Abstract
Fishes are capable of regenerating sensory hair cells in the inner ear after acoustic trauma. However, a time course of auditory hair cell regeneration has not been established for zebrafish. Adult zebrafish (Danio rerio) were exposed to a 100 Hz pure tone at 179 dB re 1 μPa RMS for 36 hours and then allowed to recover for 0 to 14 days before morphological analysis. Hair cell bundle loss and recovery were determined using phalloidin to visualize hair bundles. Cell proliferation was quantified through bromodeoxyuridine (BrdU) labeling. Immediately following sound exposure, zebrafish saccules exhibited significant hair bundle damage (eg., splayed, broken, and missing stereocilia) and loss (ie., missing bundles and lesions in the epithelia) in the caudal region. Hair bundle counts increased over the course of the experiment, reaching pre-treatment levels at 14 days post-sound exposure (dpse). Low levels of proliferation were observed in untreated controls, indicating that some cells of the zebrafish saccule are mitotically active in the absence of a damaging event. In sound-exposed fish, cell proliferation peaked two dpse in the caudal region, and to a lesser extent in the rostral region. This proliferation was followed by an increase in numbers of cuticular plates with rudimentary stereocilia and immature-like hair bundles at 7 and 14 dpse, suggesting that at least some of the saccular cell proliferation resulted in newly formed hair cells. This study establishes a time course of hair cell bundle regeneration in the zebrafish inner ear and demonstrates that cell proliferation is associated with the regenerative process.
Keywords: hair cell, regeneration, zebrafish, saccule, inner ear, phalloidin, BrdU
1. Introduction
Mammalian cochlear hair cells are damaged or lost due to loud or prolonged noise-exposure (Lim, 1976; Lindeman and Bredberg, 1972; Stockwell et al., 1969), ototoxic drugs (Lim, 1976; Theopold, 1977), and age (Coleman, 1976; Keithley and Feldman, 1982). Limited hair cell regeneration has been reported in mammalian utricular sensory epithelium via mitosis in vitro (Warchol et al., 1993; Zheng et al., 1997) and via non-mitotic hair cell replacement in vivo (Rubel et al., 1995). Hair cell replacement has also been reported in cultured embryonic mouse cochleae and neonatal supporting cell cultures (Kelley et al., 1995; White et al., 2006); however, hair cell regeneration does not appear to take place in the sensory epithelium of the intact postnatal cochlea (Roberson and Rubel, 1994) in mammals, leading to permanent deafness.
In contrast, non-mammalian vertebrates such as fish and birds spontaneously regenerate hair cells in both the vestibular and auditory portions of the inner ear (Cotanche, 1987b; Corwin and Cotanche, 1988; Ryals and Rubel, 1988; Lombarte et al., 1993; Weisleder and Rubel, 1993; Smith et al., 2006). Following noise- or drug-induced trauma in the avian ear, supporting cells appear capable of giving rise to new hair cells through mitosis (Corwin and Cotanche, 1988; Hashino and Salvi, 1993; Raphael, 1992; Ryals and Rubel, 1988; Stone and Cotanche, 1994) or direct transdifferentiation (Adler et al., 1996; Baird et al., 1996; Baird et al., 2000; Roberson et al., 1996; 2004; Taylor and Forge, 2005). Post-embryonic hair cell production has been observed in the lateral line neuromast organs of amphibians and fish (Stone, 1937; Wright, 1947; Tester and Kendall, 1969; Corwin, 1986; Corwin et al., 1989). Hair cell regeneration has been reported in the saccule and lateral line of urodele amphibians (Jones and Corwin, 1996; Taylor and Forge, 2005), and in the crista ampullaris of lizards (Avallone et al., 2003).
Similarly, hair cell regeneration occurs in fishes. This has been demonstrated in the goldfish saccule (Smith et al., 2006), the oscar utricle and lagena (Lombarte et al., 1993), and the zebrafish lateral line (Harris et al., 2003). Although hair cell regeneration has been reported inthe zebrafish lateral line exposed to copper sulfate or aminoglycosides (Song et al., 1995; Harris et al, 2003; Hernandez et al., 2007), the regenerative abilities of the zebrafish inner ear hair cells have not been characterized.
The zebrafish (Danio rerio) is an emerging model organism for vertebrate inner ear development and deafness (Whitfield, 2002), and genetic diseases associated with hearing loss such as Usher 1B syndrome (Ernest et al., 2000), Long QT syndrome (Arnaout et al., 2007) and Branchio-oto-renal syndrome (Kozlowski et al., 2005). The zebrafish is a useful model organism because the basic structure and function of the fish inner ear is similar to that of other vertebrates (Popper and Fay, 1999) and mammals share homologous genes with zebrafish that are known to affect inner ear structure and/or function. For instance, the zebrafish Mariner mutant possesses a missense mutation in the gene encoding Myosin VIIA and presents functional and morphological hair cell defects that are similar to those found in mice defective in Myosin VIIA (Ernest et al., 2000). Foxi1(aka Fkh10), a gene expressed in otic precursor cells, is necessary for normal inner ear development in both mice (Hulander et al., 1998; 2003) and zebrafish (Solomon et al., 2003). Atoh1 (atonal homolog 1), a gene also known as Math1, is a key regulator of differentiation of precursor cells that become hair cells in mice (Bermingham et al., 1999; Zheng and Gao, 2000). The presence of zebrafish atoh1 homologs are prominent during development and are necessary for hair cell fate selection in the lateral line and inner ears of zebrafish (Itoh and Chitnis, 2001; Millimaki et al., 2007). Since zebrafish share inner ear developmental and differentiation genes with mammals, examination of gene expression in the zebrafish during naturally occurring hair cell regeneration may uncover targets for genetic manipulation in mammals to provide new ways to treat deafness.
The purpose of this study was to establish the time course and extent of hair cell regeneration in the zebrafish saccule in response to acoustic trauma, in preparation for subsequent work exploring gene expression patterns during this process. In addition, we wanted to determine whether hair cell bundle recovery is associated with mitosis in the adult zebrafish saccule, as mitosis is associated with hair cell regeneration following acoustic trauma in the avian basilar papilla (Corwin and Cotanche, 1988; Hashino and Salvi, 1993; Raphael, 1992; Ryals and Rubel, 1988; Stone and Cotanche, 1994). We chose to examine the saccule specifically since, of the three otolithic organs (utricle, saccule, and lagena), the saccule has been most fully characterized as a sound detector in fishes (reviewed in Popper and Fay, 1973, 1999).
2. Materials and Methods
2.1. Experimental animals
Wild Type adult breeder zebrafish were obtained from a commercial supplier (Segrest Farms, Gibsonton, FL) and maintained in 170-L flow-through aquaria under conditions of constant temperature (25 °C) and a 12-h light/12-h dark schedule. Fish total lengths ranged from 36 to 44 mm. All work was done under the supervision of the Institutional Animal Care and Use Committee of Western Kentucky University.
2.2. Acoustic exposure
Zebrafish were exposed to a 100 Hz tone at a source level of 179 dB re 1 μPa root mean squared (RMS) at 1 cm directly above the center of the speaker. A preliminary study had shown that this stimulus was capable of producing significant hair bundle damage in the saccule. The sound was generated by a B&K Precision function generator (4017A) connected to a 5.3 amp/200 watt Audiosource monoblock amplifier and University Sound UW-30 underwater speaker placed in a 19-L sound exposure chamber. A total of 56 fish were sound-exposed for 36 hours at 24.5–25 °C. Three sound exposure experiments were performed. One for phalloidin labeling and two for BrdU labeling. Fish in the bromodeoxyuridine (BrdU)-labeling experiment were divided into two groups. Following sound exposure, fish in the first group were allowed to recover for 0, 2, and 4 days before dissection, and fish in the second group were allowed to recover 1, 3, and 10 days before dissection. Two groups of sound-exposed fish were needed because the process used to label BrdU incorporation was too time-consuming to allow consecutive daily dissections.
2.3. Phalloidin labeling
Hair bundles were quantified through visualization of stereocilia stained with Alexa Fluor 488-conjugated phalloidin at 0, 2, 7, and 14 days post- sound exposure (dpse), plus controls (n = 5 per group). Phalloidin binds with actin, a primary component of hair stereociliary bundles and cuticular plates, permitting visual identification of hair cells. Fish were sacrificed in a solution of tricaine methanesulfonate (MS-222, Argent, Redmond, WA), a fish anesthetic, and the heads were removed and fixed with 4% paraformaldehyde overnight. After rinsing 4 × 10 min in 0.1M phosphate buffered saline solution (PBS), the inner ears were dissected out of the head, the saccules isolated from the ears, and excess tissue was trimmed away to allow the saccules to lie flat. Saccules were then placed in concavity wells and incubated in 1:100 fluorescein phalloidin (Invitrogen) in PBS at room temperature in a dark box for 30 minutes. Following incubation, saccules were mounted on glass slides using Prolong Gold Antifade reagent with DAPI (Invitrogen), which allows visualization of nuclei. Slides were viewed under a Zeiss Axioplan 2 epifluorescent microscope with FITC and DAPI filters. Images were captured using an AxioCam MRm camera under 5, 10, 20, and 100X objectives. Images were analyzed with Zeiss Axiovision 4.4 software. Saccular length was determined for each sample, and 2500 μm2 sampling squares were placed along the center length of the saccule at 5, 25, 50, and 75% of the length from the rostral tip of the saccule (Fig. 1). The hair cell bundle densities at the most caudal tip of saccules (100% of length measured from the rostral tip) were too high to accurately quantify; therefore, we chose not to attempt quantification of hair bundle density in this area of the saccule. Phalloidin-labeled hair bundles fluoresced green under the FITC filter, allowing their number to be counted in each square. Due to extensive DAPI staining of nuclei through multiple layers of cells, we abandoned attempts to quantify numbers of nuclei. However, we did note qualitative decreases in DAPI staining in areas of the saccular epithelia that were missing hair bundles.
Figure 1.
Hair cell bundle count locations on the zebrafish saccule. Hair cell counts were sampled at four predetermined locations: 5, 25, 50, and 75% of the total saccular length, as measured from the rostral tip. A 2500 μm2 box was placed at each sampling area and labeled hair cell bundles were counted within each box to determine hair cell density. D= dorsal, R= rostral; scale bar = 100μm.
Two non-overlapping 100X images totaling 12,000 μm2 were obtained from the caudal portion of each phalloidin-stained saccule at 75% of the length from the rostral tip, where hair bundle counts had previously been obtained. These images were visually scanned for abnormal features visible via phalloidin staining. Since phalloidin binds with f-actin in cell membranes, cuticular plates, and hair bundles, we were able to observe some abnormal features on the surface of sound-exposed saccular epithelia, as well as differences in hair bundle appearance.
The abnormal features we identified included short hair bundles (between a quarter and half of the length of neighboring bundles), thin hair bundles (≤ half the width of neighboring bundles), immature-like hair bundles (very short, compact hair bundles less than one quarter of the length of neighboring mature hair bundles), scars (regions where supporting cells had expanded surfaces that formed adjoining boundaries) and bundle-less cuticular plates with emerging strands of stereocilia (rudimentary hair cell features). The numbers of short hair bundles, thin hair bundles, immature-like hair bundles and bundle-less cuticular plates were counted for each image. We also observed putative lesions in the epithelia, which were presumably areas in which damaged hair cells had been ejected from the epithelia. Lesions were defined as areas that were much darker than the surrounding cells and bounded on at least three sides by lighter-colored cells. It was sometimes difficult to determine whether a dark area near the edge of the image was caused low illumination or a missing cell. Additionally, it was not possible to determine how many missing cells each lesion represented, as lesions differed in size. In order to avoid subjective decision-making concerning the origins of each putative lesion, we counted all dark areas bounded on at least three sides by lighter- colored cells, relying on the assumption that the occurrence of dark areas caused by low illumination would be consistently random across samples.
2.4. BrdU labeling
Fish were exposed to the acoustic stimulus and allowed to recover for 0, 1, 2, 3, 7 and 10 days following sound exposure. Controls were placed in the treatment setup but no sound was administered (n=6 for each exposure group and controls). Cell proliferation in saccules of these fish was then quantified through visualization of cells labeled for BrdU, which is a synthetic thymidine analog that is incorporated into cellular DNA during S-phase. BrdU (Sigma-Aldritch, St. Louis, MO) was dissolved into normal Ringer’s solution (Westerfield, 1994) at a concentration of 5 mg BrdU/ml. Fish were injected intraperitoneally with 0.02 ml BrdU/Ringer’s solution and then placed in a separate tank. After four hours, the fish were euthanized with an overdose of MS-222. The heads were removed and placed in 4% paraformaldehyde overnight at 4°C. The heads were then rinsed 4 × 10 min in 0.1M PBS and the inner ears dissected out under a stereomicroscope. The saccules were isolated from the ears and excess tissue was trimmed away to allow the saccules to lie flat. The saccules were bathed in 1N HCL for one hour at 37°C to denature DNA, 0.1M borate buffer (pH 8.5) for 10 min to neutralize tissue pH, and washed 3 × 10 min in PBS. Saccules were incubated overnight at 4°C in mouse monoclonal anti-BrdU antibody (Invitrogen, Carlsbad, CA) diluted to 1:100 in 1% BSA/0.5% Triton X-100/PBS. Saccules were washed 3 × 10 min and incubated for 30 min at room temperature in 1:500 Alexa Fluor 568–conjugated rabbit anti-mouse antibody (Invitrogen) in PBS. Saccules were again washed 3 × 10 min in PBS and mounted with Prolong Gold Antifade reagent with DAPI (Invitrogen). The slides were cover-slipped and viewed under an Zeiss Axioplan 2 epifluorescent microscope with rhodamine and DAPI filters. Images were captured with an AxioCam MRm camera and analyzed with Zeiss Axiovision 4.4 software. Alexa Fluor 568-labeled cells were counted in both the rostral and caudal halves of the saccule to quantify cell proliferation.
2.5. Data analysis
Preliminary analyses showed no statistical differences between right and left saccules in terms of numbers of hair bundles, abnormal features, or BrdU-labeling, so data from both ears were pooled for all subsequent analyses. Differences in hair bundle density along the rostral-caudal axis of control saccules was tested using analysis of variance (ANOVA) with counting box location as the independent variable. Hair bundle loss and recovery were tested using a separate ANOVA for each sampling location (5, 25, 50, and 75% of saccule from rostral tip; Fig. 1) for phalloidin-labeled cells, with recovery dpse as the independent variable.
The data for thin hair bundles were square root normalized, and the effect of dpse on numbers of thin hair bundles, short hair bundles, and putative lesions were tested using separate ANOVAs. The effect of dpse on numbers of immature-like bundles and bundleless cuticular plates was analyzed using separate Kruskal-Wallis analysis of variance tests.
The effect of recovery on cell proliferation in the fish saccule was tested using separate ANOVAs for the caudal and rostral portions of the saccule, with dpse as the independent factor. Tukey’s post-hoc test was used to make pairwise comparisons between days when significant main effects were found through ANOVAs. Wilcoxon’s signed rank test was used to make pairwise comparisons when significant main effects were revealed by the Kruskal-Wallis tests.
3. Results
3.1. Hair bundle density varies along the length of the saccule
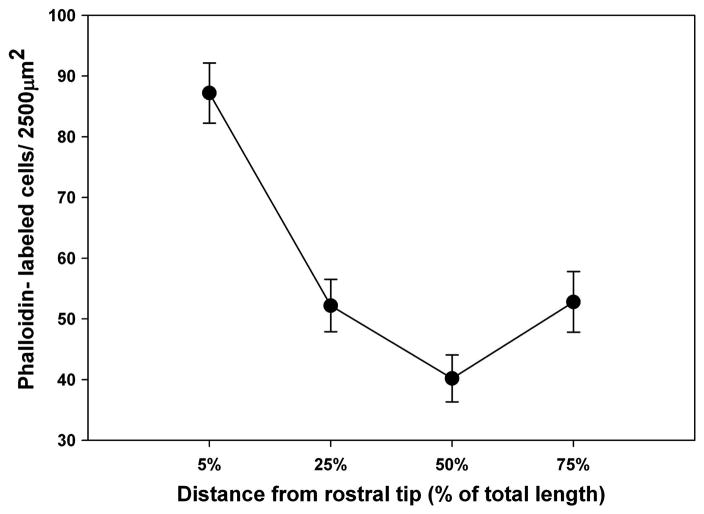
Hair cell bundle density among control fish varied significantly by percent distance from the rostral tip of the saccule (p ≤ 0.001; Fig. 2). Hair bundle density changed in a graduated manner across the length of the saccule, with the greatest density occurring near the rostral tip, decreasing toward the center, and increasing again in the caudal region.
Figure 2.
Mean (± SE) hair cell bundle density across control zebrafish saccules as a function of distance from the rostral tip of the saccule (n = 5).
3.2. Auditory hair bundle loss and recovery
Phalloidin staining revealed hair bundles that had survived the sound exposure, and structural changes that occurred at the epithelial surface following acoustic damage and subsequent repair, including evidence of new hair bundles.
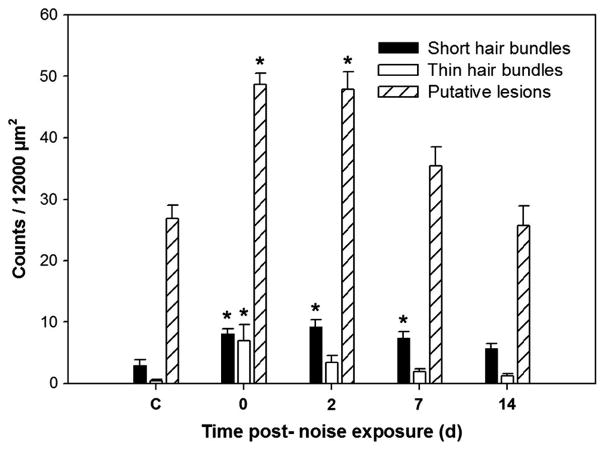
Immediately following cessation of the sound exposure, saccular epithelia exhibited ragged, splayed, and fractured stereocilia, and reduced stereocilia density indicating hair bundle damage. A variety of abnormal structures were observed in the region of hair bundle loss over the time course of recovery, including short hair bundles, thin hair bundles, putative lesions, immature-like hair bundles, and bundle-less cuticular plates (Fig. 3). Short hair bundles, thin hair bundles, lesions, and scars formed by expanded supporting cells were most often observed immediately following sound exposure and at 2 dpse. Numbers of short hair bundles, thin hair bundles, and putative lesions varied significantly by dpse (p = 0.001, 0.029, and p ≤ 0.001, respectively; Fig. 4). Short hair bundles were defined in this study as being ≤ half the length of neighboring cells. The stereocilia of many short hair bundles at 0 and 2 dpse were more splayed, ragged, and disordered (Fig 3a) than short hair bundles on 7 and 14 dpse (Fig. 3f), suggesting that there were two types of short hair bundles represented by the short hair bundle counts--damaged bundles and new bundles. Short hair bundle counts were highest 2 dpse, but counts were significantly greater than control on days 0, 2, and 7 (p =0.004, 0.001, and 0.017, respectively) with no other significant differences found between time points.
Figure 3.
Phalloidin visualization of morphological changes in hair cells of the zebrafish caudal saccule following acoustic damage. (a, c, e) Hair cells observed immediately following sound exposure (day 0). Many hair bundles in the caudal saccule are splayed, disordered, and exhibit broken stereocilia. Note thin hair bundle characterized by fractured, sparse, and fused stereocilia (arrow, a). Stereocilia in the short hair bundle (arrowhead, a) are noticeably shorter than those of neighboring hair bundles, suggesting that tips have been lost through acoustic overexposure. Lesions are also present in the caudal saccule (arrow, e). (b, d, f) Hair cells observed after 7 days of recovery. Cuticular plates (arrow, b), immature-like hair bundles (arrows, d), and compact, well-ordered short hair bundles (arrow, f) occur in the acoustically- damaged region. Bar = 5 μm.
Figure 4.
Mean (± SE) short hair cell bundle, thin hair cell bundle, and putative lesion counts as a function of days post-sound exposure (dpse) in the caudal region (75% of the total saccular length as measured from the rostral tip) (* p < 0.05; n = 5).
Thin hair bundles appeared to possess fused stereocilia and/or fewer stereociliary fibers than most other hair bundles. Thin hair bundle counts were significantly greater 0 dpse compared to controls (p = 0.029) with no other significant differences found between time points. Lesions were observed most often in saccules collected immediately following cessation of sound exposure and were significantly greater than controls at 0 and 2 dpse (p ≤ 0.001), subsequently decreasing over time. Lesion counts were significantly greater on day 0 than on days 7 and 14 (p = 0.003 and p ≤ 0.001, respectively), and significantly greater on day 2 than on day 14 (p ≤0.001), indicating that the number of lesions decreased over the time course of recovery. Although we noted regions of the sound-exposed caudal saccular epithelium where supporting cells had expanded surfaces forming scars, we chose to quantify only cuticular plates with absent or rudimentary emerging stereocilia because these structures stained most readily with phalloidin and were reliably visible. Additionally, the arrangement of hair cells is not as orderly and predictable in fishes as it is in other vertebrates, making it more difficult to determine with certainty whether the arrangement of a particular group of supporting cells was truly a scar.
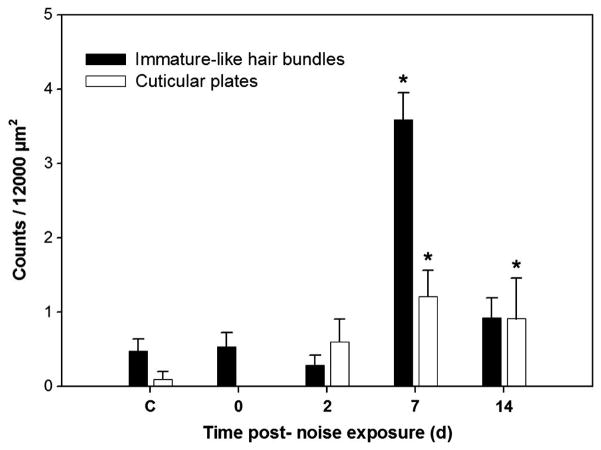
Zebrafish surviving for 7 and 14 dpse exhibited evidence of hair cell recovery-specifically, bundle-less cuticular plates, immature-like hair bundles, and an increase in hair bundle density. Numbers of both bundle-less cuticular plates and immature-like bundles significantly differed over time (p = 0.006 and p ≤ 0.001, respectively; Fig. 5). Bundle-less cuticular plates with rudimentary stereocilia-like structures were not evident in phalloidin-stained samples taken immediately following sound exposure, but were found at subsequent time points. Cuticular plate counts were highest at 7 dpse, and were significantly greater than both control and day 0 (p = 0.007, and 0.011, respectively). Cuticular plate counts were also significantly greater than controls on day 14 (p = 0.039).
Figure 5.
Mean (± SE) immature-like hair cell bundle and cuticular plate counts as a function of days post-sound exposure (dpse) in the caudal region (75% of the total saccular length as measured from the rostral tip) (* p < 0.05; n = 5).
Immature-like hair bundles were characterized as being approximately a quarter of the length of neighboring mature bundles but longer than the emerging stereocilia associated with bundle-less cuticular plates. Immature-like hair bundles were compact, and appeared to possess fewer stereocilia than larger neighboring cells. Immature-like hair bundles were observed at all time points, but the greatest numbers of them were observed after a week of recovery from sound exposure. Immature-like hair bundle counts were significantly greater at 7 dpse than all other time points (p ≤0.005 for all) and were also significantly greater on day 14 than day 2 (p = 0.042).
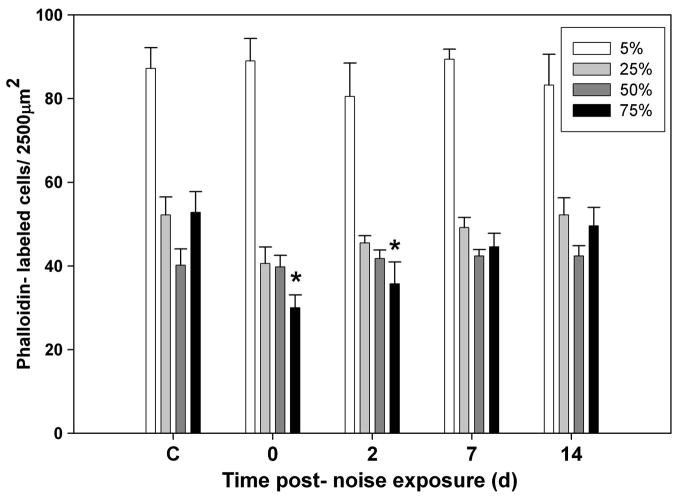
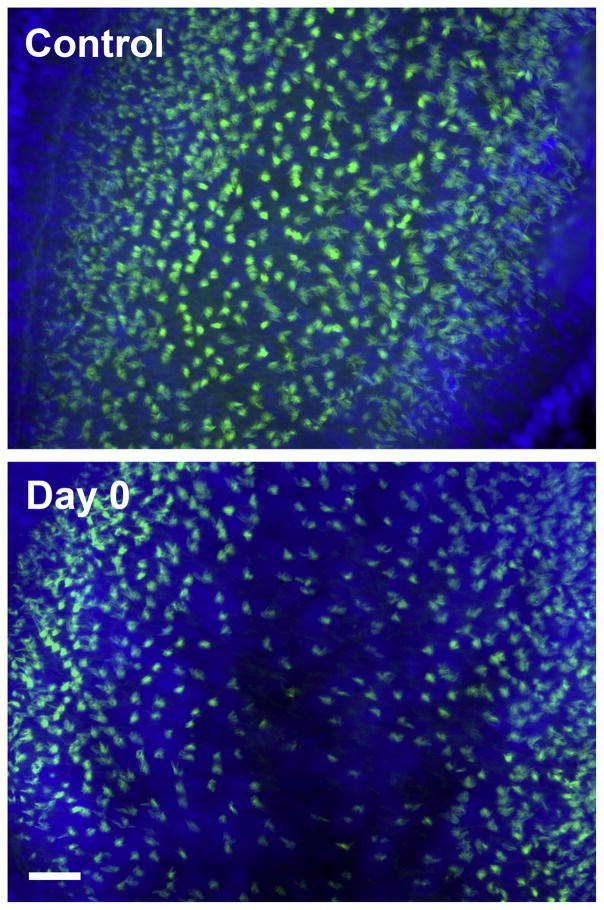
We observed that hair bundle density significantly decreased (43%) in the caudal region (at 75% of the total distance from the rostral tip) following sound exposure (p ≤ 0.001; Fig. 6) and increased by days 7 and 14. There was also a slight reduction in bundle density in the central rostral region (at 25% of the total distance from the rostral tip) immediately following sound exposure, although this trend was not significantly different than controls. Specifically, hair bundle densities in the caudal region were significantly lower than controls at 0 and 2 dpse (p ≤ 0.001 and p = 0.003 respectively). No significant differences were found for other regions or time points. Areas of the caudal epithelium exhibiting hair bundle loss also showed patches of reduced nuclear staining immediately following sound exposure and 2 dpse, indicating that whole cells were probably lost (Fig. 7; see the web version of this paper at www.sciencedirect.com to view the color rendering of this image, which better illustrates DAPI staining in the saccules). Hair bundle density in the caudal region was not significantly different from controls at 7 and 14 dpse, suggesting that hair cell recovery had occurred.
Figure 6.
Mean (± SE) hair cell bundle density as a function of days post-sound exposure (dpse) at 5, 25, 50, and 75% of the total saccular length as measured from the rostral tip (* p ≤ 0.01 for days 0 and 2, compared to control).
Figure 7.
Hair cell bundles in the caudal saccules of control and sound-exposed zebrafish immediately following sound exposure (0 dpse). The reader is referred to the web version of this paper at www.sciencedirect.com for this figure in color, which shows green phalloidin staining (for hair cell bundles) and blue DAPI staining (for nuclei). Scale bar = 20 μm.
3.3. Cell proliferation
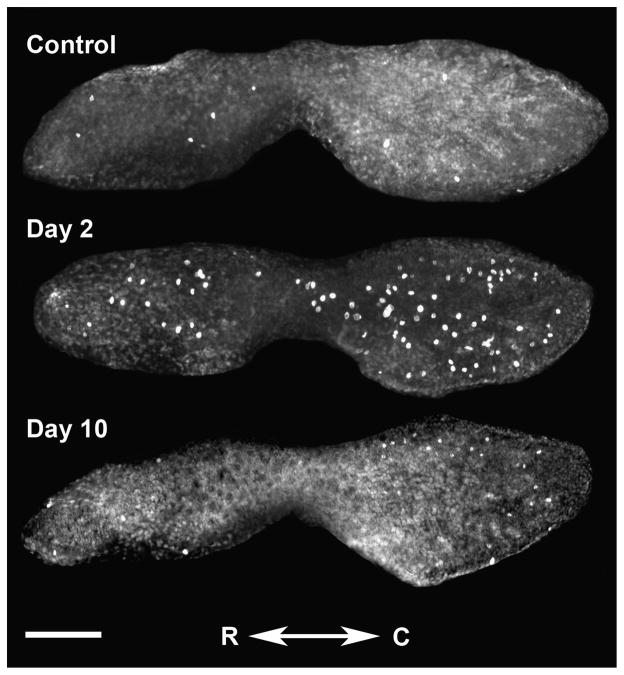
Control saccules treated for BrdU detection were observed to contain labeled cells within the sensory epithelium (Fig. 8). The mean (±SE) number of BrdU-labeled cells in control saccules was 6.6 (±0.7), with labeling being approximately equal between rostral and caudal halves of the saccule. Although there was some variability between individuals, labeled cells were most frequently found near the rostral tip, the narrow portion of the tissue between the caudal and rostral halves of the saccule, and the edges of the epithelium in the caudal half of the saccule. Labeled cells were found in other, more central locations of the saccule as well.
Figure 8.
BrdU-labeled proliferating cells in a control saccule and in saccules dissected for 2 and 10 days post-noise exposure. C = caudal, R = rostral; scale bar = 100 μm.
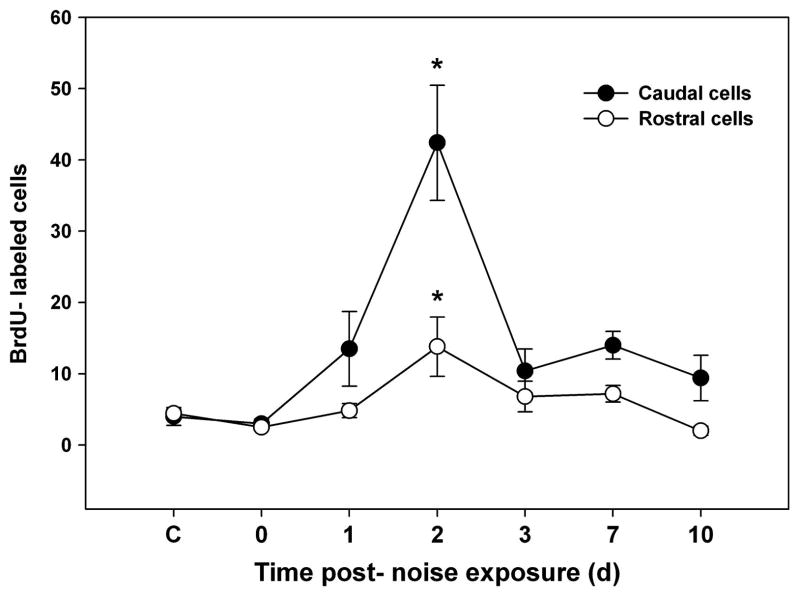
Among animals exposed to the acoustic stimulus, BrdU labeling was significantly greater than controls only at 2 dpse (p ≤ 0.001); however labeling was much greater in the caudal compared to the rostral half (p ≤ 0.001; Figs. 8 & 9). Proliferating cells at 2 dpse were noted over most of the caudal half of the saccule, in the narrow region between the caudal and rostral halves, and at the rostral tip. Additionally, some fish exhibited BrdU-labeled cells in the center of the rostral half of the saccule. On subsequent days, proliferation declined in the central regions of the saccule. By day ten, BrdU-labeled cells were found primarily near the edges of the caudal and rostral halves of the saccule.
Figure 9.
Mean (± SE) BrdU-labeled zebrafish saccular cells as a function of time following sound exposure. (* p ≤ 0.001; n = 6).
4. Discussion
Our study characterizes some of the structural changes that take place following acoustic damage to, and during recovery of, the zebrafish saccule, and demonstrates that zebrafish are capable of restoring auditory hair cell bundle density to control levels within fourteen days following sound exposure. We also show that increased cell proliferation takes place during the time course of hair bundle recovery, and is greatest in the same region where significant hair bundle loss occurs, suggesting that the mitotic production of new cells is part of the recovery process in the zebrafish saccule following sound exposure. Hair bundle recovery also occurs in the saccule of the goldfish Carrasius auratus (an otophysan fish closely related to zebrafish) following noise-induced damage (Smith et al., 2006). The present study documents a similar phenomenon in the zebrafish model and extends the finding to include full hair bundle recovery, which was not demonstrated in goldfish since the time frame investigated was shorter than the current study (8 vs. 14 days). Interestingly, full recovery of the ciliary bundles of utricles and lagenae in gentamicin-treated oscars (Astronotus ocellatus) occurs within a similar time frame (approximately 10 days; Lombarte et al., 1993) as the hair bundle recovery found in this study.
4.1. Location of damage
We observed that exposure to the 100 Hz tone produced significant hair bundle loss only in the caudal region of the zebrafish saccule, immediately and 2 dpse, and a non-significant but noticeable bundle loss in the central portion of the rostral region (25% of saccular length from the rostral tip) 0 and 2 dpse. Enger (1981) found that low frequency tones primarily damaged hair cells in the caudal region of the saccule of cod (Gadus morhua). Additionally, Enger observed that higher frequency tones produced hair cell damage in the rostral region, and suggested that coarse frequency discrimination occurred peripherally in the fish saccule. Previous studies in our lab have shown that goldfish exposed to intense low frequency tones (100 Hz) exhibit hair cell damage in the caudal portion of the saccule, while those exposed to high frequency tones (4000 Hz) exhibit damage to the rostral portion of the saccule (Michael Smith, unpublished data). Our results with zebrafish are consistent with these previous studies.
Further work will be needed to determine whether tonotopic mapping of frequencies takes place in the zebrafish saccule. Whether or not tonotopy is responsible for the caudal localization of significant damage seen in our study, the minimal bundle loss observed in the rostral region may have been due to the high sound intensity that was used. Cotanche et al. (1987) found that chicks, which have a tonotopically organized basilar papilla, exhibited a secondary lesion site in the basilar papilla when exposed to high sound intensities, while lower intensities produced only a single lesion site.
4.2. Hair cell loss and scar formation
In a noise-exposure study performed with goldfish, Smith et al. (2006) observed that hair bundle counts were not significantly different from hair cell nuclear counts from the hair cell layer in either control or noise-exposed saccules. In that study, the number of nuclei present in the saccule decreased following noise exposure, indicating disappearance of the entire hair cell following acoustic trauma. Although DAPI staining in our study was too diffuse to quantify cell loss, we found that the region of the saccule in which hair bundles were missing also exhibited patches devoid of DAPI staining, which strongly suggests that nuclei were lost. This same region of the saccule showed a significant increase in small, hole-like lesions (visualized with phalloidin) at 0 and 2 days following cessation of sound exposure, also suggesting that whole cells were lost in the zebrafish saccule following acoustic exposure. Two basic types of hair cell loss have been observed following acoustic injury. Investigators have noted that severely damaged hair cells may be ejected from the epithelium (Cotanche, 1987b; Cotanche et al., 1987; Cotanche and Dopyera, 1990) or disintegrate in situ (Raphael, 1993). In our study, it appears that hair cell ejection was a likely source of hair cell loss, given the presence of lesions in the saccular epithelium.
Holes in the reticular lamina have been observed following exposure to intense noise and ototoxic drugs (Baird et al., 1993; Baird et al., 1996; Bohne, 1972, 1976; Bohne and Rabbitt, 1983; Lim, 1976), similar to those seen in our study with zebrafish. As part of the healing process, supporting cells expand to fill spaces that were previously occupied by hair cells or concurrently occupied by dying hair cells (Cotanche and Dopyera, 1990; Forge, 1985; Marsh et al., 1990; Raphael and Altschuler, 1991a, 1992) forming scars that preserve the integrity of the luminal membrane (Raphael and Altschuler, 1991a) and protect the sensory epithelium from leakage of endolymph (Bohne and Rabbitt, 1983). Scars appear rapidly in the auditory epithelium following damaging events- as early as 9 hours in the mammalian cochlea following exposure to ototoxic drugs (Raphael and Altschuler, 1991b). In the mammalian organ of Corti, scars formed by supporting cells are permanent (Bohne, 1976; Hawkins and Johnson, 1981; Raphael and Altschuler, 1991a). However, scar formations in the inner ears of non-amniotes are transient; supporting cells can directly transdifferentiate into hair cells or proliferate and produce daughter cells that differentiate into hair cells (Balak et al., 1990; Jones and Corwin, 1996; Raphael, 1992; Tsue et al, 1994; Warchol and Corwin, 1996).
4.3. Cell proliferation
Stone and Cotanche (1994) found that chicks surviving for long periods after noise exposure and BrdU injection exhibit BrdU-labeled hair cells, suggesting that some of the daughter cells of precursor cells differentiate into hair cells. Supporting cells serve as mitotic precursor cells in the avian cochlea (Tsue et al, 1994; Warchol and Corwin, 1996) the fish saccule (Wilkins et al., 1999) and the lateral line systems of axolotls (Balak et al., 1990; Jones and Corwin, 1996), suggesting that the ability of supporting cells to serve as precursor cells for hair cell replacement may be a conserved feature in multiple species and organs.
The greatest number of proliferating cells seen in our study was observed in the caudal region of the saccule, the same region that exhibited significant hair bundle loss (Figs. 6 and 8). Similarly, Warchol and Corwin (1996) noted that regenerative proliferation occurred within or near (<200μm) lesion sites created by laser microbeam in hatchling avian cochleae. In chicks, acoustic damage induces a spatio-temporal pattern of cell proliferation that adheres to the spatio-temporal pattern of hair cell loss as well (Hashino and Salvi, 1993). Others have observed that supporting cells exhibiting clear signs of direct transdifferentiation (DT) or DNA synthesis are located near the area of hair cell loss (Corwin and Cotanche, 1988; Raphael, 1992; Ryals and Rubel, 1988), suggesting that supporting cells respond largely to local damage-related signaling.
The acoustically-exposed zebrafish in our study exhibited a spike of BrdU labeling at 2 dpse. Smith et al. (2006) reported significant apoptosis in noise-exposed goldfish saccules from 0 to 2 dpse. Cell proliferation in the fish saccule may be initiated by a reduction in cell density following apoptosis, as cultured pieces of chick utricle exhibit proliferative rates that are inversely related to local cell density (Warchol, 2002). Given that regenerative proliferation occurs locally, it seems likely that diffusible mitogens from the lesion site prompt proliferation (for a review of possible signals, see Stone and Cotanche, 2007). Atoh1, a proneural transcription factor necessary for hair cell fate determination, has been detected in nuclei of the supporting cell layer in avian cochleas as early as 15h post-ototoxin treatment, prior to clear demonstration of hair cell damage, demonstrating that signaling for hair cell replacement occurs very early following a damaging stimulus (Cafaro et al., 2007).
Hair cell proliferation may occur in the absence of damage. Fishes are capable of producing additional hair cells postembryonically as the animal continues to grow (Corwin, 1981, 1983). In our study, a low level of BrdU labeling was seen in the saccules of untreated control zebrafish, consistent with a previous study in which non-acoustically exposed adult zebrafish exhibited hair cells labeled for BrdU, five days after BrdU injection (Higgs et al., 2002). BrdU-labeled hair cells have also been identified in thin sections of control goldfish saccules (Lanford et al., 1996). In contrast, auditory hair cells and supporting cells in the post-embryonic chick basilar papilla/cochlea are quiescent in the absence of a damaging stimulus (Corwin and Cotanche, 1988; Katayama and Corwin, 1989).
Onset of damage-related mitosis appears to be rapid in the larval zebrafish lateral line. Proliferative cells identified 12 h following neomycin treatment gave rise to new hair cells 24–48 h following exposure (Harris et al., 2003), while we saw a spike in mitotic activity much later in the adult saccule at 48 h following acoustic overexposure. Given that the onset and rate of mitosis may be different in larvae than in adults, it is difficult to generalize about the origin of new hair cells in the saccule based on time post-sound exposure. However, it seems likely that DT of non-dividing precursor cells, or repair of hair bundles, are modes of cell recovery responsible for the early increase in hair bundle density we observed at 2 dpse (see discussion below), and mitosis that occurred at approximately 2 dpse supplied additional daughter cells that differentiated into hair cells by days 7 and 14.
4.4. Cellular repair and direct transdifferentiation
Transdifferentiation of supporting cells into hair cells may account for part of the increase in hair bundle density we observed; however, hair bundle repair may have also played a role. Hair cells that do not undergo apoptosis may repair stereociliary bundles, as demonstrated in mammalian utricular cultures exposed to gentamicin (Zheng et al., 1999), and in cochlear cultures exposed to mechanical injury (Sobkowicz et al., 1996). However, it appears that surviving hair cells do not always regenerate stereociliary bundles following injury. In the chick cochlea, surviving hair cells with damaged or lost stereocilia have been observed even after 10 days of recovery following acoustic trauma (Cotanche, 1987b). Chick regenerating hair cells go through the same sequence of developmental steps as hair cells in the embryonic ear (Cotanche, 1987a, 1987b; Tilney et al., 1986). We noted that immature-like hair bundles in sound-exposed saccules were indistinguishable from putative new hair bundles in control saccules (personal observation), suggesting that the process of stereociliary bundle regeneration or repair following acoustic trauma takes a similar course as hair bundle formation seen during natural hair cell addition in the zebrafish.
In chicks continuously infused with mitotic markers, about one third of new hair cells were not labeled for mitotic markers (Roberson et al., 1996), indirect evidence for direct transdifferentiation (DT), a change in cell fate that does not involve mitosis (Beresford, 1990). Studies of DT point to supporting cells as the precursor cells for hair cell replacement in the lateral line and inner ear. Some regenerating cells in the inner ear show an intermediate morphology, possessing features of both hair cells and supporting cells (Forge et al., 1998; Li and Forge, 1997; Steyger et al., 1997). However, in the bullfrog saccule, sublethally damaged hair cells take on morphological and immunological markers of supporting cells, and a number of apparently immature hair cells possess autophagic vacuoles, suggesting that some of the transitional cells that could be identified as former supporting cells were actually damaged hair cells (Baird et al, 2000).
Further support for DT has been demonstrated in studies using mitotic blocking (Adler and Raphael, 1996; Adler et al., 1997; Baird et al., 2000). For example, chicks administered mitotic blockers show hair cell recovery through DT after exposure to aminoglycoside antibiotics (Baird et al., 2000), and acoustic overstimulation (Adler and Raphael, 1996; Adler et al., 1997). New hair cells arising through DT are observed 24–48 hrs earlier than hair cells produced via mitosis, and hair cell production after 6 d is usually mitotic in chicks exposed to gentamicin (Roberson et al., 2004). Although DT has been demonstrated to occur early in the process of hair cell recovery, mitotic replacement of hair cells in the avian auditory epithelium can occur early as well. Raphael (1992) detected proliferation in chick basilar papillae 24 h following noise exposure and many pairs of BrdU-labeled cells at 36 h. However, BrdU-labeled chick supporting cells appear in sizable numbers at about 3 d post-damage (Bhave et al., 1995), indicating that DT is most likely the main source of early hair cell replacement. We found that the peak of BrdU labeling occurs at 2 dpse, one day earlier than in the chick, perhaps illustrating species differences in the onset of mitosis in the inner ear.
4.5. Hair bundle damage
We noted that exposure to excessive sound produced structural changes such as fusion, splaying, elongation, and fracturing in surviving zebrafish saccular hair cell bundles. These traits have also been observed in the stereocilia of inner and/or outer hair cells of mammals following noise exposure (Bredberg et al., 1970; Gao et al., 1992; Liberman, 1987; Mulroy and Curley, 1982), indicating that the effects of acoustic trauma on hair bundles are similar among species as evolutionally divergent as mammals and fish. It is not completely clear whether the decline in prevalence of damaged hair bundles seen over time in our study was due to ejection of old hair cells followed by development of new hair cells or stereociliary repair, but it is possible that both mechanisms are involved.
4.6. Functional recovery
Fishes are capable of restoring hearing function after exposure to intense noise (Amoser and Ladich, 2003; Scholik and Yan, 2001, 2002; Smith et al., 2004; Smith et al., 2006; Wysocki and Ladich, 2005). For example, Scholik and Yan (2001) determined that hearing recovery in fathead minnows exposed to white noise depended on the duration of noise exposure. Smith et al. (2004) found that goldfish exposed to a loud white noise stimulus for 21 d exhibited significant hearing loss, but hearing recovered to control levels by 14 d post-noise exposure. It is interesting to note that 14 d is also the time needed for recovery of hair bundle density in the current zebrafish study. It remains to be determined whether full hair bundle recovery is required for full hearing recovery in the zebrafish, but Smith et al. (2006) found that in goldfish, temporary threshold shifts declined to within 4 dB of control levels at seven days post-noise exposure, even though full hair cell recovery had not yet occurred. Saunders et al. (1992) demonstrated that full hair cell replacement was not required for recovery of near normal hearing function in the chick. Factors such as tip link regeneration, which plays a role in the rapid recovery of hearing levels observed in chicks (Husbands et al., 1999), and mechanical changes at the cellular level (i.e. cell length and stiffness; Chan et al., 1998) may be at least partially responsible for early hearing recovery, rather than cell regeneration.
4.7. Summary
Zebrafish have served as a useful model for investigating the development and function of the vertebrate inner ear (reviewed in Whitfield, 2002), and may be a useful model for regeneration in the inner ear as well. The current study shows that the zebrafish saccule is capable of regenerating lost hair cell bundles, and that this regeneration is at least partially mediated via cell proliferation. The presented time course of sound-induced hair cell damage and regeneration in the zebrafish inner ear will provide a framework for future studies examining gene expression during the process of hair cell regeneration in zebrafish.
Supplementary Material
Suppl. Fig. 1. Hair cell bundle count locations on the zebrafish saccule. Hair cell counts were sampled at four predetermined locations: 5, 25, 50, and 75% of the total saccular length, as measured from the rostral tip. A 2500 μm2 box was placed at each sampling area and labeled hair cell bundles were counted within each box to determine hair cell density. D= dorsal, R= rostral; scale bar = 100 μm.
Suppl. Fig. 2. Hair cell bundles in the caudal saccules of control and sound-exposed zebrafish immediately following sound exposure (0 dpse). Hair cell bundles were labeled with phalloidin (green) and cell nuclei were labeled with DAPI (blue). Scale bar = 20 μm.
Acknowledgments
The authors wish to thank Amanda Webb for help with maintenance of fish used in this experiment and John Andersland, who assisted with the microscope setup. This research was supported by NIH grant P20 RR-16481, an NSF Kentucky EPSCoR grant, and a WKU faculty summer fellowship to M.E.S.
Abbreviations
- ANOVA
analysis of variance
- BrdU
bromodeoxyuridine
- cm
centimeters
- d
days
- DAPI
4′,6-diamidino-2-phenylindole
- dB
decibels
- DT
direct transdifferentiation
- dpse
days post-sound exposure
- FITC
fluorescein isothiocyanate
- h
hour
- HCL
hydrochloric acid
- Hz
hertz
- L
liter
- μm
micron
- M
molar
- mm
millimeter
- min
minute
- μPa
microPascal
- MS-222
tricaine methanesulfonate
- n
number (ie., sample size)
- N
normal
- PBS
phosphate buffered saline
- RMS
root mean square
References
- Adler HJ, Komeda M, Raphael Y. Further evidence for supporting cell conversion in the damaged avian basilar papilla. Int J Dev Neurosci. 1997;15:375–385. doi: 10.1016/s0736-5748(96)00098-6. [DOI] [PubMed] [Google Scholar]
- Adler HJ, Raphael Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neurosci Lett. 1996;205:17–20. doi: 10.1016/0304-3940(96)12367-3. [DOI] [PubMed] [Google Scholar]
- Amoser S, Ladich F. Diversity in noise-induced temporary hearing loss in otophysine fishes. J Acoust Soc Am. 2003;113:2170–9. doi: 10.1121/1.1557212. [DOI] [PubMed] [Google Scholar]
- Arnaout R, Ferrer T, Huisken J, Spitzer K, Stainier DY, Tristani-Firouz IM, Chi NC. Zebrafish model for human long QT syndrome. Proc Natl Acad Sci U S A. 2007;104:11316–21. doi: 10.1073/pnas.0702724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avallone B, Porritiello M, Esposito D, Mutone R, Balsamo G, Marmo F. Evidence for hair cell regeneration in the crista ampullaris of the lizard Podarcis sicula. Hear Res. 2003;178:79–88. doi: 10.1016/s0378-5955(03)00040-6. [DOI] [PubMed] [Google Scholar]
- Baird RA, Burton MD, Fashena DS, Naeger RA. Hair cell recovery in mitotically blocked cultures of the bullfrog saccule. Proc Natl Acad Sci U S A. 2000;97:11722–11729. doi: 10.1073/pnas.97.22.11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird RA, Steyger PS, Schuff N. Mitotic and nonmitotic hair cell regeneration in the bullfrog vestibular otolith organs. Ann NY Acad Sci. 1996;781:59–70. doi: 10.1111/j.1749-6632.1996.tb15693.x. [DOI] [PubMed] [Google Scholar]
- Baird RA, Torres MA, Schuff NR. Hair cell regeneration in the bullfrog vestibular otolith organs following aminoglycoside toxicity. Hear Res. 1993;65:164–174. doi: 10.1016/0378-5955(93)90211-i. [DOI] [PubMed] [Google Scholar]
- Balak KJ, Corwin JT, Jones JE. Regenerated hair cells can originate from supporting cell progeny: evidence from phototoxicity and laser ablation experiments in the lateral line system. J Neurosci. 1990;10:2502–12. doi: 10.1523/JNEUROSCI.10-08-02502.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresford WA. Direct transdifferentiation: can cells change their phenotype without dividing? Cell Differ Dev. 1990;29:81–93. doi: 10.1016/0922-3371(90)90026-s. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bhave SA, Stone JS, Ruble EW, Coltrera MD. Cell cycle progression in gentamicin-damaged avian cochleas. J Neurosci. 1995;15:4618–28. doi: 10.1523/JNEUROSCI.15-06-04618.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohne BA. Locations of small cochlear lesions by phase contrast microscopy prior to thin sectioning. Laryngoscope. 1972;82:1–16. doi: 10.1002/lary.5540820101. [DOI] [PubMed] [Google Scholar]
- Bohne BA. Healing of the noise damaged inner ear. In: Hirsh SK, Eldredge DH, Hirsh IJ, Silverman SR, editors. Hearing and Davis: Essays Honoring Hallowell Davis. Washington University Press; Saint Louis: 1976. pp. 85–96. [Google Scholar]
- Bohne BA, Rabbitt KD. Holes in the reticular lamina after noise exposure: Implication for continuing damage in the organ of Corti. Hear Res. 1983;11:41–53. doi: 10.1016/0378-5955(83)90044-8. [DOI] [PubMed] [Google Scholar]
- Bredberg G, Lindeman HH, Ades HW, West R, Engström H. Scanning electron microscopy of the organ of Corti. Science. 1970;170:861–863. doi: 10.1126/science.170.3960.861. [DOI] [PubMed] [Google Scholar]
- Cafaro J, Lee GS, Stone JS. Atoh1 expression defines activated progenitors as well as differentiating hair cells during avian hair cell regeneration. Dev Dyn. 2007;236:156–170. doi: 10.1002/dvdy.21023. [DOI] [PubMed] [Google Scholar]
- Chan E, Suneson A, Ulfendahl M. Acoustic trauma causes reversible stiffness changes in auditory sensory cells. Neuroscience. 1998;83:961–8. doi: 10.1016/s0306-4522(97)00446-6. [DOI] [PubMed] [Google Scholar]
- Coleman JW. Hair cell loss as a function of age in the normal cochlea of the guinea pig. Acta Otolaryngol. 1976;82:33–40. doi: 10.3109/00016487609120860. [DOI] [PubMed] [Google Scholar]
- Corwin JT. Postembryonic production and aging of inner ear hair cells in sharks. J Comp Neurol. 1981;201:541–53. doi: 10.1002/cne.902010406. [DOI] [PubMed] [Google Scholar]
- Corwin JT. Postembryonic growth of the macula neglecta auditory detector in the ray, Raja clavata: continual increases in hair cell number, neural convergence, and physiological sensitivity. J Comp Neurol. 1983;217:345–56. doi: 10.1002/cne.902170309. [DOI] [PubMed] [Google Scholar]
- Corwin JT. Regeneration and self-repair in hair cell epithelia: experimental evaluation of capacities and limitations. In: Ruben RJ, Van de Water TR, Rubel EW, editors. Biology of Change in Otolaryngology. Elsevier; New York: 1986. pp. 291–304. [Google Scholar]
- Corwin JT, Balak KJ, Borden PC. Cellular events underlying the regenerative replacement of lateral line sensory epithelia in amphibians. In: Coombs S, Görner P, Münz PH, editors. The Mechanosensory Lateral Line: Neurobiology and Evolution. Springer; New York: 1989. pp. 161–183. [Google Scholar]
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Cotanche DA. Development of hair cell stereocilia in the avian cochlea. Hear Res. 1987a;28:35–44. doi: 10.1016/0378-5955(87)90151-1. [DOI] [PubMed] [Google Scholar]
- Cotanche DA. Regeneration of hair cell stereociliary bundles in the chick cochlea following severe acoustic trauma. Hear Res. 1987b;30:181–196. doi: 10.1016/0378-5955(87)90135-3. [DOI] [PubMed] [Google Scholar]
- Cotanche DA, Dopyera CEJ. Hair cell and supporting cell responses to acoustic trauma in the chick cochlea. Hear Res. 1990;46:29–40. doi: 10.1016/0378-5955(90)90137-e. [DOI] [PubMed] [Google Scholar]
- Cotanche DA, Saunders JC, Tilney LG. Hair cell damage produced by acoustic trauma in the chick cochlea. Hear Res. 1987;25:267–286. doi: 10.1016/0378-5955(87)90098-0. [DOI] [PubMed] [Google Scholar]
- Enger PS. Frequency discrimination in teleosts -- central or peripheral? In: Tavolga WN, Popper AN, Fay RR, editors. Hearing and Sound Communication in Fishes. Springer-Verlag; NewYork: 1981. pp. 243–255. [Google Scholar]
- Ernest S, Rauch GJ, Haffter P, Geisler R, Petit C, Nicolson T. Mariner is defective in myosin VIIA: a zebrafish model for human hereditary deafness. Hum Mol Genet. 2000;9:2189–2196. doi: 10.1093/hmg/9.14.2189. [DOI] [PubMed] [Google Scholar]
- Forge A. Outer hair cell loss and supporting cell expansion following chronic gentamicin treatment. Hear Res. 1985;19:171–82. doi: 10.1016/0378-5955(85)90121-2. [DOI] [PubMed] [Google Scholar]
- Forge A, Li L. Apoptotic death of hair cells in mammalian vestibular sensory epithelia. Hear Res. 2000;139:97–115. doi: 10.1016/s0378-5955(99)00177-x. [DOI] [PubMed] [Google Scholar]
- Forge A, Li L, Nevill G. Hair cell recovery in the vestibular sensory epithelia of mature guinea pigs. J Comp Neurol. 1998;397:69–88. [PubMed] [Google Scholar]
- Gao W, Ding D, Zheng X, Ruan F, Liu Y. A comparison of changes in the stereocilia between temporary and permanent hearing losses in acoustic trauma. Hear Res. 1992;62:27–41. doi: 10.1016/0378-5955(92)90200-7. [DOI] [PubMed] [Google Scholar]
- Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio) J Assoc Res Otolaryngol. 2003;4:219–34. doi: 10.1007/s10162-002-3022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashino E, Salvi RJ. Changing spatial patterns of DNA replication in the noise-damaged chick cochlea. J Cell Sci. 1993;105:23–31. doi: 10.1242/jcs.105.1.23. [DOI] [PubMed] [Google Scholar]
- Hawkins JE, Jr, Johnson LG. Histopathology of cochlear and vestibular ototoxicity in laboratory animals. In: Lerner SA, Matz JG, Hawkins JE Jr, editors. Aminoglycoside Ototoxicity. Little, Brown and Company; Boston: 1981. pp. 175–95. [Google Scholar]
- Hernandez PP, Olivari FA, Sarrazin AF, Sandoval PC, Allende ML. Regeneration in zebrafish lateral line neuromasts: expression of the neural progenitor cell marker Sox2 and proliferation-dependent and -independent mechanisms of hair cell renewal. Dev Neurobiol. 2007;67:637–654. doi: 10.1002/dneu.20386. [DOI] [PubMed] [Google Scholar]
- Higgs DM, Souza MJ, Wilkins HR, Presson JC, Popper AN. Age- and size-related changes in the inner ear and hearing ability of the adult zebrafish (Danio rerio) J Assoc Res Otolaryngol. 2002;3:174–184. doi: 10.1007/s101620020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulander M, Wurst W, Carlsson P, Enerbaeck S. The winged helix transcription factor Fkh10 is required for normal development of the inner ear. Nat Genet. 1998;20:374–376. doi: 10.1038/3850. [DOI] [PubMed] [Google Scholar]
- Hulander M, Kiernan AE, Blomqvuist SR, Carlsson P, Samuelsson E-J, Johansson BR, Steel KP, Enerbaeck S. Lack of pendrin expression leads to deafness and expansion of the endolymphatic compartment in inner ears of Foxi1 null mutant mice. Development. 2003;130:2013–2025. doi: 10.1242/dev.00376. [DOI] [PubMed] [Google Scholar]
- Husbands JM, Steinberg SA, Kurian R, Saunders JC. Tip-link integrity on chick tall hair cell stereocilia following intense sound exposure. Hear Res. 1999;135:135–45. doi: 10.1016/s0378-5955(99)00101-x. [DOI] [PubMed] [Google Scholar]
- Itoh M, Chitnis AB. Expression of proneural and neurogenic genes in the zebrafish lateral line primordium correlates with selection of hair cell fate in neuromasts. Mech Dev. 2001;102:263–6. doi: 10.1016/s0925-4773(01)00308-2. [DOI] [PubMed] [Google Scholar]
- Jones JE, Corwin JT. Regeneration of sensory cells after laser ablation in the lateral line system: hair cell lineage and macrophage behavior revealed by time-lapse video microscopy. J Neurosci. 1996;16:649–662. doi: 10.1523/JNEUROSCI.16-02-00649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama A, Corwin JT. Cell production in the chicken cochlea. J Comp Neurol. 1989;281:129–35. doi: 10.1002/cne.902810110. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Feldman ML. Hair cell counts in an age-graded series of rat cochleas. Hear Res. 1982;8:249–62. doi: 10.1016/0378-5955(82)90017-x. [DOI] [PubMed] [Google Scholar]
- Kelley MW, Talreja DR, Corwin JT. Replacement of hair cells after laser microbeam irradiation in cultured organs of Corti from embryonic and neonatal mice. J Neurosci. 1995;15:3013–3026. doi: 10.1523/JNEUROSCI.15-04-03013.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski DJ, Whitfield TT, Hukriede NA, Lam WK, Weinberg ES. The zebrafish dog-eared mutation disrupts eya1, a gene required for cell survival and differentiation in the inner ear and lateral line. Dev Biol. 2005;277:27–41. doi: 10.1016/j.ydbio.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Presson JC, Popper AN. Cell proliferation and hair cell addition in the ear of the goldfish, Carassius auratus. Hear Res. 1996;100:1–9. doi: 10.1016/0378-5955(96)00110-4. [DOI] [PubMed] [Google Scholar]
- Li L, Forge A. Morphological evidence for supporting cell to hair cell conversion in the mammalian utricular macula. Int J Dev Neurosci. 1997;15:433–446. doi: 10.1016/s0736-5748(96)00102-5. [DOI] [PubMed] [Google Scholar]
- Li L, Nevill G, Forge A. Two modes of hair cell loss from the vestibular sensory epithelia of the guinea pig inner ear. J Comp Neurol. 1995;355:405–417. doi: 10.1002/cne.903550307. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Chronic ultrastructural changes in acoustic trauma: serial-section reconstruction of stereocilia and cuticular plates. Hear Res. 1987;26:65–88. doi: 10.1016/0378-5955(87)90036-0. [DOI] [PubMed] [Google Scholar]
- Lim DJ. Ultrastructural cochlear changes following acoustic hyperstimulation and ototoxicity. Ann Otol Rhinol Laryngol. 1976;85:740–51. doi: 10.1177/000348947608500604. [DOI] [PubMed] [Google Scholar]
- Lindeman HH, Bredberg G. Scanning electron microscopy of the organ of Corti after intense auditory stimulation: effects on stereocilia and cuticular surface of hair cells. Eur Arch Oto-Rhino-L. 1972;203:1–15. doi: 10.1007/BF00344558. [DOI] [PubMed] [Google Scholar]
- Lombarte A, Yan HY, Popper AN, Chang JS, Platt C. Damage and regeneration of hair cell ciliary bundles in a fish ear following treatment with gentamicin. Hear Res. 1993;64:166–74. doi: 10.1016/0378-5955(93)90002-i. [DOI] [PubMed] [Google Scholar]
- Manley GA, Brix J, Kaiser A. Developmental stability of the tonotopic organization of the chick’s basilar papilla. Science. 1987;23:655–656. doi: 10.1126/science.3603046. [DOI] [PubMed] [Google Scholar]
- Marsh RR, Xu L, Moy JP, Saunders JC. Recovery of the basilar papilla following intense sound exposure in the chick. Hear Res. 1990;46:229–238. doi: 10.1016/0378-5955(90)90004-9. [DOI] [PubMed] [Google Scholar]
- Millimaki BB, Sweet EM, Dahson MS, Riley BB. Zebrafish atoh1 genes: classic proneural activity in the inner ear and regulation by Fgf and Notch. Development. 2007;134:295–305. doi: 10.1242/dev.02734. [DOI] [PubMed] [Google Scholar]
- Mulroy MJ, Curley FJ. Stereociliary pathology and noise-induced threshold shift: a scanning electron microscopic study. Scan Electron Micros. 1982;4:1753–1762. [PubMed] [Google Scholar]
- Popper AN, Fay RR. Sound detection and processing by teleost fishes: a critical review. J Acoust Soc Am. 1973;53:1515–1529. doi: 10.1121/1.1913496. [DOI] [PubMed] [Google Scholar]
- Popper AN, Fay RR. The auditory periphery in fishes. In: Fay RR, Popper AN, editors. Comparative Hearing: Fish and Amphibians. Springer-Verlag; New York: 1999. pp. 43–100. [Google Scholar]
- Raphael Y. Evidence for supporting cell mitosis in response to acoustic trauma in the avian inner ear. J Neurocytol. 1992;21:663–71. doi: 10.1007/BF01191727. [DOI] [PubMed] [Google Scholar]
- Raphael Y. Reorganization of the chick basilar papilla after acoustic trauma. J Comp Neurol. 1993;330:521–532. doi: 10.1002/cne.903300408. [DOI] [PubMed] [Google Scholar]
- Raphael Y, Altschuler RA. Reorganization of cytoskeletal and junctional proteins during cochlear hair cell degeneration. Cell Motil Cytoskel. 1991a;18:215–27. doi: 10.1002/cm.970180307. [DOI] [PubMed] [Google Scholar]
- Raphael Y, Altschuler RA. Scar formation after drug-induced cochlear insult. Hear Res. 1991b;51:173–184. doi: 10.1016/0378-5955(91)90034-7. [DOI] [PubMed] [Google Scholar]
- Raphael Y, Altschuler RA. Early microfilament reorganization in injured auditory epithelia. Exp Neurol. 1992;115:32–36. doi: 10.1016/0014-4886(92)90217-e. [DOI] [PubMed] [Google Scholar]
- Radeloff A, Smolders JWT. Brain-derived neurotrophic factor treatment does not improve functional recovery after hair cell regeneration in the pigeon. Acta Oto-Laryngol. 2006;126:452–459. doi: 10.1080/00016480500437344. [DOI] [PubMed] [Google Scholar]
- Roberson DW, Alosi JA, Cotanche DA. Direct transdifferentiation gives rise to the earliest new hair cells in regenerating avian auditory epithelium. J Neurosci Res. 2004;78:461–471. doi: 10.1002/jnr.20271. [DOI] [PubMed] [Google Scholar]
- Roberson DW, Kreig CS, Rubel EW. Light microscopic evidence that direct transdifferentiation gives rise to new hair cells in regenerating avian auditory epithelium. Audit Neurosci. 1996;2:195–205. doi: 10.1002/jnr.20271. [DOI] [PubMed] [Google Scholar]
- Roberson DW, Rubel EW. Cell division in the gerbil cochlea after acoustic trauma. Am J Otol. 1994;15:28–34. [PubMed] [Google Scholar]
- Rubel EW, Dew LA, Roberson DW, Warchol ME, Corwin JT, Forge A, Li L, Nevill G. Mammalian vestibular hair cell regeneration. Science. 1995;267:701–707. doi: 10.1126/science.7839150. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Cortunix quail. Science. 1988;240:1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- Saunders JC, Adler HJ, Pugliano FA. The structural and functional aspects of hair cell regeneration in the chick as a result of exposure to intense sound. Exp Neurol. 1992;115:13–17. doi: 10.1016/0014-4886(92)90213-a. [DOI] [PubMed] [Google Scholar]
- Scholik AR, Yan HY. Effects of underwater noise on auditory sensitivity of a cyprinid fish. Hear Res. 2001;152:17–24. doi: 10.1016/s0378-5955(00)00213-6. [DOI] [PubMed] [Google Scholar]
- Scholik AR, Yan HY. The effects of noise on the auditory sensitivity of the bluegill sunfish, Lepomis macrochirus. Comp Biochem Physiol A. 2002;133:43–52. doi: 10.1016/s1095-6433(02)00108-3. [DOI] [PubMed] [Google Scholar]
- Smith ME, Kane AS, Popper AN. Noise-induced stress response and hearing loss in goldfish (Carrasius auratus) J Exp Biol. 2004;207:427–435. doi: 10.1242/jeb.00755. [DOI] [PubMed] [Google Scholar]
- Smith ME, Coffin AB, Miller DL, Popper AN. Anatomical and functional recovery of the goldfish (Carassius auratus) ear following noise exposure. J Exp Biol. 2006;209:4193–202. doi: 10.1242/jeb.02490. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, August BK, Slapnick SM. Post-traumatic survival and recovery of the auditory sensory cells in culture. Acta Otolaryngol. 1996;116:257–62. doi: 10.3109/00016489609137836. [DOI] [PubMed] [Google Scholar]
- Solomon KS, Kudoh T, Dawid IB, Fritz A. Zebrafish foxi1 mediates otic placode formation and jaw development. Development. 2003;130:929–940. doi: 10.1242/dev.00308. [DOI] [PubMed] [Google Scholar]
- Song J, Yan HY, Popper AN. Damage and recovery of hair cells in fish canal (but not superficial) neuromasts after gentamicin exposure. Hear Res. 1995;91:63–71. doi: 10.1016/0378-5955(95)00170-0. [DOI] [PubMed] [Google Scholar]
- Steyger PS, Burton M, Hawkins JR, Schuff NR, Baird BA. Calbindin and parvalbumin are early markers of non-mitotically regenerating hair cells in the bullfrog vestibular otolith organs. Int J Dev Neurosci. 1997;15:417–732. doi: 10.1016/s0736-5748(96)00101-3. [DOI] [PubMed] [Google Scholar]
- Stockwell CW, Ades HW, Engström H. Patterns of hair cell damage after intense auditory stimulation. Ann Oto Rhinol Laryn. 1969;78:1144–68. doi: 10.1177/000348946907800602. [DOI] [PubMed] [Google Scholar]
- Stone JS, Choi YS, Woolley SM, Yamashita H, Rubel EW. Progenitor cell cycling during hair cell regeneration in the vestibular and auditory epithelia of the chick. J Neurocytol. 1999;28:863–76. doi: 10.1023/a:1007022205821. [DOI] [PubMed] [Google Scholar]
- Stone JS, Cotanche DA. Identification of the timing of S phase and the patterns of cell proliferation during hair cell regeneration in the chick cochlea. J Comp Neurol. 1994;341:50–67. doi: 10.1002/cne.903410106. [DOI] [PubMed] [Google Scholar]
- Stone JS, Cotanche DA. Hair cell regeneration in the avian auditory epithelium. Int J Dev Biol. 2007;51:633–647. doi: 10.1387/ijdb.072408js. [DOI] [PubMed] [Google Scholar]
- Stone LS. Further experimental studies of the development of lateral-line sense organs in the amphibians observed in living preparations. J Comp Neurol. 1937;68:83–115. [Google Scholar]
- Taylor RR, Forge A. Hair cell regeneration in sensory epithelia from the inner ear of a urodele amphibian. J Comp Neurol. 2005;484:105–20. doi: 10.1002/cne.20450. [DOI] [PubMed] [Google Scholar]
- Tester AL, Kendall JI. Morphology of the lateralis canal system in the shark genus Carcharinus. Pac Sci. 1969;23:1–16. [Google Scholar]
- Theopold HM. Comparative surface studies of ototoxic effects of various aminoglycoside antibiotics on the organ of Corti in the guinea pig. A scanning electron microscopic study. Acta Otolaryngol. 1977;84:57–64. doi: 10.3109/00016487709123942. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Tilney MS, Saunders JC, DeRosier DJ. Actin filaments, stereocilia and hair cells of the bird cochlea. III The development and differentiation of hair cells and stereocilia in embryos. Dev Biol. 1986;116:100–118. doi: 10.1016/0012-1606(86)90047-3. [DOI] [PubMed] [Google Scholar]
- Tsue TT, Watling DL, Weisleder P, Coltrera MD, Rubel EW. Identification of hair cell progenitors and intermitotic migration of their nuclei in the normal and regenerating avian inner ear. J Neurosci. 1994;14:140–52. doi: 10.1523/JNEUROSCI.14-01-00140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Frisch K. The sense of hearing in fish. Nature. 1938;141:8–11. [Google Scholar]
- Warchol ME. Cell density and N-cadherin interactions regulate cell proliferation in the sensory epithelia of the inner ear. J Neurosci. 2002;22:2607–2616. doi: 10.1523/JNEUROSCI.22-07-02607.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol ME, Corwin JT. Regenerative proliferation in organ cultures of the avian cochlea: identification of the initial progenitors and determination of the latency of the proliferative response. J Neurosci. 1996;16:5466–5477. doi: 10.1523/JNEUROSCI.16-17-05466.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science. 1993;259:1619–1622. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- Weisleder P, Rubel EW. Hair cell regeneration after streptomycin toxicity in the avian vestibular epithelium. J Comp Neurol. 1993;331:97–110. doi: 10.1002/cne.903310106. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. University of Oregon Press; Eugene, OR: 1994. [Google Scholar]
- White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441:984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- Whitfield TT. Zebrafish as a model for hearing and deafness. J Neurobiol. 2002;53:157–171. doi: 10.1002/neu.10123. [DOI] [PubMed] [Google Scholar]
- Wilkins HR, Presson JC, Popper AN. Proliferation of vertebrate inner ear supporting cells. J Neurobiol. 1999;39:527–535. doi: 10.1002/(sici)1097-4695(19990615)39:4<527::aid-neu6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Wright MR. Regeneration and degeneration experiments on lateral line nerves and sense organs in anurans. J Exp Zool. 1947;105:221–257. doi: 10.1002/jez.1401050206. [DOI] [PubMed] [Google Scholar]
- Wysocki LE, Ladich F. Effects of noise exposure on click detection and the temporal resolution ability of the goldfish auditory system. Hear Res. 2005;201:27–36. doi: 10.1016/j.heares.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Helbig C, Gao WQ. Analysis of rat vestibular hair cell development and regeneration using calretinin as an early marker. J Neurosci. 1997;17:8270–8282. doi: 10.1523/JNEUROSCI.17-21-08270.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Keller G, Gao WQ. Immunocytochemical and morphological evidence for intracellular self-repair as an important contributor to mammalian hair cell recovery. J Neurosci. 1999;19:2161–70. doi: 10.1523/JNEUROSCI.19-06-02161.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Fig. 1. Hair cell bundle count locations on the zebrafish saccule. Hair cell counts were sampled at four predetermined locations: 5, 25, 50, and 75% of the total saccular length, as measured from the rostral tip. A 2500 μm2 box was placed at each sampling area and labeled hair cell bundles were counted within each box to determine hair cell density. D= dorsal, R= rostral; scale bar = 100 μm.
Suppl. Fig. 2. Hair cell bundles in the caudal saccules of control and sound-exposed zebrafish immediately following sound exposure (0 dpse). Hair cell bundles were labeled with phalloidin (green) and cell nuclei were labeled with DAPI (blue). Scale bar = 20 μm.