SUMMARY
Ligand-mediated dimerization has emerged as a universal mechanism of growth factor receptor activation. Recent structural studies have shown that neurotrophins interact with dimers of the p75 neurotrophin receptor (p75NTR), but the actual mechanism of receptor activation has remained elusive. Here we show that p75NTR forms disulphide-linked dimers independently of neurotrophin binding through the highly conserved Cys257 in its transmembrane domain. Mutation of Cys257 abolished neurotrophin-dependent receptor activity but did not affect downstream signaling by the p75NTR/NgR/Lingo-1 complex in response to MAG, indicating the existence of distinct, ligand-specific activation mechanisms for p75NTR. FRET experiments revealed a close association of p75NTR intracellular domains that was transiently disrupted by conformational changes induced upon NGF binding. Although mutation of Cys257 did not alter the oligomeric state of p75NTR, the mutant receptor was no longer able to propagate conformational changes to the cytoplasmic domain upon ligand binding. We propose that neurotrophins activate p75NTR by a novel mechanism involving rearrangement of disulphide-linked receptor subunits.
INTRODUCTION
The neurotrophins are a family of neurotrophic factors that control multiple aspects of nervous system development and function, including neurogenesis, neuronal differentiation, cell survival, neurite outgrowth, target innervation and synaptic plasticity (Bibel and Barde, 2000). Four neurotrophins are present in mammals: nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and neurotrophin-4 (NT-4). The neurotrophins are synthesized as a pre-pro-peptide from which the mature portion is released by proteolytic cleavage. The mature forms of neurotrophins interact with two distinct receptors, a cognate member of the Trk receptor tyrosine kinase family and the common p75 neurotrophin receptor (p75NTR), a member of the Tumor Necrosis Factor Receptor (TNFR) superfamily of death receptors (Friedman and Greene, 1999; Kaplan and Miller, 2000; Lee et al., 2001a; Patapoutian and Reichardt, 2001). On the other hand, unprocessed neurotrophins (pro-neurotrophins) are thought to display selectivity for p75NTR over Trk receptors (Lee et al., 2001b) with the aid of the co-receptor sortilin (Nykjaer et al., 2004). While this interaction is thought to preferentially lead to cell death (Lee et al., 2001b), cell survival is mediated by Trk signaling (Patapoutian and Reichardt, 2001). A key issue in neurotrophin research is the elucidation of the molecular mechanisms underlying each of the physiological actions of the neurotrophins in different cell types, throughout development, and in pathological situations.
Unlike Trk receptors, p75NTR lacks catalytic activity. The intracellular region of p75NTR contains a flexible juxtamembrane segment followed by a globular domain known as the death domain (Liepinsh et al., 1997). Signal transduction by p75NTR is thought to proceed via ligand-dependent recruitment and release of cytoplasmic effectors to and from the receptor. Over 20 different intracellular interactors of p75NTR have been identified to date (Barker, 2004; Bronfman and Fainzilber, 2004; Dechant and Barde, 2002; Roux and Barker, 2002), but this wealth of interactions has not translated in a comparable understanding of receptor function. Some of the major downstream signaling events triggered by p75NTR in response to neurotrophins include activation of NF-κB (Carter et al., 1996), c-jun kinase (JNK) (Friedman, 2000; Yoon et al., 1998), and caspases (Troy et al., 2002). In addition, p75NTR can also activate the small GTPase RhoA (Yamashita et al., 1999), but this requires a different set of ligands derived from myelin, such as myelin-associated glycoprotein (MAG) and Nogo (Wang et al., 2002; Wong et al., 2002; Yamashita et al., 2002), and two different co-receptors: a lipid-anchored ligand-binding subunit known as the Nogo receptor (NgR) (Fournier et al., 2001) and Lingo-1 (Mi et al., 2004). In addition, p75NTR is also known to undergo proteolytic cleavage upon activation by several of its ligands or induction of membrane metalloproteases with phorbol esters (Jung et al., 2003; Kanning et al., 2003). The release of the p75NTR intracellular domain following intramembrane cleavage by γ-secretase is thought to be required for activation of some signaling pathways, such as NRIF-mediated neuronal death of sympathetic neurons (Kenchappa et al., 2006).
Receptor oligomerization has been recognized as a crucial step in the activation of many plasma membrane receptors. In receptors carrying intrinsic kinase activity, ligand-mediated dimerization stabilizes the active conformation of the kinase domain by receptor trans-phosphorylation (Schlessinger, 2002). In non-catalytic receptors, such as cytokine and death receptors, oligomerization of intracellular domains results in recruitment of adaptors or activation of intracellularly bound effectors (Ashkenazi and Dixit, 1999; Constantinescu et al., 1999). However, while necessary, receptor oligomerization may not always be sufficient for signaling. Several studies have indicated that some cytokine and growth factor receptors exist in a pre-assembled dimeric complex prior to ligand stimulation, and that receptor activation results from a ligand-mediated change in the relative orientation of the two receptor protomers (Jiang and Hunter, 1999; Seubert et al., 2003).
Although p75NTR was discovered over 20 years ago, and has since been intensively studied, its mechanism of activation has remained mysterious. Numerous studies have reported crosslinking of radiolabeled neurotrophins to p75NTR dimers and higher-order oligomers (Hempstead et al., 1991; Mahadeo et al., 1994; Rydén et al., 1997b), demonstrating the ability of these ligands to directly bind oligomeric forms of this receptor. Based on this evidence, and the dimeric nature of neurotrophins, it has been assumed that these ligands activate p75NTR by classical ligand-mediated dimerization. A recent study has brought support to this notion by solving the x-ray crystal structure of a 2:2 complex between NT-3 and p75NTR (Gong et al., 2008). In the same work, a 2:2 complex between NGF and p75NTR could also be purified, and it was shown that the glycosylated extracellular domain of p75NTR is monomeric in solution in the absence of ligand, suggesting a general model of p75NTR dimerization by all neurotrophins. In the present study, we investigated and characterized endogenous determinants of receptor dimerization. We discovered that full-length p75NTR forms disulphide-linked dimers in the absence of neurotrophins through the highly conserved Cys257 in its transmembrane domain. Functional analysis of a p75NTR mutant lacking Cys257 revealed the functional importance of disulphide-mediated receptor dimerization and suggested a novel mechanism of p75NTR activation by neurotrophins.
RESULTS
Transmembrane Cys257 mediates formation of disulphide-linked p75NTR dimers
Surface expression of p75NTR was assessed in transfected cells following biotinylation of cell surface proteins, immunoprecipitation and gel electrophoresis under non-reducing and reducing conditions. This analysis revealed that wild type p75NTR forms disulphide-linked dimers in the plasma membrane of transfected cells (Figure 1A). At high levels of expression in COS-7 cells, comparable amounts of cell surface p75NTR monomers and disulphide-linked dimers could be detected under non-reducing conditions (Figure 1A). However, the proportion of disulphide-linked p75NTR dimers in transfected cells depended upon the level of expression of the receptor. At moderate overexpression levels, less than 10% of wild type p75NTR was disulphide-linked (Figure 1B). NGF treatment had no effect on the proportion of disulphide-linked p75NTR dimers (data not shown). Since neither the extracellular (Gong et al., 2008; He and Garcia, 2004) or intracellular (Liepinsh et al., 1997) domains of p75NTR have been reported to form disulphide-linked dimers, we turned our attention to the transmembrane domain of the receptor, which contains a Cys residue (Cys257) that is absolutely conserved in all p75NTR orthologs known to date, from echinoderms to mammals (Bothwell, 2006) (Figure 1C). Mutation of Cys257 to Alanine (C257A) abolished the ability of p75NTR to form disulphide-linked dimers (Figure 1A), indicating that such dimers are generated by disulphide bonding of two p75NTR molecules through Cys257 in the transmembrane domain. Disulphide-linked dimers could also be detected to varying degrees on the surface of cells endogenously expressing p75NTR, such as PC12 pheochromocytoma, RN22 Schwannoma and sympathetic neurons from the rat superior cervical ganglion (SCG) (Figure 1D), as well as in total extracts of newborn cortex, hippocampus and cerebellum (Figure 1E). Interestingly, in the latter cases the majority of p75NTR was disulphide-linked. Of note, a previous study found high levels of disulphide-linked p75NTR dimers in one melanoma cell line (A875) but not in another (HS294) (Grob et al., 1985). Importantly, the C257A mutation did not affect the ability of p75NTR to bind NGF (Figure 1F), nor its capacity to undergo γ-secretase-dependent intramembrane cleavage upon stimulation with the phorbol ester PMA (Figure 1G).
Figure 1. Transmembrane Cys257 mediates formation of disulphide-linked p75NTR dimers.
(A) Cell surface expression of disulphide-linked p75NTR dimers (dim) and monomers (mon) in transfected COS-7 cells visualized by Neutravidin probing of p75NTR immunoprecipitates under non-reducing (−DTT) and reducing (+DTT) conditions. In non-reducing conditions, p75NTR dimers run somewhat higher, and monomers lower, than their predicted molecular weights.
(B) Cell surface expression of disulphide-linked p75NTR dimers (dim) and monomers (mon) in COS-7 cells transfected with different amounts of wild type p75NTR. Immunoprecipitates were electrophoresed under non-reducing conditions and p75NTR was visualized by Neutravidin probing of p75NTR immunoprecipitates. Results are expressed as mean dimer/monomer ratio.
(C) Alignment of p75NTR transmembrane domain sequences from vertebrate and invertebrate species.
(D) Cell surface expression of endogenous disulphide-linked p75NTR dimers (dim) and monomers (mon) in PC12 cells, RN22 Schwannoma cells and SCG sympathetic neurons visualized by Neutravidin probing of p75NTR immunoprecipitates. Control, untransfected COS cells.
(E) Expression of endogenous disulphide-linked p75NTR dimers (dim) and monomers (mon) in extracts of newborn rat cerebellum (cblm), cortex (ctx) and hippocampus (hc) visualized by immunoblotting of p75NTR immunoprecipitates under reducing and non-reducing conditions. The control lane represents a mock immunoprecipitate of the cblm extract.
(F) Binding of 125I-NGF to wild type and C257A p75NTR analyzed by chemical crosslinking. Samples were run under reducing conditions. For each construct, binding counts were normalized to levels of expression as assessed by immunoblotting (IB). Results are expressed as mean ± SD of three independent determinations.
(G) γ-secretase-dependent intramembrane cleavage of C257A p75NTR following stimulation with PMA in transfected COS cells. The proteasome inhibitor epoxomycin was used to prevent degradation of CTF and ICD fragments. Mutant and wild type (wt) p75NTR molecules were recovered by immunoprecipitation and visualized by immunoblotting. The γ-secretase inhibitor DAPT blocked the generation of p75NTR intracellular domain (ICD). CTF, carboxy terminal fragment.
Cys257 is essential for p75NTR signaling in response to neurotrophins
The ability of native p75NTR to form disulphide-linked dimers prompted the question of its possible significance to p75NTR activation and downstream signaling. We therefore investigated the effects of the C257A mutation on the ability of the receptor to recruit intracellular effectors and activate downstream pathways in response to NGF. Unlike wild type p75NTR, the C257A mutant was unable to interact with NRIF, either in the presence or absence of NGF (Figure 2A). Although a weak interaction could be observed between the C257A mutant and TRAF6, this was not increased by NGF treatment, which on the other hand readily promoted TRAF6 recruitment to wild type receptors (Figure 2B). These data suggested that, although wild type p75NTR is present as both monomers and disulphide-linked dimers at the cell membrane, only the latter appear to be capable of recruiting downstream effectors such as NRIF and TRAF6 in a ligand-dependent manner. In order to address this directly, we tested the ability of NRIF and TRAF6 to differentially interact with monomers and disulphide-linked dimers of wild type p75NTR by analyzing pull-down products under reducing and non-reducing conditions. We found that NRIF interacted exclusively with disulphide-linked p75NTR dimers, but not at all with monomers, and NGF treatment could readily increase this interaction (Figure 2C). On the other hand, although TRAF6 could pull-down both p75NTR monomers and dimers, NGF treatment only stimulated TRAF6 binding to p75NTR dimers, but not monomers (Figure 2D). Also in sympathetic neurons from rat SCG, NRIF interacted only with p75NTR disulphide-linked dimers, not with monomers, and BDNF treatment strongly stimulated NRIF recruitment to the receptor (Figure 2E).
Figure 2. Cys257 is essential for recruitment of NRIF and TRAF6 to p75NTR in response to NGF.
(A) Binding of NRIF to wild type and mutant p75NTR in transfected HEK293 cells assayed by immunoprecipitation (IP) and immunoblotting (IB).
(B) Binding of TRAF6 to wild type and mutant p75NTR in transfected HEK293 cells.
(C)Binding of NRIF to wild type and mutant p75NTR in transfected HEK293 cells. Prior to gel electrophoresis, the pull-down sample was splitted in two equal parts, one was boiled in sample buffer, the other was treated with 1M DTT prior to boiling. The migration of p75NTR dimers and monomers is indicated.
(D) Binding of TRAF6 to wild type and mutant p75NTR in transfected HEK293 cells analyzed as in panel (C).
(E) Binding of NRIF to wild type and mutant p75NTR in rat SCG neurons. NRIF only interacts with p75NTR disulphide-linked dimers, not monomers.
We also examined three different downstream readouts of p75NTR activity in transfected fibroblasts and 293 cells, including activation of NF-κB (Figure 3A), caspase-3 (Figure 3B), and induction of cell death (Figure 3C) in response to NGF. In all cases, the C257A mutant was unresponsive to stimulation, indicating that Cys257 is required for neurotrophin-dependent p75NTR signaling and downstream biological effects.
Figure 3. Cys257 is essential for p75NTR signaling to NF-κB, caspase-3 and cell death in response to NGF.
(A) NF-κB activity in transfected M23 fibroblasts in the presence and absence of NGF.
(B) Activation of caspase-3 visualized with a cleavage-specific antibody in HEK293 cells transfected with p75NTR constructs in response to NGF. Reprobing controls for p75NTR and GAPDH are shown. Independent experiments confirmed comparable expression of transfected p75NTR constructs.
(C) Cell death assay in HEK293 cells transfected with p75NTR constructs in response to NGF. Results are expressed as mean ± SD of three independent experiments, each performed in duplicate.
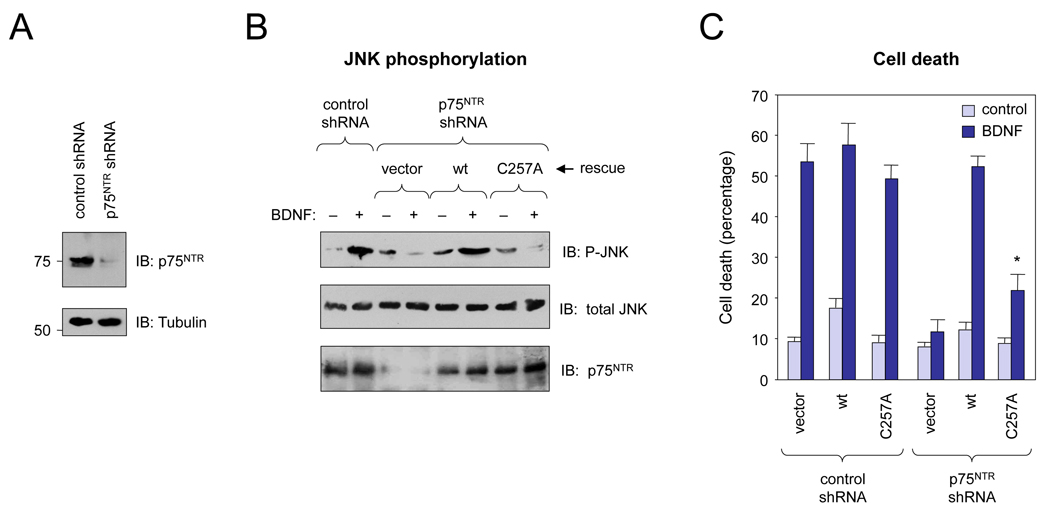
In order to probe the functional importance of Cys257 for p75NTR signaling in a more physiological context, we developed two shRNA constructs directed to 3’ UTR sequences of the rat p75NTR mRNA to knock-down endogenous p75NTR expression in neurons. When introduced together into primary cultures of rat SCG neurons, these shRNAs effectively suppressed endogenous p75NTR expression, while a control shRNA had no effect (Figure 4A). As expected, control shRNA did not affect the ability of BDNF to induce JNK phosphorylation (Figure 4B) and cell death (Figure 4C) in SCG neurons, which express p75NTR and TrkA but not TrkB. In contrast, shRNAs directed against p75NTR abolished both responses, indicating their dependence on p75NTR signaling (Figures 4B and 4C). Under those conditions, BDNF-mediated JNK phosphorylation and cell death could be restored by introduction of a wild type p75NTR expression construct that is insensitive to our p75NTR shRNAs (Figures 4B and 4C). In contrast to the wild type construct, the C257A mutant was unable to restore either JNK phosphorylation or cell death in response to BDNF in SCG neurons (Figures 4B and 4C). Together, these results demonstrate that Cys257 is essential for p75NTR signaling in response to neurotrophins, and support the notion that disulphide-linked p75NTR dimers are the active, neurotrophin-sensitive receptor species in neurons.
Figure 4. Cys257 is essential for p75NTR signaling in SCG neurons.
(A) Downregulation of p75NTR expression in rat SCG neurons following transfection of p75NTR shRNAs.
(B) Assay of JNK phosphorylation in SCG neurons in response to BDNF. SCG neurons were transfected with the indicated shRNA constructs. In rescue experiments, wild type p75NTR or C257A p75NTR constructs insensitive to p75NTR shRNAs were also introduced by DNA transfection. JNK phosphorylation was assessed by immunoblotting of total cell lysates.
(C) Cell death in SCG neurons transfected with p75NTR shRNAs in response to BDNF. Rescue experiments with wild type and C257A p75NTR constructs were performed as above. *, p<0.05 vs. wild type, n=3.
Cys257 is not required for downstream signaling by the p75NTR/NgR/Lingo-1 complex in response to MAG
The importance of Cys257 in neurotrophin-dependent p75NTR signaling prompted the question of its role in the activation of p75NTR by other types of ligands. We therefore tested the ability of wild type and mutant p75NTR to recruit RhoGDI and to increase RhoA activity in response to the myelin-derived ligand MAG. These activities require the presence of two additional receptor subunits, NgR and Lingo-1. Interestingly, cells expressing p75NTR, NgR and Lingo-1 responded equally well to stimulation with MAG in both RhoGDI recruitment to p75NTR (Figure 5A) and RhoA activity (Figure 5B) regardless of whether they received wild type or the C257A mutant. Thus, and in contrast to activation by neurotrophins, Cys257 is not required for the ability of p75NTR to signal in response to the non-neurotrophin ligand MAG, suggesting the existence of mechanistic differences in the activation of p75NTR by different ligands.
Figure 5. Cys257 is not required for downstream signaling by the p75NTR/NgR/Lingo-1 complex in response to MAG.
(A) Binding of RhoGDI to wild type and C257A p75NTR in COS-7 cells co-transfected with NgR and Lingo-1 and stimulated with MAG-Fc.
(B) RhoA activity in COS-7 cells transfected with wild type and mutant p75NTR in the presence of NgR and Lingo-1 after stimulation with MAG-Fc for 30 min. Results are expressed as mean ± SD relative to wild type without MAG treatment. *, p<0.05 vs. control, n=3.
Cys257 allows disulphide-linked p75NTR dimers to propagate conformational changes to intracellular domains following NGF binding
How does Cys257 contribute to p75NTR signaling in response to neurotrophins? Its localization in the transmembrane domain suggested that it may play a role in the mechanism of receptor activation, either by regulating p75NTR oligomerization, or by allowing the propagation of conformational changes induced by neurotrophin binding. Although Cys257 clearly contributes to the formation of disulphide-linked p75NTR dimers, it remained unclear whether this was the primary determinant of receptor oligomerization. In particular, the ability of the C257A mutant to bind NGF at normal levels (Figure 1E) suggested that the mutation may not have disrupted the oligomeric state of p75NTR at the cell surface, as this would have been expected to affect ligand binding affinity. In order to examine the possible role of non-covalent interactions in p75NTR oligomerization we performed chemical crosslinking studies of wild type and mutant cell surface receptors in living COS-7 cells. Following chemical crosslinking of cell surface proteins, p75NTR was immunoprecipitated from cell lysates, and then subjected to SDS/PAGE under reducing conditions, so that only oligomeric complexes stabilized by chemical crosslinking would be visualized. As expected, chemical crosslinking allowed the detection of wild type receptor dimers (Figure 6A), demonstrating that p75NTR indeed forms dimers at the surface of living cells in the absence of ligand. As chemical crosslinkers have only a limited efficiency, the actual proportion of receptor dimers and monomers at the cell surface can not be determined with this method. NGF binding did not alter the proportion of p75NTR dimers detected by chemical crosslinking in transfected cells (Figure 6A). Surprisingly, however, mutation of Cys257 did not affect the proportion of p75NTR dimers detected after chemical crosslinking, either in the absence or presence of NGF (Figure 6A). This indicated that p75NTR may still oligomerize in the absence of Cys257 through non-covalent interactions.
Figure 6. Cell surface p75NTR dimerization is mediated by covalent and non-covalent interactions between transmembrane domains.
(A) Wild type and C257A p75NTR dimers analyzed by cell surface chemical crosslinking under reducing conditions. Cell lysates were immunoprecipitated with anti-p75NTR antibodies; immunoblot was probed with anti-HA antibodies.
(B) Alignment of p75NTR transmembrane domains highlighting the AxxxG self-association motif and Gly266.
(C) ToxCAT assay of self-association of wild type and mutant p75NTR transmembrane domains. Wild type and mutant transmembrane domains from glycophorin A (GpATM) were used as positive and negative controls, respectively.
(D) Wild type and mutant p75NTR dimers analyzed by cell surface chemical crosslinking as above.
Inspection of the p75NTR transmembrane domain, revealed the presence of a relatively well conserved AxxxG266 motif (Figure 6B), which is characteristic of self-associating transmembrane domains, including those from integrins and glycophorin A (GpA) (Kubatzky et al., 2001). Interactions between isolated transmembrane domains can be studied in a biological membrane using the bacterial ToxCAT system (Russ and Engelman, 1999) (see Experimental Procedures for further details). Dimerization of wild type and mutant p75NTR transmembrane domains was assessed along with wild type GpA transmembrane domain, which is known for its strong self-association, and a GpA point mutant (G83I) that disrupts homodimerization, as positive and negative controls, respectively. The transmembrane domain of p75NTR was found to homodimerize in this system to about 60% of the level shown by GpA (Figure 6C). Mutation of Cys257 had only a small effect on transmembrane homodimerization (Figure 6C). In contrast, mutation of Gly266 to Ile (G266I), which is analogous to the G83I in GpA, abolished self-association of the p75NTR transmembrane domain (Figure 6C). When introduced in full-length p75NTR together with C257A, the G266I mutation diminished the formation of p75NTR dimers at the plasma membrane (Figure 6D). Together, these data indicate that p75NTR homodimers can be stabilized at the cell surface by both covalent and non-covalent interactions between transmembrane domains, and that the primary function of Cys257 is not in the formation of receptor dimers.
We then considered the possibility that Cys257 may instead contribute to p75NTR signaling by mediating the propagation of conformational changes induced by neurotrophin binding. We reasoned that conformational changes involving alterations in the relative positions of p75NTR intracellular domains may be detected by fluorescence resonance energy transfer (FRET). Unlike methods based on enzyme complementation, in FRET, the transfer of energy between two chromophores depends both on the distance between them and their relative orientation, and it is therefore more suitable to quantitatively assess conformational rearrangements of receptor subunits in a complex. We chose homo-FRET over other more conventional FRET techniques because of its greater sensitivity and the advantage of using a single spectral variant to detect interactions between identical molecules (Squire et al., 2004). Homo-FRET can be detected as a decrease in steady-state fluorescence anisotropy that results from energy transfer between identical fluorophores. Monomeric Enhanced Green Fluorescent Protein (EGFP1) displays high anisotropy (i.e. low FRET), while a concatenated EGFP trimer (EGFP3) shows very low anisotropy (i.e. very high FRET), and they were used as negative and positive controls, respectively (Figures 7A, 7B and 7E). Wild type and C257A p75NTR were tagged at the C-terminus with monomeric EGFP (see Experimental Procedures) and transfected in COS-7 cells. Anisotropy and total fluorescence were measured at different locations in the plasma membrane of transfected cells. At basal conditions, wild type p75NTR–EGFP displayed lower anisotropy than EGFP1 (i.e. higher FRET) at the plasma membrane (Figures 7C and 7E), in agreement with its oligomeric state in living cells. Using the anisotropy value of EGFP1 as baseline, the FRET level of wild type p75NTR–EGFP could be estimated to about 25% that of EGFP3 (Figure 7E), which gives a very strong FRET signal. Mutant C257A p75NTR–EGFP also showed lower anisotropy than EGFP1 at the cell surface, albeit not as much as wild type p75NTR (Figures 7D and 7E), which is in accordance with our crosslinking studies showing that this mutant retains the ability to form dimers at the plasma membrane. Interestingly, both p75NTR-EGFP constructs appeared mainly monomeric at intracellular locations (Figures 7C and 7D), suggesting that oligomerization occurs upon transit to the plasma membrane.
Figure 7. Analysis of conformational changes in p75NTR intracellular domains by anisotropy microscopy.
(A–D) Steady-state anisotropy in transfected cells. Examples of areas used for anisotropy measurements are boxed and shown as high magnification insets. Monomeric EGFP (EGFP1, high anisotropy, low FRET) and a concatenated EGFP trimer (EGFP3, low anisotropy, high FRET) were used as controls. The calibration bar of the look-up table is shown below.
(E) Steady-state anisotropy of wild type and C257A p75NTR-EGFP in COS-7 cells. The anisotropy value of EGFP1 was arbitrarily set to zero and used as baseline for the histogram. Bars show average ± SD (n=8–11 cells for EGFP and 22–30 cells for p75NTR).
(F) Representative examples of anisotropy traces after addition of NGF or medium in cells expressing wild type or C257A p75NTR-EGFP. A peak in anisotropy was observed after NGF addition in all cells expressing wild type p75NTR that were examined (n=30) regardless of their initial baseline anisotropy level.
(G) Anisotropy change after addition of NGF or medium (control). The difference in anisotropy before and after addition of NGF (i.e. peak minus baseline value) or medium was calculated for wild type and C257A p75NTR-EGFP. Results are expressed as average ± SD (n=15 to 17 cells examined). *, p<0.0001 vs. C257A.
NGF was added to cells expressing wild type and C257A p75NTR–EGFP and changes in anisotropy were evaluated by time-lapse microscopy. Acute addition of NGF produced an increase in anisotropy (i.e. decrease in FRET) at the plasma membrane of cells expressing wild type p75NTR–EGFP (Figure 7E). This anisotropy rise was observed in all cells examined (n=30), showed a consistent peak 2–3 minutes after NGF addition, and lasted for 3 to 10 minutes, waning towards the end of the recording (15 min) (Figures 7F and 7G). In contrast, NGF had no effect on cell surface anisotropy in cells expressing the mutant C257A p75NTR–EGFP (n=22 cells examined) (Figures 7F and 7G). These results indicate that NGF induces a decrease in FRET in wild type p75NTR–EGFP at the plasma membrane, indicating that receptor activation by neurotrophins involves conformational changes in the relative position or orientation of p75NTR intracellular domains. The inability of NGF to induce any change in FRET in cells expressing C257A p75NTR–EGFP correlates with the loss of neurotrophin-dependent p75NTR signaling in this mutant, and supports a role for Cys257 in the propagation of conformational changes induced by neurotrophin binding.
DISCUSSION
In this study, we have investigated the mechanism of activation of the p75 neurotrophin receptor. The first major finding of this work is the ability of native p75NTR to form disulphide-linked dimers through the conserved Cys257 in the transmembrane domain. The second is the unexpected requirement of this cysteine residue for the recruitment of intracellular effectors and downstream signaling by p75NTR in response to neurotrophins, but not in response to other ligands, such as MAG. The third is the ability of the p75NTR dimer to undergo a conformational change in response to NGF, and the essential role of Cys257 in this process. Based on these observations, we propose a novel mechanism of receptor activation involving ligand-induced rearrangement of disulphide-linked receptor subunits.
While it is relatively straightforward to visualize how receptor kinases may become activated upon ligand binding (Schlessinger, 2002), this is less obvious for non-catalytic receptors. Since these signal by selective interactions with intracellular effectors, the question that arises is how ligand binding regulates those interactions. Clearly, something ought to change in the receptor after ligand binding that makes its intracellular domain more or less prone to interact with downstream effectors. Ligand binding may change the oligomerization state of the receptor —by, for example, inducing dimers— leading to cooperative binding or release of downstream effectors. Although this has been the prevalent model for p75NTR activation to date, our results show that the active p75NTR species pre-exists as a disulphide-linked dimer which is essential for downstream signaling in response to neurotrophins, obviating a classical ligand-mediated dimerization mechanism. Although an earlier study using artificially deglycosylated p75NTR extracellular domain had suggested that NGF may activate a preformed receptor dimer by inducing its dissociation into monomers (He and Garcia, 2004), more recent work has clearly shown that glycosylated p75NTR extracellular domain is monomeric in the absence of ligand, and that NGF interacts with p75NTR dimers, not monomers (Gong et al., 2008). Another possible mechanism for receptor activation is that ligand binding changes the relative orientation of receptor subunits in a dimeric or multimeric receptor complex. In some cytokine and growth factor receptors, this is achieved through the relative rotation of their transmembrane domains within the plane of the membrane (Moriki et al., 2001; Seubert et al., 2003). In the case of p75NTR, however, the disulphide link formed by Cys257 in the transmembrane domain clearly restricts the possibilities for relative movement of receptor subunits.
Our FRET experiments indicate that the two intracellular domains of the p75NTR dimer are in close proximity under basal conditions. This is in agreement with data from a recent study using a β-gal complementation strategy (Wehrman et al., 2007). Unlike FRET, however, enzyme complementation gives an all-or-none response at the single molecule level, and is hence less suited to reveal subtle conformational changes that do not alter the oligomeric state of the receptor. In our experiments, we observed a consistent decrease in FRET upon NGF binding to wild type p75NTR. This could either result from a separation of p75NTR intracellular domains or changes in their relative orientation due to rotation of receptor subunits. Although these two possibilities are not mutually exclusive, it is difficult to envision how changes in the relative orientation of intracellular domains can be brought about by ligand binding to the extracellular region of a dimeric receptor that is covalently crosslinked at the plasma membrane. As explained above, a disulphide bridge linking the transmembrane regions of two receptor subunits would clearly prevent the propagation of rotational movements from the extracellular to the intracellular domains. Thus, we believe that the decrease in FRET observed after NGF binding most likely reflects the separation of p75NTR intracellular domains. Importantly, and in contrast to the wild type receptor, we found no evidence of NGF-mediated conformational changes in the intracellular domain of the C257A p75NTR mutant. Based on these observations, we propose that neurotrophin binding produces a scissors-like movement of disulphide-linked p75NTR subunits with the Cys257–Cys257 disulphide link acting as the fulcrum, thereby altering the relative proximity of intracellular domains (Figure 8). In this model, Cys257 would function as the pin in the scissors: in its absence, relative movements at one end can not be propagated to the other. Unlike normal scissors, however, our results suggest that as the extracellular domains come closer together on binding to the neurotrophin dimer, the intracellular domains separate, not unlike a “snail-tong” mechanism (Figure 8). This type of movement could be achieved if the p75NTR protein were to be kinked, as opposed to straight, relative to the plane of the plasma membrane, in the vicinity of Cys257. Proline residues are known to introduce kinks that may vary from 5 to 50 degrees, and are common in transmembrane helices (Yohannan et al., 2004). Thus, the highly conserved Pro254, three residues upstream of Cys257 (see Figure 1C), seems like a good candidate for such function. Although not as well conserved, an unusual Pro triad is also present in the intracellular juxtamembrane region of p75NTR, 20 residues away from the plasma membrane (Pro295-Pro296-Pro297). This portion of p75NTR is not known to be helical in vivo, and has been characterized by nuclear magnetic resonance as unstructured in solution (Liepinsh et al., 1997). The conformational changes inferred from our FRET experiments require a relatively rigid structure connecting the extra and intracellular domains, and suggest that the juxtamembrane region of p75NTR may be stabilized in vivo by interaction with other intracellular components.
Figure 8. The “snail-tong” mechanism of p75NTR activation in response to neurotrophins.
Hypothetical schematic of p75NTR in the cell membrane before and after neurotrophin binding (adapted from Gong et al. (2008) and Liepinsh et al. (1997)). The approximate position of Cys257 is indicated. Arrows denote the postulated “snail-tong” movement of p75NTR subunits initiated by ligand binding: closing onto the neurotrophin dimer in the outside, opening in the inside.
The conformational changes observed upon NGF binding showed a characteristic maximum at 2–3 minutes after NGF addition and lasted for an additional 3 to 10 minutes. This kinetics is consistent with the onset of p75NTR internalization and downstream signaling, which requires at least 15 min after ligand stimulation (Bronfman et al., 2003). The separation of intracellular domains induced by NGF binding may facilitate the recruitment of downstream effectors by exposing determinants important for their interaction with the receptor. This may be particularly important for receptors with relatively small intracellular domains, such as p75NTR. Weak homotypic interactions between these domains may keep the receptor in a closed, inactive state, and could potentially explain the residual amount of dimers observed after simultaneous mutation of Cys257 and Gly266. Although the p75NTR death domain was found to be monomeric in solution up to 2.5 mM (Liepinsh et al., 1997), such interactions might be detected in crystallization experiments. Even though it is unclear at present why the conformational change observed is not sustained for longer periods of time, the variation observed in its duration could reflect interactions with different effector molecules or activation of different downstream signaling events. That p75NTR activation involves separation of intracellular domains is consistent with a previous study using chimeric intracellular domains artificially tethered to the plasma membrane that were induced to dimerize by addition of a dimeric drug (Wang et al., 2000). In this work, dimerization inhibited the proapoptotic effect of the constructs, from which the authors inferred that neurotrophin-mediated receptor dimerization should likewise silence p75NTR activity, a conclusion that was at odds with the bulk of studies in the field. Our results indicating that p75NTR is already dimeric before ligand binding and that p75NTR activation by NGF involves the separation of intracellular domains reconcile those results and suggest that this is in fact the way in which neurotrophins activate this receptor.
Previous work has shown that p75NTR can enhance Trk responses to neurotrophins, particularly when these are present at limiting concentrations. For example, sensory and sympathetic neurons from p75NTR knock-out mice show reduced resposiveness to NGF (Lee et al., 1994), and a mutant NGF unable to bind p75NTR displays lower binding affinity and reduced biological activity in PC12 cells and sensory and sympathetic neurons (Rydén et al., 1997a). This positive modulatory role of p75NTR on neurotrophin activities mediated by Trk receptors has been attributed to receptor-receptor interactions and/or ligand concentration or presentation effects. It is unclear at present whether these activities require disulphide-linked dimerization of p75NTR. Given the proposed role for the TrkA and p75NTR transmembrane domains in their functional interaction (Esposito et al., 2001), it is possible that formation of high-affinity NGF binding sites may be sensitive to conformational changes mediated by Cys257.
The fact that p75NTR signaling in response to MAG does not require Cys257 suggests that myelin-derived ligands activate p75NTR by a different mechanism. Although our results indicate that disulphide-linked dimerization is important for p75NTR activation by neurotrophins, the stoichiometry of the complex formed by p75NTR, NgR and Lingo-1 needs to be further investigated. The lack of involvement of Cys257 in signaling by MAG opens an opportunity to specifically disrupt neurotrophin-specific p75NTR effects in loss-of-function studies. Intriguingly, we found that the proportion of cell surface disulphide-linked p75NTR dimers varied in different cell types and with different levels of receptor expression. As only such dimers are able to respond to neurotrophins, changes in their proportion or abundance at the cell surface, e.g. between different cell types or in response to external stimuli or redox states, could determine the relative degree of p75NTR responsiveness to different ligands.
Cys257 represents a critical element in the activation mechanism of p75NTR by neurotrophins and is absolutely conserved in all vertebrate and invertebrate p75NTR molecules isolated so far (Bothwell, 2006). We propose that neurotrophins neither activate p75NTR by inducing receptor dimers or disassembly into monomers, as previously suggested, but through a novel mechanism involving the rearrangement of disulphide-linked receptor subunits. Many receptors in the TNFR superfamily —to which p75NTR belongs— bear intramembrane cysteines, including Fas, DR6, RANK, RELT, BCM, TACI, CD30, CD40 and receptors for TNF, lymphotoxin beta and TRAIL. The “snail-tong” mechanism described here could therefore represent a general way by which receptors bearing intramembrane cysteines are activated.
EXPERIMENTAL PROCEDURES
Plasmids, antibodies, proteins and chemicals
Rat p75NTR was expressed from the pCDNA3 vector backbone (Invitrogen) using a full-length coding sequence flanked by an N-terminal hemagglutinin (HA) epitope tag. Mutations were introduced using QuickChange (Stratagene) and verified by DNA sequencing. Plasmids to express RIP2, TRAF6, NRIF, sortilin, Lingo-1 and RhoGDI were previously described. EGFP plasmid was from Clontech. Luciferase reporter plasmid for NF-κB was from Promega. The origin of antibodies were as follows: MC192 anti-p75NTR from Phil Barker; anti-HA from Roche; anti-myc, anti-phospho and-total JNK, cleavage-specific anti-caspase-3, anti-RhoA and anti-RhoGDI from Cell Signaling; anti-tubulin from Sigma; anti-RIP2 from Santa Cruz. NGF was purchased from Alomone Labs, MAG-Fc and Nogo peptide from R&D. NGF was typically applied at 100 ng/ml for 30 minutes unless otherwise indicated. MAG-Fc was used at 25 µg/ml for 30 minutes. PMA was used at 200 nM for 1 h. Epoxomycin (1 µM) and DAPT (2 µM) were applied 1.5 h prior to PMA. All compounds were from Sigma.
Cell transfection and tissue culture
COS-7 cells were transfected with polyethylenimine (PEI). HEK293 and M23 cells were transfected with Lipofectamine 2000 (Gibco). M23 is a clonal derivative of MG87 cells (Eketjäll et al., 1999), originally derived from mouse NIH3T3 fibroblasts. Cells were typically used on the 2nd day after transfection for short-term signaling assays, at which point cell death was still low or undetectable. We found that different signaling assays worked best in different cell lines: RhoA and RhoGDI in COS-7, NF-κB in M23 and P-JNK and caspase-3 in HEK293 cells. This may be related to the specific complement of downstream effectors expressed by each cell type. Cell lines were cultured under standard conditions and primary neurons in serum-free, N2-supplemented DMEM:F12 medium (Gibco).
Sympathetic neuron culture and p75NTR shRNAs
Sympathetic neurons from P4 rat SCGs were cultured and transfected with control or p75NTR shRNAs alone or co-transfected with wild type or mutant p75NTR as previously described (Kenchappa et al., 2006; Palmada et al., 2002). The target sequence of the first p75NTR shRNA was ACGGACCTATCTGAGCTGAAA, the second was ATGGCGTGACTTTCAGGGAAA. Control shRNA was targeted against EGFP sequences. Three days after transfection neurons were treated with 200 ng/ml of BDNF for 1 hr and lysed in NP-40 buffer (10% glycerol, 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% NP-40,, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride (PMSF), 2 mg/ml leupeptin and aprotinin) to assess JNK activation. For cell survival assay the cells were fixed in 4% paraformaldehyde 48 hrs after BDNF treatment and scored for apoptosis as previously described (Kenchappa et al., 2006).
Biotinylation, immunoprecipitation, immunoblotting and chemical crosslinking
Cell surface proteins were biotinylated with Sulfo-NHS-LC-Biotin (Pierce). Cells were lysed in buffer containing 1% Triton X-100, 60 mM octyl-glucosyde, 10 mM iodoacetamide and protease inhibitors (Roche). For reducing conditions, immunoprecipitates were boiled in sample buffer containing 1M DTT. Gels were blotted to PVDF membranes (Amersham). Biotinylated proteins were detected using Neutravidin conjugated to Alkaline Phosphatase (Sigma). Filters were developed by chemifluoresence (Amersham) and scanned on a STORM840 fluorimager (MolDynamics). Radioiodination of NGF was done with lactoperoxidase (Sigma), and EDAC/SulfoNHS (Pierce) was used as chemical crosslinker. Autoradiography was done on a STORM840 phosphorimager. Quantifications of immunoblots and autoradiograms were done with ImageQuant software (MolDynamics).
Assays of NF-kB, RhoA and cell death
NF-κB activity was assayed using a luciferase reporter kit (Promega). NGF was added 2 days after transfection and left for 24 h prior to cell lysis. RhoA activity was evaluated using a kit from Cytoskeleton. Cell death was assessed by the TUNEL method using kits from Roche and Biocolor. NGF was added 2 days after transfection and left for another day in serum free-medium prior to assay of cell death.
ToxCAT assay of self-association of transmembrane domains
The reporter system exploits the ability of the ToxR transcription activator of the Vibrio Cholerae pathogen to bind the cholera toxin (ctx) gene promoter only when dimerized. A transmembrane segment of interest fused to ToxR is delivered to the bacterial inner membrane by fusion to maltose-binding protein (MBP). Varying amounts of ToxR dimers will be formed in the cytosol in direct proportion to the oligomerization ability of the TM domain. Binding of dimerized ToxR to the ctx DNA element triggers expression of a chloramphenicol transferase (cat) gene reporter and production of CAT protein, which can then be quantified by a CAT-ELISA kit (Roche Diagnostics) as described by McClain et al. (McClain et al., 2003). Expression levels of different ToxR-TM-MBP chimeras were determined by Western blotting. The ToxR and ToxCAT systems have been previously applied to demonstrate self-association of a range of TM domains from glycophorin A (GpA) (Kubatzky et al., 2001), integrin αIIb (Li et al., 2004), ErbB1 to 4 (Mendrola et al., 2002), and the Epo receptor (Kubatzky et al., 2001).
Anisotropy microscopy and FRET
Anisotropy microscopy was done as described by Squire et al. (Squire et al., 2004) in transiently transfected COS-7 cells. Images were acquired 15–24 h post-transfection, using a Olympus IX81 inverted microscope (Olympus, Germany) equipped with a MT20 illumination system. A linear dichroic polarizer (Meadowlark Optics, Frederick, Colorado, US) was placed in the illumination path of the microscope, and two identical polarizers were placed in an external filter wheel at orientations parallel and perpendicular to the polarization of the excitation light. The fluorescence was collected via a 20× 0.7 NA air objective, and parallel and polarized emission images were acquired sequentially on an Orca CCD camera (Hamamatsu Photonics, Japan). Data acquisition was controlled by the CellR software supplied by the microscope manufacturer. NGF (or vehicle) was added 1 min after the start of the time-lapse at a concentration of 100 ng/ml. Anisotropy values were extracted from image stacks of 30 images acquired in both parallel and perpendicular emission modes every 30 sec for a time period of 15 min after NGF addition. For each consrtuct, 2 areas per field of view were measured in 4 independent trasnfections performed in duplicate or triplicate. Fluorescence intensity and anisotropy images were calculated as described by Squire et al. (Squire et al., 2004). Wild type and C257A p75NTR constructs were tagged at the C-terminus with a monomeric version of EGFP (Clontech) carrying the A207K mutation that disrupts EGFP dimerization. A cytosolic EGFP monomer (EGFP1) gave a mean anisotropy value of 0.295 ± 0.005 (no FRET), and a concatenated EGFP trimer (EGFP3) gave a value of 0.2369 ± 0.013 (maximal FRET), so thresholds for image analysis (done with Image J software) were set between 0.2 and 0.35 (Figure 7A to D).
ACKNOWLEDGEMENTS
We thank Phil Barker for MC192 antibody, Moses Chao, Toshihide Yamashita, Anders Nykjær and Daniel Lee for RIP2, RhoGDI, sortilin and Lingo-1 expression plasmids, and Neil McDonald for fruitful discussions. This work was supported by grants from the Swedish Foundation for Strategic Research (CEDB), the Swedish Research Council (33X-10908-10A and Linné program), the Vth Framework Program of the European Union (QLG3-CT-1999-00573), and the National Institutes of Health (NIH 1 R01 MH071624-01A2). R.S.K. and B.D.C. were supported by the National Institutes of Health (R01 NS038220), A.R. and G.S. by Cancer Research UK, M.B. by the National Institutes of Health (NIH 5 R01NS47348), I.M. by the Spanish Ministry of Education (BFU2006-08542), and A.S by the Marie Curie RTN “ENDOCYTE” from the European Union FP6 Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–260. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- Barker PA. p75NTR is positively promiscuous: novel partners and new insights. Neuron. 2004;42:529–533. doi: 10.1016/j.neuron.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–2937. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- Bothwell M. Evolution of the neurotrophin signaling system in invertebrates. Brain Behav Evol. 2006;68:124–132. doi: 10.1159/000094082. [DOI] [PubMed] [Google Scholar]
- Bronfman FC, Fainzilber M. Multi-tasking by the p75 neurotrophin receptor: sortilin things out? EMBO Rep. 2004;5:867–871. doi: 10.1038/sj.embor.7400219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfman FC, Tcherpakov M, Jovin TM, Fainzilber M. Ligand-induced internalization of the p75 neurotrophin receptor: a slow route to the signaling endosome. J Neurosci. 2003;23:3209–3220. doi: 10.1523/JNEUROSCI.23-08-03209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BD, Kaltschmidt C, Kaltschmidt B, Offenhäuser N, Böhm-Matthaei R, Baeuerle PA, Barde YA. Selective activation of NF-kappa B by nerve growth factor through the neurotrophin receptor p75. Science. 1996;272:542–545. doi: 10.1126/science.272.5261.542. [DOI] [PubMed] [Google Scholar]
- Constantinescu SN, Ghaffari S, Lodish HF. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends Endocrinol Metab. 1999;10:18–23. doi: 10.1016/s1043-2760(98)00101-5. [DOI] [PubMed] [Google Scholar]
- Dechant G, Barde YA. The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system. Nat Neurosci. 2002;5:1131–1136. doi: 10.1038/nn1102-1131. [DOI] [PubMed] [Google Scholar]
- Eketjäll S, Fainzilber M, Murray-Rust J, Ibáñez CF. Distinct structural elements in GDNF mediate binding to GFRa1 and activation of the GFRa1-c-Ret receptor complex. EMBO J. 1999;18:5901–5910. doi: 10.1093/emboj/18.21.5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito D, Patel P, Stephens RM, Perez P, Chao MV, Kaplan DR, Hempstead BL. The cytoplasmic and transmembrane domains of the p75 and Trk A receptors regulate high affinity binding to nerve growth factor. J Biol Chem. 2001;276:32687–32695. doi: 10.1074/jbc.M011674200. [DOI] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Friedman WJ. Neurotrophins induce death of hippocampal neurons via the p75 receptor. J Neurosci. 2000;20:6340–6346. doi: 10.1523/JNEUROSCI.20-17-06340.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman WJ, Greene LA. Neurotrophin signaling via Trks and p75. Exp Cell Res. 1999;253:131–142. doi: 10.1006/excr.1999.4705. [DOI] [PubMed] [Google Scholar]
- Gong Y, Cao P, Yu HJ, Jiang T. Crystal structure of the neurotrophin-3 and p75NTR symmetrical complex. Nature. 2008;454:789–793. doi: 10.1038/nature07089. [DOI] [PubMed] [Google Scholar]
- Grob PM, Ross AH, Koprowski H, Bothwell M. Characterization of the human melanoma nerve growth factor receptor. J Biol Chem. 1985;260:8044–8049. [PubMed] [Google Scholar]
- He XL, Garcia KC. Structure of nerve growth factor complexed with the shared neurotrophin receptor p75. Science. 2004;304:870–875. doi: 10.1126/science.1095190. [DOI] [PubMed] [Google Scholar]
- Hempstead BL, Martin-Zanca D, Kaplan DR, Parada LF, Chao MV. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991;350:678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Jiang G, Hunter T. Receptor signaling: when dimerization is not enough. Curr Biol. 1999;9:R568–R571. doi: 10.1016/s0960-9822(99)80357-1. [DOI] [PubMed] [Google Scholar]
- Jung KM, Tan S, Landman N, Petrova K, Murray S, Lewis R, Kim PK, Kim DS, Ryu SH, Chao MV, Kim TW. Regulated intramembrane proteolysis of the p75 neurotrophin receptor modulates its association with the TrkA receptor. J Biol Chem. 2003;278:42161–42169. doi: 10.1074/jbc.M306028200. [DOI] [PubMed] [Google Scholar]
- Kanning KC, Hudson M, Amieux PS, Wiley JC, Bothwell M, Schecterson LC. Proteolytic processing of the p75 neurotrophin receptor and two homologs generates C-terminal fragments with signaling capability. J Neurosci. 2003;23:5425–5436. doi: 10.1523/JNEUROSCI.23-13-05425.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Kenchappa RS, Zampieri N, Chao MV, Barker PA, Teng HK, Hempstead BL, Carter BD. Ligand-dependent cleavage of the P75 neurotrophin receptor is necessary for NRIF nuclear translocation and apoptosis in sympathetic neurons. Neuron. 2006;50:219–232. doi: 10.1016/j.neuron.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Kubatzky KF, Ruan W, Gurezka R, Cohen J, Ketteler R, Watowich SS, Neumann D, Langosch D, Klingmuller U. Self assembly of the transmembrane domain promotes signal transduction through the erythropoietin receptor. Curr Biol. 2001;11:110–115. doi: 10.1016/s0960-9822(01)00018-5. [DOI] [PubMed] [Google Scholar]
- Lee FS, Kim AH, Khursigara G, Chao MV. The uniqueness of being a neurotrophin receptor. Curr Opin Neurobiol. 2001a;11:281–286. doi: 10.1016/s0959-4388(00)00209-9. [DOI] [PubMed] [Google Scholar]
- Lee KF, Davies AM, Jaenisch R. p75-deficient embryonic dorsal root sensory and neonatal sympathetic neurons display a decreased sensitivity to NGF. Development. 1994;120:1027–1033. doi: 10.1242/dev.120.4.1027. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001b;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Li R, Gorelik R, Nanda V, Law PB, Lear JD, DeGrado WF, Bennett JS. Dimerization of the transmembrane domain of Integrin alphaIIb subunit in cell membranes. J Biol Chem. 2004;279:26666–26673. doi: 10.1074/jbc.M314168200. [DOI] [PubMed] [Google Scholar]
- Liepinsh E, Ilag LL, Otting G, Ibañez CF. NMR structure of the death domain of the p75 neurotrophin receptor. EMBO J. 1997;16:4999–5005. doi: 10.1093/emboj/16.16.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadeo D, Kaplan L, Chao MV, Hempstead BL. High affinity nerve growth factor binding displays a faster rate of association than p140trk binding. Implications for multi-subunit polypeptide receptors. J Biol Chem. 1994;269:6884–6891. [PubMed] [Google Scholar]
- McClain MS, Iwamoto H, Cao P, Vinion-Dubiel AD, Li Y, Szabo G, Shao Z, Cover TL. Essential role of a GXXXG motif for membrane channel formation by Helicobacter pylori vacuolating toxin. J Biol Chem. 2003;278:12101–12108. doi: 10.1074/jbc.M212595200. [DOI] [PubMed] [Google Scholar]
- Mendrola JM, Berger MB, King MC, Lemmon MA. The single transmembrane domains of ErbB receptors self-associate in cell membranes. J Biol Chem. 2002;277:4704–4712. doi: 10.1074/jbc.M108681200. [DOI] [PubMed] [Google Scholar]
- Mi S, Lee X, Shao Z, Thill G, Ji B, Relton J, Levesque M, Allaire N, Perrin S, Sands B, et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- Moriki T, Maruyama H, Maruyama IN. Activation of preformed EGF receptor dimers by ligand-induced rotation of the transmembrane domain. J Mol Biol. 2001;311:1011–1026. doi: 10.1006/jmbi.2001.4923. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- Palmada M, Kanwal S, Rutkoski NJ, Gustafson-Brown C, Johnson RS, Wisdom R, Carter BD. c-jun is essential for sympathetic neuronal death induced by NGF withdrawal but not by p75 activation. J Cell Biol. 2002;158:453–461. doi: 10.1083/jcb.200112129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11:272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- Roux PP, Barker PA. Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol. 2002;67:203–233. doi: 10.1016/s0301-0082(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Russ WP, Engelman DM. TOXCAT: a measure of transmembrane helix association in a biological membrane. Proc Natl Acad Sci U S A. 1999;96:863–868. doi: 10.1073/pnas.96.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydén M, Hempstead B, Ibá ñ ez CF. Differential modulation of neuron survival during development by nerve growth factor binding to the p75 neurotrophin receptor. J Biol Chem. 1997a;272:16322–16328. doi: 10.1074/jbc.272.26.16322. [DOI] [PubMed] [Google Scholar]
- Rydén M, Hempstead BL, Ibañez CF. Differential modulation of neuron survival during development by nerve growth factor binding to the p75 neurotrophin receptor. J Biol Chem. 1997b;272:16322–16328. doi: 10.1074/jbc.272.26.16322. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–672. doi: 10.1016/s0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- Seubert N, Royer Y, Staerk J, Kubatzky KF, Moucadel V, Krishnakumar S, Smith SO, Constantinescu SN. Active and inactive orientations of the transmembrane and cytosolic domains of the erythropoietin receptor dimer. Mol Cell. 2003;12:1239–1250. doi: 10.1016/s1097-2765(03)00389-7. [DOI] [PubMed] [Google Scholar]
- Squire A, Verveer PJ, Rocks O, Bastiaens PI. Red-edge anisotropy microscopy enables dynamic imaging of homo-FRET between green fluorescent proteins in cells. J Struct Biol. 2004;147:62–69. doi: 10.1016/j.jsb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Troy CM, Friedman JE, Friedman WJ. Mechanisms of p75-mediated death of hippocampal neurons Role of caspases. J Biol Chem. 2002;277:34295–34302. doi: 10.1074/jbc.M205167200. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Rabizadeh S, Tasinato A, Sperandio S, Ye X, Green M, Assa-Munt N, Spencer D, Bredesen DE. Dimerization-dependent block of the proapoptotic effect of p75(NTR) J Neurosci Res. 2000;60:587–593. doi: 10.1002/(SICI)1097-4547(20000601)60:5<587::AID-JNR3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Wang KC, Kim JA, Sivasankaran R, Segal RA, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- Wehrman T, He X, Raab B, Dukipatti A, Blau H, Garcia KC. Structural and mechanistic insights into nerve growth factor interactions with the TrkA and p75 receptors. Neuron. 2007;53:25–38. doi: 10.1016/j.neuron.2006.09.034. [DOI] [PubMed] [Google Scholar]
- Wong ST, Henley JR, Kanning KC, Huang KH, Bothwell M, Poo MM. A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat Neurosci. 2002;5:1302–1308. doi: 10.1038/nn975. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Higuchi H, Tohyama M. The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J Cell Biol. 2002;157:565–570. doi: 10.1083/jcb.200202010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Tucker KL, Barde YA. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron. 1999;24:585–593. doi: 10.1016/s0896-6273(00)81114-9. [DOI] [PubMed] [Google Scholar]
- Yohannan S, Faham S, Yang D, Whitelegge JP, Bowie JU. The evolution of transmembrane helix kinks and the structural diversity of G protein-coupled receptors. Proc Natl Acad Sci U S A. 2004;101:959–963. doi: 10.1073/pnas.0306077101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SO, Casaccia-Bonnefil P, Carter B, Chao MV. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J Neurosci. 1998;18:3273–3281. doi: 10.1523/JNEUROSCI.18-09-03273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]