Abstract
The neurotrophins (NTs) have recently been shown to elicit pronounced effects on quantal neurotransmitter release at both central and peripheral nervous system synapses. Due to their activity-dependent release, as well as the subcellular localization of both protein and receptor, NTs are ideally suited to modify the strength of neuronal connections by “fine-tuning” synaptic activity through direct actions at presynaptic terminals. Here, using BDNF as a prototypical example, the authors provide an update of recent evidence demonstrating that NTs enhance quantal neurotransmitter release at synapses through presynaptic mechanisms. The authors further propose that a potential target for NT actions at presynaptic terminals is the mechanism by which terminals retrieve synaptic vesicles after exocytosis. Depending on the temporal demands placed on synapses during high-frequency synaptic transmission, synapses may use two alternative modes of synaptic vesicle retrieval, the conventional slow endosomal recycling or a faster rapid retrieval at the active zone, referred to as “kiss-and-run.” By modulating Ca2+ microdomains associated with voltage-gated Ca2+ channels at active zones, NTs may elicit a switch from the slow to the fast mode of endocytosis of vesicles at presynaptic terminals during high-frequency synaptic transmission, allowing more reliable information transfer and neuronal signaling in the central nervous system.
Keywords: BDNF, Docked vesicles, Fusion pore, Hippocampus, mEPSC, Poisson stimulation, Quantal release, SNARE proteins, Synaptic vesicles, TrkB, Voltage-gated Ca2+ channels
Experience-dependent changes in the efficacy of fast synaptic transmission are thought to be a mechanism by which an organism adapts and changes in response to interactions with its environment. How, where, and under what conditions these changes in synaptic strength occur has been under intense speculation for more than half a century. Since the development of Katz's seminal quantal theory of neurotransmitter release (Katz 1969), a host of molecules have been evaluated for their ability to modulate synaptic strength by enhancing quantal neurotransmitter release. Advances in our current knowledge and technology have permitted for more precise delineation of pre- and postsynaptic sites of action with regard to enhanced synaptic transmission, although these sites are not always mutually exclusive. Recently, neurotrophins (NT) have emerged as candidates to mediate those modulatory actions at synapses because of their pronounced effects on quantal neurotransmitter release in both the central and peripheral nervous system.
The mammalian NTs, nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin 4/5 (NT-4/5) have all been shown to be essential for neuronal viability and differentiation, as well as synaptic plasticity in various brain regions relevant to learning and memory (for review, see McAllister and others 1999; Schinder and Poo 2000; Tyler and others 2002). Two types of plasma membrane receptors mediate NT action: high-affinity tyrosine kinase receptors (Trk) and low-affinity pan-neurotrophin receptors (p75). Specific Trk receptors have preferential affinities for one or more of the NTs: TrkA for NGF, TrkB for BDNF and NT-4/5, and TrkC for NT-3 (Chao 1992). For the purpose of this Neuroscience Up-date, we use BDNF as a prototypical example to review the actions of NTs at presynaptic terminals. With regard to their subcellular localization, BDNF and its TrkB receptor have been found in both pre- and postsynaptic compartments (for review, see Murer and others 2001). Furthermore, BDNF can undergo both retro- and anterograde transport and signaling at the synapse in an activity-dependent manner (Kohara and others 2001). Indeed, BDNF exerts its actions on hippocampal synaptic physiology by acting pre-, post-, and perisynaptically (for review, see Poo 2001). The implications of activity-dependent bi-directional release and signaling at synapses make NTs ideally suited to modify the strength of neuronal connectivity by “fine-tuning” synaptic activity through direct actions at synapses. This is of particular importance for those synapses recruited by high levels of neuronal activity, providing a rapid switch from a low to highly active synapse. Not only are the mechanisms of plasticity during development similar to those proposed to occur during plasticity in the adult nervous system, but biologically similar molecular signals are often conserved to serve similar functions in a diversity of roles, enhancing the evolutionary advantage they confer. NTs appear to represent such molecular signals, by initially participating in the establishment of neuronal connections during development, as well as continuing to modify those neuronal networks long after their formation.
In the present Neuroscience Update, we review the evidence of the actions of BDNF at presynaptic terminals and propose a model for the mechanism of BDNF effects on quantal neurotransmitter release.
BDNF Acts at Presynaptic Terminals to Increase Quantal Neurotransmitter Release
BDNF and NT-3 were first reported to rapidly enhance synaptic transmission at cultured Xenopus neuromuscular junctions through a presynaptic mechanism (Lohof and others 1993). It was subsequently demonstrated that BDNF increases the frequency of spontaneous synaptic transmission, as well as reduces the number of synaptic failures in acute slices of visual cortex, further supporting a presynaptic site of action (Carmignoto and others 1997). BDNF increases the frequency of AMPA receptor-mediated miniature excitatory postsynaptic currents (mEPSCs) without affecting their amplitudes in cultured hippocampal neurons (Lessmann and others 1994; Lessmann and Heumann 1998; Li and others 1998; Collin and others 2001), as well as in hippocampal slice cultures (Fig. 1; Tyler and Pozzo-Miller 2001), an effect indicative of an increase in quantal release probability without modulation of quantal content or postsynaptic AMPA receptors. Expression of a noncatalytic truncated TrkB receptor in presynaptic hippocampal neurons, but not in postsynaptic ones, prevents BDNF-induced increases in spontaneous neurotransmitter release in vitro (Li and others 1998). Furthermore, in cultured hippocampal neurons, BDNF decreases paired-pulse facilitation, a simple and reliable measure of presynaptic properties with few assumptions (Foster and McNaughton 1991; Schulz and others 1994), as well as the coefficient of variation of evoked EPSCs, another robust indicator of presynaptic properties, such as neurotransmitter release probability (Pr) and the number of release sites (Lessmann and Heumann 1998; Berninger and others 1999; Schinder and others 2000). Synapses that initially have a low Pr, show marked potentiation in response to BDNF, whereas synapses with high Pr show little potentiation in response to BDNF (Lessmann and Heumann 1998; Berninger and others 1999). This differential synaptic enhancement by BDNF could be affected by distinct differences in presynaptic calcium channel modulation and/or expression, as well as alterations in calcium release from internal stores, but most likely reflects overall differences in synapse maturity, in that Trk signaling has been shown to play a role in synapse maturation (Martinez and others 1998).
Fig. 1.
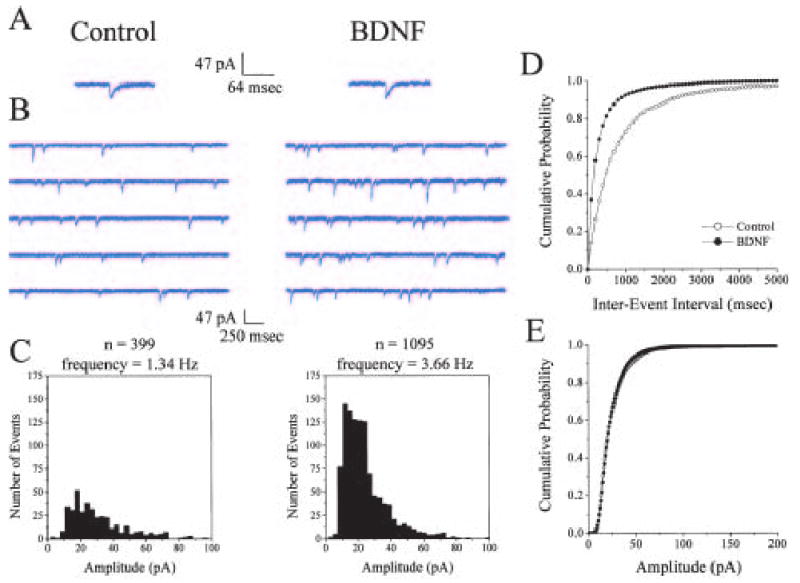
BDNF increases the frequency of AMPA-mediated miniature excitatory postsynaptic currents (mEPSCs) without affecting their amplitude. Whole-cell voltage-clamp recordings were performed in CA1 pyramidal neurons from hippocampal slice cultures treated for 5 days in vitro with serum-free control media (left A, B, and C), or BDNF (250 ng/ml; right A, B, and C). A, Representative unitary AMPA-mediated mEPSCs. BDNF treatment did not affect the kinetics or amplitude of AMPA mEPSCs. B, Representative continuous records of membrane currents showing AMPA mEPSC events from control- (left) or BDNF- (right) treated slice cultures. C, Frequency histogram distributions (5pA bins) of events recorded from the control- (left) and BDNF- (right) treated cell in 10- to 30-s epochs. Total number of events recorded and frequency of mEPSCs are shown above each frequency histogram distribution. D, Cumulative probability distribution of interevent (mEPSC) intervals. E, cumulative probability distribution of mEPSC amplitude from six cells each obtained from control (open circles) and BDNF (filled circles) treated hippocampal slice cultures. (Modified from Tyler and Pozzo-Miller 2001.)
Modern optical imaging techniques combined with the use of amphipathic fluorescent probes, such as the styryl dye FM1-43, have been used to directly detect the exocytosis and endocytosis of synaptic vesicles at small presynaptic nerve terminals (Betz and others 1992), greatly enhancing our understanding of the vesicular trafficking events underlying neurotransmitter release (for review, see Cochilla and others 1999). The use of styryl dyes eliminates the dependence of monitoring presynaptic function at small central terminals exclusively by the electrophysiological detection of postsynaptic events. Many fundamental properties of presynaptic terminals have been characterized using styryl dyes, including the size of the total recycling population of vesicles, the size of the readily releasable pool, the rate of endocytosis, and the rate of spontaneous vesicle undocking. Furthermore, styryl dyes incorporate into vesicles in a quantal manner that is proportional to Pr (reviewed by Harata and others 2001). It has been demonstrated using this optical imaging approach that BDNF enhances vesicle exocytosis from presynaptic terminals of embryonic cortical neurons in vitro, in a manner consistent with an increase in Pr (Bradley and Sporns 1999), supporting the hypothesis that BDNF enhances neurotransmitter release by facilitating vesicle mobilization and/or fusion at presynaptic active zones.
Mechanisms by Which BDNF Increases Quantal Neurotransmitter Release
It is well established that neurotransmitter release is directly modulated by aspects of presynaptic calcium signaling, including action potential duration, Ca2+ transients mediated by presynaptic voltage-dependent Ca2+ channels, and Ca2+-dependent interactions among synaptic vesicle proteins of the SNARE complex (for review, see Llinás and others 1995; Augustine 2001). Stoop and Poo (1996) demonstrated that BDNF-induced enhancement of synaptic transmission at cultured Xenopus neuromuscular junctions is correlated with an augmentation of presynaptic Ca2+ influx from extracellular space. Although it has been proposed that NT-induced enhancement of neurotransmitter release is due to enhanced Ca2+ signaling in the presynaptic nerve terminal (Stoop and Poo 1995, 1996), very little is known regarding the modulation of presynaptic calcium transients by BDNF. BDNF has been shown to selectively up-regulate the functional expression of non-L-type (N-, P/Q-, and R-type) Ca2+ channels in hippocampal pyramidal neurons (Baldelli and others 2000), as well as in rat embryo motoneurons (Baldelli and others 1999). These channel types are known to contribute to fast synaptic transmission at excitatory hippocampal synapses (Gasparini and others 2001). Taken together, these observations suggest that Ca2+ channels known to be involved in neurotransmitter release at small central synapses are a likely site for the initial modulatory action of BDNF at presynaptic terminals.
Another mechanism by which NTs could enhance quantal neurotransmitter release is to increase the size of the readily releasable pool of quanta, or its morphological correlate, the vesicles docked at the active zone (Schikorski and Stevens 1997, 2001). Synaptic fatigue during sustained high-frequency stimulation (HFS) is thought to result from the depletion of the readily releasable pool of synaptic vesicles (Model and others 1975; Dickinson-Nelson and Reese 1983; Zucker 1989; Dobrunz and Stevens 1997). BDNF has been shown to prevent synaptic fatigue during HFS in hippocampal slices from developing rats (Figurov and others 1996; Gottschalk and others 1998), whereas slices from BDNF knockout mice exhibit more pronounced synaptic fatigue than those from their wild-type littermates (Pozzo-Miller and others 1999). Additionally, disruption of endogenous BDNF/TrkB signaling elicits greater synaptic fatigue at CA3-CA1 synapses in hippocampal slices (Figurov and others 1996; Pozzo-Miller and others 1999). Thus, prevention of synaptic fatigue during HFS increases synaptic reliability during the sort of protocols used to induce hippocampal long-term potentiation (LTP), and may represent a mechanism by which BDNF promotes LTP in slices from young rats (Figurov and others 1996). Quantitative electron microscopy of docked synaptic vesicles (DV) at the active zone of hippocampal CA3-CA1 synapses lends support to the hypothesis that BDNF modulates the readily releasable pool of vesicles. Corresponding to the findings of pronounced synaptic fatigue during HFS, BDNF knockout mice had fewer DVs than their wild-type littermates (Pozzo-Miller and others 1999); similarly, it was shown that TrkB knockout mice also have fewer DVs at those CA1 synapses (Martinez and others 1998). Subsequently, it has been demonstrated that BDNF increases the number of DVs at excitatory synapses in the CA1 region of hippocampal slice cultures (Fig. 2; Tyler and Pozzo-Miller 2001) and in cultured embryonic hippocampal neurons (Collin and others 2001). Prior to the finding that BDNF increases the number of docked synaptic vesicles, the only manipulation that had been shown to increase the physiologically defined readily releasable pool of quanta was phorbol ester activation of PKC (Stevens and Sullivan 1998; Waters and Smith 2000). Interestingly, TrkB receptor activation by BDNF leads to PKC activation via the PLCγ-DAG signaling pathway (Segal and Greenberg 1996).
Fig. 2.
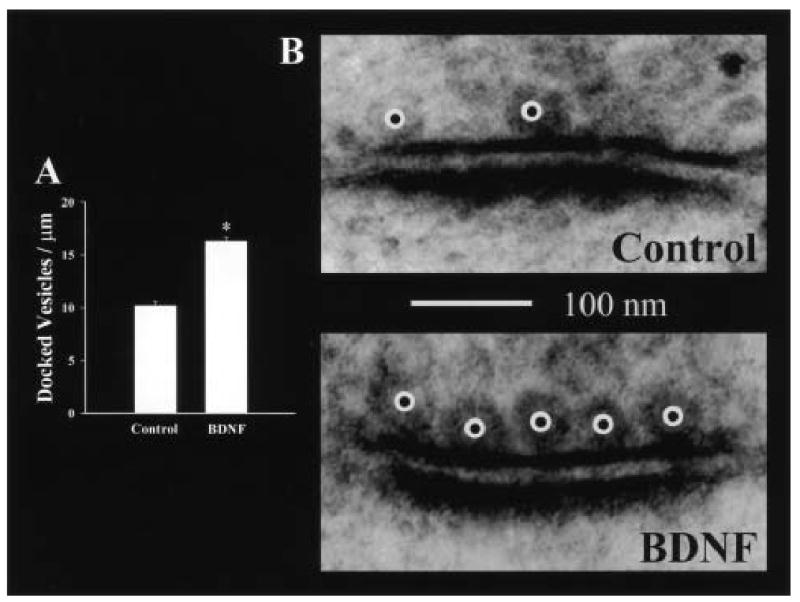
BDNF increases the number of docked synaptic vesicles at the active zones of CA3-CA1 excitatory synapses. A, Histogram plot of the number of docked synaptic vesicles per μm of active zone. Data are illustrated as mean ± SEM (control n = 98 synapses, BDNF n = 101 synapses; asterisk indicates P < 0.05). Interestingly, the effect on DV was observed in the absence of an observed effect on the total number of reserve pool vesicles. B, Representative electron micrographs of synapses on dendritic spines within CA1 stratum radiatum from serum-free control (top), and slice cultures treated with BDNF (250 ng/ml; bottom) for 5 days in vitro. Morphologically docked synaptic vesicles are indicated with a white circle superimposed with a black circle. (Modified from Tyler and Pozzo-Miller 2001.)
The molecular targets of the NT signaling cascade involved in neurotransmitter release are beginning to be identified. Some of these targets include synaptic vesicle trafficking proteins as well as the proteins involved in the formation of the SNARE complex. In fact, BDNF has been shown to increase the expression of several SNARE proteins, which play a fundamental role in the formation of synaptic vesicle docking (Tartaglia and others 2001). The expression of several vesicular-SNAREs (v-SNAREs; synaptophysin, synapsin-I, and synaptotagmin-I) and target-SNAREs (t-SNAREs; SNAP-25 and syntaxin-1) is reduced in several hippocampal regions in TrkC and, to a greater extent, in TrkB knockout mice (Martinez and others 1998). BDNF enhances glutamate release from synaptosomes dependent on MAPK phosphorylation of synapsin-I (Jova-novic and others 2000). Because one of the major functions of synapsins is to regulate the trafficking of synaptic vesicles between distinct pools within presynaptic terminals (Greengard and others 1993), it seems likely that BDNF is facilitating synaptic vesicle docking through the modulation of these presynaptic vesicle proteins (Pozzo-Miller and others 1999). BDNF treatment of hippocampal slices from BDNF knockout mice not only rescues the pronounced synaptic fatigue that occurs during HFS, but it also rescues the expression levels of the reduced synaptic vesicle proteins, synaptobrevin and synaptophysin (Pozzo-Miller and others 1999). Furthermore, it has recently been shown that rab3A, a small GTP-binding vesicular trafficking protein involved in the mobilization of synaptic vesicles from the reserve pool to the active zone, is necessary for BDNF enhancement of hippocampal synaptic transmission (Thakker-Varia and others 2001). Thus, it appears as though NT signaling plays a fundamental role in both developmental and adult activity-dependent synaptic plasticity through transcriptional and/or translational regulation of proteins, such as those of the SNARE complex, involved in vesicle mobilization, docking, and fusion at the active zone of presynaptic terminals.
Although it has become widely accepted that NTs increase the probability of neurotransmitter release, the temporal aspects of the signaling mechanisms underlying this enhanced neurotransmitter release remain under intense investigation. Although NTs have been shown to enhance the machinery known to be involved in neurotransmitter release (e.g., increased DVs, Ca2+ channel expression, and SNAREs protein expression), the majority of these mechanisms require hours to days before they can be observed. The increase in neurotransmitter release mediated by NTs is, however, evident within minutes. Perhaps a lack of detection sensitivity prevents the precise characterization of the temporal aspect of NT-induced enhancement of neurotransmitter release. Considering the phenomenal speed at which neurotransmitter release occurs (Llinás and others 1995), we propose that the initial site of NT action is likely to involve a direct modulation of the Ca2+ signal that triggers either full vesicle fusion or the formation and expansion rate of the flickering fusion pore required for the exocytosis of neurotransmitter from synaptic vesicles.
The Role of Endocytosis in Maintaining Synaptic Transmission at Central Synapses
The small presynaptic terminals of neurons in the CNS may contain only ∼200 synaptic vesicles (Schikorski and Stevens 1997), of which, only ∼15%–20% on average are normally recycled (Murthy and Stevens 1998, 1999; Harata and others 2001). Yet, neurons from cortical structures recorded in vivo can fire short bursts of spikes at rates of up to 200 Hz during behavioral tasks (Buzsaki 1989; Buracas and others 1998; McAdams and Maunsell 1999; Carandini and Ferster 2000). Therefore, a critical determinant of the ability of small, central synapses to maintain synaptic transmission during high-frequency activity is the rate at which synaptic vesicles can be recycled to a release competent state. The first and, perhaps, rate-limiting step in this process is endocytosis of depleted vesicles, which can occur via two mechanisms with vastly different kinetics. The process of conventional endocytosis is likely to prevail during low-frequency synaptic activity; in this mode, synaptic vesicles completely fuse with the plasma membrane (Heuser and Reese 1973), are slowly retrieved through endosomal compartments, and eventually (within 30–60 s; Ryan and others 1993; Liu and Tsien 1995) are refilled with neurotransmitter to become release-competent. On the other hand, during high-frequency activity, presynaptic terminals may engage a faster vesicle retrieval locally at the active zone; in this “kiss-and-run” retrieval mode originally proposed by Bruno Ceccarelli (Ceccarelli and others 1973), synaptic vesicles empty their contents without complete fusion with the plasma membrane and are immediately (within ∼1 s) refilled and reused (Pyle and others 2000; Stevens and Williams 2000).
Obviously, a switch from conventional to “kiss-and-run” endocytosis would permit synaptic transmission to be maintained during periods of high-frequency activity. A test of this hypothesis is illustrated in Figure 3. Excitatory postsynaptic potentials (EPSPs) evoked by stimulation in layer 4 were recorded from pyramidal cells in layer 2/3 of acute slices from the primary visual cortex (S.P.P. & M. J. Friedlander, unpublished observations; methods described in [Perrett and others 2001]). The figure shows representative examples of the synaptic depression that occurs during 5 Hz stimulus trains presented for 3 min (i.e., exactly 900 stimuli in each case). The only difference between the traces is that stimuli are presented at constant intervals (i.e., 200 ms interstimulus interval during constant stimulation) for the closed circles, whereas for the open circles stimuli are presented at Poisson-distributed intervals (Poisson stimulation). Poisson-distributed intervals were chosen to approximate the natural spiking patterns of cortical neurons (Abbott and others 1997; Perrett and others 2001), which fire in bursts followed by quiescent periods (Shadlen and Newsome 1998; Stevens and Zador 1998). Much less synaptic depression is induced by Poisson stimulation than by constant stimulation, and the rate of recovery from that depression is significantly faster following Poisson stimulation. We believe this occurs because the stimulus bursts inherent in the Poisson pattern recruit the “kiss-and-run” mode of endocytosis, but the evenly spaced stimuli of the constant stimulus pattern do not. The critical assumption we make is that, in the absence of endocytosis, 900 stimuli should completely deplete synaptic vesicles at these small central synapses (Pyle and others 2000; Harata and others 2001). Under this assumption, the large differences in rates of synaptic depression and recovery observed must be due to differences in the rate at which vesicles are recycled. Differential recruitment of the conventional and kiss-and-run endocytosis during the two stimulus patterns is the most straightforward explanation for these differences.
Fig. 3.
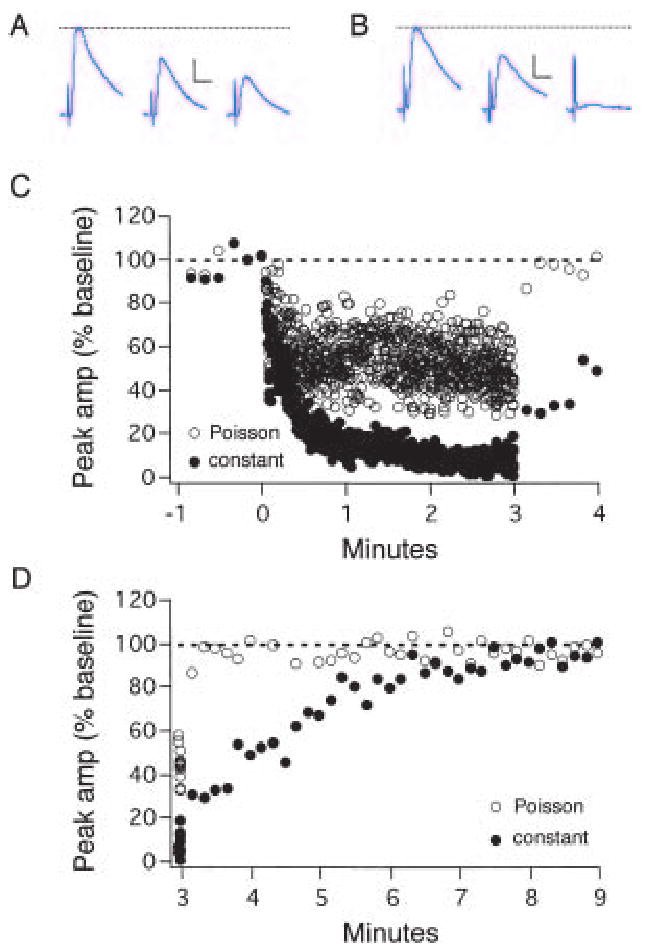
Stimulation with Poisson-distributed intervals induces much less synaptic depression than stimulation with constant intervals. Representative examples are shown in which synaptic depression was induced by a 5 Hz stimulus train (3 min, 900 stimuli, initiated at time 0) with either Poisson-distributed intervals (A; C, open circles) or constant intervals (B; C, filled circles) between stimuli. Stimulation at constant intervals of 10 s was delivered before each train to determine the baseline excitatory postsynaptic potential (EPSP) amplitude and following each train to assess the rate of recovery from synaptic depression. A, Average of 10 EPSP traces taken (from left to right) immediately preceding, at the beginning, and at the end of the 5 Hz Poisson train. B, Average of 10 traces taken (from left to right) immediately preceding, at the beginning, and at the end of the 5 Hz constant train. Note that there is effectively a failure of synaptic transmission by the end of the train. C, Plot of the peak amplitudes of EPSPs relative to baseline during and immediately following Poisson (open circles) and constant (filled circles) 5 Hz stimulus trains. During Poisson stimulation, EPSP amplitudes quickly declined during the first 30 s of the train before stabilizing at ∼50% of baseline amplitude. During constant stimulation, EPSP amplitudes declined throughout the first 2 min of the train before stabilizing at ∼10% of baseline amplitude. D, Following the Poisson train synaptic strength recovered to baseline within 20 sec, whereas it took 480 s for complete recovery from synaptic depression following the constant train. Dashed lines denote average baseline EPSP amplitude. Scale bars = 2 mV, 10 sec (S. P. Perrett and M. J. Friedlander, unpublished observations).
Why might Poisson stimulation preferentially recruit kiss-and-run endocytosis relative to constant stimulation? Recent studies indicate that, like exocytosis, endocytosis is also regulated by Ca2+ (Abenavoli and others 2001). Indeed, larger Ca2+ influx into presynaptic terminals triggers the faster kiss-and-run mode of endocytosis (Pyle and others 2000; Neves and others 2001; Sankaranarayanan and Ryan 2001). Therefore, Poisson stimulation could induce less synaptic depression because the high-frequency bursts of stimuli that occur during the train would elevate Ca2+ levels beyond the threshold for the recruitment of kiss-and-run endocytosis. During constant stimulation, this Ca2+ threshold would not be exceeded and, therefore, endocytosis would occur in the slower conventional mode, leading to greater synaptic depression. In a similar manner, NTs could modulate endocytosis by triggering the kiss-and-run mode through its aforementioned effects on presynaptic Ca2+ levels, an exciting possibility under current investigation in our laboratory.
Concluding Remarks
NTs enhance neurotransmitter release by modulating critical properties of presynaptic terminals, which allow them to reliably follow high rates of synaptic transmission. Because a flickering fusion pore during kiss-and-run endocytosis of synaptic vesicles is more efficient to prevent synaptic fatigue during high-frequency spiking, we propose that BDNF switches the mode of vesicle retrieval from conventional, slow full fusion to the faster kiss-and-run mode by modulating presynaptic voltage-dependent Ca2+ channels. The initial effects of BDNF on presynaptic voltage-gated Ca2+ channels are likely mediated by direct TrkB-dependent phosphorylation. Subsequently, this would lead to increased docking sites and enhanced vesicle mobilization from the reserve pool to the active zone mediated by SNARE and synaptic vesicle trafficking proteins. Increased docking sites will allow for more vesicles docked at the active zone and cause an overlap of Ca2+ microdomains (Fig. 4), further promoting the kiss-and-run mode of vesicle retrieval. In our working model, BDNF allows presynaptic terminals to follow high-frequency synaptic activity during short bouts of enhanced neuronal firing, like those observed during behavioral information acquisition and consolidation (Buzsaki 1989). The modulation of neurotransmitter release by NTs has therapeutic implications for the improvement of the memory impairments observed in several neurodegenerative disorders, such as Alzheimer's disease.
Fig. 4.
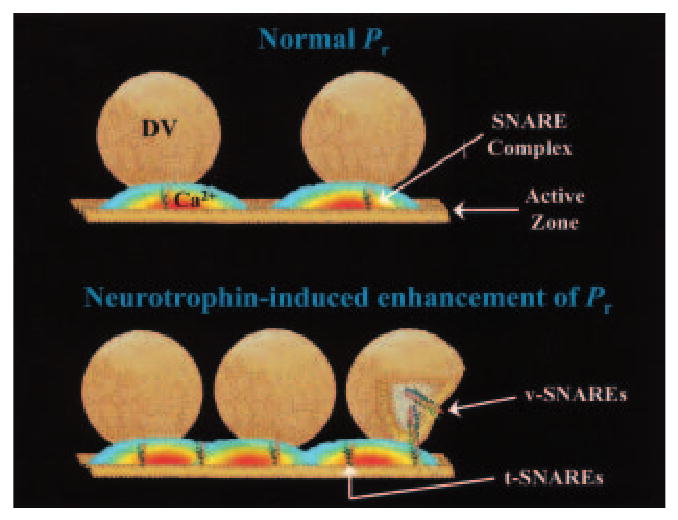
Working model for the mechanism by which NT-Trk signaling enhances quantal neurotransmitter release. The model depicted is an oversimplified illustration of mechanisms known to play a fundamental role in neurotransmitter release at fast chemical synapses. The upper panel illustrates SNARE-mediated synaptic vesicles docking at the presynaptic active zone. The Ca2+ signal is illustrated as an isoconcentration surface depicting the Ca2+ microdomain that triggers vesicle fusion, and neurotransmitter release (after Simon and Llinás 1985). The lower panel depicts the same release site after activation of the signaling cascades triggered by NT binding to Trk receptors. The docked vesicle at far right presents a cutaway view for display purposes. This illustration shows an increase in both v-SNARE and t-SNARE proteins, an increase in the number of DVs, and the occurrence of overlapping Ca2+ microdomains. Following NT-Trk signaling, an increase in the SNARE proteins associated with synaptic vesicle mobilization and targeting increases the number of docking sites, as well as the probability of a vesicle occupying that site. Direct NT modulation of Ca2+ channels responsible for the appearance of a Ca2+ microdomain results in the higher Ca2+ levels necessary to switch from the conventional slow mode of vesicle retrieval to the faster and more reliable “kiss and run” endocytotic pathway.
Acknowledgments
The work reviewed here has been supported by NIH grants RO1-NS40593, MRRC P30-HD38985, PO1-HD38760, EY-12782, NRSA-09770, and by AMGEN for the generous supply of BDNF.
References
- Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997;275:220–4. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- Abenavoli A, Montagna M, Malgaroli A. Calcium: the common theme in vesicular cycling. Nat Neurosci. 2001;4:117–8. doi: 10.1038/83924. [DOI] [PubMed] [Google Scholar]
- Augustine GJ. How does calcium trigger neurotransmitter release? Curr Opin Neurobiol. 2001;11:320–6. doi: 10.1016/s0959-4388(00)00214-2. [DOI] [PubMed] [Google Scholar]
- Baldelli P, Forni PE, Carbone E. BDNF, NT-3 and NGF induce distinct new Ca2+ channel synthesis in developing hippocampal neurons. Eur J Neurosci. 2000;12:4017–32. doi: 10.1046/j.1460-9568.2000.00305.x. [DOI] [PubMed] [Google Scholar]
- Baldelli P, Magnelli V, Carbone E. Selective up-regulation of P- and R-type Ca2+ channels in rat embryo motoneurons by BDNF. Eur J Neurosci. 1999;11:1127–33. doi: 10.1046/j.1460-9568.1999.00523.x. [DOI] [PubMed] [Google Scholar]
- Berninger B, Schinder AF, Poo MM. Synaptic reliability correlates with reduced susceptibility to synaptic potentiation by brain-derived neurotrophic factor. Learn Mem. 1999;6:232–42. [PMC free article] [PubMed] [Google Scholar]
- Betz WJ, Mao F, Bewick GS. Activity-dependent fluorescent staining and destaining of living vertebrate motor nerve terminals. J Neurosci. 1992;12:363–75. doi: 10.1523/JNEUROSCI.12-02-00363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley J, Sporns O. BDNF-dependent enhancement of exocytosis in cultured cortical neurons requires translation but not transcription. Brain Res. 1999;815:140–9. doi: 10.1016/s0006-8993(98)01112-3. [DOI] [PubMed] [Google Scholar]
- Buracas GT, Zador AM, DeWeese MR, Albright TD. Efficient discrimination of temporal patterns by motion-sensitive neurons in primate visual cortex. Neuron. 1998;20:959–69. doi: 10.1016/s0896-6273(00)80477-8. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551–70. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Carandini M, Ferster D. Membrane potential and firing rate in cat primary visual cortex. J Neurosci. 2000;20:470–84. doi: 10.1523/JNEUROSCI.20-01-00470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignoto G, Pizzorusso T, Tia S, Vicini S. Brain-derived neurotrophic factor and nerve growth factor potentiate excitatory synaptic transmission in the rat visual cortex. J Physiol. 1997;498:153–64. doi: 10.1113/jphysiol.1997.sp021848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B, Hurlbut WP, Mauro A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1973;57:499–524. doi: 10.1083/jcb.57.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV. Neurotrophin receptors: a window into neuronal differentiation. Neuron. 1992;9:583–93. doi: 10.1016/0896-6273(92)90023-7. [DOI] [PubMed] [Google Scholar]
- Cochilla AJ, Angleson JK, Betz WJ. Monitoring secretory membrane with FM1-43 fluorescence. Annu Rev Neurosci. 1999;22:1–10. doi: 10.1146/annurev.neuro.22.1.1. [DOI] [PubMed] [Google Scholar]
- Collin C, Vicario-Abejon C, Rubio ME, Wenthold RJ, McKay RD, Segal M. Neurotrophins act at presynaptic terminals to activate synapses among cultured hippocampal neurons. Eur J Neurosci. 2001;13:1273–82. doi: 10.1046/j.0953-816x.2001.01500.x. [DOI] [PubMed] [Google Scholar]
- Dickinson-Nelson A, Reese TS. Structural changes during transmitter release at synapses in the frog sympathetic ganglion. J Neurosci. 1983;3:42–52. doi: 10.1523/JNEUROSCI.03-01-00042.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–9. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Foster TC, McNaughton BL. Long-term enhancement of CA1 synaptic transmission is due to increased quantal size, not quantal content. Hippocampus. 1991;1:79–91. doi: 10.1002/hipo.450010108. [DOI] [PubMed] [Google Scholar]
- Gasparini S, Kasyanov AM, Pietrobon D, Voronin LL, Cherubini E. Presynaptic R-type calcium channels contribute to fast excitatory synaptic transmission in the rat hippocampus. J Neurosci. 2001;21:8715–21. doi: 10.1523/JNEUROSCI.21-22-08715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk W, Pozzo-Miller LD, Figurov A, Lu B. Presynaptic modulation of synaptic transmission and plasticity by brain-derived neurotrophic factor in the developing hippocampus. J Neurosci. 1998;18:6830–9. doi: 10.1523/JNEUROSCI.18-17-06830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P, Valtorta F, Czernik AJ, Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993;259:780–5. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Harata N, Pyle JL, Aravanis AM, Mozhayeva M, Kavalali ET, Tsien RW. Limited numbers of recycling vesicles in small CNS nerve terminals: implications for neural signaling and vesicular cycling. Trends Neurosci. 2001;24:637–43. doi: 10.1016/s0166-2236(00)02030-0. [DOI] [PubMed] [Google Scholar]
- Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973;57:315–44. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Czernik AJ, Fienberg AA, Greengard P, Sihra TS. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat Neurosci. 2000;3:323–9. doi: 10.1038/73888. [DOI] [PubMed] [Google Scholar]
- Katz B. The release of neural transmitter substances. Liverpool, UK: Liverpool University; 1969. [Google Scholar]
- Kohara K, Kitamura A, Morishima M, Tsumoto T. Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. Science. 2001;291:2419–23. doi: 10.1126/science.1057415. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Gottmann K, Heumann R. BDNF and NT-4/5 enhance glutamatergic synaptic transmission in cultured hippocampal neurones. Neuroreport. 1994;6:21–5. doi: 10.1097/00001756-199412300-00007. [DOI] [PubMed] [Google Scholar]
- Lessmann V, Heumann R. Modulation of unitary glutamatergic synapses by neurotrophin-4/5 or brain-derived neurotrophic factor in hippocampal microcultures: presynaptic enhancement depends on pre-established paired-pulse facilitation. Neuroscience. 1998;86:399–413. doi: 10.1016/s0306-4522(98)00035-9. [DOI] [PubMed] [Google Scholar]
- Li YX, Zhang Y, Lester HA, Schuman EM, Davidson N. Enhancement of neurotransmitter release induced by brain-derived neurotrophic factor in cultured hippocampal neurons. J Neurosci. 1998;18:10231–40. doi: 10.1523/JNEUROSCI.18-24-10231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Tsien RW. Properties of synaptic transmission at single hippocampal synaptic boutons. Nature. 1995;375:404–8. doi: 10.1038/375404a0. [DOI] [PubMed] [Google Scholar]
- Llinás R, Sugimori M, Silver RB. The concept of calcium concentration microdomains in synaptic transmission. Neuropharmacology. 1995;34:1443–51. doi: 10.1016/0028-3908(95)00150-5. [DOI] [PubMed] [Google Scholar]
- Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363:350–3. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- Martinez A, Alcantara S, Borrell V, Del Rio JA, Blasi J, Otal R, et al. TrkB and TrkC signaling are required for maturation and synaptogenesis of hippocampal connections. J Neurosci. 1998;18:7336–50. doi: 10.1523/JNEUROSCI.18-18-07336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JH. Effects of attention on the reliability of individual neurons in monkey visual cortex. Neuron. 1999;23:765–73. doi: 10.1016/s0896-6273(01)80034-9. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- Model PG, Highstein SM, Bennett MV. Depletion of vesicles and fatigue of transmission at a vertebrate central synapse. Brain Res. 1975;98:209–28. doi: 10.1016/0006-8993(75)90002-5. [DOI] [PubMed] [Google Scholar]
- Murer MG, Yan Q, Raisman-Vozari R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer's disease and Parkinson's disease. Prog Neurobiol. 2001;63:71–124. doi: 10.1016/s0301-0082(00)00014-9. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Stevens CF. Synaptic vesicles retain their identity through the endocytic cycle. Nature. 1998;392:497–501. doi: 10.1038/33152. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Stevens CF. Reversal of synaptic vesicle docking at central synapses. Nat Neurosci. 1999;2:503–7. doi: 10.1038/9149. Available from java/Propub/neuro/nn0699_503.fulltextjava/Propub/neuro/nn_503.abstract. [DOI] [PubMed]
- Neves G, Gomis A, Lagnado L. Calcium influx selects the fast mode of endocytosis in the synaptic terminal of retinal bipolar cells. Proc Natl Acad Sci U S A. 2001;98:15282–7. doi: 10.1073/pnas.261311698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett SP, Dudek SM, Eagleman D, Montague PR, Friedlander MJ. LTD induction in adult visual cortex: role of stimulus timing and inhibition. J Neurosci. 2001;21:2308–19. doi: 10.1523/JNEUROSCI.21-07-02308.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, et al. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J Neurosci. 1999;19:4972–83. doi: 10.1523/JNEUROSCI.19-12-04972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyle JL, Kavalali ET, Piedras-Renteria ES, Tsien RW. Rapid reuse of readily releasable pool vesicles at hippocampal synapses. Neuron. 2000;28:221–31. doi: 10.1016/s0896-6273(00)00098-2. [DOI] [PubMed] [Google Scholar]
- Ryan TA, Reuter H, Wendland B, Schweizer FE, Tsien RW, Smith SJ. The kinetics of synaptic vesicle recycling measured at single presynaptic boutons. Neuron. 1993;11:713–24. doi: 10.1016/0896-6273(93)90081-2. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Calcium accelerates endocytosis of vSNAREs at hippocampal synapses. Nat Neurosci. 2001;4:129–36. doi: 10.1038/83949. [DOI] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF. Quantitative ultrastructural analysis of hippocampal excitatory synapses. J Neurosci. 1997;17:5858–67. doi: 10.1523/JNEUROSCI.17-15-05858.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF. Morphological correlates of functionally defined synaptic vesicle populations. Nat Neurosci. 2001;4:391–5. doi: 10.1038/86042. [DOI] [PubMed] [Google Scholar]
- Schinder AF, Berninger B, Poo M. Postsynaptic target specificity of neurotrophin-induced presynaptic potentiation. Neuron. 2000;25:151–63. doi: 10.1016/s0896-6273(00)80879-x. [DOI] [PubMed] [Google Scholar]
- Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23:639–45. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- Schulz PE, Cook EP, Johnston D. Changes in paired-pulse facilitation suggest presynaptic involvement in long-term potentiation. J Neurosci. 1994;14:5325–37. doi: 10.1523/JNEUROSCI.14-09-05325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci. 1996;19:463–89. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J Neurosci. 1998;18:3870–96. doi: 10.1523/JNEUROSCI.18-10-03870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SM, Llinás RR. Compartmentalization of the submembrane calcium activity during calcium influx and its significance in transmitter release. Biophys J. 1985;48:485–98. doi: 10.1016/S0006-3495(85)83804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CF, Sullivan JM. Regulation of the readily releasable vesicle pool by protein kinase C. Neuron. 1998;21:885–93. doi: 10.1016/s0896-6273(00)80603-0. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Williams JH. “Kiss and run” exocytosis at hippocampal synapses. Proc Natl Acad Sci U S A. 2000;97:12828–33. doi: 10.1073/pnas.230438697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CF, Zador AM. Input synchrony and the irregular firing of cortical neurons. Nat Neurosci. 1998;1:210–7. doi: 10.1038/659. [DOI] [PubMed] [Google Scholar]
- Stoop R, Poo MM. Potentiation of transmitter release by ciliary neurotrophic factor requires somatic signaling. Science. 1995;267:695–9. doi: 10.1126/science.7839148. [DOI] [PubMed] [Google Scholar]
- Stoop R, Poo MM. Synaptic modulation by neurotrophic factors: differential and synergistic effects of brain-derived neurotrophic factor and ciliary neurotrophic factor. J Neurosci. 1996;16:3256–64. doi: 10.1523/JNEUROSCI.16-10-03256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia N, Du J, Tyler WJ, Neale E, Pozzo-Miller L, Lu B. Protein synthesis dependent and independent regulation of hippocampal synapses by brain-derived neurotrophic factor. J Biol Chem. 2001;276:37585–93. doi: 10.1074/jbc.M101683200. [DOI] [PubMed] [Google Scholar]
- Thakker-Varia S, Alder J, Crozier RA, Plummer MR, Black IB. Rab3A is required for brain-derived neurotrophic factor-induced synaptic plasticity: transcriptional analysis at the population and single-cell levels. J Neurosci. 2001;21:6782–90. doi: 10.1523/JNEUROSCI.21-17-06782.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factors signaling in hippocampal-dependent learning. Learn Mem. 2002;9:224–37. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Pozzo-Miller LD. BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci. 2001;21:4249–58. doi: 10.1523/JNEUROSCI.21-12-04249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters J, Smith SJ. Phorbol esters potentiate evoked and spontaneous release by different presynaptic mechanisms. J Neurosci. 2000;20:7863–70. doi: 10.1523/JNEUROSCI.20-21-07863.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]


