Abstract
Pediatric low-grade gliomas are the most common tumors of the central nervous system in children, accounting for almost 50% of all childhood brain tumors. They are a heterogeneous group of tumors with different histologic subtypes. Most treatment studies address low-grade gliomas as a single entity, depriving us of histology-specific treatment outcomes. This is mostly due to a lack of understanding of tumor biology at the molecular level. Pediatric low-grade gliomas are not benign, and most incompletely resected tumors will progress and negatively affect quality of life. The advancements made in understanding sporadic pilocytic astrocytoma and neurofibromatosis 1-associated pilocytic astrocytoma in particular have paved the way for potential targeted therapy and biological stratification. Such progress in pilocytic astrocytoma needs to be consolidated and expanded to other histologic varieties of pediatric low-grade gliomas.
Keywords: gliomas, pilocytic astrocytoma, pediatric, therapy, neurofibromatosis, tuberous sclerosis, low-grade
INTRODUCTION
Low-grade gliomas (LGGs) include a wide spectrum of grade I and grade II tumors, according to the World Health Organization 2007 classification.1 Table I lists the characteristics of these tumors, but this review will focus on the tumors most common in children.
Table I.
WHO 2007 classification
| Tumor Type | Grade |
|---|---|
| Astrocytic tumors | |
| Subependymal giant cell astrocytoma | I |
| Pilocytic astrocytoma | I |
| Pilomyxoid astrocytoma | II |
| Diffuse astrocytoma | II |
| Pleomorphic xanthoastrocytoma | II |
| Protoplasmic astrocytoma | II |
| Oligodendroglial and oligoastrocytic tumors | |
| Oligodendroglioma | II |
| Oligoastrocytoma | II |
| Neuronal and mixed neuronal-glial | |
| Ganglioglioma | I |
| Desmoplastic infantile ganglioglioma | I |
| Dysembryoplastic neuroepithelial tumor | I |
| Central neurocytoma | II |
| Astroblastoma | * |
The classification is according the World Health Organization 2007 central nervous system tumors classification.
The grade for astroblastoma was undetermined in the 2007 publication.
If completely surgically resected, LGGs do not require further therapy. Unfortunately, total resection is not attainable in many of these tumors that are centrally located, so adjuvant treatments such as radiation therapy and chemotherapy were introduced. The initial study of chemotherapy in pediatric LGG in 1985 utilized vincristine and actinomycin D.2 Various regimens have emerged since then, with variable progression-free survival (PFS) curves that have not plateaued.3 Such regimens have been used for all LGG and did not reflect a biological understanding of LGG.
Radiotherapy is an effective treatment with better PFS results than chemotherapy alone; 5-year PFS rates are 38% for chemotherapy alone and 68% for chemotherapy plus radiotherapy.4
Better targeted therapy is needed, and this can be achieved through a better understanding of the genetics and biology of the histologic subtypes that constitute LGG.
WHY WE NEED NEW OPTIONS FOR THERAPY
LGGs are the most common brain tumors in children, and pilocytic astrocytoma (PA) alone accounts for 21–23% of all pediatric brain tumors.5, 6 Although complete surgical resection is the preferred therapeutic option, it is not always possible to achieve gross total resection in eloquent areas such the diencephalon. In a multicenter study of 198 low-grade chiasmatic hypothalamic tumors, gross total resection was achieved in only 5 cases.7 Radiotherapy is another effective treatment but with significant morbidities, such as neuroendocrine-cognitive deficits, vasculopathy, and second tumors, especially in patients with neurofibromatosis 1 (NF1).8–10 Chemotherapy is widely used after surgery in an effort to avoid or defer radiation therapy, especially in young children. Some of the most common regimens used are carboplatin/vincristine, thioguanine/procarbazine/lomustine or CCNU/vincristine (TPCV), and temozolomide. In three studies utilizing these regimens, only 4 patients (2.6%) achieved complete radiologic response: 4/78 (5%) on carboplatin/vincristine, 0/42 (0%) on TPCV, and 0/30 (0%) on temozolomide.11–13 A further concern is that chemotherapy is not without complications. Carboplatin allergy, which can affect as many as 42% of patients, significantly limits delivery of an effective chemotherapy regimen.14 The randomized Children’s Oncology Group A 9952 trial showed 5-year PFS of 35% using carboplatin/vincristine and 48% using TPCV (p=0.11), thus demonstrating no clear superiority between the 2 commonly used regimens.15
The choice of therapy for patients whose disease progresses on chemotherapy is an area of ongoing research. The preference of most treating physicians is to try to delay the use of definitive radiation therapy until the child is past the first decade of life. Recent studies have identified vinblastine as an effective second-line treatment for recurrent or refractory LGG.16, 17 Current studies are seeking to determine the feasibility of delivering vinblastine in combination with carboplatin.
LGGs are not benign tumors. They can negatively affect quality of life significantly, even in cerebellar PA treated with surgery alone: these patients are still at risk for cognitive and adaptive impairment, in addition to language, memory, attention, and spatial function problems.18, 19 In patients with optical pathway gliomas (OPGs), the quality of life can be compromised further due to effects on vision and the endocrine system.20, 21 Visual outcomes in patients with OPGs have not been well documented, with most reports just focusing on limited aspects such as visual acuity or visual field alone.21, 22 More detailed outcome measures such as contrast sensitivity and color vision are rarely addressed, and when such outcomes are documented, it is likely that the visual outcome will be much worse.20
LGGs can also, although rarely, metastasize or transform into high-grade gliomas.23, 24 Most important, LGGs can recur even if resected completely.25 In one study, the PFS in 278 patients was 55% at 5 years and 42% at 10 years.26 In the Children’s Oncology Group A 9952 trial, the 5-year PFS for children without NF1 was 41.7%. This high rate of progression exposes these children to different lines of treatment and more side effects.
HOW CAN WE ACHIEVE NEW OPTIONS FOR THERAPY FOR LGG?
Improved pathologic classification
The pathologic classification of LGG can be challenging. In the Children’s Cancer Group CCG-945 high-grade glioma study, 70/250 patients (28%) were reclassified as having LGG on further central pathologic review.4 These patients were exposed to unnecessary aggressive therapy that could have been avoided. In other studies, unclassified or “not otherwise specified” LGGs were predominant, constituting 42% and 32% of patients enrolled in the protocol, respectively, in two series.26, 27
To better understand some of the rare types of LGGs, it is pivotal that we investigate them as such. Launching international studies may facilitate accrual of larger numbers of different types, allowing better conclusions to be reached. The “Montevideo initiative” for childhood leukemia launched in 1995 in an attempt to understand the biology and heterogeneity of rare leukemia subgroups is a good model to follow for rare LGGs.28 A similar example at the national level in pediatric brain tumors is the NF1 OPG task force.29 Such an initiative is responsible for many of the advancements seen in NF1-associated PA (NF1-PA).
Better understanding of the behavior of LGG
There is still much we do not understand about the behavior of LGG. Metastasis is a real phenomenon estimated to afflict 3–5% of patients at diagnosis and 7–10% at progression.21, 24, 30, 31 Most of the literature consists of case reports, with the largest series reporting only 13, 11, 8, and 6 cases.24, 31–33 Even the etiology of metastasis in LGG is poorly understood. Tabori et al32 identified amplification of the epidermal growth factor receptor gene in 6/6 (100%) cases of metastatic LGG.
Another poorly understood and understudied event in LGG is transformation to high-grade glioma. Malignant transformation is a controversial issue, with some investigators doubting that it occurs independent of radiation therapy in PA.34 Our group documented that malignant transformation can occur in patients who have not been treated with radiotherapy.23 Five of the 11 patients in this study did not receive prior radiotherapy. Three of those 5 patients had undergone gross total resection before the malignant transformation. In 9 patients (82%), tissue was available for molecular studies before and after malignant transformation. The overexpression of p53 and deletion of phosphatase and tensin homolog (PTEN) were more frequently seen after malignant transformation. Our data confirmed that malignant transformation is exceedingly rare in children with PA. On the other hand, the 15-year risk of malignant transformation among children with World Health Organization grade II LGG was 6.7% in our study.
At the other extreme is spontaneous regression in pediatric LGG with and without associated NF1.35, 36 Radiologically, the regression presents as a decrease in tumor size or a change in signal and clinically can be associated with improved symptoms. The biology of this phenomenon is poorly understood. An interesting study by Tabori et al37 identified shortening telomeres as an explanation for the growth arrest seen in some pediatric LGG. In their series, they did not find any telomerase activity or alternative lengthening of telomeres in 56 LGG samples.
Many studies have been launched in an attempt to identify clinical and biological risk factors for progression in LGG. Age of less than 5 years at diagnosis was found by most researchers to be associated with poorer 5-year PFS.15, 27 Others found that infants and young children, less than 2 years, had worse PFS.7, 21, 38, 39 On the other hand, one study found that children younger than 5 years had a better prognosis.12 Other factors such as NF1 presence, location, quality of response to chemotherapy, extent of resection, and pathologic grade have been suggested as risk factors by different studies with contradicting findings.26, 27, 38–40
The MIB-1 labeling index, a measure of the proliferation rate in a specific tumor, was one of the earliest methods used to identify the risk of progression in LGG. To date, the importance of the MIB-1 labeling index is controversial, with one study showing it to be a good predictor of tumor progression and others showing no such correlation.41, 42
In an interesting study, tumor vascularity and angiogenesis were found to predict progression in OPGs.43 In addition, Wong et al44 found that PA can be divided into two subgroups based on the differential expression of genes involved in cell adhesion, cell motility, angiogenesis, and nerve ensheathment. The group overexpressing gene products associated with cell motility, adhesion, and angiogenesis but underexpressing gene products associated with nerve ensheathment such as proteolipid protein and myelin basic protein was associated with more aggressive behavior and incomplete resection.44
Chromosomal abnormalities were the focus of the earliest genetic studies done on LGGs, particularly PA. Many numerical and structural abnormalities were identified, with chromosomes 5, 7, 8, and 17 being the most frequently affected.45–48 In one study, the deletion of 17p (p53 site) was associated with rapid recurrence in 4 cases of PA regardless of aggressive treatment.49
Individualized understanding for each tumor type
Pilocytic astrocytoma
Most of the literature regarding the biology of LGGs is focused on PAs, which are the most common type.5, 6, 26, 27 PAs are a heterogeneous group with a wide range of patterns that vary by location and sometimes even within the same lesion. Sporadic PA differs from NF1-PA in clinical behavior and natural history.50, 51
Sporadic PA
We have addressed some of genetic aberrations involved in subgrouping sporadic PA into more aggressive and less aggressive tumors.44, 49 As mentioned earlier, chromosomes 7 and 8 are among the most common chromosomal abnormalities identified in sporadic PA.47, 48 The importance of such chromosomal abnormalities was demonstrated by identifying the matrilin-2 gene (MATN2) and band 7q34 on chromosomes 8 and 7, respectively.52–55
Matrilin-2 is an extracellular matrix protein. The extracellular matrix is involved in creating the microenvironment that influences growth, proliferation, and differentiation. An interesting finding in regard to matrilin-2 was the differential the three-tier expression of the matrilin-2 gene and protein.55 First, matrilin-2 was overexpressed in sporadic PA compared with NF1- PA. Second, matrilin-2 was overexpressed in 14 of 15 (93%) supratentorial PA compared with 10 of 19 (53%) infratentorial PA. Third, matrilin-2 was overexpressed in tumors with aggressive behavior, whereas all 4 recurrent and 5 fatal tumors showed increased levels of matrilin-2.
On chromosome 7, band 7q34 had been intensively studied.52–54 Band 7q34 gain in these tumors included part of the BRAF gene locus that encodes the kinase domain.52 BRAF is a downstream effector in the mitogen-activated protein kinase (MAPK) pathway that is activated in some cases of sporadic PA.54 The mechanism of BRAF activation was found to be duplication of 7q34 in 53% or an activating mutation in 6% of sporadic PA.54 Such activation was proved by showing overexpression of CCND1, a downstream target of the MAPK pathway, and increased phosphorylation of extracellular signal-regulated kinase (ERK) 1/2, an immediate downstream target of BRAF when phosphorylated. Another interesting finding in this study was that the duplication at the 7q34 band was associated more with supratentorial location, recurrence, and incomplete resection. Moreover, 7q34 duplication was specific to PA and was negative in all 28 ganglioglioma (GG) samples and 10 pleomorphic xanthoastrocytomas.
Similar findings were confirmed by Bar et al,52 who found 7q34 gain in 17/25 (68%) cases of sporadic PA. In this study, there were 20 infratentorial and 5 supratentorial tumors. The gain was again associated with location, but all 17 tumors with the 7q34 gain were in the infratentorial group, which contrasts with what Pfister et al54 found. This inconsistency between studies may be due to sample size.
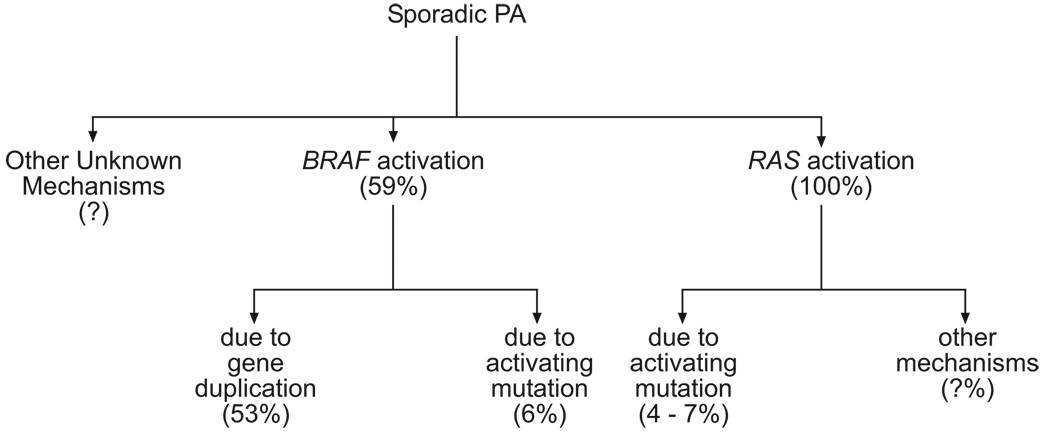
Another possible genetic aberration involved in the pathogenesis of sporadic PA is activation of the phosphatidylinositol-3 kinase (PI3K) pathway.56, 57 In one study, Ras was found to be activated in all 21 sporadic PA cases tested by demonstrating phosphorylation of its downstream effector, AKT kinase.57 However, the mechanism of activation was found to be an activating mutation in K-RAS in only one tumor (5%). The same group confirmed this finding in one other tumor (7%) out of 15 sporadic PA samples.56 A summary of putative genetic abnormalities that may be responsible for the tumorigenesis of sporadic PA is provided in Figure 1. Understanding such genetic aberrations in sporadic PA my allow us to better biologically target these tumors.
Figure 1.
Some putative mechanisms of sporadic PA tumorigenesis. RAS activation was found in 21/21 tumors, but the mechanism of such activation was identified in only a small fraction in these tumors, and it was due to an activating mutation. This leaves most of these without identified mechanisms that account for RAS activation. Another potential etiologic mechanism for PA is BRAF activation due to gene duplication or an activating mutation. In most sporadic PA, we still do not know the biological events leading to their formation. (Note: the percentages in brackets are specific for the tumor investigated in each study and not for PA in general.)
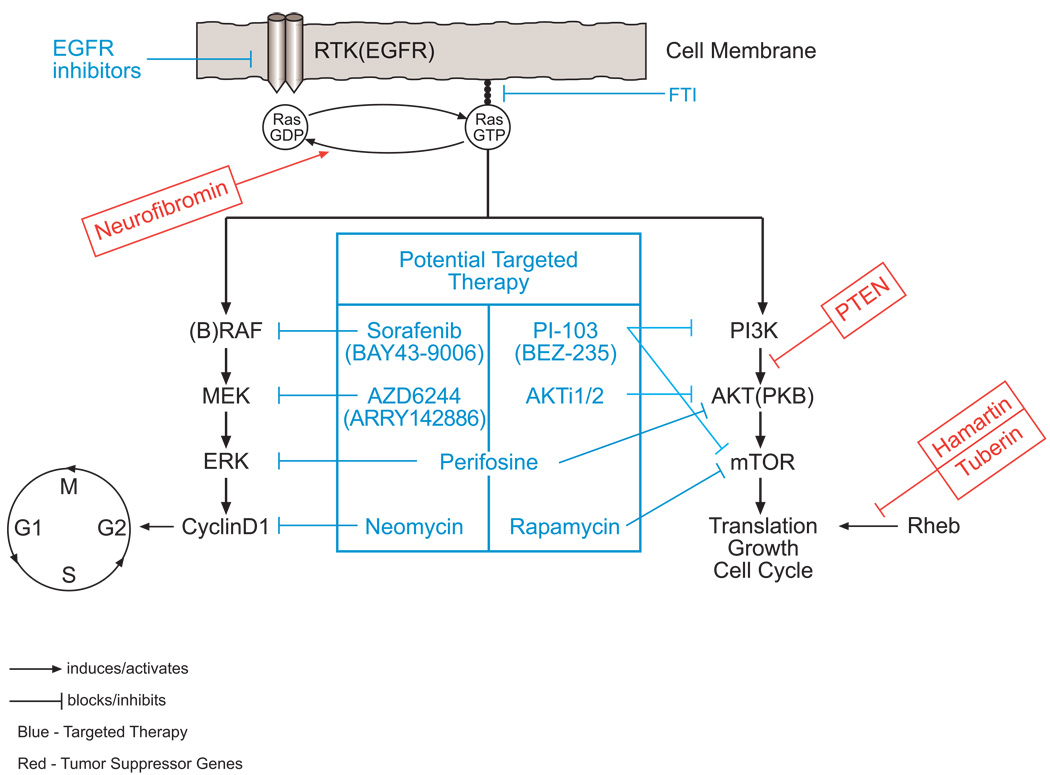
Decoding mechanisms of the MAPK and PI3K pathways in sporadic PA and NF1-PA may help guide us in selecting potential targeted therapies (Figure 2). This can include RAF inhibitors, such as urea derivatives.58, 59 One such compound, BAY 43–9006 (sorafenib), which inhibits C-RAF and B-RAF, is being investigated in clinical trials and has been approved for renal cell cancer.60 Among other therapeutic targets under investigation in vitro are the following:
Inhibiting cyclin D1 by neomycin, which has shown efficacy in glioma cell lines.61
Inhibiting ERK and mitogen-activated protein kinase kinase (MEK) using compounds such as AZD6244 and perifosine, which also inhibits Akt in the PI3K pathway.62, 63
Inhibiting AKT1/2 using AKTi1/2, which is effective against cancer cell lines with activated AKT.64
Inhibiting PI3K and mammalian target of rapamycin (mTOR) with the dual inhibitor PI-103, which has shown efficacy in preclinical trials on malignant glioma cell lines.65 It also can be a putative targeted therapy in PA when the PI3K pathway is activated.
Figure 2.
Activation of a receptor tyrosine kinase leads to converting RAS from the inactive GDP-bound form to the active GTP-bound form, which localizes to the cellular membrane. The activation of RAS starts a cascade via the RAF/MEK/ERK or PI3K/AKT/mTOR pathways. Many tumor suppressors affect different steps in the cascade, and their loss leads to hyperactivation of the pathway. First, neurofibromin converts the GTP-bound active RAS into the GDP-bound inactive form. Second, PTEN is a lipid phosphatase that counteracts PI3K by dephosphorylating its second messengers. Third, the hamartin/tuberin complex, which is a GTP-activating protein, inhibits mTOR by inactivating Rheb. Different small molecules and compounds are being studied as potential targeted therapies by blocking different steps in the cascade in both pathways.
NF1-PA
NF1 is an autosomal dominant disease that affects 1 in 3500 individuals.66 Those affected are at risk for many central nervous system tumors, with OPG being the most common, occurring in 15–20% of NF1 patients. NF1-PA differs from sporadic PA in clinical behavior and genetic signature.
The loss of NF1 on 17q is the main genetic difference between the two types.67, 68 The NF1 gene product, neurofibromin, is a negative regulator of RAS by exerting guanosine-5’-triphosphate (GTP)ase activity on the GTP-bound RAS and converting it to guanosine-5’-diphosphate (GDP)-bound RAS.69 As seen in Figure 2, the constitutively activated Ras will trigger both the MAPK (RAF/RAS/MEK/ERK) and the PI3K (RAS/PI3K/AKT/mTOR) pathways.70 Normally, RAS can be activated by a receptor tyrosine kinase such as epidermal growth factor receptor (Figure 2). For RAS to perform its functions, it must localize to the cell membrane. This is done by adding hydrophobic molecules through the process of prenylation.71 Using Nf1-deficient mice, Dasgupta et al72 found specific activation of K-RAS responsible for glioma formation in vitro and in vivo.
One of the downstream targets of the PI3K pathway is mTOR, a serine/threonine kinase involved in protein synthesis and growth that can be activated by AKT, as in Figure 2.73, 74 Neurofibromin is a negative regulator of mTOR via RAS, and its loss causes hyperactivation of mTOR.75 In an interesting study, rapamycin, an mTOR inhibitor, was administered in an NF1 mouse model and yielded a positive response in a dose-dependent manner.76 In addition to mTOR inhibitors, other inhibitors of the RAS/PI3K/AKT/mTOR pathway can be putative targeted therapies, as mentioned earlier in connection with sporadic PA (Figure 2).
Subependymal giant cell astrocytoma and PA in tuberous sclerosis complex
Another mechanism to activate mTOR is through RAS homolog enriched in brain (Rheb).77 As shown in Figure 2, the TSC1/2 (tuberin/hamartin) complex inactivates Rheb. The loss of this complex, as in tuberous sclerosis complex, results in hyperactivation of mTOR.78 The mTOR inhibitor rapamycin is again a rational option for targeted therapy. In 5 patients with a clinical diagnosis of tuberous sclerosis complex who developed subependymal giant cell astrocytoma (n=4) or PA (n=1) with documented progressive lesions, rapamycin was administered.79 In all 5 patients, lesions and PA decreased in size.
Pilomyxoid astrocytoma
First reported as a separate entity in 1999,80 pilomyxoid astrocytoma is closely related to PA, with bipolar cells in a myxoid matrix but no Rosenthal fibers or eosinophilic granular bodies. It occurs in the very young (less than 1 year old) with hypothalamic/chiasmatic predilection and carries a worse prognosis with a 1-year PFS of only 37.8%.80 Reports about the genetics of pilomyxoid astrocytoma are scarce, consisting of only one case report of an NF1 patient and another report of disruption of the BCR gene in a child.81, 82
Dysembryoplastic neuroepithelial tumor
First reported by Daumas-Duport83 in 1988 on a combined series of 39 patients from the United States and France, dysembryoplastic neuroepithelial tumors are cortical lesions that occur in young individuals with longstanding refractory seizures. Pathologically, these tumors are composed of glial nodules, cortical dysplasia, and specific glioneuronal elements.83 Initial reports showed no recurrence even after incomplete resection. However, later reports showed one case of recurrence after gross total resection and another case of malignant transformation 14 years after resection with no chemotherapy or radiotherapy.84, 85 Genetically, we know very little about this tumor so far. Case reports have shown these tumors in association with NF1 and XYY syndromes.86, 87 Other studies showed a lack of 1p and 19q deletions and p53 mutations.88,89 A unique study by Prayson’s group90 investigated some of the apoptosis-associated proteins such as Bcl-2, bcl-x, and bax in 18 cases. They found these genes differentially expressed between the oligodendroglial and neuronal components. The significance of such findings is not yet known.
Ganglioglioma
GG is a well differentiated, indolent, mixed neuronal-glial tumor composed of mature ganglion cells and abnormal glial cells. It is also associated with a long history of seizure. One of the initial studies on the genetics of GG investigated 13 tumors (9 temporal and 4 at other sites) from 120 total cases of pediatric brain tumors.91 Only 2 non-temporal cases showed abnormalities, with both involving inversion 1q. Later publications included an unusual case report on spinal GG in an NF2 patient and 2 reports of metastatic GGs.92–94 Genetic analysis on both metastatic cases was performed and showed inversion of chromosome 7 and loss of 17p.93, 94 A recent large study investigated 61 cases of GG, 19 of which were in patients 18 years old or younger.95 In the study, 2 groups of GG were identified. The first group had a complete gain of chromosome 7 with additional gains in 5, 8, and 12. The second group had mostly normal chromosomes, with occasional losses on chromosome 9 and 22q. It should be noted that the second group was similar to the 11 of 13 GG cases with normal cytogenetics mentioned above.91 Another interesting finding in this study was the anaplastic recurrence of 2/61 (3%) as GG grade III.95 These two tumors had genetic aberrations similar to those of high-grade gliomas. Moreover, these genetic aberrations were present on the primary tumor as GG grade I at diagnosis.
There is a distinct entity called dysplastic gangliocytoma of the cerebellum or Lhermitte-Duclos disease that is associated with Cowden disease. It can occur in infancy and adulthood. A PTEN inactivating mutation is the genetic basis for this syndrome.96 PTEN is a tumor suppressor that inhibits the phosphorylation of PI3K. Such inactivation causes hyperactivation in the PI3K/Akt/mTOR pathway (Figure 2). Interestingly, PTEN mutations were identified only in adult-onset Lhermitte-Duclos disease and not in the pediatric cases.97
CONCLUSION
In the last decade, we have come to know somewhat more about PAs. We still know very little about other types of LGGs. We believe that the future of research in pediatric LGGs will provide a better understanding of the biology and genetics of each type. Furthermore, within each type, there will be additional stratification based on genetic signatures that provide clues to clinical behavior, such as recurrence, metastasis, or a more indolent natural history. Such understanding may allow us to stratify treatment accordingly. For this to occur, multicenter and multination studies are needed to provide enough numbers for basic and clinical research for each group of LGGs.
Acknowledgments
This work was supported in part by the Cancer Center Support (CORE) Grant P30 CA21765 from the National Institutes of Health, by the American Lebanese Syrian Associated Charities (ALSAC), by the Pediatric Brain Tumor Foundation, by the Noyes Brain Tumor Foundation, by the Ryan McGhee Foundation, and by Musicians Against Childhood Cancer (MACC). The authors would like to thank David Galloway for the scientific editing he provided for this manuscript.
Abbreviations
- LGG
low-grade glioma
- PFS
progression-free survival
- OPG
optic pathway glioma
- NF1
neurofibromatosis 1
- PA
pilocytic astrocytoma
- NF1-PA
neurofibromatosis 1-associated pilocytic astrocytoma
- ERK
extracellular signal-regulated kinase
- MAPK
mitogen-activated protein kinase
- MEK
mitogen-activated protein kinase kinase
- PI3K
phosphatidylinositol-3 kinase
- mTOR
mammalian target of rapamycin
- GG
ganglioglioma
- GTP
guanosine-5’-triphosphate
- GDP
guanosine-5’-diphosphate
- PTEN
phosphatase and tensin homolog
- Rheb
Ras homolog enriched in brain
References
- 1.Kleihues PDNL, Wiestler OD, Burger P, Scheithauer B. WHO grading of tumours of the central nervous system. In: Louis DN, Ohgaki H, Wiestler OD, Cavene WK, editors. WHO Classification of Tumors of the Central Nervous System. Lyon, France: International Agency for Research on Cancer; 2007. pp. 10–11. [Google Scholar]
- 2.Rosenstock JG, Packer RJ, Bilaniuk L, Bruce DA, Radcliffe JL, Savino P. Chiasmatic optic glioma treated with chemotherapy. A preliminary report. J Neurosurg. 1985;63:862–866. doi: 10.3171/jns.1985.63.6.0862. [DOI] [PubMed] [Google Scholar]
- 3.Perilongo G. Considerations on the role of chemotherapy and modern radiotherapy in the treatment of childhood low grade glioma. J Neurooncol. 2005;75:301–307. doi: 10.1007/s11060-005-6754-8. [DOI] [PubMed] [Google Scholar]
- 4.Fouladi M, Hunt DL, Pollack IF, Dueckers G, Burger PC, Becker LE, et al. Outcome of children with centrally reviewed low-grade gliomas treated with chemotherapy with or without radiotherapy on Children's Cancer Group high-grade glioma study CCG-945. Cancer. 2003;98:1243–1252. doi: 10.1002/cncr.11637. [DOI] [PubMed] [Google Scholar]
- 5.Rickert CH, Paulus W. Epidemiology of central nervous system tumors in childhood and adolescence based on the new WHO classification. Childs Nerv Syst. 2001;17:503–511. doi: 10.1007/s003810100496. [DOI] [PubMed] [Google Scholar]
- 6.States CBTRotU. CBTRUS. Statistical Report. Hinsdale, IL: Primary Brain Tumors in the United States, 1998–2002. 2005
- 7.Gnekow AK, Kortmann RD, Pietsch T, Emser A. Low grade chiasmatic-hypothalamic glioma-carboplatin and vincristin chemotherapy effectively defers radiotherapy within a comprehensive treatment strategy -- report from the multicenter treatment study for children and adolescents with a low grade glioma -- HIT-LGG 1996 -- of the Society of Pediatric Oncology and Hematology (GPOH) Klin Padiatr. 2004;216:331–342. doi: 10.1055/s-2004-832355. [DOI] [PubMed] [Google Scholar]
- 8.Sharif S, Ferner R, Birch JM, Gillespie JE, Gattamaneni HR, Baser ME, et al. Second primary tumors in neurofibromatosis 1 patients treated for optic glioma: substantial risks after radiotherapy. J Clin Oncol. 2006;24:2570–2575. doi: 10.1200/JCO.2005.03.8349. [DOI] [PubMed] [Google Scholar]
- 9.Pierce SM, Barnes PD, Loeffler JS, McGinn C, Tarbell NJ. Definitive radiation therapy in the management of symptomatic patients with optic glioma. Survival and long-term effects. Cancer. 1990;65:45–52. doi: 10.1002/1097-0142(19900101)65:1<45::aid-cncr2820650111>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 10.Donahue B. Short- and long-term complications of radiation therapy for pediatric brain tumors. Pediatr Neurosurg. 1992;18:207–217. doi: 10.1159/000120664. [DOI] [PubMed] [Google Scholar]
- 11.Prados MD, Edwards MS, Rabbitt J, Lamborn K, Davis RL, Levin VA. Treatment of pediatric low-grade gliomas with a nitrosourea-based multiagent chemotherapy regimen. J Neurooncol. 1997;32:235–241. doi: 10.1023/a:1005736104205. [DOI] [PubMed] [Google Scholar]
- 12.Packer RJ, Ater J, Allen J, Phillips P, Geyer R, Nicholson HS, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86:747–754. doi: 10.3171/jns.1997.86.5.0747. [DOI] [PubMed] [Google Scholar]
- 13.Gururangan S, Fisher MJ, Allen JC, Herndon JE, Quinn JA, Reardon DA, et al. Temozolomide in children with progressive low-grade glioma. NeuroOncol. 2007;9:161–168. doi: 10.1215/15228517-2006-030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lafay-Cousin L, Sung L, Carret AS, Hukin J, Wilson B, Johnston DL, et al. Carboplatin hypersensitivity reaction in pediatric patients with low-grade glioma: a Canadian Pediatric Brain Tumor Consortium experience. Cancer. 2008;112:892–899. doi: 10.1002/cncr.23249. [DOI] [PubMed] [Google Scholar]
- 15.Ater J, Holmes E, Zhou T, Mazewski C, Roberts W, Vezina G, et al. Results of COG protocol A9952: A randomized phase 3 study of two chemotherapy regimens for incompletely resected low-grade glioma in young children. NeuroOncol. 2008;10:451–452. [Google Scholar]
- 16.Bouffet E, Jakacki R, Goldman S, Hargrave D, Shroff M, Hukin J, et al. Phase II study of weekly vinblastine in recurrent/refractory pediatric low-grade gliomas. NeuroOncol. 2008:450. doi: 10.1200/JCO.2011.34.5843. [DOI] [PubMed] [Google Scholar]
- 17.Lafay-Cousin L, Holm S, Qaddoumi I, Nicolin G, Bartels U, Tabori U, et al. Weekly vinblastine in pediatric low-grade glioma patients with carboplatin allergic reaction. Cancer. 2005;103:2636–2642. doi: 10.1002/cncr.21091. [DOI] [PubMed] [Google Scholar]
- 18.Aarsen FK, Paquier PF, Reddingius RE, Streng IC, Arts WF, Evera-Preesman M, et al. Functional outcome after low-grade astrocytoma treatment in childhood. Cancer. 2006;106:396–402. doi: 10.1002/cncr.21612. [DOI] [PubMed] [Google Scholar]
- 19.Beebe DW, Ris MD, Armstrong FD, Fontanesi J, Mulhern R, Holmes E, et al. Cognitive and adaptive outcome in low-grade pediatric cerebellar astrocytomas: evidence of diminished cognitive and adaptive functioning in National Collaborative Research Studies (CCG 9891/POG 9130) J Clin Oncol. 2005;23:5198–5204. doi: 10.1200/JCO.2005.06.117. [DOI] [PubMed] [Google Scholar]
- 20.Dalla VP, Opocher E, Pinello ML, Calderone M, Viscardi E, Clementi M, et al. Visual outcome of a cohort of children with neurofibromatosis type 1 and optic pathway glioma followed by a pediatric neuro-oncology program. NeuroOncol. 2007;9:430–437. doi: 10.1215/15228517-2007-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fouladi M, Wallace D, Langston JW, Mulhern R, Rose SR, Gajjar A, et al. Survival and functional outcome of children with hypothalamic/chiasmatic tumors. Cancer. 2003;97:1084–1092. doi: 10.1002/cncr.11119. [DOI] [PubMed] [Google Scholar]
- 22.Grabenbauer GG, Schuchardt U, Buchfelder M, Rodel CM, Gusek G, Marx M, et al. Radiation therapy of optico-hypothalamic gliomas (OHG)--radiographic response, vision and late toxicity. Radiother Oncol. 2000;54:239–245. doi: 10.1016/s0167-8140(00)00149-3. [DOI] [PubMed] [Google Scholar]
- 23.Broniscer A, Baker SJ, West AN, Fraser MM, Proko E, Kocak M, et al. Clinical and molecular characteristics of malignant transformation of low-grade glioma in children. J Clin Oncol. 2007;25:682–689. doi: 10.1200/JCO.2006.06.8213. [DOI] [PubMed] [Google Scholar]
- 24.Gajjar A, Bhargava R, Jenkins JJ, Heideman R, Sanford RA, Langston JW, et al. Low-grade astrocytoma with neuraxis dissemination at diagnosis. J Neurosurg. 1995;83:67–71. doi: 10.3171/jns.1995.83.1.0067. [DOI] [PubMed] [Google Scholar]
- 25.Bowers DC, Krause TP, Aronson LJ, Barzi A, Burger PC, Carson BS, et al. Second surgery for recurrent pilocytic astrocytoma in children. Pediatr Neurosurg. 2001;34:229–234. doi: 10.1159/000056027. [DOI] [PubMed] [Google Scholar]
- 26.Fisher PG, Tihan T, Goldthwaite PT, Wharam MD, Carson BS, Weingart JD, et al. Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood Cancer. 2008;51:245–250. doi: 10.1002/pbc.21563. [DOI] [PubMed] [Google Scholar]
- 27.Gajjar A, Sanford RA, Heideman R, Jenkins JJ, Walter A, Li Y, et al. Low-grade astrocytoma: a decade of experience at St. Jude Children's Research Hospital. J Clin Oncol. 1997;15:2792–2799. doi: 10.1200/JCO.1997.15.8.2792. [DOI] [PubMed] [Google Scholar]
- 28.Pui CH, Ribeiro RC. International collaboration on childhood leukemia. Int J Hematol. 2003;78:383–389. doi: 10.1007/BF02983810. [DOI] [PubMed] [Google Scholar]
- 29.Listernick R, Louis DN, Packer RJ, Gutmann DH. Optic pathway gliomas in children with neurofibromatosis 1: consensus statement from the NF1 Optic Pathway Glioma Task Force. Ann Neurol. 1997;41:143–149. doi: 10.1002/ana.410410204. [DOI] [PubMed] [Google Scholar]
- 30.Perilongo G, Garre ML, Giangaspero F. Low-grade gliomas and leptomeningeal dissemination: a poorly understood phenomenon. Childs NervSyst. 2003;19:197–203. doi: 10.1007/s00381-003-0733-1. [DOI] [PubMed] [Google Scholar]
- 31.Hukin J, Siffert J, Cohen H, Velasquez L, Zagzag D, Allen J. Leptomeningeal dissemination at diagnosis of pediatric low-grade neuroepithelial tumors. NeuroOncol. 2003;5:188–196. doi: 10.1215/S1152-8517-02-00029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabori U, Rienstein S, Dromi Y, Leider-Trejo L, Constantini S, Burstein Y, et al. Epidermal growth factor receptor gene amplification and expression in disseminated pediatric low-grade gliomas. J Neurosurg. 2005;103:357–361. doi: 10.3171/ped.2005.103.4.0357. [DOI] [PubMed] [Google Scholar]
- 33.Mamelak AN, Prados MD, Obana WG, Cogen PH, Edwards MS. Treatment options and prognosis for multicentric juvenile pilocytic astrocytoma. J Neurosurg. 1994;81:24–30. doi: 10.3171/jns.1994.81.1.0024. [DOI] [PubMed] [Google Scholar]
- 34.Parsa CF, Givrad S. Juvenile pilocytic astrocytomas do not undergo spontaneous malignant transformation: grounds for designation as hamartomas. Br J Ophthalmol. 2008;92:40–46. doi: 10.1136/bjo.2007.125567. [DOI] [PubMed] [Google Scholar]
- 35.Parsa CF, Hoyt CS, Lesser RL, Weinstein JM, Strother CM, Muci-Mendoza R, et al. Spontaneous regression of optic gliomas: thirteen cases documented by serial neuroimaging. Arch Ophthalmol. 2001;119:516–529. doi: 10.1001/archopht.119.4.516. [DOI] [PubMed] [Google Scholar]
- 36.Rozen WM, Joseph S, Lo PA. Spontaneous regression of low-grade gliomas in pediatric patients without neurofibromatosis. Pediatr Neurosurg. 2008;44:324–328. doi: 10.1159/000134925. [DOI] [PubMed] [Google Scholar]
- 37.Tabori U, Vukovic B, Zielenska M, Hawkins C, Braude I, Rutka J, et al. The role of telomere maintenance in the spontaneous growth arrest of pediatric low-grade gliomas. Neoplasia. 2006;8:136–142. doi: 10.1593/neo.05715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laithier V, Grill J, Le Deley MC, Ruchoux MM, Couanet D, Doz F, et al. Progression-free survival in children with optic pathway tumors: dependence on age and the quality of the response to chemotherapy--results of the first French prospective study for the French Society of Pediatric Oncology. J Clin Oncol. 2003;21:4572–4578. doi: 10.1200/JCO.2003.03.043. [DOI] [PubMed] [Google Scholar]
- 39.Opocher E, Kremer LC, Da DL, van dWMD, Viscardi E, Caron HN, et al. Prognostic factors for progression of childhood optic pathway glioma: a systematic review. Eur J Cancer. 2006;42:1807–1816. doi: 10.1016/j.ejca.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 40.Fernandez C, Figarella-Branger D, Girard N, Bouvier-Labit C, Gouvernet J, Paz PA, et al. Pilocytic astrocytomas in children: prognostic factors--a retrospective study of 80 cases. Neurosurgery. 2003;53:544–553. doi: 10.1227/01.neu.0000079330.01541.6e. [DOI] [PubMed] [Google Scholar]
- 41.Bowers DC, Gargan L, Kapur P, Reisch JS, Mulne AF, Shapiro KN, et al. Study of the MIB-1 labeling index as a predictor of tumor progression in pilocytic astrocytomas in children and adolescents. J Clin Oncol. 2003;21:2968–2973. doi: 10.1200/JCO.2003.01.017. [DOI] [PubMed] [Google Scholar]
- 42.Giannini C, Scheithauer BW, Burger PC, Christensen MR, Wollan PC, Sebo TJ, et al. Cellular proliferation in pilocytic and diffuse astrocytomas. J Neuropathol Exp Neurol. 1999;58:46–53. doi: 10.1097/00005072-199901000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Bartels U, Hawkins C, Jing M, Ho M, Dirks P, Rutka J, et al. Vascularity and angiogenesis as predictors of growth in optic pathway/hypothalamic gliomas. J Neurosurg. 2006;104:314–320. doi: 10.3171/ped.2006.104.5.314. [DOI] [PubMed] [Google Scholar]
- 44.Wong KK, Chang YM, Tsang YT, Perlaky L, Su J, Adesina A, et al. Expression analysis of juvenile pilocytic astrocytomas by oligonucleotide microarray reveals two potential subgroups. Cancer Res. 2005;65:76–84. [PubMed] [Google Scholar]
- 45.Jones DT, Ichimura K, Liu L, Pearson DM, Plant K, Collins VP. Genomic analysis of pilocytic astrocytomas at 0.97 Mb resolution shows an increasing tendency toward chromosomal copy number change with age. J Neuropathol Exp Neurol. 2006;65:1049–1058. doi: 10.1097/01.jnen.0000240465.33628.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von DA, Louis DN, Menon AG, von AK, Petersen I, Ellison D, et al. Deletions on the long arm of chromosome 17 in pilocytic astrocytoma. Acta Neuropathol. 1993;86:81–85. doi: 10.1007/BF00454903. [DOI] [PubMed] [Google Scholar]
- 47.White FV, Anthony DC, Yunis EJ, Tarbell NJ, Scott RM, Schofield DE. Nonrandom chromosomal gains in pilocytic astrocytomas of childhood. Hum Pathol. 1995;26:979–986. doi: 10.1016/0046-8177(95)90087-x. [DOI] [PubMed] [Google Scholar]
- 48.Zattara-Cannoni H, Gambarelli D, Lena G, Dufour H, Choux M, Grisoli F, et al. Are juvenile pilocytic astrocytomas benign tumors? A cytogenetic study in 24 cases. Cancer Genet Cytogenet. 1998;104:157–160. doi: 10.1016/s0165-4608(97)00455-x. [DOI] [PubMed] [Google Scholar]
- 49.Willert JR, Daneshvar L, Sheffield VC, Cogen PH. Deletion of chromosome arm 17p DNA sequences in pediatric high-grade and juvenile pilocytic astrocytomas. Genes Chromosomes Cancer. 1995;12:165–172. doi: 10.1002/gcc.2870120303. [DOI] [PubMed] [Google Scholar]
- 50.Astrup J. Natural history and clinical management of optic pathway glioma. Br J Neurosurg. 2003;17:327–335. doi: 10.1080/02688690310001601216. [DOI] [PubMed] [Google Scholar]
- 51.Listernick R, Darling C, Greenwald M, Strauss L, Charrow J. Optic pathway tumors in children: the effect of neurofibromatosis type 1 on clinical manifestations and natural history. J Pediatr. 1995;127:718–722. doi: 10.1016/s0022-3476(95)70159-1. [DOI] [PubMed] [Google Scholar]
- 52.Bar EE, Lin A, Tihan T, Burger PC, Eberhart CG. Frequent Gains at Chromosome 7q34 Involving BRAF in Pilocytic Astrocytoma. J Neuropathol Exp Neurol. 2008 doi: 10.1097/NEN.0b013e3181845622. [DOI] [PubMed] [Google Scholar]
- 53.Deshmukh H, Yeh TH, Yu J, Sharma MK, Perry A, Leonard JR, et al. High-resolution, dual-platform aCGH analysis reveals frequent HIPK2 amplification and increased expression in pilocytic astrocytomas. Oncogene. 2008;27:4745–4751. doi: 10.1038/onc.2008.110. [DOI] [PubMed] [Google Scholar]
- 54.Pfister S, Janzarik WG, Remke M, Ernst A, Werft W, Becker N, et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118:1739–1749. doi: 10.1172/JCI33656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma MK, Watson MA, Lyman M, Perry A, Aldape KD, Deak F, et al. Matrilin-2 expression distinguishes clinically relevant subsets of pilocytic astrocytoma. Neurology. 2006;66:127–130. doi: 10.1212/01.wnl.0000188667.66646.1c. [DOI] [PubMed] [Google Scholar]
- 56.Janzarik WG, Kratz CP, Loges NT, Olbrich H, Klein C, Schafer T, et al. Further evidence for a somatic KRAS mutation in a pilocytic astrocytoma. Neuropediatrics. 2007;38:61–63. doi: 10.1055/s-2007-984451. [DOI] [PubMed] [Google Scholar]
- 57.Sharma MK, Zehnbauer BA, Watson MA, Gutmann DH. RAS pathway activation and an oncogenic RAS mutation in sporadic pilocytic astrocytoma. Neurology. 2005;65:1335–1336. doi: 10.1212/01.wnl.0000180409.78098.d7. [DOI] [PubMed] [Google Scholar]
- 58.Wu S, Guo W, Fang B. Development of small-molecule inhibitors of Raf. Recent Patents Anti-Infect Drug Disc. 2006;1:241–246. doi: 10.2174/157489106777452665. [DOI] [PubMed] [Google Scholar]
- 59.Garnett MJ, Marais R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell. 2004;6:313–319. doi: 10.1016/j.ccr.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 60.Pratilas CA, Solit DB. Therapeutic strategies for targeting BRAF in human cancer. Rev Recent Clin Trials. 2007;2:121–134. doi: 10.2174/157488707780599393. [DOI] [PubMed] [Google Scholar]
- 61.Cuevas P, az-Gonzalez D, Dujovny M. Antiproliferative action of neomycin is associated with inhibition of cyclin D1 activation in glioma cells. Neurol Res. 2003;25:691–693. doi: 10.1179/016164103101202165. [DOI] [PubMed] [Google Scholar]
- 62.Adjei AA, Cohen RB, Franklin W, Morris C, Wilson D, Molina JR, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol. 2008;26:2139–2146. doi: 10.1200/JCO.2007.14.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Momota H, Nerio E, Holland EC. Perifosine inhibits multiple signaling pathways in glial progenitors and cooperates with temozolomide to arrest cell proliferation in gliomas in vivo. Cancer Res. 2005;65:7429–7435. doi: 10.1158/0008-5472.CAN-05-1042. [DOI] [PubMed] [Google Scholar]
- 64.She QB, Chandarlapaty S, Ye Q, Lobo J, Haskell KM, Leander KR, et al. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoSONE. 2008;3:e3065. doi: 10.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fan QW, Knight ZA, Goldenberg DD, Yu W, Mostov KE, Stokoe D, et al. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Listernick R, Ferner RE, Liu GT, Gutmann DH. Optic pathway gliomas in neurofibromatosis-1: controversies and recommendations. AnnNeurol. 2007;61:189–198. doi: 10.1002/ana.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kluwe L, Hagel C, Tatagiba M, Thomas S, Stavrou D, Ostertag H, et al. Loss of NF1 alleles distinguish sporadic from NF1-associated pilocytic astrocytomas. J Neuropathol Exp Neurol. 2001;60:917–920. doi: 10.1093/jnen/60.9.917. [DOI] [PubMed] [Google Scholar]
- 68.Wimmer K, Eckart M, Meyer-Puttlitz B, Fonatsch C, Pietsch T. Mutational and expression analysis of the NF1 gene argues against a role as tumor suppressor in sporadic pilocytic astrocytomas. J Neuropathol Exp Neurol. 2002;61:896–902. doi: 10.1093/jnen/61.10.896. [DOI] [PubMed] [Google Scholar]
- 69.Xu GF, O'Connell P, Viskochil D, Cawthon R, Robertson M, Culver M, et al. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990;62:599–608. doi: 10.1016/0092-8674(90)90024-9. [DOI] [PubMed] [Google Scholar]
- 70.Lau N, Feldkamp MM, Roncari L, Loehr AH, Shannon P, Gutmann DH, et al. Loss of neurofibromin is associated with activation of RAS/MAPK and PI3-K/AKT signaling in a neurofibromatosis 1 astrocytoma. J Neuropathol Exp Neurol. 2000;59:759–767. doi: 10.1093/jnen/59.9.759. [DOI] [PubMed] [Google Scholar]
- 71.Casey PJ, Solski PA, Der CJ, Buss JE. p21ras is modified by a farnesyl isoprenoid. Proc Natl Acad Sci USA. 1989;86:8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dasgupta B, Li W, Perry A, Gutmann DH. Glioma formation in neurofibromatosis 1 reflects preferential activation of K-RAS in astrocytes. Cancer Res. 2005;65:236–245. [PubMed] [Google Scholar]
- 73.Sandsmark DK, Pelletier C, Weber JD, Gutmann DH. Mammalian target of rapamycin: master regulator of cell growth in the nervous system. HistolHistopathol. 2007;22:895–903. doi: 10.14670/HH-22.895. [DOI] [PubMed] [Google Scholar]
- 74.Sato T, Umetsu A, Tamanoi F. Characterization of the Rheb-mTOR signaling pathway in mammalian cells: constitutive active mutants of Rheb and mTOR. Methods Enzymol. 2008;438:307–320. doi: 10.1016/S0076-6879(07)38021-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dasgupta B, Yi Y, Chen DY, Weber JD, Gutmann DH. Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1-associated human and mouse brain tumors. Cancer Res. 2005;65:2755–2760. doi: 10.1158/0008-5472.CAN-04-4058. [DOI] [PubMed] [Google Scholar]
- 76.Hegedus B, Banerjee D, Yeh TH, Rothermich S, Perry A, Rubin JB, et al. Preclinical cancer therapy in a mouse model of neurofibromatosis-1 optic glioma. Cancer Res. 2008;68:1520–1528. doi: 10.1158/0008-5472.CAN-07-5916. [DOI] [PubMed] [Google Scholar]
- 77.Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 78.Swiech L, Perycz M, Malik A, Jaworski J. Role of mTOR in physiology and pathology of the nervous system. Biochim Biophys Acta. 2008;1784:116–132. doi: 10.1016/j.bbapap.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 79.Franz DN, Leonard J, Tudor C, Chuck G, Care M, Sethuraman G, et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol. 2006;59:490–498. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- 80.Tihan T, Fisher PG, Kepner JL, Godfraind C, McComb RD, Goldthwaite PT, et al. Pediatric astrocytomas with monomorphous pilomyxoid features and a less favorable outcome. J Neuropathol Exp Neurol. 1999;58:1061–1068. doi: 10.1097/00005072-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 81.Khanani MF, Hawkins C, Shroff M, Dirks P, Capra M, Burger PC, et al. Pilomyxoid astrocytoma in a patient with neurofibromatosis. Pediatr Blood Cancer. 2006;46:377–380. doi: 10.1002/pbc.20391. [DOI] [PubMed] [Google Scholar]
- 82.Melendez B, Fiano C, Ruano Y, Hernandez-Moneo JL, Mollejo M, Martinez P. BCR gene disruption in a pilomyxoid astrocytoma. Neuropathology. 2006;26:442–446. doi: 10.1111/j.1440-1789.2006.00712.x. [DOI] [PubMed] [Google Scholar]
- 83.Daumas-Duport C, Scheithauer BW, Chodkiewicz JP, Laws ER, Jr, Vedrenne C. Dysembryoplastic neuroepithelial tumor: a surgically curable tumor of young patients with intractable partial seizures. Report of thirty-nine cases. Neurosurgery. 1988;23:545–556. doi: 10.1227/00006123-198811000-00002. [DOI] [PubMed] [Google Scholar]
- 84.Maher CO, White JB, Scheithauer BW, Raffel C. Recurrence of dysembryoplastic neuroepithelial tumor following resection. PediatrNeurosurg. 2008;44:333–336. doi: 10.1159/000138372. [DOI] [PubMed] [Google Scholar]
- 85.Hammond RR, Duggal N, Woulfe JM, Girvin JP. Malignant transformation of a dysembryoplastic neuroepithelial tumor. Case report. J Neurosurg. 2000;92:722–725. doi: 10.3171/jns.2000.92.4.0722. [DOI] [PubMed] [Google Scholar]
- 86.Lellouch-Tubiana A, Bourgeois M, Vekemans M, Robain O. Dysembryoplastic neuroepithelial tumors in two children with neurofibromatosis type 1. Acta Neuropathol. 1995;90:319–322. doi: 10.1007/BF00296517. [DOI] [PubMed] [Google Scholar]
- 87.Krossnes BK, Wester K, Moen G, Mork SJ. Multifocal dysembryoplastic neuroepithelial tumour in a male with the XYY syndrome. NeuropatholApplNeurobiol. 2005;31:556–560. doi: 10.1111/j.1365-2990.2005.00680.x. [DOI] [PubMed] [Google Scholar]
- 88.Perry A, Fuller CE, Banerjee R, Brat DJ, Scheithauer BW. Ancillary FISH analysis for 1p and 19q status: preliminary observations in 287 gliomas and oligodendroglioma mimics. Front Biosci. 2003;8:a1–a9. doi: 10.2741/896. [DOI] [PubMed] [Google Scholar]
- 89.Fujisawa H, Marukawa K, Hasegawa M, Tohma Y, Hayashi Y, Uchiyama N, et al. Genetic differences between neurocytoma and dysembryoplastic neuroepithelial tumor and oligodendroglial tumors. J Neurosurg. 2002;97:1350–1355. doi: 10.3171/jns.2002.97.6.1350. [DOI] [PubMed] [Google Scholar]
- 90.Prayson RA. Bcl-2, bcl-x, and bax expression in dysembryoplastic neuroepithelial tumors. Clin Neuropathol. 2000;19:57–62. [PubMed] [Google Scholar]
- 91.Bhattacharjee MB, Armstrong DD, Vogel H, Cooley LD. Cytogenetic analysis of 120 primary pediatric brain tumors and literature review. Cancer Genet Cytogenet. 1997;97:39–53. doi: 10.1016/s0165-4608(96)00330-5. [DOI] [PubMed] [Google Scholar]
- 92.Sawin PD, Theodore N, Rekate HL. Spinal cord ganglioglioma in a child with neurofibromatosis type 2. Case report and literature review. J Neurosurg. 1999;90:231–233. doi: 10.3171/spi.1999.90.2.0231. [DOI] [PubMed] [Google Scholar]
- 93.Jay V, Squire J, Blaser S, Hoffman HJ, Hwang P. Intracranial and spinal metastases from a ganglioglioma with unusual cytogenetic abnormalities in a patient with complex partial seizures. Childs Nerv Syst. 1997;13:550–555. doi: 10.1007/s003810050136. [DOI] [PubMed] [Google Scholar]
- 94.Wacker MR, Cogen PH, Etzell JE, Daneshvar L, Davis RL, Prados MD. Diffuse leptomeningeal involvement by a ganglioglioma in a child. Case report. J Neurosurg. 1992;77:302–306. doi: 10.3171/jns.1992.77.2.0302. [DOI] [PubMed] [Google Scholar]
- 95.Hoischen A, Ehrler M, Fassunke J, Simon M, Baudis M, Landwehr C, et al. Comprehensive characterization of genomic aberrations in gangliogliomas by CGH, array-based CGH and interphase FISH. Brain Pathol. 2008;18:326–337. doi: 10.1111/j.1750-3639.2008.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nelen MR, Kremer H, Konings IB, Schoute F, van Essen AJ, Koch R, et al. Novel PTEN mutations in patients with Cowden disease: absence of clear genotype-phenotype correlations. Eur J Hum Genet. 1999;7:267–273. doi: 10.1038/sj.ejhg.5200289. [DOI] [PubMed] [Google Scholar]
- 97.Zhou XP, Marsh DJ, Morrison CD, Chaudhury AR, Maxwell M, Reifenberger G, et al. Germline inactivation of PTEN and dysregulation of the phosphoinositol-3-kinase/Akt pathway cause human Lhermitte-Duclos disease in adults. Am J Hum Genet. 2003;73:1191–1198. doi: 10.1086/379382. [DOI] [PMC free article] [PubMed] [Google Scholar]