) Meth.Mol.Biol. 1999;96:131–136. doi: 10.1385/1-59259-258-9:131. [DOI] [PubMed] [Google Scholar]
- 16.Petrova TV, Makinen T, Makela TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Yla-Herttuala S, Alitalo K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 hemeobox transcription factor. EMBO J. 2002;21:4593–4599. doi: 10.1093/emboj/cdf470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu Y, Varughese J, Brown LF, Mulliken JB, Bischoff J. Increased Tie2 expression, enhanced response to angiopoietin-1, and dysregulated angiopoietin-2 expression in hemangioma-derived endothelial cells. Am. J. Path. 2001;159:2271–2280. doi: 10.1016/S0002-9440(10)63077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller AM, Cronen C, Kupferwasser LI, Oelert H, Muller KM, Kirkpatrick CJ. Expression of endothelial cell adhesion molecules on heart valves: up-regulation in degeneration as well as acute endocarditis. J.Pathol. 2000;191:54–60. doi: 10.1002/(SICI)1096-9896(200005)191:1<54::AID-PATH568>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 19.Ghaisas NK, Foley JB, O’Briain S, Crean P, Kelleher D, Walsh M. Adhesion molecules in nonrheumatic aortic valve disease: endothelial expression, serum levels and effects of valve replacement. J. Amer. Col. Cardiol. 2000;36:2257–2262. doi: 10.1016/s0735-1097(00)00998-0. [DOI] [PubMed] [Google Scholar]
- 20.Bourdeau A, Dumont DJ, Letarte M. A murine model of hereditary hemorrhagic telangiectasia. J. Clin. Invest. 1999;104:1343–1351. doi: 10.1172/JCI8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galvin KM, Donavan MJ, Lynch CA, Meyer RI, Paul RJ, Lorenz JN, Fairchild-Huntress V, Dixon KL, Dunmore JH, Gimbrone MJ, Falb D, Huszar D. A role for Smad6 in development and homeostasis of the cardiovascular system. Nature Medicine. 2000;24:171–174. doi: 10.1038/72835. [DOI] [PubMed] [Google Scholar]
- 22.Lakkis MM, Epstein JA. Neurofibromin modulation of ras activity is required for normal endocardial-mesenchymal transformation in the developing heart. Dev. 1998;125:4359–4367. doi: 10.1242/dev.125.22.4359. [DOI] [PubMed] [Google Scholar]
- 23.Chen B, Bronson RT, Klamen LD, Hampton TG, Wang JF, Green PJ, Magnuson T, Douglas PS, Morgan JP, Neel BG. Mice mutant for Egfr and Shp2 have defective cardiac semilunar valvulogenesis. Nature Genetics. 2000;24:296–299. doi: 10.1038/73528. [DOI] [PubMed] [Google Scholar]