Abstract
Purpose
To assess the neuroprotective effect of virally-mediated over-expression of ciliary derived neurotrophic factor (CNTF) and brain-derived neurotrophic factor (BDNF) in experimental rat glaucoma.
Methods
Laser-induced glaucoma was produced in one eye of 224 Wistar rats after injection of adeno-associated viral vectors (type 2) containing either CNTF, BDNF or both, using saline injected eyes and uninjected glaucoma eyes as controls. IOP was measured with the TonoLab and semi-automated optic nerve axon counts were performed by masked observers. IOP exposure over time was adjusted in multivariate regression analysis to calculate the effect of CNTF and BDNF.
Results
By multivariate regression, CNTF had a significant protective effect, with 15% less RGC axon death (p < 0.01). Both combined CNTF—BDNF and BDNF over-expression alone had no statistically significant improvement in RGC axon survival. By Western blot, there was a quantitative increase in CNTF and BDNF expression in retinas exposed to single viral vectors carrying each gene, but no increase with sequential injection of both vectors.
Conclusion
These data confirm that CNTF can exert a protective effect in experimental glaucoma. The reason for a lack of observed effect with the BDNF overexpression groups is unclear, but may be a function of the level of neurotrophin expression achieved.
Introduction
Glaucoma is the second leading cause of bilateral blindness worldwide1,2 and death of retinal ganglion cells (RGC) is a principal pathological finding in glaucoma3. RGC death by apoptosis has been detected in human glaucoma eyes4,5, in human optic neuropathy6, and in animal models of glaucoma and optic nerve injury7,8,9,10,11,12. In an effort to complement intraocular pressure reducing therapies, which are the mainstay of current treatment for glaucoma, a number of investigators have been pursuing development of neuroprotective strategies that seek to directly promote the survival and health of RGCs. Among the approaches that have been reported to promote RGC survival in rat glaucoma models are inhibition of nitric oxide synthase13,14, stimulation of heat shock protein production15, blockade of N-methyl-d-aspartate (NMDA) receptors16, treatment with alpha-adrenergic agonists17,18, treatment with T cells or copaxone19, overexpression of a caspase inhibitor20, and modulation of neurotrophin expression21.
We have been concentrating on modulation of neurotrophin expression because there is substantial evidence that neurotrophin withdrawal plays a central role in glaucoma damage. Loss of physiological neurotrophin levels (particularly brain-derived neurotrophic factor, BDNF) is consistent with known events in the clinical and pathological aspects of glaucoma22. Obstruction of anterograde and retrograde axonal transport at the optic nerve head23,24,25 occurs in human glaucoma and probably is one of the initiators of survival and death mechanisms that affect both RGC axons and cell bodies. BDNF moves from the brain to RGC on the trkB receptor, and its retrograde transport is obstructed in acute and chronic glaucoma models. Trophic dependence of fetal and adult rat RGC neurotrophic support is established26 and overexpression of BDNF delays RGC death in experimental glaucoma21. BDNF is not only delivered retrogradely from target central neurons27, but is also produced by RGC28 and retinal astrocytes29.
The specific BDNF receptor, trkB, is present on RGC dendrites and cell bodies, so BDNF can have an effect when presented intraretinally30, as well as by retrograde transport. Acting through the trkB receptor, it leads to phosphorylation of c-jun by jun kinase31 and may activate phosphoinositol 3-kinase, preventing caspase 3 from being activated32. TrkB levels are dependent upon excitation state and cyclic AMP levels33. BDNF may also have a proapoptotic effect through binding to the p75 NT receptor28, as well as indirect effects on other neurons or glia34,35.
Sustained increase in retinal BDNF is neuroprotective in multiple optic nerve injury models, but the presence and magnitude of the beneficial effect of neurotrophins on injured RGC may depend on the delivery method, dose, and on the model. Single BDNF intravitreal injections confer no protection in experimental glaucoma36, nor in experimental retinal detachment37, but BDNF injection and virally-mediated overexpression of BDNF slow RGC death38,39,40 and increase RGC regeneration41 after optic nerve transection. Repeated BDNF injections alone or by injection combined with additional measures prevent some experimental glaucoma injury42,43. Yet, neuronal exposure to exogenous BDNF has been reported to downregulate its trkB receptor44 and to be toxic as well as beneficial45,46. While optic nerve transection downregulates the trkB receptor, overexpression of the trkB receptor delays RGC death after optic nerve transection47.
Ciliary-derived neurotrophic factor (CNTF), first discovered by Adler and colleagues48, has neuroprotective properties in various experimental injuries to retinal and central neurons49,50,51,52,53,54,55, including by intravitreal injection in experimental glaucoma56. It is a secreted protein affecting neurons through a heterotrimeric membrane receptor57. CNTF is expressed in cells of all retinal layers and in retinal pigment epithelium58 as well as in optic nerve head59. Endogenous retinal expression of CNTF increases with retinal and optic nerve injury60,61, but may decrease with experimental IOP elevation62. Virally-mediated over-expression of CNTF is protective for RGC after optic nerve injury63,64 and for photoreceptors in retinal degeneration models65,66,67, but depresses electroretinographic potentials at some dose levels. Human clinical trials are underway with intravitreal capsules containing immortalized pigment epithelial cells expressing CNTF68.
Some reports with BDNF found more RGC preservation when it was combined with other interventions. BDNF supplemented by a free radical scavenger was protective in rat glaucoma, but neither agent alone was effective42. After optic nerve transection, regeneration into a nerve graft was greater with CNTF treatment alone compared to BDNF alone69. Combined treatment with CNTF and BDNF was superior to either alone in the rescue of rd photoreceptors70 and after laser retinal treatment71. Both inhibition of free radicals and inhibition of nitric oxide synthase are known to potentiate the beneficial effect of BDNF72. The potentially neuroprotective effect of BDNF and CNTF were studied further here in a rat glaucoma model, by overexpressing CNTF or BDNF alone or by overexpressing both in combination.
Methods
Comparison of BDNF, CNTF, BDNF plus CNTF or Saline
A total of 224 adult male Wistar rats (400–425g) began the study, of which 38 died prior to sacrifice for the following reasons: 18 had anesthetic-related death, 11 were euthanized for cataract after virus injection, 7 were euthanized for other ocular complications, and 2 were euthanized for overall poor health. After sacrifice, an additional 10 were unable to be used due to technical problems, leaving 176 for final analysis. All animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, using protocols approved and monitored by the Johns Hopkins University School of Medicine Animal Care and Use Committee.
The experimental groups for this protocol received one of the following 4 possible treatments: 1) overexpression of BDNF; 2) overexpression of CNTF; 3) overexpression of both BDNF and CNTF; or 4) saline injection. To produce these groups, we performed intravitreal injections of: 1) adeno-associated virus vector containing the BDNF gene and the woodchuck hepatitis enhancing element (WPRE) AAV/BDNF/WPRE (n = 52 eyes), or 2) AAV carrying the CNTF gene (n = 45), or 3) normal saline (n = 47), or 4) AAV/BDNF/WPRE followed by AAV/CNTF 2 weeks later (or vice versa; n = 47). In each group, experimental glaucoma was induced 2 weeks following the last viral vector injection. In addition, a laser only group of 33 rats was included that did not receive any injection, leading to an overall total of 224 rats.
Animals were sacrificed 4 weeks after induction of experimental glaucoma. Of the AAV/BDNF/WPRE group, 31 were used to estimate the number of surviving RGC axons and 5 were prepared for western blot analysis of BDNF expression. In the AAV/CNTF group, 29 were used for RGC axon count and 6 for western blot; in the normal saline group, 29 were used for RGC axon count and 6 for western blot; and in the combination therapy group, 34 were used for RGC axon count and 6 for western blot. In the laser only group, 30 were used to estimate the number of surviving retinal ganglion cells, for a total of 176 animals in the final analysis. The laser treatments, IOP measurements, and optic nerve counts were performed in a masked fashion.
Plasmid preparation
The CNTF plasmid66 expresses a secretory form of human CNTF which has a high affinity for the CNTF receptor alpha, using a hybrid chicken beta-actin (CBA) promoter and cytomegalovirus (CMV) enhancer element that supports expression in photoreceptors, retinal pigment epithelium and retinal ganglion cells73.
The pGFP plasmid74 was used to create the AAV/BDNF/WPRE. Flanked by AAV terminal repeats, the expression cassette of pGFP included a 1.7 kb sequence containing the hybrid CMV immediate early enhancer/chicken beta-actin (CBA) promoter. The pGFP construct had GFP cDNA downstream of the CBA promoter and incorporated the woodchuck hepatitis post-transcriptional regulatory element (WPRE)75. For AAV/BDNF/WPRE, GFP was replaced by BDNFmyc cDNA, shown previously to produce biologically active rat BDNF76,77.
Vector packaging and titering
Packing of CNTF plasmid into rAAV2 was carried out at the Retina Gene Therapy Group, University of Florida78, whereas BDNF and GFP plasmids were packaged in rAAV2 by the Johns Hopkins University Cystic Fibrosis Vector Core79. Briefly, low-passage human 293 cells were co-transfected with the given plasmid (CNTF or BDNF), and the helper plasmid pDG that contains both the AAV genes (rep and cap) and the adenovirus genes required for AAV propagation. After the cells were harvested, the virus was extracted by freezing and thawing the cells, and the supernatant was then clarified by low-speed centrifugation. Crude cell lysates containing AAV were loaded onto Iodixanol (Nycomed Pharma, Oslo, Norway) density step gradients for purification. The fraction containing AAV was further purified by column chromatography using a Pharmacia AKTA FPLC (Amersham Biosciences, Piscataway, NJ, USA). Purified AAV was concentrated and desalted by centrifugation through Biomax 100K filters (Millipore, Mississauga, Ontario, Canada) according to the manufacturer’s instructions. Viral titers were determined for each batch of virus using Real-Time PCR. The AAV/CNTF was in the order of 4.79 × 1014 physical particles per milliliter. The AAV/BDNF/WPRE was titered at 1.2 × 1013 DNase resistance particles per ml (DRPM).
Functional assay of AAV/BDNF/WPRE and AAV/CNTF
To test the bioactivity of AAV/BDNF/WPRE and AAV/CNTF, COS-7 cells were grown to 30% confluency in a 24-well culture plate in 1 ml of DMEM/F12 media per well containing 10% FBS and 1 unit/milliliter penicillin/streptomycin. One microliter of AAV/CNTF or AAV/BDNF/WPRE (physical particle titers 4.79 × 1014 and 1.2 × 1013 respectively), or one microliter of a control AAV/GFP virus (1 × 1013)79 used as a virus control, was added per well in triplicate. At 72 hours, the media was exchanged. Twenty-Four hours later the media from the triplicate samples were harvested and pooled. Media from COS-7 cells that were not exposed to virus, but had the same media exchange treatment, was used as a conditioned media control (CM).
RGCs from post-natal day 4 (P4) rats were enriched by immunoselection80 and plated at a density of 5000 cells/well in 96-well culture dishes in growth media (Neurobasal media, B-27 supplement, glutamine (2 millimolar), and penicillin/streptomycin (1 unit/milliliter)), containing forskolin at a final concentration of 2.5 micromolar. Quadruplicate wells were supplemented with either 20, or 40 microliter of the various conditioned medium, after it had been centrifuged at 14,000 rpm for 10 minutes. Two additional controls were: 1) RGC cultures grown in unsupplemented growth media and 2) RGC grown in growth media supplemented with 50 nanogram/milliliter recombinant BDNF (PeproTech, Rocky Hill, NJ). After 3 days in culture, the ganglion cells were stained with calcein AM, Hoescht and ethidium homodimer. Twenty fields per well were imaged using a Cellomics VTI array scan and the images were analyzed and quantified with the Neuronal Profiling software application (Thermo Fisher, Pittsburgh, PA)81.
Intravitreal injection
Animals were anesthetized with a cocktail of intraperitoneal ketamine (75mg/kg) and xylazine (5mg/kg) and topical 1% proparacaine eye drops. Using an operating microscope, a peritomy was made superotemporally, and a partial thickness pilot hole was made in the sclera using a 30g needle. A glass needle with a tip diameter between 30–50 microns was connected by polyethylene tubing to a 5 microliter Hamilton syringe (Hamilton Company, Reno NV) to inject virus or saline. The assembly was pre-filled with light mineral oil (Sigma Aldrich, St. Louis, MO) prior to drawing up 3.5ul of either normal saline or virus. The needle was left in place for 2 minutes to allow for dispersal of the material.
In animals receiving BDNF alone or CNTF alone, injections were given 2 weeks prior to first laser treatment for IOP elevation. In animals that received injections of both vectors (BDNF and CNTF), the first injection was 4 weeks prior to laser treatment, and the second was two weeks prior to laser, with the order of the two vectors randomly chosen. After the injections, the retina was examined using an indirect ophthalmoscope and 90 diopter lens (Volk Optical, Mentor OH) to assure the lack of retinal detachment or injury.
Experimental IOP elevation
Two weeks after the intravitreal injection of virus or saline (4 weeks after the first vector injection in the CNTF/BDNF combined group), animals were anesthetized with intraperitoneal ketamine (75mg/kg) and xylazine (5mg/kg) and topical 1% proparacaine eye drops. A 532nm diode laser was used to treat the trabecular meshwork with 40–50 spots at 50 micrometers at a power of 0.6 watt and 0.6 second duration82. IOP was measured in each eye under anesthesia with the TonoLab using the machine generated average (Colonial Medical Supply, Franconia NH). Readings were taken 1 day after laser and again immediately before re-treatment. A laser second treatment was given one week later if the IOP difference between the treated and control eye was less than 8 mmHg. Additional weekly IOP measurements were made for the first month after the induction of experimental glaucoma. We calculated the mean IOP, peak IOP, peak IOP difference and positive IOP integral, representing the area under the curve of IOP exposure in the glaucomatous eye relative to the fellow control eye over time. Expressed in units of mmHg-days, the positive integral captures the cumulative exposure of IOP compared to the control.
RGC axon counting
Animals used for nerve counts had tissues fixed by vascular perfusion with 4% paraformaldehyde in 0.1 M phosphate buffer followed by 5% glutaraldehyde in 0.1M phosphate buffer. Nerves were embedded in epoxy resin, sectioned at 1 micron and photographed with a 100x oil lens for image analysis using a Cool Snap Camera and Metamorph Image Analysis software (Molecular Devices, Downington PA). Ten random 50 × 50 micrometer fields, equivalent to a 10% sampling of the total nerve area, were counted to determine the average fiber density/mm2. This value was multiplied by the total nerve area to estimate the total fiber number, which was then compared to a pooled control to generate the percent fiber loss per animal. Mean RGC axon counts from each of the treatment groups were compared to each of the control groups by univariate regression controlling for positive IOP integral.
Biostatistical Analysis
While animals are randomly assigned to treatment groups and given uniform laser exposure, the resultant mean IOP levels over time can differ between treatment groups. This would bias the interpretation of data, potentially masking a treatment effect, or producing one that did not exist. Therefore, we calculated for every animal the mean, peak and positive integral IOP (cumulative exposure above the fellow eye over time), as described above. In regression models in which axon loss was the dependent variable, the independent variables were treatment group and IOP exposure (mean, peak, or positive integral). The model with positive integral most accurately captures the potential damaging effect of IOP on RGC and this was the model used to determine if the treatment groups differed significantly.
Western Blot Analysis
Retinas were flash frozen on dry ice and stored at − 80° C until homogenization in 300 microliters of 20mM Tris buffer with 10% sucrose and protease inhibitor (Roche Diagnostics Corporation, Indianapolis IN) by sonication for 4 seconds at 4°C. Their protein concentration was determined using a commercial assay (Bio-Rad, Hercules CA). For a given treatment group’s western blot analysis, the treated and contralateral control eye for each animal were loaded in serial order and processed in a single gel. This allowed for standardization of the processing steps within each treatment library and allowed a direct comparison between the experimental and fellow control eye. In our prior study by Martin et al21, we compared uninjected control eyes to AAV-GFP control virus treated eyes and found no increase in BDNF expression; therefore, we did not repeat those comparisons in this study. Proteins were separated on a 4–12% Bis-Tris Gel using sodium dodecylsulfate--polyacrylamide gel, transfered to a membrane with a 0.45 um pore size and blocked for 1 hour at room temperature in 5% non-fat dry milk/PBS-T. To identify CNTF, membranes were probed overnight at 4° C with goat anti-human CNTF antibody (R&D Systems, Minneapolis, MN) at 1:250 dilution followed by peroxidase-conjugated donkey anti-goat secondary (R&D Systems) at 1:5,000 dilution for 1 hour at room temperature. For BDNF, we probed at room temperature for 1 hour with rabbit polyclonal anti-BDNF N-20 (Santa Cruz Biotechnology, Santa Cruz, CA) at a 1:350 dilution, followed by peroxidase-conjugated donkey anti-rabbit secondary (GE Healthcare, Bucks, United Kingdom) at 1:20,000 dilution.
Immunoblots were detected using SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific, Waltham, MA) and BioMax autoradiographic film (Eastman Kodak, Rochester, NY). To prepare samples for probing with the second neurotrophin antibody, the membranes were washed in buffer and stripped with Restore Western Blot Stripping Buffer (Thermo Scientific) for 1 hour at room temperature prior to reprobing. Image J software was used to quantify the intensity of specific bands.
Results
Bioactivity of AAV/BDNF/WPRE and AAV/CNTF
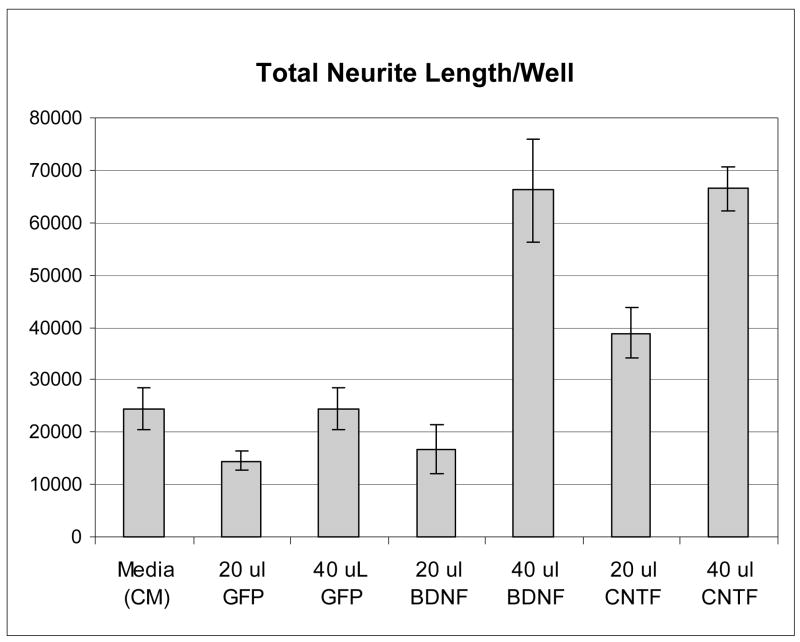
In order to insure that the lots of AAV/BDNF/WPRE and AAV/CNTF that were being used for the in vivo experiments were capable of infecting cells and producing bioactive neurotrophins, we infected COS-7 cells with the same preparations of virus used in the animal experiments and tested the conditioned medium from the infected cells, and control conditioned medium that had not been exposed to virus, in a RGC cell-based assay81. The assay involves culture of purified P4 rat RGC for 3 days with or without various test media, followed by automated image analysis to quantify neurite outgrowth. As can be seen in Figure 1, the level of total neurite length observed with the AAV/GFP virus conditioned medium was similar to that seen with the uninfected conditioned medium (CM), while both the AAV/BDNF/WPRE and AAV/CNTF CM demonstrated a dose dependent stimulation of total neurite length, thus demonstrating that the viruses could indeed produce bioactive neurotrophins.
Figure 1.
Expressed BDNF and CNTF proteins are active on cultured RGCs. COS-7 cells were infected with AAV/GFP, AAV/BDNF/WPRE, or AAV/CNTF. Supernatant from the infected cells was then tested for activity on primary RGC cultures from P4 rats. After 3 days in culture, the cells were analyzed for neurite outgrowth. Total neurite outgrowth per well is shown on the Y axis.
IOP exposure
Analysis of the laser-treated rats revealed that the IOP exposure among the experimental groups was not significantly different, in terms of mean IOP, peak IOP and positive IOP integral difference of the experimental and controls eyes (P > 0.05, ANOVA; Table 1,2).
Table 1.
IOP exposure in glaucoma and control eyes
| Treatment Group (n) | Mean IOP (mmHg) | Peak IOP (mmHg) | ||
|---|---|---|---|---|
| Glaucoma | Control | Glaucoma | Control | |
| AAV/BDNF (n=31) | 17.7 (3.2) | 10.1 (1.9) | 45.1 (9.5) | 9.8 (2.5) |
| AAV/CNTF (n =29) | 18.2 (4.7) | 9.5 (1.4) | 42.8 (10.4) | 8.5 (1.6) |
| AAV/CNTF + AAV/BDNF (n=34) | 16.6 (3.5) | 10.0 (1.7) | 43.3 (11.1) | 9.18 (2.4) |
| Saline (n=29) | 16.3 (3.2) | 9.8 (2.0) | 40.2 (11.5) | 9.0 (2.0) |
| Laser Only (n=30) | 17.3 (3.6) | 10.7 (2.7) | 44.2 (13.2) | 9.3(2.0) |
Data are mean (standard deviation) for each treatment group. Mean IOP is the average IOP after induction of experimental glaucoma. Peak IOP is the IOP of the experimental and control eye on the day that the IOP was most elevated post laser treatment.
Table 2.
IOP exposure and percent RGC axon loss
| Treatment Group (n) | % RGC Loss | IOP exposure | Ratio RGC loss/IOP |
|---|---|---|---|
| AAV/CNTF (n=29) | 39.7 (26.3) | 205.1 (110.0) | 0.19 |
| AAV/CNTF + AAV/BDNF (n=34) | 44.2 (25.5) | 161.3 (67.9) | 0.27 |
| AAV/BDNF (n=31) | 57.9 (21.3) | 183.9 (52.9) | 0.32 |
| Saline (n=29) | 48.9 (26.5) | 154.0 (64.7) | 0.32 |
| No injection (n=30) | 53.9 (27.4) | 163.9 (73.1) | 0.33 |
| Prior Study: AAV/BDNF (n= 27) | 32.3 (23.0) | 214.5 (73.8) | 0.15 |
Data are mean (standard deviation) comparing experimental glaucoma eye to control eye in each animal; IOP exposure is total positive integral data in units of mm Hg—days. Ratio IOP/RGC loss divides the value in IOP exposure column by value in % RGC loss column. Prior study BDNF values are presented for comparison.
Estimation of Surviving Retinal Ganglion Cell Axons
The group with overexpression of CNTF alone had 15% less fiber loss (greater survival) than the combined control groups (saline injection with glaucoma and glaucoma only), using regression analysis in which IOP exposure was entered as an independent variable (p = 0.018; Table 2). The group with combined overexpression of both CNTF and BDNF had 7% increased survival compared to control, but this was not statistically significant (p = 0.19). BDNF overexpression alone did not change RGC axon survival (p = 0.44). We also calculated the ratio of percent RGC loss as judged from axon counts to IOP exposure in mm Hg—days. This relationship provides a method to compare the rate of axon loss as a function of IOP exposure between the various treatment groups. Note that the proportionate loss by this measure was most mitigated by CNTF treatment and was comparable for this group with the value obtained in a prior study of BDNF overexpression.
Western Blot Analysis of CNTF and BDNF Expression
Western blots for whole retina CNTF and BNDF showed significant increases for both molecules in the appropriate single vector over-expression groups, with nearly 3-fold increase in CNTF in experimental compared to control fellow eyes and almost a doubling of BDNF levels (Table 3). However, neither neurotrophin was significantly increased in the double injection group (CNTF/BDNF). Furthermore, the injection of saline led to a mild, significant increase in CNTF levels in experimental eyes, though the specific over-expression with the CNTF vector caused nearly twice the increase in CNTF as did the saline injection (p = 0.006). Values that were normalized against the amount of actin present in each specimen were not different in any substantive way from those shown without normalization.
Table 3.
Western blot assay for retinal CNTF and BDNF expression
| Neurotrophin | Treatment Group | |||
|---|---|---|---|---|
| AAV/CNTF | AAV/CNTF+AAV/BNDF | AAV/BDNF | Saline | |
| CNTF (23kD) | 2.82 (0.73)* | 1.62 (0.68) | 1.80 (0.31) | 1.64 (0.39) |
| BDNF (14kD) | 1.21 (0.33) | 1.12 (0.14) | 1.44 (0.14)* | 1.02 (0.04) |
Values are mean ratio (standard deviation) of experimental to control eye density data from blots for 6 animals in CNTF, CNTF/BDNF and saline groups and 5 animals in BDNF group.
p values significant: CNTF in CNTF group (p = 0.0017), CNTF in saline group (p = 0.01), BDNF in BDNF group (p = 0.0022).
Discussion
These experiments support past reports cited above that found CNTF to increase RGC survival after injury and represents the first report to our knowledge that over-expression of CNTF using viral vectors could have therapeutic potential to reduce RGC loss in an animal model of glaucoma. Other investigators have given CNTF by injection into the vitreous or have over-expressed the gene in other optic nerve injury models with significant neuroprotective effects. Interestingly, we found that CNTF levels in eyes that had saline injection were significantly higher than in fellow control eyes, but this increase, which was less than that seen with viral-mediated over-expression, did not measurably increase RGC survival. It has been reported that lens injury leads to increase in retinal CNTF and extends RGC life after mechanical optic nerve injury83. We excluded from analysis any rats with visible lens injury, but undetected injury or other effects of the injection procedure could have had a similar effect84.
We did not see increased RGC axon survival when CNTF and BDNF vectors were injected together, but the Western blot data suggest that there was less CNTF expression in this combined group than in the CNTF alone group. We speculate that the level of CNTF expression may have been diminished by dual injections, either by detrimental effects of the second injection, or by competition or an interaction between the viral vectors. Because we found no evidence of increased RGC axon survival in the two groups with lesser CNTF increase (CNTF—BDNF combined and saline control) and a significant effect in the CNTF alone group, our data may indicate the threshold for a short-term beneficial effect on experimental glaucoma, as they represent a dose—response comparison. The issue of the appropriate dose of CNTF for RGC neuroprotection is particularly important in light of the several reports that administration of CNTF to the retina can result in decreased retinal function as measured by both acuity (optokinetic response) and electrophysiological parameters84,. McGill et al have recently shown that lower doses of CNTF have protective effects in models of outer retinal disease without toxicity85. Clearly, the possible future use of CNTF as a human therapeutic agent will require monitoring for such functional deficits and determining the dose that best optimizes efficacy and minimizes unwanted side-effects.
The mechanisms by which CNTF acts to decrease RGC death may be similar to the effects reported in other models. These studies have found that it activates receptors on Muller cells, astrocytes86, and RGC87, leading to upregulation of Stat 366, which is known to be activated in experimental glaucoma injury88. Additional research recently found that CNTF acts to increase RGC survival both in vitro and in vivo through mechanisms involving both STAT 3 and the mitogen activated kinase pathway89. It is increasingly clear that glaucoma and other RGC injuries simultaneously upregulate both survival and death mechanisms90.
In these experiments, we did not detect a beneficial effect of BDNF over-expression, but the level of retinal BDNF was increased only 44% compared to untreated eyes. By contrast, in our previous study in which we observed a significant RGC neuroprotective effect with AAV/BDNF/WPRE, retinal BDNF levels were increased approximately 5 fold26. We had attempted to produce the same quality vector in the present work as in that prior study, but it was evident in pilot tests of the newer vector preparations that the efficiency of the AAV/GFP was less than we had observed previously. In addition, perhaps explaining the difference in neuroprotective activity and relative expression levels between the AAV/BDNF/WPRE and AAV/CNTF vectors, the BDNF vector had a lower physical particle titer than the CNTF vector and at the 20 microliter dose it also had less neurite promoting activity. This again emphasizes the importance of dose and expression effects in conducting and interpreting gene therapy studies.
Acknowledgments
This research was supported by PHS Research Grants EY 02120 (Dr. Quigley and Zack), EY01765 (Core Facility Grant, Microscopy Module and Biostatistics Core, Wilmer Eye Institute), EY13729, EY11123, EY08571 (Dr. Hauswirth), Research to Prevent Blindness, Inc. and by support of the Leonard Wagner Trust, New York, New York. We acknowledge NIH grants, and grants from the Macular Vision Research Foundation, Foundation Fighting Blindness, Juvenile Diabetes Research Foundation, Hope for Vision and for partial support of this work. W.W.H. and the University of Florida have a financial interest in the use of AAV therapies, and own equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work.
The authors thank Liudmila Cebataru of the Johns Hopkins University Cystic Fibrosis Vector Core for the production of the BDNF and GFP viruses.
References
- 1.Quigley HA, Broman A. The number of persons with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:151–156. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broman AT, Quigley HA, West SK, et al. Estimating the rate of progressive visual field damage among those with open-angle glaucoma from cross-sectional data. Invest Ophthalmol Vis Sci. 2008;49:66–76. doi: 10.1167/iovs.07-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quigley HA. Neuronal death in glaucoma. Progress Ret Eye Res. 1998;18:39–57. doi: 10.1016/s1350-9462(98)00014-7. [DOI] [PubMed] [Google Scholar]
- 4.Kerrigan LA, Zack DJ, Quigley HA, Smith SD, Pease ME. TUNEL-positive ganglion cells in human primary open angle glaucoma. Arch Ophthalmol. 1997;115:1031–5. doi: 10.1001/archopht.1997.01100160201010. [DOI] [PubMed] [Google Scholar]
- 5.Okisaka S, Murakami A, Mizukawa A, Ito J. Apoptosis in retinal ganglion cell decrease in human glaucomatous eyes. Jpn J Ophthalmol. 1997;41:84–8. doi: 10.1016/s0021-5155(97)00013-0. [DOI] [PubMed] [Google Scholar]
- 6.Levin LA, Louhab A. Apoptosis of retinal ganglion cells in anterior ischemic optic neuropathy. Arch Ophthalmol. 1996;114:488–91. doi: 10.1001/archopht.1996.01100130484027. [DOI] [PubMed] [Google Scholar]
- 7.Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36:774–86. [PubMed] [Google Scholar]
- 8.Garcia-Valenzuela E, Shareef S, Walsh J, Sharma S. Programmed cell death of retinal ganglion cells during experimental glaucoma. Exp Eye Res. 1995;6:33–44. doi: 10.1016/s0014-4835(95)80056-5. [DOI] [PubMed] [Google Scholar]
- 9.Rabacchi SA, Bonfanti L, Liu X-H, Maffei L. Apoptotic cell death induced by optic nerve lesion in the neonatal rat. J Neurosci. 1994;14:5292–5304. doi: 10.1523/JNEUROSCI.14-09-05292.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Valenzuela E, Gorczyca W, Darzynkiewicz Z, Sharma S. Apoptosis in adult retina ganglion cells after axotomy. J Neurobiol. 1994;25:431–8. doi: 10.1002/neu.480250408. [DOI] [PubMed] [Google Scholar]
- 11.Berkelaar M, Clarke DB, Wang Y-C, Bray GM, Aguayo AJ. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J Neurosci. 1994;14:4368–74. doi: 10.1523/JNEUROSCI.14-07-04368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.John SW, Smith RS, Savinova OV, Hawes NL, Chang B, Turnbull D, Davisson M, Roderick TH, Heckenlively JR. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci. 1998;39:951–62. [PubMed] [Google Scholar]
- 13.Neufeld AH, Sawada A, Becker B. Inhibition of nitric-oxide synthase 2 by aminoguanidine provides neuroprotection of retinal ganglioin cells in a rat model of chronic glaucoma. Proc Natl Acad Sci USA. 1999;96:9944–9948. doi: 10.1073/pnas.96.17.9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neufeld AH, Das S, Vora S, Gachie E, Kawai S, Manning PT, Connor JR. A prodrug of a selective inhibitor of inducible nitric oxide synthase is neuroprotective in the rat model of glaucoma. J Glaucoma. 2002;11:221–225. doi: 10.1097/00061198-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Park KH, Cozier F, Ong OC, Caprioli J. Induction of heat shock protein 72 protects retinal ganglion cells in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2001;42:1522–1530. [PubMed] [Google Scholar]
- 16.Hare W, WoldeMussie E, Lai R, Ton H, Ruiz G, Feldmann B, Wijono M, Chun T, Wheeler L. Efficacy and safety of memantine, an NMDA-type open-channel blocker, for reduction of retinal injury associated with experimental glaucoma in rat and monkey. Surv Ophthalmol. 2001;45(Suppl 3):S284–S289. doi: 10.1016/s0039-6257(01)00200-4. [DOI] [PubMed] [Google Scholar]
- 17.WoldeMussie E, Ruiz G, Wijono M, Wheeler LA. Neuroprotection of retinal ganglion cells by brimonidine in rats with laser-induced chronic ocular hypertension. Invest Ophthalmol Vis Sci. 2001;42:2849–2855. [PubMed] [Google Scholar]
- 18.Baptiste DC, Hartwick ATE, Jollimore CAB, Baldridge WH, Chauhan BC, Tremblay F, Kelly MEM. Comparison of the neuroprotective effets of adrenoceptor drugs in retinal cell culture and intact retina. Invest Ophthalmol Vis Sci. 2002;43:2666–2676. [PubMed] [Google Scholar]
- 19.Schori H, Kipnis J, Yoles E, WoldeMussie E, Ruiz G, Wheeler LA, Schwartz M. Vaccination for protection of retinal ganglion cells against death from glutamate cytotoxicity and ocular hypertension: Implications for glaucoma. PNAS. 2001;98:3398–3403. doi: 10.1073/pnas.041609498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKinnon SJ, Lehman DM, Tahzib NG, Ransom NL, Reitsamer HA, Liston P, LaCasse E, Li Q, Korneluk RG, Hauswirth WW. Baculoviral IAP repeat-containing-4 protects optic nerve axons in a rat glaucoma model. Mol Ther. 2002;5:780–787. doi: 10.1006/mthe.2002.0608. [DOI] [PubMed] [Google Scholar]
- 21.Martin KRG, Quigley HA, Zack DJ, Levkovitch-Verbin H, Kielczewski J, Valenta D, Baumrind L, Pease ME, Klein RL, Hauswirth WW. Gene therapy with brain-derived neurotrophic factor protects retinal ganglion cells in a rat glaucoma model. Invest Ophthalmol Vis Sci. 2003;44:4357–65. doi: 10.1167/iovs.02-1332. [DOI] [PubMed] [Google Scholar]
- 22.Quigley HA. Ganglion cell death in glaucoma: pathology recapitulates ontogeny. Austral N Z J Ophthalmol. 1995;23:85–91. doi: 10.1111/j.1442-9071.1995.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 23.Anderson DR, Hendrickson A. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest Ophthalmol. 1974;13:771–783. [PubMed] [Google Scholar]
- 24.Minckler DS, Bunt AH, Klock IB. Radioautographic and cytochemical ultrastructural studies of axoplasmic transport in the monkey optic nerve head. Invest Ophthalmol Vis Sci. 1978;17:33–50. [PubMed] [Google Scholar]
- 25.Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981;99:635–49. doi: 10.1001/archopht.1981.03930010635009. [DOI] [PubMed] [Google Scholar]
- 26.Meyer-Franke A, Kaplan MR, Pfrieger RW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–19. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 27.Gall CM, Gold SJ, Isackson PJ, Seroogy KB. Brain-derived neurotrophic factor and neurotrophin-3 mRNAs are expressed in ventral midbrain regions containing dopaminergic neurons. Molec Cell Neurosci. 1992;3:56–63. doi: 10.1016/1044-7431(92)90009-q. [DOI] [PubMed] [Google Scholar]
- 28.Herzog K-H, von Bartheld CS. Contributions of the optic tectum and the retina as sources of brain-derived neurotrophic factor for retinal ganglion cells in the chick embryo. J Neurosci. 1998;18:2891–2906. doi: 10.1523/JNEUROSCI.18-08-02891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moretto G, Xu RY, Walker DG, Kim SU. Co-expression of mRNA for neurotrophic factors in human neurons and glial cells in culture. J Neuropath Exp Neurol. 1994;53:78–85. doi: 10.1097/00005072-199401000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Yan Q, Radeke MJ, Matheson CR, Talvenheimo J, Welcher AA, Feinstein SC. Immunocytochemical localization of TrkB in the central nervous system of the adult rat. J Comp Neurol. 1997;378:135–157. [PubMed] [Google Scholar]
- 31.Bahr M. Live or let die—retinal ganglion cell death and survival during development and in the lesioned adult CNS. Trends Neurosci. 2000;23:483–490. doi: 10.1016/s0166-2236(00)01637-4. [DOI] [PubMed] [Google Scholar]
- 32.Kurokawa T, Katai N, Shibuki H, Kuroiwa S, Kurimoto Y, Nakayama C, Yoshimura N. BDNF diminishes caspase-2 but not c-jun immunoreactivity of neurons in retinal ganglion cell layer after transient ischemia. Invest Ophthalmol Vis Sci. 1999;40:3006–3011. [PubMed] [Google Scholar]
- 33.Meyer-Franke A, Wilkinson GA, Kruttgen A, Hu M, Munro E, Hanson MG, Reichardt LF, Barres BA. Depolarization and cAMP elevation rapidly recruit trkB to the plasma membrane of CNS neurons. Neuron. 1998;21:681–693. doi: 10.1016/s0896-6273(00)80586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fournier AE, Beer J, Arregui CO, Essagian C, Aguayo AJ, McKerracher L. Brain-derived neurotrophic factor modulates GAP-43 but not T 1 expression in injured retinal ganglion cells of adult rats. J Neurosci Res. 1997;47:561–72. [PubMed] [Google Scholar]
- 35.Hu B, Yip HK, So K-F. Localization of p75 neurotrophin receptor in the retina of the adult SD rat: An immunocytochemical study at light and electron microscopic levels. Glia. 1998;24:187–97. [PubMed] [Google Scholar]
- 36.Ko M-L, Hu D-N, Ritch R, Sharma SC, Chen C-F. Patterns of retinal ganglion cell survival after brain-derived neurotrophic factor administration in hypertensive eyes of rats. Neuroscience Letters. 2001;305:139–142. doi: 10.1016/s0304-3940(01)01830-4. [DOI] [PubMed] [Google Scholar]
- 37.Lewis GP, Linberg KA, Geller SF, Guerin CJ, Fisher SK. Effects of the neurotrophins brain-derived neurotrophic factor in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 1999;40:1530–1544. [PubMed] [Google Scholar]
- 38.DiPolo A, Aigner LJ, Dunn RJ, Bray GM, Aguayo AJ. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proc Nat Acad Sci USA. 1998;95:3978–83. doi: 10.1073/pnas.95.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mo X, Yokoyama A, Oshitari T, Negishi H, Dezawa M, Mizota A, Adachi-Usami E. Rescue of axotomized retinal ganglion cells by BDNF gene electroporation in adult rats. Invest Ophthalmol Vis Sci. 2002;43:2401–2405. [PubMed] [Google Scholar]
- 40.Chen H, Weber A. BDNF enhanced retinal ganglion cell survival in cats with optic nerve damage. Invest Ophthalmol Vis Sci. 2001;42:966–974. [PubMed] [Google Scholar]
- 41.Negishi H, Dezawa M, Oshitari T, Adachi-Usami E. Optic nerve regeneration within artificial Schwann cell graft in the adult rat. Brain Res Bull. 2001;55:409–419. doi: 10.1016/s0361-9230(01)00534-2. [DOI] [PubMed] [Google Scholar]
- 42.Ko M-L, Hu D-N, Ritch R, Sharma SC. The combined effect of brain-derived neurotrophic factor and a free radical scavenger in experimental glaucoma. Invest Ophthalmol Vis Sci. 2000;41:2967–71. [PubMed] [Google Scholar]
- 43.Klocker N, Kermer P, Weishaupt JH, Labes M, Anderhold R, Bahr M. Brain-derived neurotrophic factor-mediated neuroprotection of adult rat retinal ganglion cells in vivo does not exclusively depend on phosphatidyl-inositol-3’-kinase/protein kinase B signaling. J Neurosci. 2000;20:6962–6967. doi: 10.1523/JNEUROSCI.20-18-06962.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frank L, Ventimiglia R, Anderson K, Lindsay RM, Rudge JS. BDNF down-regulates neurotrophin responsiveness, trkB p;rotein and trkB mRNA levels in cultured rat hippocampal neurons. Eur J Neurosci. 1996;8:1220–1230. doi: 10.1111/j.1460-9568.1996.tb01290.x. [DOI] [PubMed] [Google Scholar]
- 45.Koh JY, Gwag BJ, Lobner D, Choi DW. Potentiated necrosis of cultured cortical neurons by neurotrophins. Science. 1995;268:573–575. doi: 10.1126/science.7725105. [DOI] [PubMed] [Google Scholar]
- 46.Shen S, Wiemelt AP, McMorris FA, Barres BA. Retinal ganglion cells lose trophic responsiveness after axotomy. Neuron. 1999;23:285–295. doi: 10.1016/s0896-6273(00)80780-1. [DOI] [PubMed] [Google Scholar]
- 47.Cheng L, Sapieha P, Kittlerova P, Hauswirth WW, Di Polo A. TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. J Neurosci. 2002;22:3977–3986. doi: 10.1523/JNEUROSCI.22-10-03977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adler R, Landa KB, Manthorpe M, Varon S. Cholinergic neuronotrophic factors: intraocular distribution of soluble trophic activity for ciliary neurons. Science. 1979;204:1434–1436. doi: 10.1126/science.451576. [DOI] [PubMed] [Google Scholar]
- 49.Cui Q, Lu Q, So KF, Yip HK. CNTF, not other trophic factors, promotes axonal regeneration of axotomized retinal ganglion cells in adult hamsters. Invest Ophthalmol Vis Sci. 1999;40:760–766. [PubMed] [Google Scholar]
- 50.Fischer AJ, Schmidt M, Omar G, Reh TA. BMP4 and CNTF are neuroprotective and suppress damage-induced proliferation of Muller glia in the retina. Mol Cell Neurosci. 2004;27:531–542. doi: 10.1016/j.mcn.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Honjo M, Tanibara H, Kido N, Inatani M, Okazaki K, Honda Y, et al. Expression of ciliary neurotrophic factor activated by retinal Müller cells in eyes with NMDA-and kainic acid-induced neuronal death. Invest Ophthalmol Vis Sci. 2000;41:552–560. [PubMed] [Google Scholar]
- 52.Zhang CW, Lu Q, You SW, Zhi Y, Yip HK, Wu W, et al. CNTF and BDNF have similar effects on retinal ganglion cell survival but differential effects on nitric oxide synthase expression soon after optic nerve injury. Invest Ophthalmol Vis Sci. 2005;46:1497–1503. doi: 10.1167/iovs.04-0664. [DOI] [PubMed] [Google Scholar]
- 53.Maier K, Rau CR, Storch MK, Sattler MB, Demmer I, Weissert R, et al. Ciliary neurotrophic factor protects retinal ganglion cells from secondary cell death during acute auto-immune optic neuritis in rats. Brain Pathol. 2004;14:378–387. doi: 10.1111/j.1750-3639.2004.tb00081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Unoki K, LaVail MM. Protection of the rat retina from ischemic injury by brain-derived neurotrophic factor, ciliary neurotrophic factor, and basic fibroblast growth factor. Invest Ophthalmol Vis Sci. 1994;35:907–915. [PubMed] [Google Scholar]
- 55.Watanabe M, Fukuda Y. Survival and axonal regeneration of retinal ganglion cells in adult cats. Prog Retin Eye Res. 2002;21:529–53. doi: 10.1016/s1350-9462(02)00037-x. [DOI] [PubMed] [Google Scholar]
- 56.Ji J-T, Elyaman W, Yip HK, Lee VWH, Yick L-W, Hugon J, So K-F. CNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats: the possible involvement of STAT3 pathway. Eur J Neurosci. 2004;19:265–272. doi: 10.1111/j.0953-816x.2003.03107.x. [DOI] [PubMed] [Google Scholar]
- 57.Stahl N, Yancopoulos GD. The tripartite CNTF receptor complex: activation and signaling involves components shared with other cytokines. J Neurobiol. 1994;25:1454–66. doi: 10.1002/neu.480251111. [DOI] [PubMed] [Google Scholar]
- 58.Beltran WA, Zhang Q, Kijas JW, Gu D, Rohrer H, Jordan JA, Aguirre GD. Cloning, mapping, and retinal expression of the canine ciliary neurotrophic factor receptor α (CNTFRα) Invest Ophthalmol Vis Sci. 2003;44:3642–3649. doi: 10.1167/iovs.02-0763. [DOI] [PubMed] [Google Scholar]
- 59.Liu X, Clark A, Wordinger RJ. Expression of ciliary neurotrophic factor (CNTF) and its tripartite receptor complex by cells of the human optic nerve head. Molecular Vision. 2007;13:758–763. [PMC free article] [PubMed] [Google Scholar]
- 60.Cao W, Lia F, Steindberg RH, Lavail MM. Development of normal and injury-induced gene expression of aFGF, bFGF, CNTF, BDNF, GFAP and IGF-I in the rat retina. Exp Eye Res. 2001;72:591–604. doi: 10.1006/exer.2001.0990. [DOI] [PubMed] [Google Scholar]
- 61.Valter K, Bisti S, Gargini C, DiLoreto S, Maccarone R, Cervetto L, Stone J. Time course of neurotrophic factor upregulation and retinal protection against light-induced damage after optic nerve section. Invest Ophthalmol Vis Sci. 2005;46:1748–1754. doi: 10.1167/iovs.04-0657. [DOI] [PubMed] [Google Scholar]
- 62.Yu S, Tanabe T, Yoshimura Y. A rat model of glaucoma induced by episcleral vein ligation. Exper Eye Res. 2006;83:758–770. doi: 10.1016/j.exer.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 63.van Adel BA, Kostic C, Déglon N, Ball AK, Arsenijevic Y. Delivery of ciliary neurotrophic factor via lentiviral-mediated transfer protects axotomized retinal ganglion cells for an extended period of time. Hum Gene Ther. 2003;14:103–15. doi: 10.1089/104303403321070801. [DOI] [PubMed] [Google Scholar]
- 64.Leaver SG, Cui Q, Plant GW, Arulpragasam A, Hisheh S, Verhaagen J, Harvey AR. AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene Therapy. 2006;13:1328–1341. doi: 10.1038/sj.gt.3302791. [DOI] [PubMed] [Google Scholar]
- 65.Liang F-Q, Aleman TS, Djneka NS, Duds L, Fisher KJ, Maguire AM, Jacobson SG, Bennett J. Long-term protection of retinal structure but not function using RAAV.CNTF in animal models of retinitis pigmentosa. Molec Therapy. 2001;4:461–472. doi: 10.1006/mthe.2001.0473. [DOI] [PubMed] [Google Scholar]
- 66.Bok D, Yasumura D, Matthes MT, Ruiz A, Duncan JL, Chappelow AV, Zolutukhin S, Hauswirth W, Lavail MM. Effects of adeno-associated virus-vectored ciliary neurotrophic factor on retinal structure and function in mice with a P216L rds/peripherin mutation. Exp Eye Res. 2002;74:719–735. doi: 10.1006/exer.2002.1176. [DOI] [PubMed] [Google Scholar]
- 67.Adamus G, Sugden B, Shiraga S, Timmers AM, Hauswirth WW. Anti-apoptotic effects of CNTF gene transfer on photoreceptor degeneration in experimental antibody-induced retinopathy. J Autoimmunity. 2003;21:121–129. doi: 10.1016/s0896-8411(03)00092-1. [DOI] [PubMed] [Google Scholar]
- 68.Tao W. Application of encapsulated cell technology for retinal degenerative diseases. Expert Opin Biol Ther. 2006;6:717–726. doi: 10.1517/14712598.6.7.717. [DOI] [PubMed] [Google Scholar]
- 69.Cui Q, Lu Q, So K-F, Yip HK. CNTF, not other trophic factors, promotes axonal regeneration of axotomized retinal ganglion cells in adult hamsters. Invest Ophthalmol Vis Sci. 1999;40:760–766. [PubMed] [Google Scholar]
- 70.Caffé AR, Söderpalm AK, Holmqvist I, vanVeen T. A combination of CNTF and BDNF rescues rd photoreceptors but changes rod differentiation in the presence of RPE in retinal explants. Invest Ophthalmol Vis Sci. 2001;42:275–282. [PubMed] [Google Scholar]
- 71.Paskowitz D, Donohue-Rolfe KM, Yang H, Yasumura D, Matthes MT, Hosseini K, Carolyn M, Graybea CM, Nune G, Zarbin MA, Matthew M, LaVail MM, Duncan JL. Neurotrophic factors minimize the retinal toxicity of verteporfin photodynamic therapy. Invest Ophthalmol Vis Sci. 2007;48:430–437. doi: 10.1167/iovs.06-0690. [DOI] [PubMed] [Google Scholar]
- 72.Klocker N, Cellerino A, Bahr M. Free radical scavenging and inhibition of nitric oxide synthase potentiates the neurotrophic effects of brain-derived neurotrophic factor on axotomized retinal ganglion cells in vivo. J Neurosci. 1998;18:1038–1046. doi: 10.1523/JNEUROSCI.18-03-01038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Acland GM, Aquirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, Jacobson SG, Hauswirth WW, Bennett J. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 74.Klein RL, Hamby ME, Gong Y, et al. Dose and promoter effects of adeno-associated viral vector for green fluorescent protein expression in the rat brain. Exp Neurol. 2002;176:66–74. doi: 10.1006/exnr.2002.7942. [DOI] [PubMed] [Google Scholar]
- 75.Paterna JC, Moccetti T, Mura A, et al. Influence of promoter and WHV post-transcriptional regulatory element on AAV-mediated transgene expression in the rat brain. Gene Ther. 2000;7:1304–1311. doi: 10.1038/sj.gt.3301221. [DOI] [PubMed] [Google Scholar]
- 76.Klein RL, Lewis MH, Muzyczka N, Meyer EM. Prevention of 6-hydroxydopamine-induced rotational behavior by BDNF somatic gene transfer. Brain Res. 1999;847:314–320. doi: 10.1016/s0006-8993(99)02116-2. [DOI] [PubMed] [Google Scholar]
- 77.Klein RL, Muir D, King MA, et al. Long-term actions of vector-derived nerve growth factor or brain-derived neurotrophic factor on choline acetyltransferase and Trk receptor levels in the adult rat basal forebrain. Neuroscience. 1999;90:815–821. doi: 10.1016/s0306-4522(98)00537-5. [DOI] [PubMed] [Google Scholar]
- 78.Hauswirth WW, Lewin AS, Zolotukhin S, Muzyczka N. Production and purification of recombinant adeno-associated virus. Methods Enzymol. 2000;316:743–61. doi: 10.1016/s0076-6879(00)16760-6. [DOI] [PubMed] [Google Scholar]
- 79.Zolotukhin S, Byrne BJ, Mason E, et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- 80.Kerrison JB, Zack DJ. Neurite outgrowth in retinal ganglion cell culture. Methods Mol Biol. 2007;356:427–34. doi: 10.1385/1-59745-217-3:427. [DOI] [PubMed] [Google Scholar]
- 81.Kerrison JB, Lewis RN, Otteson DC, Zack DJ. Bone morphogenetic proteins promote neurite outgrowth in retinal ganglion cells. Mol Vis. 2005;18:208–15. [PubMed] [Google Scholar]
- 82.Levkovitch-Verbin H, Quigley HA, Martin KRG, Valenta D, Kerrigan-Baumrind LA, Pease ME. Translimbal laser photocoagulation to the trabecular meshwork as a model for glaucoma in rats. Invest Ophthalmol Vis Sci. 2002;43(2):402–10. [PubMed] [Google Scholar]
- 83.Muller A, Hauk TG, Fischer D. Astrocyte-derived CNTF switches mature RGCs to a regenerative state following inflammatory stimulation. Brain. 2007;130:3308–3320. doi: 10.1093/brain/awm257. [DOI] [PubMed] [Google Scholar]
- 84.LaVail MM, Yasumura D, Mattes MT, Lau-Villacourt C, Unoki K, Sung CH, Steinber RH. Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophthal Vis Sci. 1998;39:592–602. [PubMed] [Google Scholar]
- 85.McGill TJ, Prusky GT, Douglas RM, Yasumura D, Matthes MT, Nune G, Donohue-Rolfe K, Yang H, Niculescu D, Hauswirth WW, Girman SV, Lund RD, Duncan JL, LaVail MM. Intraocular CNTF reduces vision in normal rats in a dose-dependent manner. Invest Ophthalmol Vis Sci. 2007;48:5756–5766. doi: 10.1167/iovs.07-0054. [DOI] [PubMed] [Google Scholar]
- 86.van Adel BA, Arnold JM, Phipps J, Doering LC, Ball AK. Ciliary neurotrophic factor protects retinal ganglion cells from axotomy-induced apoptosis via modulation of retinal glia in vivo. J Neurobiol. 2005;63:215–234. doi: 10.1002/neu.20117. [DOI] [PubMed] [Google Scholar]
- 87.Sarup V, Patil K, Sharma SC. Ciliary neurotrophic factor and its receptors are differentially expressed in the optic nerve transected adult rat retina. Br Res. 2004;1013:152–158. doi: 10.1016/j.brainres.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 88.Yang Z, Quigley HA, Pease ME, Yang Y, Qian J, Valenta D, Zack DJ. Changes in gene expression in experimental glaucoma and optic nerve transection: The equilibrium between protective and detrimental mechanisms. Invest Ophthalmol Vis Sci. 2007;48:5539–48. doi: 10.1167/iovs.07-0542. [DOI] [PubMed] [Google Scholar]
- 89.Lingor P, Tonges L, Pieper N, Bermel C, Barski E, Planchamp V, Bahr M. ROCK inhibition and CNTF interact on intrinsic signalling pathways and differentially regulate survival and regeneration in retinal ganglion cells. Brain. 2008;131:250–263. doi: 10.1093/brain/awm284. [DOI] [PubMed] [Google Scholar]
- 90.Levkovitch-Verbin H, Dardik R, Vander S, Nisgav Y, Kalev-Landoy M, Melamed S. Experimental glaucoma and optic nerve transection induce simultaneous upregulation of proapoptotic and prosurvival genes. Invest Ophthalmol Vis Sci. 2006;47:2491–7. doi: 10.1167/iovs.05-0996. [DOI] [PubMed] [Google Scholar]