Abstract
Purpose
TGFβ is the major mediator to induce myofibroblast differentiation in the corneal wound-healing process. Elevated cAMP can reduce TGFβ-induced fibrosis in other tissues. This study was conducted to determine whether elevated cAMP can inhibit TGFβ1-induced rabbit corneal keratocyte–myofibroblast transformation.
Methods
Primary isolated rabbit corneal keratocytes were cultured in serum-free medium. The effects of the adenylate cyclase agonist forskolin (FSK; 2 μM) on TGFβ1 (5 ng/mL)-induced α-smooth muscle actin (α-SMA) expression was examined by immunofluorescence, flow cytometry, and immunochemistry 72 hours after treatment. The effects of TGFβ+FSK on activated pSmad3, CREB binding protein (CBP), MAPKs, and RhoA were determined by coimmunoprecipitation and Western blot.
Results
FSK significantly reduced the myofibroblast phenotype and α-SMA expression induced by TGFβ1 in rabbit corneal keratocytes. TGFβ1 increased the phosphorylation of ERK and Smad3. TGFβ1-induced α-SMA expression was reduced by MEK inhibition (U0126); however, the levels of pERK, pSmad3, or the extent of the interaction between pSmad3 and CBP induced by TGFβ1 were not affected by FSK. TGFβ1 also activated RhoA and ROCK (Y27632) inhibition reduced α-SMA expression. Activation of RhoA was significantly reduced by FSK.
Conclusions
Raising cAMP by FSK treatment inhibits the TGFβ1-induced corneal myofibroblast transformation and α-SMA expression and thereby provides a promising method to control corneal fibrosis. The data suggest that cAMP-dependent inhibition does not occur by altering Smads or MAPK signaling, but possibly by reducing the activation of RhoA.
Corneal wound healing is a complex process that involves keratocyte apoptosis, activated keratocyte proliferation, myofibroblast transformation, and abnormal collagen formation.1 Myofibroblasts have a contractile and refractile phenotype distinguished by the expression of α-SMA, elevated fibronectin secretion, decreased crystallin expression,2 and altered extracellular matrix formation which dramatically changes corneal optical properties. Extensive corneal wound healing that involves myofibroblast transformation severely impairs corneal transparency and visual acuity.
Transforming growth factor (TGF)-β is a major mediator of fibrotic wound repair in the cornea and is necessary for corneal fibroblast or keratocyte transformation to the myofibroblast phenotype.3–5 TGFβ is mainly produced and stored in corneal epithelial cells. TGFβ can diffuse to the stroma when the basement membrane is broken6 and stimulate TGFβ receptors on keratocytes. TGFβ activates the type 2 TGFβ receptor and the type 2 receptor-mediated type 1 receptor, which phosphorylates the Smad transcription factors (Smad2 and Smad3) that combine with common Smad (Smad4) and enter the nucleus to exert its regulatory function. Smads are the major effectors of TGFβ signaling leading to cellular responses such as cell cycle regulation and altered extracellular matrix component expression. Smads will recruit transcriptional coactivators (e.g., CREB binding protein [CBP]/p3007) for DNA binding, which adds another dimension to regulation of the repair phenotype.
TGFβ signaling is cell-type specific and can sometimes activate Smad-independent pathways, such as mitogen-activated protein kinase (MAPK)8–11 and Rho.12 For example, in cardiac fibroblasts TGFβ-induced myofibroblast transformation includes ERK1/2 activation,13 whereas the N-terminal kinases (JNKs), the p38 kinases, and the extracellular signal–related kinases (ERKs) have been associated with TGFβ-induced α-SMA expression in human fetal lung fibroblasts.14 TGFβ can also activate the small GTP-binding protein RhoA, which has been found to mediate epithelial-to-mesenchymal transdifferentiation in several epithelial cell lines.15 Rho-associated kinase (ROCK) is the major effector of active RhoA and inhibition of ROCK inhibits the downstream effect of RhoA.16 The RhoA/Rho-kinase pathway has been shown to be an important component of TGFβ-induced effects on pulmonary endothelial cytoskeletal reorganization and barrier integrity.17 The RhoA pathway has been shown to be involved in α-SMA expression in TGFβ and serum-induced myofibroblasts in rabbit cornea.18 These examples illustrate that activation of Smad-independent pathways like MAPK and RhoA is involved in TGFβ signaling and their involvement is cell-type specific.
Downregulating myofibroblast transformation after corneal surgery or trauma could improve visual outcome. Neutralizing antibody to TGFβ can reduce corneal fibrotic wound healing in animal models19,20 and in corneal organ culture.21 Fibroblast growth factor acting through focal adhesion kinase has also been shown to downregulate α-SMA expression in corneal fibroblasts.22 In pulmonary23 and cardiac fibroblasts,13 elevated [cAMP] was shown to prevent TGFβ-induced myofibroblast transformation and collagen synthesis. Elevated cAMP in cardiac fibroblasts can inhibit TGFβ-induced transformation through ERK1/2 activation and by activating CREB, which recruits CBP1, effectively competing with Smad transcriptional complexes for binding to CBP1.13 The effect of elevated cAMP on TGFβ-induced corneal keratocyte differentiation has not been studied. We tested whether elevated cAMP could reduce keratocyte myofibroblast transformation induced by TGFβ and explored the possible involvement of CBP competition, ERK activation, and RhoA signaling pathways.
Materials and Methods
Medium, additives, Alexa Fluor 488 goat anti-mouse IgG, and collagenase were purchased from Invitrogen (Carlsbad, CA). TGFβ1 was obtained from Biosource (Camarillo, CA). TGFβ2, forskolin (FSK), Y27632 (ROCK inhibitor), and monoclonal anti-α-SMA antibody was obtained from Sigma-Aldrich Chemical Company (St. Louis, MO). Texas Red-X phalloidin was purchased from Invitrogen-Molecular Probes. Phospho-MAPK family antibody and U0126 (MEK1/2 inhibitor) were purchased from Cell Signaling Technology (Danvers, MA).
Cell Culture and Cell Treatment
New Zealand White rabbit eyes were delivered from Pel-Frez (Rogers, AR). Rabbit corneal keratocytes were cultured as previously described.24 Briefly, the epithelium was scraped off, the cornea was dissected, and the endothelium was wiped off. The entire stroma was put into DMEM with 2 mg/mL collagenase, 0.5 mg/mL hyaluronidase and antibiotics at 37°C overnight. The resultant isolated cells were washed once in DMEM and seeded in serum-free DMEM with nonessential amino acids, MEM vitamins, and sodium pyruvate onto 12-mm glass coverslips double coated with poly-L-lysine and collagen for immunocytochemistry and onto 60 or 100-mm Petri dishes for immunochemistry, pull-down assays, and flow cytometry, at 5 × 104 cells/cm2. For immunochemistry, FSK 2 μM and TGFβ1 5 ng/mL were used to treat the cells, unless otherwise stated. For 4- and 24-hour treatments, the drugs were added once. For 72-hour treatments, the drugs were added every day with medium changed every other day.
Immunocytochemistry
The cells were washed with fresh medium and fixed in medium containing 3% paraformaldehyde for 10 minutes at room temperature. The fixed cells were then permeabilized with acetone (−20°C for 5 minutes). Coverslips were dried at RT and immediately rehydrated with PBS for 5 minutes, blocked in PBS–goat serum (1:1) for 1 hour, incubated with anti-αSMA (Sigma-Aldrich) at 1:100 in blocking medium for 1 hour at RT, washed twice with PBS, and then washed in blocking medium for 10 minutes. The cells were then incubated in goat anti-mouse IgG Alexa Fluor 488 at 1:20, together with 1:200 Texas red phalloidin (Invitrogen-Molecular Probes) for 40 minutes; washed twice with PBS for 10 minutes; quickly washed with dH2O; and mounted in medium (Prolong; Invitrogen-Molecular Probes) with DAPI.
Immunochemistry
For Western blots, the cells were incubated with lysis buffer for 5 minutes, scraped, and centrifuged at 14,000g for 10 minutes. The supernatants were collected and stored at −80°C. Protein concentrations were determined by the BCA method. Samples were separated on 10% polyacrylamide-SDS gels and electroblotted onto PVDF membranes. After blocking with PBST/5% skim milk, the membrane was incubated overnight at 4°C with primary antibodies against αSMA at 1:1000, followed by peroxidase conjugated anti-mouse IgG at 1:1000 for 1 hour at room temperature. Signals were detected by chemiluminescence. Each experiment was repeated at least three times. For statistical analysis, band density was analyzed (Un-scan-it gel analysis software; Silk Scientific, Orem, UT). The density of the target band is referenced to the loading control.
Flow Cytometry
Rabbit corneal keratocytes (1.5 × 106) in 100-mm Petri dishes were detached with 0.25 mg/mL trypsin for 3 minutes, collected, and fixed in 2% paraformaldehyde for 5 minutes at RT, permeabilized in 0.02% Triton with buffered PBS, blocked with 1:1 PBS–filtered goat serum for 1 hour, stained with mouse anti-αSMA antibody (1:100) in blocking medium for 40 minutes at RT, washed twice with PBS, and stained with goat anti-mouse IgG Alexa Fluor 488 (Invitrogen-Molecular Probes) at 1:20 for 40 minutes. The cells were washed twice, and at least 5000 cells/sample were loaded in a flow cytometer (FACSCalibur; BD Bioscience, San Jose, CA) and analyzed (CellQuest software; BD Biosciences-Pharmingen) by using manual gating.
Coimmunoprecipitation (Co-IP)
Co-IP was performed both on whole-cell lysates and nuclear extracts (ExactaCruz C: sc-45040; Santa Cruz Biotechnology, Santa Cruz, CA and One-Step IP-Western Kit; GenScript Corporation, Piscataway, NJ). Keratocytes (1.2 × 106) were plated onto 100-mm plates in serum-free DMEM, as stated earlier. Monoclonal mouse anti-CBP from two companies was pooled to increase the binding efficiency. IP matrix (100 μL) with 15 μL of anti-CBP from Chemicon (Temecula, CA) and 15 μL of anti-CBP from R&D Systems (Minneapolis, MN) was incubated by end-over-end rotation overnight at 4°C, to make the IP antibody–IP matrix solution. The cells were then treated with FSK, alone or together with TGFβ1 for 4 hours. Whole-cell lysates or nuclear extracts were collected using the specified lysis buffer together with proteinase and phosphatase inhibitors, according to the manufacturer’s protocol (Pierce, Rockford, IL). The lysate was incubated with IP antibody-matrix by end-over-end rotation for 2 hours at room temperature to pull-down CBP. Eluates from the pull-down were normalized according to protein concentration, run on 10% polyacrylamide-SDS gels, and electroblotted onto nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA). The membrane was cut in half along the 100-kDa marker. The higher molecular weight half was blotted with pooled anti-CBP antibody to check the loading. The lower molecular weight half was blotted with anti-pSmad3 (R&D Systems) using Western Blot detection (One-Step; GenScript).
RhoA Activity Assay
RhoA activity was measured semiquantitatively by pull-down assay (cat. no. BK036; Cytoskeleton, Denver, CO), according to the manufacturer’s protocol. Cells in 60-mm Petri-dishes were lysed in 550 μL of lysis buffer and cleared at 10,000g for 2 minutes, and 450 μL supernatant was added to 25 μg rhotekin-RBD beads and incubated for 1 hour at 4°C. The samples were washed once with washing buffer, boiled with 5× Laemmli sample buffer, run on 12% polyacrylamide-SDS gels, electroblotted onto PVDF membranes and detected with 1:2000 mouse anti-RhoA antibody. Supernatant (35 μL) was used to detect total RhoA.
Myofibroblast Cell Counting
For keratocytes cultured on 12-mm coverslips, five random, distinct, 200× microscopic fields were photographed on each coverslip. DAPI(3) cells were counted to obtain the total cell count. DAPI(+) and αSMA(+) cells were counted as myofibroblasts. Each experimental condition had duplicate or triplicate coverslips. Data were collected from approximately 750 cells for each condition in each experiment. Experiments were repeated at least three times, giving a total of at least 2000 cells counted per condition. Data are expressed as the mean ± SE.
Statistical Analysis
Data are presented as the mean ± SE for at least three separate experiments. Student’s t-test was used for statistical analysis, with significance of differences set at P < 0.05.
Results
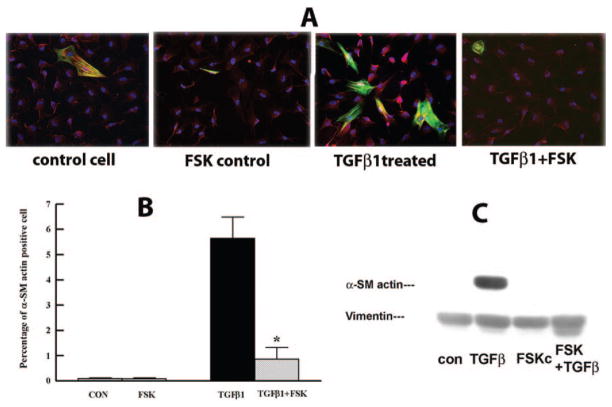
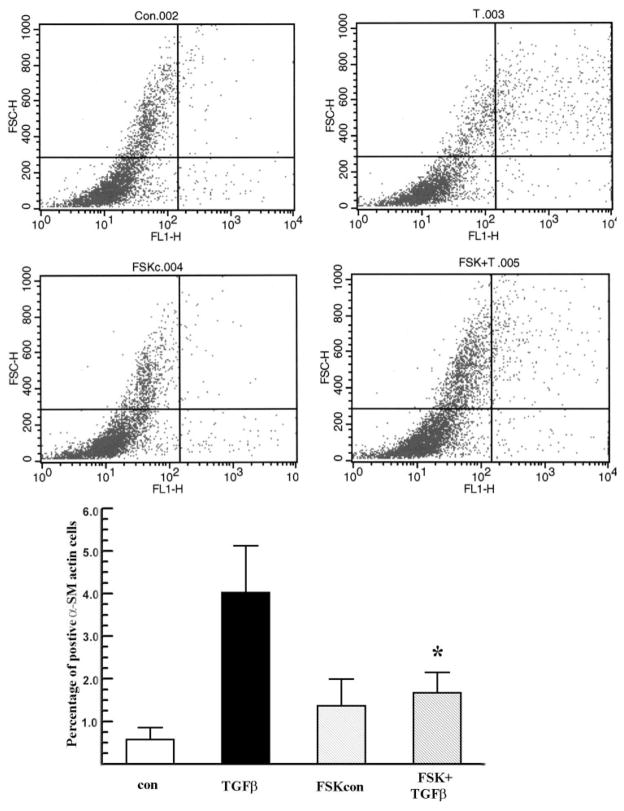
cAMP elevating reagents like PGE2 and FSK were found to inhibit the transformation of pulmonary fibroblasts to the myofibroblast phenotype.23,25 We tested whether FSK (an activator of adenylate cyclase) has an inhibitory effect on rabbit keratocyte–myofibroblast transformation induced by TGFβ1. TGFβ1 5 ng/mL induced 4.8% ± 1% of keratocytes to become myofibroblasts (Fig. 1A) at 72 hours, as observed by α-SMA immunocytochemistry. F-actin staining did not change in the presence of FSK or TGFβ in untransformed keratocytes. Figure 1B shows that myofibroblast counts were reduced by FSK from 5.7% ± 1.2% to 0.9% ± 0.4%. FSK also greatly reduced the α-SMA protein level induced by TGFβ1, as shown by Western blot (Fig. 1C). FSK by itself had no significant effect on inducing the myofibroblast phenotype or α-SMA protein level compared with control nontreated cells. To confirm our finding that FSK reduced the TGFβ1-induced myofibroblast phenotype in rabbit corneal keratocytes, we used the more objective flow cytometry analysis of α-SMA staining. Figure 2 shows that FSK significantly reduced detection of α-SMA from 4.2% ± 1.0% to 1.8% ± 0.4% in TGFβ-treated keratocytes. These results confirm that FSK is a significant inhibitor of TGFβ1-induced rabbit keratocyte myofibroblast transformation.
Figure 1.
FSK inhibits TGFβ1-induced myofibroblast transformation. Primary rabbit corneal keratocytes cultured on coverslips were treated with FSK 2 μM with or without TGFβ1 5 ng/mL for 72 hours. The cells were stained for α-SMA (green) and F-actin (red) and counterstained with DAPI (blue) or collected to detect α-SMA by Western blot. (A) Representative images from the indicated groups. Magnification, ×200. (B) Bar graph shows the percentage of α-SMA-positive cells over the total cell count. Five random fields were selected from each coverslip, at least 750 cells were counted per coverslip (n = 4). Error bars represent the SEM. *Significantly different from TGFβ, P < 0.05. (C) Representative Western blot shows α-SMA protein level under each experimental condition. Whole-cell lysates were collected 72 hours after treatment and were probed for α-SMA. Vimentin was used as the loading control.
Figure 2.
Flow cytometry analysis of α-SMA staining. Rabbit keratocytes were treated with TGFβ1, with or without FSK. The cells were stained for α-SMA 72 hours after treatment and assessed by flow cytometry. Representative flow data from each group are shown. The x-axis represents the intensity of AlexaFluor 488 (α-SMA); the y-axis shows forward scatter, which represents the size of the objects. The bar graph shows a percentage of α-SMA positive cells over total cell count in each group. Error bars represent the SEM (n = 4). *Significantly different from TGFβ, P < 0.05.
Role of pSmad3
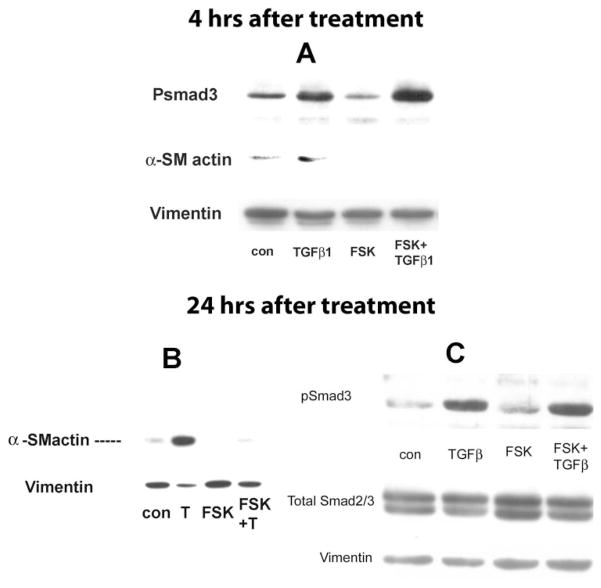
The proteins of the Smad family are the first identified substrate of type I receptor kinases and play a central role in TGFβ signaling. pSmad2/3 is considered the final step of phosphorylation and the component of the pSmad complex that translocates to the nucleus to regulate target genes. In this study, we asked whether FSK reduces α-SMA expression by reducing the pSmad3 level. Considering that phosphorylation usually occurs in a short period, we measured the pSmad3 level and corresponding α-SMA expression level using whole-cell lysates at 4 and 24 hours after treatment.
Figure 3 shows that the pSmad3 level was significantly higher in the TGFβ1-treated group relative to that in the control at 4 (Fig. 3A) and 24 (Fig. 3C) hours. FSK alone did not significantly change the baseline levels of pSmad3 and α-SMA. The pSmad3 level in the FSK+TGFβ1-treated group was at the same level as TGFβ1 alone at both 4 (Fig. 3A) and 24 (Fig. 3C) hours after treatment, whereas α-SMA levels in the FSK+TGFβ1-treated group at both 4 (Fig. 3A) and 24 (Fig. 3B) hours was greatly reduced compared with TGFβ1 alone. These results indicate that the FSK-dependent reduction in TGFβ1-induced α-SMA expression was independent of the level of pSmad3.
Figure 3.
FSK had no effect on TGFβ1-induced pSmad3 increase. The cells were treated with TGFβ1 (5 ng/mL), with or without FSK (2 μM) for 4 (A) and 24 (B, C) hours. (A) Whole-cell lysates were collected at 4 hours after treatment and examined for pSmad3 expression by Western blot. The blots were stripped and examined for α-SMA. Whole-cell lysates were collected at 24 hours after treatment and examined for α-SMA (B), pSmad3, and total Smad2/3 (C). Vimentin was used as the loading control. Blots are representative of three experiments.
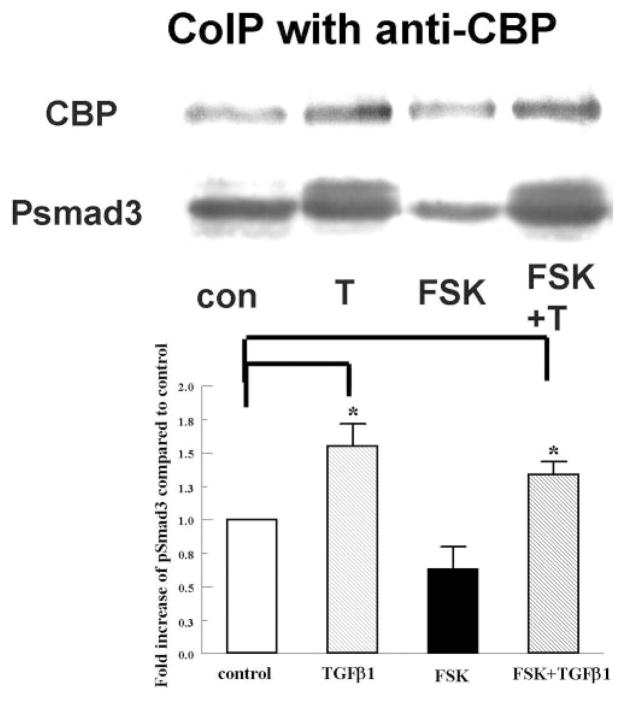
Although pSmad3 levels are not altered, it is possible that FSK interferes downstream of Smad3 phosphorylation by reducing the combination of the pSmads complex with its coactivator CBP, which is recruited when [cAMP] is elevated. In cardiac fibroblasts, increasing [cAMP] recruits cAMP-binding protein CBP/P300, effectively competing with pSmads.13 We tested the role of elevated cAMP in pSmad3 interaction with CBP in our system by coimmunoprecipitation of CBP from nuclear extracts.
Figure 4 shows that the CBP-bound pSmad3 levels in both TGFβ1 (1.6 ± 0.25-fold)- and TGFβ1+FSK (1.4 ± 0.1-fold)-treated groups were significantly increased over that in the control and there was no significant difference in phosphorylation level in nuclear extracts between these two groups. These results indicate that significant interaction of pSmads with CBP can be induced by TGFβ1, but the level of this interaction was not altered by FSK. A similar result was obtained using whole cell lysates (data not shown).
Figure 4.
FSK did not alter the interaction between pSmad3 and CBP. Nuclear extracts were collected 4 hours after the corresponding treatment and immunoprecipitated with anti-CBP antibody. Eluates were separated by SDS-PAGE and probed for pSmad3. Blots were probed for CBP as an internal control. The graph shows the increase of pSmad3, relative to CBP, in each group over the control. Error bars, SEM (n = 3). *Significantly different from the control, P < 0.05.
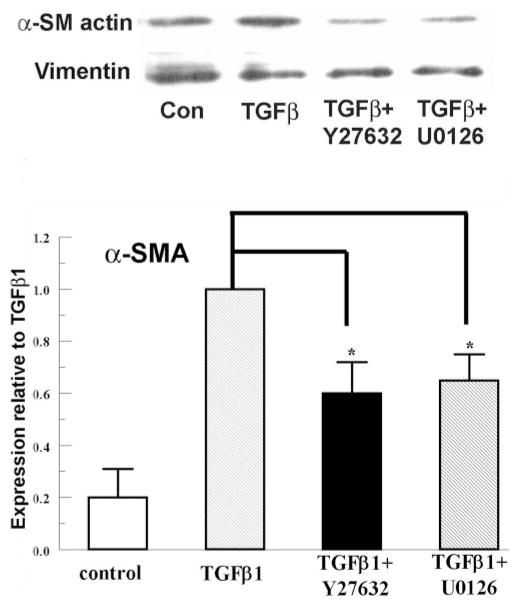
Role of pERK and RhoA/ROCK
pERK is involved in TGFβ signaling in cardiac fibroblasts,23 and the ROCK inhibitor (Y27632) has been demonstrated to reduce TGFβ1-induced α-SMA in corneal myofibroblasts.18 We tested this involvement in rabbit keratocytes. Figure 5 shows that the ROCK (Y27632) and MEK1/2 (U0126) inhibitors significantly reduced the α-SMA expression (to 62% ± 14% and 68% ± 11%, respectively), compared with TGFβ1 alone. These results confirm that pERK and RhoA/ROCK signaling is involved in TGFβ1-induced α-SMA expression in rabbit keratocytes, which leads to further testing for possible modulation by cAMP.
Figure 5.
pERK, ROCK inhibitors, reduced TGFβ1-induced α-SMA in rabbit keratocytes. Y-27632 10 μM or U0126 10 μM was added to rabbit keratocytes 20 minutes before TGFβ1. Whole cell lysates were collected 4 hours after treatment and probed for α-SMA by Western analysis. Vimentin was used as the loading control. The graph shows α-SMA expression relative to TGFβ1. Error bars, SEM (n = 3). *Significantly different from TGFβ, P < 0.05.
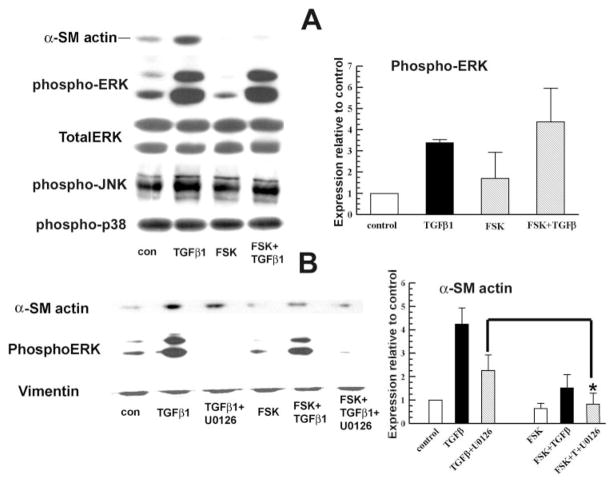
In mammalian cells, three mitogen-activated protein kinase families (ERK, p38 kinase, and JNK) have been clearly characterized. MAPK signaling plays an important role in cell proliferation, differentiation, and apoptosis.26 TGFβ can activate MEK/ERK signaling in mesangial cells.27 Studies in cardiac fibroblasts showed that increased cAMP markedly blocks TGFβ-mediated activation of collagen synthesis and α-SMA expression via the inhibition of ERK1/2 and JNK phosphorylation.13 To examine possible interactions with cAMP, we measured α-SMA and phospho-ERK, -JNK, and -p38 levels 4 hours after treatment of keratocytes with TGFβ1, with and without FSK.
Figure 6A shows that pERK was significantly higher in TGFβ1-treated cells than in control cells at 4 hours after treatment. This increase in ERK phosphorylation was not changed significantly by adding FSK to TGFβ1. These results, together with the inhibition of α-SMA expression by the MEK1/2 inhibitor, suggest that ERK plays an important positive role in TGFβ-induced α-SMA expression, but that this activation is not altered by FSK. Figure 6B shows that the addition of FSK to TGFβ1+U0126 treated cells produced a further significant reduction in α-SMA expression, suggesting that the inhibition of α-SMA by U0126 and FSK are independent. Last, Figure 6 indicates that there was no change in pJNK or phospho-p38 levels in all experimental groups at 4 hours after treatment. These results suggest that FSK inhibition of TGFβ1-induced α-SMA expression is not activated directly through MAPK signaling in rabbit keratocytes.
Figure 6.
FSK inhibition of TGFβ1-induced α-SMA was independent of MAPK activation. Whole-cell lysates were collected at 4 hours after treatment. (A) Western blot for pERK, pJNK, and phospho-p38. Total ERK was used as an internal control. The graph shows the relative increase of pERK in each group over the control. Error bars, SEM (n = 3). (B) MEK inhibitor U0126 10 μM was applied 20 minutes before TGFβ1, with or without FSK. Whole cell lysates were collected 4 hours after treatment and probed for α-SMA and pERK. Vimentin was detected as the loading control. Graph shows the relative change of α-SMA over the TGFβ1 group. Error bars, SEM (n = 3). *Significantly different from TGFβ+U0126, P < 0.05.
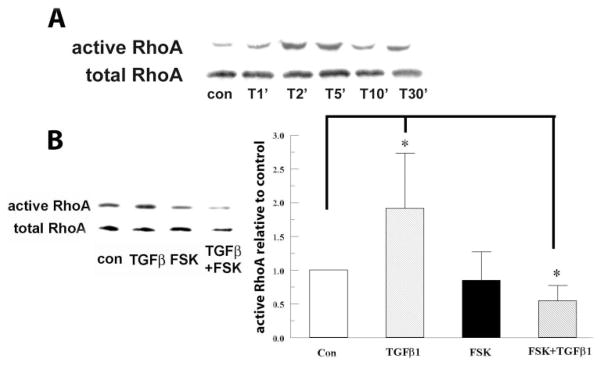
The induction of RhoA by TGFβ has been shown to be rapid and transient in an epithelial cell line,28 but persistent in vascular smooth muscle cells (VSMCs).29 The induction profile in corneal fibroblasts and keratocytes has not been documented yet. In the present study, we asked whether FSK could reduce TGFβ1-induced RhoA activation in rabbit corneal keratocytes.
Figure 7A shows that on TGFβ1 stimulation, RhoA peaked between 2 and 5 minutes and returned to the control level at ~10 minutes in rabbit keratocytes. TGFβ1-induced a 1.92 ± 0.83-fold increase in active RhoA compared with the control; whereas FSK reduced TGFβ1-induced active RhoA to the control level (Fig. 7B) at 5 minutes after TGFβ1 stimulation.
Figure 7.
RhoA was involved in FSK inhibition. (A) TGFβ1 5 ng/mL was added to rabbit keratocytes for the indicated durations. The cells were lysed, GTP-bound RhoA was pulled down and assayed by Western blot with anti-RhoA antibody. Total RhoA from whole-cell lysates was detected as the internal control. The blot is representative of three experiments. (B) The cells were treated with FSK 2 μM and TGFβ1 5 ng/mL for 5 minutes. The graph represents change of active RhoA/total RhoA relative to control. Error bars, SEM (n = 3). *Significantly different from control, P < 0.05.
Discussion
Induced scar formation during fibrotic wound healing can compromise visual performance after corneal transplantation, corneal trauma, infection, or refractive surgery. Efforts have been made to control the fibrotic repair (e.g., neutralizing antibody to TGFβ,19 or application of mitomycin C).30 Whereas several studies7,31,32 have shown that increases in cAMP can inhibit TGFβ-induced fibrosis, our study is the first report that this inhibitory effect occurs during keratocyte-to-myofibroblast transdifferentiation. Our results show that FSK significantly reduced the myofibroblast phenotype at 72 hours (Figs. 1, 2) and α-SMA elevation at 4 (Fig. 3A) and 24 (Fig. 3B) hours after TGFβ1 treatment, consistent with previous reports showing that cAMP reduces the fibrotic phenotype in cardiac13,31 and pulmonary23 fibroblasts. These results suggest that agents that increase cAMP may provide a new approach to interfere with fibrotic corneal wound healing to prevent scar formation.
Inhibition of TGFβ-induced myofibroblast transformation and collagen synthesis by cAMP in cardiac cells is not via reducing the translocation of pSmad3/4 to the nucleus, but by increased competition for CBP1 and P300.13 In our study, TGFβ induced the phosphorylation of Smad3 in the keratocytes, and FSK did not change the level of pSmad3 (Fig. 3). In contrast to cardiac cells, however, co-IP experiments showed that the interaction of pSmad3 and CBP1 from both whole cell lysates and nuclear extracts induced by TGFβ1 was not influenced by FSK in rabbit keratocytes (Fig. 4). We did not examine common Smad (Smad4) specifically since Smad4, which combines with Smad3, is generally not considered essential for TGFβ signaling, because cells lacking Smad4 still have a TGFβ response.33 Also, inhibitory Smad6/7 binds to the type 1 receptor and prevents recruitment of phosphorylation of Smad2/3, which is upstream of phosphorylation of Smad3. Since Smad3 was unaffected, Smad6/7 is unlikely to play a role in FSK inhibition as well. Overall, Smad signaling does not appear to be involved in FSK mediated inhibition of the rabbit corneal keratocyte to myofibroblast transformation.
TGFβ can also have Smad-independent effects that play important roles in myofibroblast formation. Again, in cardiac fibroblasts TGFβ1 induces pERK activation, which is significantly reduced when cellular cAMP is elevated.13 In rabbit keratocytes, we found that TGFβ significantly increases the levels of pERK, but not pJNK or p38 at 4 hours after treatment. The MEK1/2 inhibitor (U0126) reduced TGFβ-induced α-SMA expression by 38% (Fig. 5), suggesting an important role for ERK that has not been previously shown in corneal keratocytes. However, our results indicate that FSK did not change the pERK level induced by TGFβ (Fig. 6A) and that FSK can further reduce α-SMA expression in TGFβ1+U0126 –treated cells (Fig. 6B). Overall, we conclude that pERK is involved in TGFβ1-induced α-SMA expression, but that ERK phosphorylation is not affected by cAMP and cannot explain the reduction in α-SMA produced by increased cAMP.
Active GTP-RhoA can bind to many effector proteins involved in regulation of cytoskeletal dynamics34 and is a critical mediator of changes in cytoskeletal organization. RhoA activation is also involved in α-SMA expression. RhoA participates in TGFβ1-induced α-SMA expression in pulmonary-derived fibroblasts35 and ROCK inhibition reduces the α-SMA expression in the transition from rabbit corneal fibroblast to myofibroblast.18 We also showed that the ROCK inhibitor Y-27632 significantly reduced TGFβ1-induced α-SMA in rabbit keratocytes (Fig. 5) which is consistent with previous findings that α-SMA content is partially attenuated36 by Y-27632 in corneal myofibroblasts. TGFβ1 5 ng/mL significantly increased active RhoA (Figs. 7A, 7B) in rabbit keratocytes, but the increase was rapid and short lived (Fig. 7A). FSK significantly reduced initial RhoA activation induced by TGFβ1 compared with control levels (Fig. 7B), suggesting that cAMP can modulate Rho/ROCK-dependent α-SMA expression. Indeed, previous reports suggest that PKA phosphorylation of Ser188 of RhoA inhibits RhoA association with Rho Kinase.37,38 Further experiments are needed to determine the details of this interaction in keratocytes.
In summary, our results show that increasing cAMP reduced TGFβ1-induced α-SMA expression and myofibroblast transdifferentiation in rabbit keratocytes. This reduction was not associated with changes in pSmad3, pERK, pJNK, or p38. Initial experiments suggest that the FSK-induced reduction of TGFβ-induced αSMA expression is through GTP-Rho. Last, the results of our study suggest that agents that increase cAMP can be used to diminish the fibrotic response in vivo and serve as a possible alternative to the current use of cytotoxic agents.
Acknowledgments
Supported by National Institutes of Health Grant R01 EY008834. Submitted for publication June 17, 2008; revised August 7 and 29 and September 23, 2008; accepted December 4, 2008.
The authors thank Christiane Hassel for help in acquiring and analyzing flow cytometry data at the Indiana University Flow Cytometry Core Facility.
Footnotes
Disclosure: D. Xing, None; J.A. Bonanno, None
References
- 1.Mohan RR, Hutcheon AE, Choi R, et al. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- 2.Pei Y, Reins RY, McDermott AM. Aldehyde dehydrogenase (ALDH) 3A1 expression by the human keratocyte and its repair phenotypes. Exp Eye Res. 2006;83:1063–1073. doi: 10.1016/j.exer.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Jester JV, Ho-Chang J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77:581–592. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- 4.Kurosaka H, Kurosaka D, Kato K, Mashima Y, Tanaka Y. Transforming growth factor-beta 1 promotes contraction of collagen gel by bovine corneal fibroblasts through differentiation of myofibroblasts. Invest Ophthalmol Vis Sci. 1998;39:699–704. [PubMed] [Google Scholar]
- 5.Masur SK, Dewal HS, Dinh TT, Erenburg I, Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci U S A. 1996;93:4219–4223. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stramer BM, Zieske JD, Jung JC, Austin JS, Fini ME. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest Ophthalmol Vis Sci. 2003;44:4237–4246. doi: 10.1167/iovs.02-1188. [DOI] [PubMed] [Google Scholar]
- 7.Schiller M, Verrecchia F, Mauviel A. Cyclic adenosine 3′,5′-monophosphate-elevating agents inhibit transforming growth factor-beta-induced SMAD3/4-dependent transcription via a protein kinase A-dependent mechanism. Oncogene. 2003;22:8881–8890. doi: 10.1038/sj.onc.1206871. [DOI] [PubMed] [Google Scholar]
- 8.Engel ME, McDonnell MA, Law BK, Moses HL. Interdependent SMAD and JNK signaling in transforming growth factor-beta-mediated transcription. J Biol Chem. 1999;274:37413–37420. doi: 10.1074/jbc.274.52.37413. [DOI] [PubMed] [Google Scholar]
- 9.Lee MK, Pardoux C, Hall MC, et al. TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J. 2007;26:3957–3967. doi: 10.1038/sj.emboj.7601818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartsough MT, Mulder KM. Transforming growth factor beta activation of p44mapk in proliferating cultures of epithelial cells. J Biol Chem. 1995;270:7117–7124. doi: 10.1074/jbc.270.13.7117. [DOI] [PubMed] [Google Scholar]
- 11.Hanafusa H, Ninomiya-Tsuji J, Masuyama N, et al. Involvement of the p38 mitogen-activated protein kinase pathway in transforming growth factor-beta-induced gene expression. J Biol Chem. 1999;274:27161–27167. doi: 10.1074/jbc.274.38.27161. [DOI] [PubMed] [Google Scholar]
- 12.Atfi A, Djelloul S, Chastre E, Davis R, Gespach C. Evidence for a role of Rho-like GTPases and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in transforming growth factor beta-mediated signaling. J Biol Chem. 1997;272:1429–1432. doi: 10.1074/jbc.272.3.1429. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Sun SQ, Hassid A, Ostrom RS. cAMP inhibits transforming growth factor-beta-stimulated collagen synthesis via inhibition of extracellular signal-regulated kinase 1/2 and Smad signaling in cardiac fibroblasts. Mol Pharmacol. 2006;70:1992–2003. doi: 10.1124/mol.106.028951. [DOI] [PubMed] [Google Scholar]
- 14.Hu Y, Peng J, Feng D, et al. Role of extracellular signal-regulated kinase, p38 kinase, and activator protein-1 in transforming growth factor-beta1-induced alpha smooth muscle actin expression in human fetal lung fibroblasts in vitro. Lung. 2006;184:33–42. doi: 10.1007/s00408-005-2560-5. [DOI] [PubMed] [Google Scholar]
- 15.Bhowmick NA, Ghiassi M, Bakin A, et al. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishizaki T, Naito M, Fujisawa K, et al. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 1997;404:118–124. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- 17.Clements RT, Minnear FL, Singer HA, Keller RS, Vincent PA. RhoA and Rho-kinase dependent and independent signals mediate TGF-beta-induced pulmonary endothelial cytoskeletal reorganization and permeability. Am J Physiol Lung Cell Mol Physiol. 2005;288:L294–L306. doi: 10.1152/ajplung.00213.2004. [DOI] [PubMed] [Google Scholar]
- 18.Anderson S, DiCesare L, Tan I, Leung T, SundarRaj N. Rho-mediated assembly of stress fibers is differentially regulated in corneal fibroblasts and myofibroblasts. Exp Cell Res. 2004;298:574–583. doi: 10.1016/j.yexcr.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Møller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Neutralizing antibody to TGFbeta modulates stromal fibrosis but not regression of photoablative effect following PRK. Curr Eye Res. 1998;17:736–747. [PubMed] [Google Scholar]
- 20.Thom SB, Myers JS, Rapuano CJ, Eagle RC, Jr, Siepser SB, Gomes JA. Effect of topical anti-transforming growth factor-beta on corneal stromal haze after photorefractive keratectomy in rabbits. J Cataract Refract Surg. 1997;23:1324–1330. doi: 10.1016/s0886-3350(97)80110-1. [DOI] [PubMed] [Google Scholar]
- 21.Carrington LM, Albon J, Anderson I, Kamma C, Boulton M. Differential regulation of key stages in early corneal wound healing by TGF-beta isoforms and their inhibitors. Invest Ophthalmol Vis Sci. 2006;47:1886–1894. doi: 10.1167/iovs.05-0635. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg RS, Bernstein AM, Benezra M, Gelman IH, Taliana L, Masur SK. FAK-dependent regulation of myofibroblast differentiation. FASEB J. 2006;20:1006–1008. doi: 10.1096/fj.05-4838fje. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Ostrom RS, Insel PA. cAMP-elevating agents and adenylyl cyclase overexpression promote an antifibrotic phenotype in pulmonary fibroblasts. Am J Physiol Cell Physiol. 2004;286:C1089–C1099. doi: 10.1152/ajpcell.00461.2003. [DOI] [PubMed] [Google Scholar]
- 24.Jester JV, Barry-Lane PA, Cavanagh HD, Petroll WM. Induction of alpha-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea. 1996;15:505–516. [PubMed] [Google Scholar]
- 25.Kolodsick JE, Peters-Golden M, Larios J, Toews GB, Thannickal VJ, Moore BB. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E. prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am J Respir Cell Mol Biol. 2003;29:537–544. doi: 10.1165/rcmb.2002-0243OC. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 27.Hayashida T, Poncelet AC, Hubchak SC, Schnaper HW. TGF-beta1 activates MAP kinase in human mesangial cells: a possible role in collagen expression. Kidney Int. 1999;56:1710–1720. doi: 10.1046/j.1523-1755.1999.00733.x. [DOI] [PubMed] [Google Scholar]
- 28.Masszi A, Di Ciano C, Sirokmany G, et al. Central role for Rho in TGF-beta1-induced alpha-smooth muscle actin expression during epithelial-mesenchymal transition. Am J Physiol Renal Physiol. 2003;284:F911–F924. doi: 10.1152/ajprenal.00183.2002. [DOI] [PubMed] [Google Scholar]
- 29.Chen S, Crawford M, Day RM, et al. RhoA modulates Smad signaling during transforming growth factor-beta-induced smooth muscle differentiation. J Biol Chem. 2006;281:1765–1770. doi: 10.1074/jbc.M507771200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Netto MV, Mohan RR, Ambrosio R, Jr, Hutcheon AE, Zieske JD, Wilson SE. Wound healing in the cornea: a review of refractive surgery complications and new prospects for therapy. Cornea. 2005;24:509–522. doi: 10.1097/01.ico.0000151544.23360.17. [DOI] [PubMed] [Google Scholar]
- 31.Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc Natl Acad Sci U S A. 2005;102:437–442. doi: 10.1073/pnas.0408704102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mishra R, Cool BL, Laderoute KR, Foretz M, Viollet B, Simonson MS. AMP-activated protein kinase inhibits transforming growth factor-beta-induced Smad3-dependent transcription and myofibroblast transdifferentiation. J Biol Chem. 2008;283:10461–10469. doi: 10.1074/jbc.M800902200. [DOI] [PubMed] [Google Scholar]
- 33.Sirard C, Kim S, Mirtsos C, et al. Targeted disruption in murine cells reveals variable requirement for Smad4 in transforming growth factor beta-related signaling. J Biol Chem. 2000;275:2063–2070. doi: 10.1074/jbc.275.3.2063. [DOI] [PubMed] [Google Scholar]
- 34.Mackay DJ, Hall A. Rho GTPases. J Biol Chem. 1998;273:20685–20688. doi: 10.1074/jbc.273.33.20685. [DOI] [PubMed] [Google Scholar]
- 35.Harvey KA, Paranavitana CN, Zaloga GP, Siddiqui RA. Diverse signaling pathways regulate fibroblast differentiation and transformation through Rho kinase activation. J Cell Physiol. 2007;211:353–363. doi: 10.1002/jcp.20939. [DOI] [PubMed] [Google Scholar]
- 36.Harvey SA, Anderson SC, SundarRaj N. Downstream effects of ROCK signaling in cultured human corneal stromal cells: microarray analysis of gene expression. Invest Ophthalmol Vis Sci. 2004;45:2168–2176. doi: 10.1167/iovs.03-1218. [DOI] [PubMed] [Google Scholar]
- 37.Busca R, Bertolotto C, Abbe P, et al. Inhibition of Rho is required for cAMP-induced melanoma cell differentiation. Mol Biol Cell. 1998;9:1367–1378. doi: 10.1091/mbc.9.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong JM, Leung T, Manser E, Lim L. cAMP-induced morphological changes are counteracted by the activated RhoA small GTPase and the Rho kinase ROKalpha. J Biol Chem. 1998;273:22554–22562. doi: 10.1074/jbc.273.35.22554. [DOI] [PubMed] [Google Scholar]