Abstract
The K88 fimbrial adhesin enables certain strains of enterotoxigenic Escherichia coli to adhere to the porcine small intestine. In this study, the ability of the K88 adhesin to bind to glycosphingolipids was monitored by modified enzyme-linked immunosorbent assay and thin-layer chromatography overlay binding analysis. The binding of the K88 adhesin to glycosphingolipid-coated microtiter plates was saturable, with 50% maximal binding occurring with gangliotriaosylceramide, gangliotetraosylceramide, and lactosylceramide at 67 +/- 21, 117 +/- 21, and 73 +/- 22 pM, respectively. Thin-layer chromatography overlay binding analysis demonstrated that serotype O8:K87:K88ab:H19 E. coli bound to hydroxylated galactosylceramide, gangliotriaocylceramide, gangliotetraosylceramide, and lactosylceramide but not to globotriaosylceramide, nonhydroxylated galactosylceramide, glucosides, glucosylceramide, or a mixture of ceramides. The K88 adhesin did not bind by either assay to globoside, the Forssman glycolipid, GM1, GM2, GM3, GD1a, GD2, GD3, GQ1b, or GT1b. The binding pattern observed with the K88 adhesin suggests that beta 1-linked galactosyl residues are the minimum determinant required for binding, provided they are presented correctly. It is suggested that beta 1-linked galactose residues may form the molecular basis of both glycoprotein and glycolipid receptors for the K88 fimbrial adhesin in the porcine small intestine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger E. G., Buddecke E., Kamerling J. P., Kobata A., Paulson J. C., Vliegenthart J. F. Structure, biosynthesis and functions of glycoprotein glycans. Experientia. 1982 Oct 15;38(10):1129–1162. doi: 10.1007/BF01959725. [DOI] [PubMed] [Google Scholar]
- Bock K., Karlsson K. A., Strömberg N., Teneberg S. Interaction of viruses, bacteria and bacterial toxins with host cell surface glycolipids. Aspects on receptor identification and dissection of binding epitopes. Adv Exp Med Biol. 1988;228:153–186. doi: 10.1007/978-1-4613-1663-3_7. [DOI] [PubMed] [Google Scholar]
- Conway P. L., Welin A., Cohen P. S. Presence of K88-specific receptors in porcine ileal mucus is age dependent. Infect Immun. 1990 Oct;58(10):3178–3182. doi: 10.1128/iai.58.10.3178-3182.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidels L., Proia R. L., Hart D. A. Membrane receptors for bacterial toxins. Microbiol Rev. 1983 Dec;47(4):596–620. doi: 10.1128/mr.47.4.596-620.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. A., Jones G. W., Sellwood R. An attempt to identify the intestinal receptor for the K88 adhesin by means of a haemagglutination inhibition test using glycoproteins and fractions from sow colostrum. J Gen Microbiol. 1975 Feb;86(2):228–240. doi: 10.1099/00221287-86-2-228. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A., Strömberg N. Overlay and solid-phase analysis of glycolipid receptors for bacteria and viruses. Methods Enzymol. 1987;138:220–232. doi: 10.1016/0076-6879(87)38019-x. [DOI] [PubMed] [Google Scholar]
- Laux D. C., McSweegan E. F., Williams T. J., Wadolkowski E. A., Cohen P. S. Identification and characterization of mouse small intestine mucosal receptors for Escherichia coli K-12(K88ab). Infect Immun. 1986 Apr;52(1):18–25. doi: 10.1128/iai.52.1.18-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J. W., Krogfelt K. A., Krivan H. C., Cohen P. S., Laux D. C. Characterization and identification of a porcine small intestine mucus receptor for the K88ab fimbrial adhesin. Infect Immun. 1991 Jan;59(1):91–96. doi: 10.1128/iai.59.1.91-96.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne D., Moore N., Lambert P. Use of an enzyme-linked immunosorbent assay (ELISA) to measure the expression of the K88 fimbrial antigen by enterotoxigenic Escherichia coli (ETEC). J Immunol Methods. 1993 Feb 26;159(1-2):283–289. doi: 10.1016/0022-1759(93)90169-8. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Sellwood R. The interaction of the K88 antigen with porcine intestinal epithelial cell brush borders. Biochim Biophys Acta. 1980 Oct 1;632(2):326–335. doi: 10.1016/0304-4165(80)90090-2. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Linggood M. A. Observations on the pathogenic properties of the K88, Hly and Ent plasmids of Escherichia coli with particular reference to porcine diarrhoea. J Med Microbiol. 1971 Nov;4(4):467–485. doi: 10.1099/00222615-4-4-467. [DOI] [PubMed] [Google Scholar]
- Staley T. E., Wilson I. B. Soluble pig intestinal cell membrane components with affinities for E. coli K88+ antigen. Mol Cell Biochem. 1983;52(2):177–189. doi: 10.1007/BF00224926. [DOI] [PubMed] [Google Scholar]
- Stirm S., Orskov F., Orskov I., Mansa B. Episome-carried surface antigen K88 of Escherichia coli. II. Isolation and chemical analysis. J Bacteriol. 1967 Feb;93(2):731–739. doi: 10.1128/jb.93.2.731-739.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamizawa K., Iwamori M., Kozaki S., Sakaguchi G., Tanaka R., Takayama H., Nagai Y. TLC immunostaining characterization of Clostridium botulinum type A neurotoxin binding to gangliosides and free fatty acids. FEBS Lett. 1986 Jun 9;201(2):229–232. doi: 10.1016/0014-5793(86)80614-7. [DOI] [PubMed] [Google Scholar]
- Willemsen P. T., de Graaf F. K. Age and serotype dependent binding of K88 fimbriae to porcine intestinal receptors. Microb Pathog. 1992 May;12(5):367–375. doi: 10.1016/0882-4010(92)90099-a. [DOI] [PubMed] [Google Scholar]
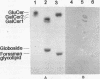
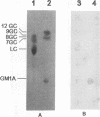
- Wilson R. A., Francis D. H. Fimbriae and enterotoxins associated with Escherichia coli serogroups isolated from pigs with colibacillosis. Am J Vet Res. 1986 Feb;47(2):213–217. [PubMed] [Google Scholar]