Synopsis
Alzheimer’s disease (AD) affects millions worldwide. Currently, there are no treatments that prevent or slow AD. Like other neurodegenerative diseases, AD is characterized by protein misfolding in the brain. This process and associated brain damage begins years prior to the substantial neurodegeneration that accompanies dementia. Studies utilizing new neuroimaging techniques and fluid biomarkers suggest that AD pathology can be detected pre-clinically. These advances should enable novel clinical trial design and early mechanism-based therapeutic intervention.
AD begins as a pathological process years before the onset of dementia
With the emergence of disease-modifying strategies for the treatment of AD, impetus to diagnose the condition in its early ‘preclinical’ stages – before significant brain damage occurs – has intensified. Fortunately, advances in technology and in our perspective on what defines AD may soon make such antecedent diagnosis possible.
Since their first description in 19071, ‘senile’ plaques and neurofibrillary tangles (NFT) have remained the hallmark histopathological features of AD, and are employed by three sets of diagnostic histological criteria (Khachaturian, CERAD, and NIA/Reagan) 2-4. Historically, they have also been associated with the dementia caused by the disease. It is clear, however, that these lesions begin to accrue in significant amounts in many ‘cognitively normal’ elderly individuals5. To reconcile these incongruent inferences/observations, several tacit hypotheses have been spawned that persist even today: (1) that AD cannot be diagnosed in the absence of cognitive impairment/dementia, (2) that plaques and NFT increase in healthy aging, and (3) that AD and aging can be distinguished by quantitative (rather than qualitative) assessment of plaque and NFT burden6. Nevertheless, a growing body of evidence now supports a different philosophy regarding the onset of AD: independent of cognitive status, amyloid plaques and NFT actually define (but do not fully represent) the disease process, which also involves inflammation as well as neuronal, axonal, and synaptic loss and dysfunction.
Consistently, neuropathological studies involving large numbers of non-demented subjects have identified significant AD pathology in the brains of older individuals7, 8. Neocortical cerebral amyloid deposits have been identified in approximately 50% of brains from individuals over 758. In contrast, the prevalence of AD dementia does not reach 50% until age 85 or more9. It appears that the onset of very mild dementia is correlated best not with plaque or NFT burden, but with significant synaptic and neuronal loss6, 10. Together, these data support the concept of ‘pre-clinical’ AD, a phase during which plaques, and subsequently, NFT, accumulate for ~10-15 years before the synaptic and neuronal loss they accompany manifest as cognitive decline (Fig. 1) 6. This concept fits with genetic, biochemical, and animal model data which demonstrate that the aggregation of the amyloid-β (Aβ) peptide plays a necessary role11, especially in the preclinical phase of AD, and that tau aggregation, which occurs in bulk later, drives neurodegeneration just prior to and during the clinical phase. Given these points, we are ready to consider the significance of proposed biomarkers in their proper pathophysiological context. However, a point of clinical context must also be addressed.
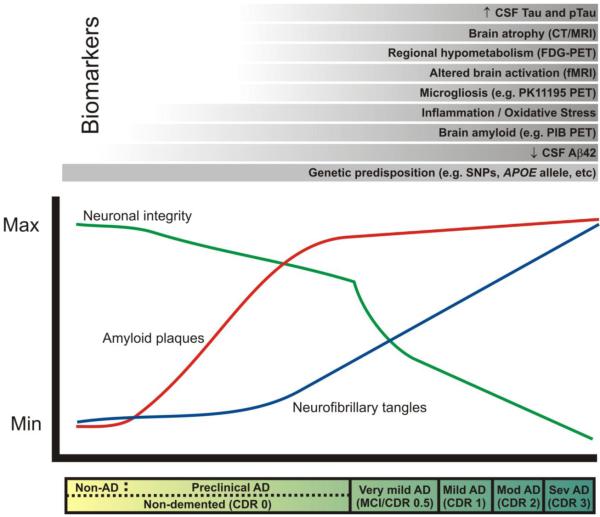
Figure 1. Biomarkers and AD: proposed changes in biomarkers in relation to time course of pathological and clinical stages.
The clinical stages of AD, marked by progressive dementia described as ‘very mild/mild cognitive impairment’ (MCI), ‘mild,’ ‘moderate,’ and ‘severe,’ correspond with Clinical Dementia Rating (CDR) scores of 0.5, 1, 2, and 3, respectively (see bar below plot). These stages are associated with abundant amyloid plaques (red line), the gradual accumulation of neurofibrillary tangles (blue line), synaptic and neuronal loss in certain brain regions (green line). In the ‘preclinical’ stage of AD, Aβ42 peptide forms amyloid plaques in the brains of non-demented individuals (CDR 0) for approximately 10-15 years, and damages neuronal processes and synapses. Eventually, dramatic neuronal losses occur in association with dementia onset. AD biomarker research seeks to measure changes in the structure and function of the brain (e.g. atrophy, regional activity changes and hypometabolism, amyloid plaque and NFT formation, microgliosis, inflammation, oxidative stress) that might be useful for diagnosis and prognosis during this ‘preclinical’ phase of AD, before irreversible neuronal loss occurs. These changes can be measured by radiological imaging modalities (e.g. computed tomography [CT], magnetic resonance imaging [MRI], functional magnetic resonance imaging [fMRI], and positron emission tomography [PET] with various imaging contrast agents) and/or by biochemical examination of cerebrospinal fluid (CSF). The most promising biomarkers to date are listed above the plot in chronological order from bottom to top according to the earliest stage of the pathological process at which they seem to show utility. A reduced concentration of CSF Aβ42 may provide the earliest definitive evidence of AD pathology in the brain. Genetic variations (e.g. single nucleotide polymorphisms [SNPs]) may also be considered biomarkers that allow the earliest possible estimation of risk. PIB, Pittsburgh compound B. (Modified, with permission, from Craig-Schapiro R., et al., Biomarkers of Alzheimer’s Disease, Neurobiol. Dis., 2009;35(2):128-140).
The early clinical manifestations that are believed to be due to AD (decline in memory and executive functioning) are referred to in different ways by clinicians. Some report this syndrome as very mild dementia of the Alzheimer type12. Others prefer the term mild cognitive impairment (MCI)13. A combination of clinical methods, laboratory tests, and cognitive testing are utilized to most accurately determine the presence or absence of cognitive impairment and its cause (Box 1). As many of the biomarkers that provide insight into the underlying pathology of AD are not yet used in day-to-day patient care, these diagnoses reflect attempts to describe clinical syndromes in the absence of definitive proof of the underlying pathology. While diagnosis, prevention, and treatment of dementia are of ultimate importance, the goal of biomarker research is to identify and monitor the underlying pathology and suggest prognosis. It is important to note up front, that while this review focuses on biomarkers, the use of genetics combined with biomarkers will likely provide additive diagnostic and prognostic information. Detailed discussion of genetics of AD is beyond the scope of this review. The most up to date information of AD genetics can be found at the Alzheimer Research Forum (http://www.alzgene.org).
Box 1. Assessment of cognitive impairment and dementia.
Dementia is an acquired syndrome in which there is a decline in memory and thinking that is sufficient to interfere with everyday performance. Some individuals demonstrate deficits either in memory alone or in memory and other cognitive domains that are indicative of an abnormality but are not yet severe enough to be termed “dementia”. Most people who go on to develop dementia go through a transitional stage that some term very mild dementia and others term mild cognitive impairment (MCI) or ‘cognitively impaired no dementia’ (CIND). There are many different entities which can lead to cognitive impairment and dementia, including a variety of neurodegenerative disorders, vascular damage, infections, tumors, and other causes. AD is the most common cause of cognitive impairment and dementia in people over the age of 65. Determination that acquired cognitive impairment or dementia is present, and diagnosis of its likely cause, is based on clinical history (especially from a reliable informant), neurological and psychiatric examinations, and certain laboratory tests. The assembled information allows assessment of whether an individual’s faculties have declined relative to their past abilities (intra-individual change) and can be used for determining the level of dementia (very mild, mild, moderate, or severe) as well as the most likely diagnosis.
Formal cognitive testing can aid these determinations, and is particularly useful for clinical situations in which cognitive symptoms and signs are subtle or confounded by other medical factors such as depression. A variety of neuropsychological tests can be used to accurately assess the different cognitive domains, including orientation, intellect, language, memory, attention, concentration, executive function, visual/perceptual abilities, sensorimotor function, mood, and personality75. Serial neuropsychological evaluations are also very useful for tracking the progress of an individual over time relative to an established baseline. Currently, the diagnosis of dementia due to AD can only be confirmed with certainty at autopsy. However, many of the biomarkers reviewed herein, when utilized together with clinical evaluation and cognitive testing, can assist with differential diagnosis and prognosis. To determine the presence of diseases such as AD prior to the emergence of cognitive impairment or dementia as detected through neuropsychological testing or by clinicians, antecedent biomarkers will be required.
Imaging atrophy and aggregates of amyloid-β and tau
Although cellular resolution has not yet been achieved, recent advances in functional and molecular neuroimaging have provided insights into brain structure and physiology, allowing for the study of specific proteins and protein aggregates in ways that are difficult or impossible to achieve at autopsy. Moreover, neuroimaging biomarker studies can immediately correlate data with structure and (dys)function.
The characteristic patterns of cortical and hippocampal volume loss in advanced AD are well known but difficult to quantify with precision at postmortem examination. Atrophy is particularly difficult to measure in the early stages of AD, when it superficially resembles the inconspicuous volume loss commonly observed among elderly individuals without neurodegeneration (so-called ‘healthy’ aging). Distinguishing such subtle differences is not a problem for high resolution quantitative magnetic resonance imaging (MRI). Applied longitudinally, this technique can distinguish the global atrophy rates of healthy aging vs. AD 14, and predict progression from normal cognition to MCI15 and MCI to AD on the basis of regional volume losses and ventricular expansion over time (Fig. 2b,c)16. However, atrophy and cognitive decline occur in most neurodegenerative disorders, and while volume changes in certain brain regions may be suggestive of, or consistent with, AD, they do not reveal the underlying pathology.
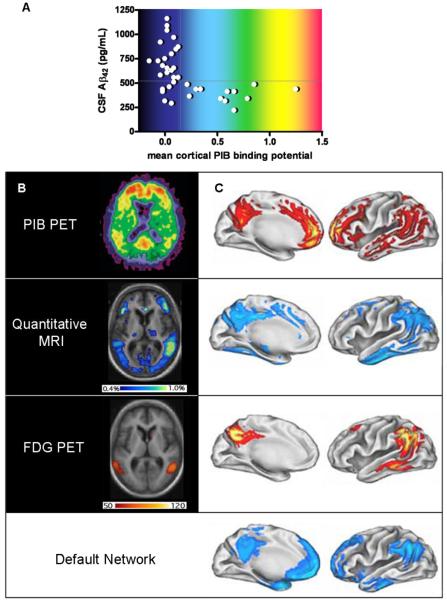
Figure 2. Imaging biomarkers.
A, Relationship of PIB PET to CSF Aβ42 levels in cognitively normal individuals. Subjects with mean cortical PIB binding potentials > 0.16 (calculated from an average of PIB retention in the prefrontal cortex, the lateral temporal cortex, the precuneus and the gyrus rectus, divided by PIB retention in the cerebellar cortex) are considered ‘positive’ and uniformly have low CSF Aβ42 (<500pg/mL); PIB ‘negative’ subjects with low Aβ42 may have non-fibrillar (diffuse) Aβ42 deposits that do not retain PIB; whether this latter group will be more likely to develop dementia than PIB ‘negative’ subjects with high CSF Aβ42 is not yet known. B, Axial (horizontal) view of AD brain, imaged to quantify amyloid (PIB PET, above), annual rates of regional atrophy (Quantitative MRI, middle), and hypometabolism (FDG PET, below) in relation to disease severity. The intensity of the PIB binding potential is depicted using a color scale (approximated by the colors in A) in which red reflects greatest PIB retention, and black and dark blue reflect least PIB retention. The regional extent of atrophy is depicted colorimetrically, with rates ranging from 0.4% per year (dark blue) to 1% per year (yellow/green). Regional hypometabolism is also depicted colorimetrically, with red and yellow representing greater and lesser hypometabolism, respectively. The units of this scale reflect the slope of the regression between hypometabolism and dementia severity as measured by mini mental status examination; high slope suggests a steeper decline in metabolism in relation to decreasing cognitive ability. C, Illustrations of left hemi-brain surfaces (medial, left; lateral, right), allowing comparison of averaged anatomic signal maps for amyloid (top), atrophy (second from top), hypometabolism (third from top), and ‘default network’ activity (bottom). Regional amyloid load (PIB binding potential) is depicted as percentage increase of PIB binding potential over that of the brainstem, ranging from 5% (red) to 40% (yellow/white). Colorimetric scales for atrophy and hypometabolism are as in B. The color scale for regional default-network activity shows the degree of association, ranging from greatest association with default-network activity (light blue) to least statistically significant association (darker blue). (Panels A and B [top view]: modified, with permission, from ref. 21.; panels B [remaining views] and C: modified, with permission, from ref. 27).
Patterns of plaque and NFT formation in AD have been thoroughly studied. Until recently, however, antemortem examination of these pathological changes has been nearly impossible. Within the last decade, a number of radiological contrast compounds have been developed that specifically bind and highlight pathological structures in the CNS, including amyloid plaques, neurofibrillary tangles, activated microglia, and reactive astrocytes.
So far, five compounds ([18F]FDDNP, 18F-BAY94-9172, 11C-SB-13, 11C-BF-227, 11C-PIB) have been reported as probes for imaging amyloid plaques in humans. One of these, [18F] FDDNP, may be retained by neurofibrillary tangles 17, but no agent that selectively binds to aggregates of tau has yet been described. Such a discovery would be a major advance for molecular imaging.
Of the amyloid-binding compounds, 11C-PIB, short for “Pittsburgh Compound-B”, has been the most extensively studied and applied in AD research. Uptake of PIB can be measured by positron emission tomography (PET). In individuals with AD, increased retention of PIB shows a very specific pattern that is restricted to brain regions typically associated with amyloid deposition (Fig. 2b,c)18. Interestingly, an appreciable number of cognitively normal individuals over age 60 show a PIB signal pattern indistinguishable from that of AD subjects, suggesting that PIB PET can detect a preclinical stage of AD19. When PIB PET and levels of CSF Aβ42 peptide were measured at the same time in individuals ranging from the cognitively normal to those with AD, it showed two discrete groups: PIB-‘negative’ individuals, and PIB-‘positive’ ones. Without exception, the ‘positive’ PIB group had low CSF Aβ42 levels (Fig. 2a)20. This finding is consistent with the idea that soluble Aβ42 is retained in the brain once plaques are formed. Larger longitudinal studies of cognitively normal subjects, comparing those with amyloid to those without amyloid, will be required to evaluate whether the presence of amyloid confers a greater risk of “conversion” to dementia.
Complementing these radiological studies of amyloid deposition, other PET labeling agents have been developed to image inflammation as reflected by activated microglia and reactive astrocytes that surround plaques. Among the changes that microglia undergo upon activation, increased expression of the peripheral benzodiazepine receptor has been exploited as a target for radiological compounds [11C]-DAA110621, [11C]-vinpocetine22 and [11C] (R)-PK11195. Only the third compound has been reported in human AD studies, in conjunction with PIB. In these studies of individuals with MCI or AD, PK11195 and PIB signals showed similar anatomic patterns, but their levels did not show regional correlation23; among AD subjects, there was an inverse correlation between PK11195 signal and cognitive performance. These findings suggest that microgliosis occurs concomitantly with amyloid deposition and may play a direct role in cognitive dysfunction. However, better imaging agents are needed to visualize microglial activation in AD. Like microglia, astrocytes show changes upon association with plaques, including elevation of monoamine oxidase B activity24, but studies employing MAO-B inhibitors as radiotracers in AD are just emerging25. Larger studies will be needed to understand whether imaging inflammation may inform subject selection for clinical trials, contribute to predictions of prognosis, and allow monitoring of response to therapy.
Watching the mind at work
Neurons of the medial temporal lobe (MTL) and hippocampus are particularly susceptible to loss in AD, and their loss appears to coincide with the onset of clinically significant cognitive impairment. Functional MRI (fMRI) is believed to provide a measure of synaptic activity. Accordingly, the MTL memory system appears to show hypoactivation by fMRI in the clinical stages of AD. However, these same structures appear to show hyperactivation in MCI, when memory is only mildly impaired, and neuronal loss is less severe; this increased activity may represent an attempt to compensate for functional inefficiencies26.
Although the MTL memory system appears to be selectively vulnerable in AD, other regions of the cortex (e.g. posterior cingulate, precuneus, temporo-parietal, and medial frontal) also experience amyloid deposition, gliosis, and atrophy during the long preclinical phase (Fig. 2)27. Accordingly, these cortical regions also show abnormalities in MCI and mild AD by fMRI 26 and SPECT (single photon emission computed tomography, which measures regional blood flow) that can be used to predict progression from MCI to AD28.
As might be expected for brain regions that display atrophy, neuronal loss, and reduced perfusion, these cortical regions also show evidence of reduced glucose metabolism in AD, as measured by fluoro-deoxyglucose (FDG)-PET (Fig. 2b,c)27. Additionally, although its accuracy has not been thoroughly compared to other biomarkers assessed in the same subjects, FDG-PET has been reported to predict conversion from cognitive normalcy to MCI 29 and from MCI to AD30. Additionally, FDG-PET has been used to demonstrate the effects of pharmacological agents, correlated with level of cognitive impairment31. Therefore, these imaging measures of synaptic activity (fMRI), perfusion (SPECT), and glucose metabolism (FDG-PET) may serve as biomarkers to guide diagnosis, predict and monitor progression, and might be used in the future to evaluate responses to therapy.
The Default Network
Although imaging studies have contributed tremendously to our understanding of AD by comparing AD subjects and age matched controls, valuable insights into AD pathophysiology have also arisen through the study of young, healthy volunteers. In 2001, reflecting on numerous functional imaging studies over the past four decades, Gusnard and Raichle published their hypothesis of a “default” mode of human brain activity32 that is engaged during internally focused tasks such as remembering past events, imagining the future, and considering the perspectives of others (comprehensively reviewed in33). In 2005, Buckner and colleagues demonstrated the remarkable correlation between the neuroanatomic substrates of the default network (MTL and hippocampus, medial prefrontal association cortex, posterior cingulate cortex, retrosplenial cortex, inferior parietal cortex, lateral temporal lobe) and the anatomic distributions of amyloid deposition, atrophy, glucose metabolism, and blood flow in AD (Fig. 2c)27. The authors suggested that young adult activity and metabolism patterns might be conducive to amyloid deposition in AD. Indeed, Aβ production is dependent on synaptic activity34, 35, and those regions of the default network that show high resting metabolism are also those that are most affected in AD. It seems clear that any substantive insight into the etiological connection between default network metabolism and AD pathology will contribute seminally to our understanding of AD.
Aβ and tau as AD Biomarkers
Short of brain biopsy or directly placing a microdialysis catheter into the brain36, CSF and plasma represent the most direct and convenient means to study the biochemical changes occurring in the CNS. Therefore, these fluids are the most attractive resources for ongoing AD biomarker research. Implicated by biochemical and immunohistological studies of AD brain tissue, the major protein constituents of the pathology of AD (Aβ40, Aβ42, tau, and phosphorylated forms of tau [p-tau]) have emerged as the current leading diagnostic and prognostic fluid biomarkers.
Aβ is a secreted peptide of unknown physiological function that is cleaved from the amyloid precursor protein (APP) by the sequential activities of beta- and gamma-secretase enzymes. The majority of Aβ is produced in the brain, but it effluxes into the CSF and plasma, appearing at relatively high and low levels, respectively. Aβ occurs in multiple forms ranging from 38 to 43 amino acids in length. Among these, Aβ40 is the most abundant species, but Aβ42 appears to be essential for initiating Aβ aggregation, and is considered central to the amyloid cascade hypothesis of AD11. Likely reflecting their roles in the pathogenesis of AD, Aβ42 has emerged as a more useful biomarker for AD than its shorter counterpart, Aβ40.
Although the finding is initially counter-intuitive, mean CSF Aβ42 is significantly reduced in AD subjects relative to age-matched controls (Fig. 3b)37; this phenomenon is thought to result from deposition of the peptide in plaques, preventing its transit from the brain into the CSF (the ‘amyloid sink’ hypothesis). In support of this hypothesis, comparisons of antemortem CSF Aβ42 levels with PET PIB scan results or postmortem measurements of brain Aβ load show that virtually all individuals with fibrillar Aβ deposits have low concentrations of CSF Aβ42, independent of cognitive status (Fig. 2a) 20, 38, 39. Thus, CSF Aβ42 may serve as both a diagnostic biomarker for AD and a surrogate biomarker for amyloid deposition.
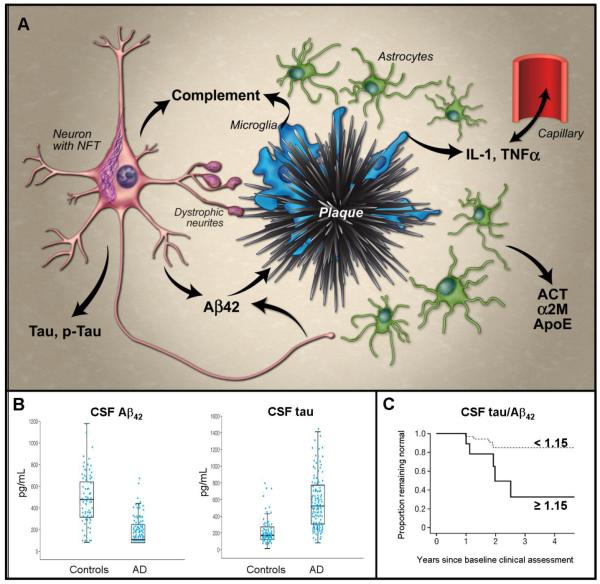
Figure 3. Fluid biomarkers.
A, Abridged schematic histological representation of fluid biomarkers in relation to AD pathology. Produced by neurons, Aβ42 becomes deposited in plaques, which activate microglia. Microglia release cytokines (e.g. IL-1β, TNFα) that appear to cross the blood brain barrier and also activate astrocytes, inducing production of α-1-antichymotrypsin (ACT) and α-2-macroglobulin (α2M). Microglia and neurons also produce complement factors that can be activated by Aβ aggregates, and cause synapse loss. Tau becomes hyperphosphorylated and aggregates into neurofibrillary tangles (NFT) in neurons and dystrophic neurites around plaques; its mechanism of release from neurons is uncertain. Lipid peroxidation in neurons leads to isoprostane formation (not shown). Most factors entering the extracellular space migrate into the CSF; Aβ42 preferentially partitions into plaques. B, Representative data demonstrating utility of CSF levels of Aβ42 (left) and tau (right) to distinguish groups of AD subjects and age-matched controls – boxes represent 25th, 50th and 75th percentiles of the data; length of box is interquartile range; lower and upper whiskers represent the 25th and, respectively, 75th percentiles plus or minus 1.5 times the interquartile range. (Reproduced, with permission, from Sunderland, T., et al., Decreased beta-amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA. 289,2094-2103 (2003)). C, Ratio of CSF levels of tau and Aβ42 as predictors of conversion from cognitive normalcy to MCI/dementia using Kaplan-Meier estimates of rates of conversion with a cut-off value of 1.15 (representing the top 15% of distribution values). More than 80% of subjects with low tau and high Aβ42 (tau/ Aβ42 < 1.15; dashed line) remain cognitively normal four years after baseline assessment; among those with high tau and low Aβ42 (tau/ Aβ42 ≥ 1.15; solid line), approximately 30% remain cognitively normal. (Reproduced, with permission, from ref. 38).
The utility of CSF Aβ42 may not end with diagnosis and surrogacy, however. In several longitudinal studies, especially when combined with CSF tau or p-tau, CSF Aβ42 has exhibited a capacity to predict progression from cognitive normalcy or MCI to MCI/DAT(Fig. 3c)40-42. Low CSF Aβ42 may also serve as a harbinger of amyloid deposition itself; recently, we have identified a unique class of individuals who demonstrate low CSF Aβ42 but show no evidence of amyloid on PET PIB scans (Fig. 2a)38. One of these individuals recently came to autopsy and demonstrated widespread diffuse – but minimal fibrillar– amyloid plaque deposits (N. Cairns, A. Fagan, and D. Holtzman, unpublished observations). Thus, CSF Aβ42 may have utility as a biomarker for diagnosis, plaque burden, and prognosis, and indeed, may provide the very earliest clue to identify preclinical AD (as defined by the emergence of Aβ deposition).
Plasma Aβ42’s utility as a biomarker for AD is less compelling. Indeed, levels of Aβ42 in CSF and plasma show no apparent correlation 38, 43, 44. Some studies suggest that the ratio of plasma Aβ40/Aβ42 are somewhat predictive of conversion from cognitively normal to MCI and AD 45, 46. Nevertheless, the plasma Aβ markers do not appear to approach the predictive value of CSF Aβ42.
Tau is a cytosolic protein predominantly expressed in neurons, wherein its primary function appears to be regulation of microtubule stability within the axon. This function is regulated by several different post translational modifications, principally phosphorylation of numerous serine and threonine residues. In AD, hyperphosphorylated tau often fills the dystrophic neurites of neuritic plaques, and is the principle component of the paired helical filaments that constitute NFT (Fig. 3a). The precise forms of tau that appear in the CSF, and the mechanism(s) by which they arrive are not entirely understood, but recent studies47 demonstrate that virtually all domains of the protein are represented, and it is widely assumed (but not proven) that the major sources of tau and phosphorylated tau (p-tau) in AD CSF are neuronal injury/death and neurofibrillary tangles.
Like Aβ42, tau and p-tau have emerged as important CSF biomarkers for AD. Mean tau and p-tau levels are higher in the CSF of AD subjects relative to age-matched controls (Fig. 3b)48. These increases appear in the setting of formed fibrillar amyloid deposits39 (consistent with the amyloid cascade hypothesis), and correlate with both neuritic plaque density and Braak NFT stage39. With Aβ42, tau and p-tau show utility as prognostic biomarkers for “conversion” or progression to dementia from cognitive normalcy or MCI (Fig. 3c)40-42.
Together, these studies suggest that longitudinal measurements of CSF Aβ42 and tau may allow clinicians to monitor and even predict the progress of AD pathology throughout its entire course. Of course, the pathophysiology of AD involves many more processes than Aβ deposition and NFT formation. APP is cleaved by beta and gamma secretase complexes. Once released as monomers, Aβ may form oligomers that are cytotoxic and/or neuromodulatory. To form plaques, Aβ must accumulate. Once Aβ forms oligomers and amyloid deposits, microglial cells become activated and migrate towards plaques as they form49. Astrocytes become reactive, and numerous inflammatory mediators, signaling molecules, oxidative processes, complement cascades, and modulators of protease and protein folding activities are released. Dendrites and axons in the vicinity of plaques become dystrophic as transport processes malfunction. Brain metabolism changes27, and amyloid deposits in vessel walls. In addition to NFTs, neurons show many other changes. Synapses are lost. Neurons die. Each of these changes – and others not mentioned and not yet recognized – may represent a therapeutic target, and may also cause changes in the composition of the CSF and plasma. Recognizing this potential, many research groups continue to search for other fluid biomarkers that may complement or improve the well-established utility of CSF Aβ42 and tau.
Oxidative Stress and Inflammation
Increasing evidence implicates oxidative damage as a mediator of toxicity in AD. By-products of lipid peroxidation (isoprostanes) and RNA oxidation (8-hydroxy-guanine) have shown the most promise as biomarkers. Produced by free-radical mediated peroxidation of polyunsaturated fatty acids, isoprostanes in CSF are increased in AD50, 51, and may predict development of MCI and AD52. The utility of plasma isoprostanes as biomarkers for AD is less promising; some53 but not all studies54 have found elevated levels in AD versus controls. One other oxidative marker, 8-hydroxy-guanine, representing oxidized RNA, has been reported to be elevated in AD CSF in one small study55 and is worthy of further research.
Inflammation, represented by plaque-associated microglia and astrocytes, is also implicated in AD pathology. In addition to any immediate role these inflammatory cells may play, their secreted products – including acute phase proteins such as α-1-antichymotrypsin (ACT)56 and α-2-macroglobulin (α2M)57,activated factors of the classical pathway of complement58, and cytokines such as IL-1β and TNF-α – persist within plaques. Although the contribution of these deposited inflammatory mediators to the pathophysiology of AD is not entirely clear, there is evidence to suggest an important role.
Appearing in CSF in proportion to cognitive impairment, ACT has emerged as a potential biomarker for AD 59 though its utility in plasma – and that of α2M – remains unclear59-62. By comparison, fluid biomarker studies of complement factors (C3a and C1q) have been inconclusive 63, 64, as have more traditional fluid biomarker studies of cytokines65.
In contrast, supporting a role for cytokines in AD, several groups have measured their spontaneous production by mononuclear cells obtained from the peripheral blood (PBMCs). This approach is relevant to AD because perivascular macrophages and microglia derived from circulating monocytes may participate in intraparenchymal inflammation in the brain66. In one report, PBMCs isolated from AD subjects produced larger amounts of cytokines than PBMCs from controls67; in another study of over 600 participants, increased PBMC production of either IL-1β or TNF-α was associated with increased hazard ratios for developing AD65.
Other recent work, measuring 120 signaling proteins in plasma, suggests that sporadic AD may be accompanied – and diagnosed at very early stages – by a systemic inflammatory state characterized by a panel of 18 signaling molecules68. If confirmed, this biomarker discovery68 demonstrates the potential power of multiple biomarkers incorporated into a panel to diagnose AD accurately, and also illustrates the value of a less-targeted, ‘unbiased,’ high-throughput approach to biomarker discovery.
Proteomics
Ongoing advances in mass spectrometry and protein handling technology continue to broaden the methodological diversity and increase the sensitivity of proteomic analyses; as many as several thousand proteins can now be measured in a sample of human CSF. Complementing the ‘directed’ fluid biomarker investigations discussed above, several groups have pursued ‘unbiased’ proteomics to discover novel AD biomarkers empirically in CSF61, 69-71. Employing different techniques, these studies have generated multiple lists of candidate biomarker proteins that show individual and collective ability to ‘recognize’ and classify samples appropriately. A comprehensive discussion of these proteomic studies is beyond the scope of this review; however, a few observations are worthy of note.
First, the wide array of proteins that distinguish the AD CSF proteome reflect the pathological changes already known to occur in the AD brain. Therefore, novel candidate biomarkers without known relevance to AD may provide clues into pathophysiological processes that are underappreciated or are not yet recognized. Second, in our experience, post translational modification – in particular, limited proteolysis in vivo – appears to be common among CSF proteins showing differential abundance in AD. Yet, when proteins are identified by mass spectrometric evaluation of trypsinized fragments, such modifications may be overlooked. This issue has received relatively little attention, despite its critical importance to the design of assays that might be used in validation experiments for these biomarkers. Third, and finally, most proteomics studies reported to date have compared individuals with AD to age-matched non-demented controls (or to those with non-AD dementias), and are therefore designed to identify diagnostic biomarkers. To advance predictive or surrogate biomarker discovery using the power of unbiased proteomics, more studies should compare groups of AD subjects that differ by rate of progression, presence of amyloid and tau deposits, or other relevant characteristics.
CNS protein metabolism and development of novel treatments
As treatments that target the production or clearance of specific molecules involved in the pathophysiology of AD come to clinical trial, the capacity to monitor relevant fluid biomarkers metabolism before, during and after drug administration would be very helpful for verifying treatment effect and optimizing dosage. To this end, a new in vivo technique has been developed to measure the production and clearance rates of CSF proteins in human subjects. In this technique, a stable (non-radioactive) isotope–labeled amino acid (e.g. 13C6-leucine) is administered intravenously, and becomes incorporated into newly synthesized proteins. CSF and plasma are sampled via intrathecal and intravenous catheters. Using mass spectrometry (LC-MS/MS) to compare labeled vs. unlabeled protein over time and/or at different doses of a candidate therapeutic agent, very precise synthesis, clearance, and dose-response curves can be developed. This technique was first applied to determine the synthesis and clearance rates of Aβ in the CNS72, and was used more recently in a randomized, double-blind, placebo-controlled study to demonstrate the pharmacokinetic/pharmodynamic relationship between an Aβ synthesis inhibitor and the absolute rate of CNS Aβ synthesis73. The potential of this technique is that it automatically labels all newly synthesized proteins when utilizing the appropriate precursor; as such, it should allow for evaluation of other proteins relevant to AD (and other neurodegenerative diseases) and the metabolism of multiple biomarkers simultaneously.
Future Directions
Advances in technology, knowledge and perspective now promise the tools to diagnose, monitor, and predict the course of sporadic AD, even before clinical symptoms begin. Equally important: parallel advances promise treatments for AD that will make such information far more valuable. Nevertheless, achieving these goals will require a great deal more work. More effective radiographic biomarkers are needed to monitor CNS inflammation and tau pathology, as well as other neurodegenerative features. Likewise, fluid biomarkers that can distinguish AD from other dementias and can provide even better prognostic information are needed. Once identified, biomarker assays must be standardized in forms that are amenable for use in existing clinical laboratories, and evaluated in suitably large sample sets. To facilitate such biomarker validation studies and provide sufficient statistical power, longitudinally-followed cohorts of study participants (including individuals with non-AD dementias), complete with uniformly collected and stored specimen collections, must continue to grow in size and number; a successful example of this is the AD neuroimaging initiative (ADNI). Finally, once this work has identified a satisfactory panel of antecedent AD biomarkers, and one or more effective treatments for AD has been approved, appropriate clinical guidelines must be developed to support and encourage widespread clinical testing.
In the more distant future, we may be able to evaluate risk for AD even earlier in life. Just as biomarker data from well-characterized, longitudinally-followed cohorts of study participants may be interpreted to guide diagnosis, estimate prognosis, and monitor response to treatments, they will also be used to identify genetic markers that are associated with particular biomarker values. In comparison with genetic studies of AD that rely on less precise diagnoses that are clinically based, genetic studies based on quantitative endophenotype data can provide greater statistical power74. Indeed, quantitative biomarker data has already been used with success in genetic studies of AD. Elevated CSF tau and p-tau levels have been found to associate with single nucleotide polymorphisms (SNPs) in the MAPT gene (from which tau protein is produced). Likewise, CSF Aβ levels have been found to associate with polymorphisms in several genes74. In this way, by “converting” endophenotype data derived from fluid and imaging biomarkers to novel genetic biomarkers, it may be possible to identify individuals at greater risk of developing AD and, in the near future, provide treatment options even before a single plaque has formed.
Acknowlegements
We thank Dr. John Cirrito and MedPic, the art and design center at Washington University School of Medicine for assistance with graphic design. This work was supported by NIH grants P01-AG026276; PO1-AG03991, P50AG00568125, and T32NS007205.
References
- 1.Alzheimer A. (Translated by Jarvik, L.a.G., H.). ber eine eignartige Erkrankung der Hirnrinde. (About a peculiar disease of the cerebral cortex.) Alzheimer Dis. Assoc. Disorders. 1987;1:7–8. [PubMed] [Google Scholar]
- 2.Khachaturian ZS. Diagnosis of Alzheimer’s disease. Arch Neurol. 1985;42:1097–105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 3.Mirra SS, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 4.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–7. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Crystal H, et al. Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology. 1988;38:1682–7. doi: 10.1212/wnl.38.11.1682. [DOI] [PubMed] [Google Scholar]
- 6.Morris J, Price J. Pathologic correlates of nondemented aging, mild cognitive impairment, and early stage Alzheimer’s disease. J Mol Neurosci. 2001;17:101–118. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- 7.Price JL, et al. Neuropathology of nondemented aging: Presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 9.Evans D, et al. Prevalence of Alzheimer’s disease in a community population of older persons. Higher than previously reported. JAMA. 1989;262:2551–2556. [PubMed] [Google Scholar]
- 10.Gomez-Isla T, et al. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardy JS, D J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–6. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 12.Morris JC. The Clinical Dementia Rating (CDR). Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 13.Petersen R, et al. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 14.Fox N, Warrington E, Rossor M. Serial magnetic resonance imaging of cerebral atrophy in preclinical Alzheimer’s disease. Lancet. 1999;353:2125. doi: 10.1016/S0140-6736(99)00496-1.Five of 28 cognitively normal individuals at risk of autosomal-dominant early-onset AD (ADEO-AD) developed “probable AD” within ~4 years of baseline measurement, and could be distinguished from others at risk for ADEO-AD and from controls by a mean rate of global cerebral atrophy of 1.5% per year compared to 0.1% and 0.2% per year, respectively.
- 15.Carlson NE, et al. Trajectories of brain loss in aging and the development of cognitive impairment. Neurology. 2008;70:828–33. doi: 10.1212/01.wnl.0000280577.43413.d9. [DOI] [PubMed] [Google Scholar]
- 16.Devanand DP, et al. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. 2007;68:828–36. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- 17.Small GW, et al. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med. 2006;355:2652–63. doi: 10.1056/NEJMoa054625. [DOI] [PubMed] [Google Scholar]
- 18.Klunk W, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009.This first study of amyloid-imaging PET tracer compound PIB in humans, describes both the retention of PIB in areas of AD brains known to contain amyloid, and an inverse relationship between PIB PET signal and cerebral glucose metabolism, measured by FDG-PET.
- 19.Mintun M, et al. [11C]PIB in a nondemented population: Potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–52. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 20.Fagan A, et al. Inverse relation between in vivo amyloid imaging load and CSF Aβ42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730.This study illustrates an inverse relationship between mean cortical retention of amyloid-imaging PET tracer compound PIB and CSF Aβ42 levels among both demented and non-demented subjects, suggesting that brain amyloid deposition results in low CSF Aβ42 and that amyloid imaging and CSF Aβ42 might serve as antecedent biomarkers of preclinical AD.
- 21.Zhang MR, et al. Development of a new radioligand, N-(5-fluoro-2-phenoxyphenyl)-N-(2-[18F]fluoroethyl-5-methoxybenzyl)acetami de, for pet imaging of peripheral benzodiazepine receptor in primate brain. J Med Chem. 2004;47:2228–35. doi: 10.1021/jm0304919. [DOI] [PubMed] [Google Scholar]
- 22.Vas A, et al. Functional neuroimaging in multiple sclerosis with radiolabelled glia markers: preliminary comparative PET studies with [11C]vinpocetine and [11C]PK11195 in patients. J Neurol Sci. 2008;264:9–17. doi: 10.1016/j.jns.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Edison P, et al. Microglia, amyloid, and cognition in Alzheimer’s disease: An [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol Dis. 2008;32:412–9. doi: 10.1016/j.nbd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura S, et al. Expression of monoamine oxidase B activity in astrocytes of senile plaques. Acta Neuropathol. 1990;80:419–25. doi: 10.1007/BF00307697. [DOI] [PubMed] [Google Scholar]
- 25.Hirvonen J, et al. Assessment of MAO-B occupancy in the brain with PET and [11C]-L-deprenyl-D2: a dose-finding study with a novel MAO-B inhibitor, EVT 301. Clin Pharmacol Ther. 2009;85:506–12. doi: 10.1038/clpt.2008.241. [DOI] [PubMed] [Google Scholar]
- 26.Dickerson BC, Sperling RA. Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer’s disease: Insights from functional MRI studies. Neuropsychologia. 2008;46:1624–1635. doi: 10.1016/j.neuropsychologia.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckner R, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: Evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005.Using five in vivo neuroimaging methods, this groundbreaking study illustrates the similarities among the anatomical distributions of atrophy, reduced glucose metabolism, and amyloid deposits seen in AD, and of the ‘default network’, suggesting that young adult brain activity and metabolism patterns may be conducive to cortical amyloid deposition.
- 28.Hirao K, et al. The prediction of rapid conversion to Alzheimer’s disease in mild cognitive impairment using regional cerebral blood flow SPECT. Neuroimage. 2005;28:1014–21. doi: 10.1016/j.neuroimage.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 29.de Leon MJ, et al. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET) Proc Natl Acad Sci U S A. 2001;98:10966–71. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chetelat G, et al. Mild Cognitive Impairment - Can FDG-PET Predict Who is to Rapidly Convert to Alzheimer’s Disease. Neurology. 2003;60:1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 31.Kadir A, A N, Almkvist O, Wall A, Forsberg A, Engler H, Hagman G, Lärksäter M, Winblad B, Zetterberg H, Blennow K, Långström B, Nordberg A. Effect of phenserine treatment on brain functional activity and amyloid in Alzheimer’s disease. Annals of Neurology. 2008;63:621–31. doi: 10.1002/ana.21345. [DOI] [PubMed] [Google Scholar]
- 32.Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 33.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 34.Kamenetz F, et al. APP processing and synaptic function. Neuron. 2003;37:925–37. doi: 10.1016/s0896-6273(03)00124-7. [DOI] [PubMed] [Google Scholar]
- 35.Cirrito JR, et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–22. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 36.Brody DL, et al. Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science. 2008;321:1221–4. doi: 10.1126/science.1161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Motter R, et al. Reduction of β-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer’s disease. Ann. Neurol. 1995;38:643–648. doi: 10.1002/ana.410380413. [DOI] [PubMed] [Google Scholar]
- 38.Fagan AM, et al. Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65:176–83. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tapiola T, et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009;66:382–9. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 40.Fagan A, et al. Cerebrospinal fluid tau/Aβ42 ratio as a prediction of cognitive decline in nondemented older adults. Arch. Neurol. 2007;64:343–49. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 41.Hansson O, et al. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–34. doi: 10.1016/S1474-4422(06)70355-6.This is the first report illustrating the utility of CSF biomarkers Aβ42, tau, and phospho-tau in predicting progression of patients from mild cognitive impairment to dementia attributed clinically to Alzheimer’s disease.
- 42.Li G, et al. CSF tau/Aβ42 ratio for increased risk of mild cognitive impairment: A follow-up study. Neurology. 2007;69:631–39. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- 43.Mehta P, Pirttila T, Patrick B, Barshatzky M, Mehta S. Amyloid β protein 1-40 and 1-42 levels in matched cerebrospinal fluid and plasma from patients with Alzheimer disease. Neurosci Lett. 2001;304:102–6. doi: 10.1016/s0304-3940(01)01754-2. [DOI] [PubMed] [Google Scholar]
- 44.Vanderstichele H, et al. Standardization of measurement of beta-amyloid(1-42) in cerebrospinal fluid and plasma. Amyloid. 2000;7:245–58. doi: 10.3109/13506120009146438. [DOI] [PubMed] [Google Scholar]
- 45.Graff-Radford N, et al. Association of low plasma Aβ42/Aβ40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007;64:354–62. doi: 10.1001/archneur.64.3.354. [DOI] [PubMed] [Google Scholar]
- 46.Mayeux R, et al. Plasma amyloid beta-peptide 1-42 and incipient Alzheimer’s disease. Ann Neurol. 1999;46:412–6. doi: 10.1002/1531-8249(199909)46:3<412::aid-ana19>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 47.Portelius E, Zetterberg H, Gobom J, Andreasson U, Blennow K. Targeted proteomics in Alzheimer’s disease: focus on amyloid-beta. Expert Rev Proteomics. 2008;5:225–37. doi: 10.1586/14789450.5.2.225. [DOI] [PubMed] [Google Scholar]
- 48.Vandermeeren M, et al. Detection of tau proteins in normal and Alzheimer’s disease cerebrospinal fluid with a sensitive sandwich enzyme-linked immunosorbant assay. J. Neurochem. 1993;61:1828–1834. doi: 10.1111/j.1471-4159.1993.tb09823.x. [DOI] [PubMed] [Google Scholar]
- 49.Meyer-Luehmann M, S.-J T, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–4. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montine TJ, et al. The magnitude of brain lipid peroxidation correlates with the extent of degeneration but not with density of neuritic plaques or neurofibrillary tangles or with APOE genotype in Alzheimer’s disease patients. Am J Pathol. 1999;155:863–8. doi: 10.1016/S0002-9440(10)65185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pratico D, V MYL, Trojanowski JQ, Rokach J, Fitzgerald GA. Increased F2-isoprostanes in Alzheimer’s disease: evidence for enhanced lipid peroxidation in vivo. FASEB J. 1998;12:1777–83. doi: 10.1096/fasebj.12.15.1777. [DOI] [PubMed] [Google Scholar]
- 52.de Leon M, et al. Longitudinal CSF isoprostane and MRI atrophy in the progression to AD. J Neurol Sci. 2007 Nov 14; doi: 10.1007/s00415-007-0610-z. [DOI] [PubMed] [Google Scholar]
- 53.Pratico D, et al. Increased 8,12-iso-iPF2alpha-VI in Alzheimer’s disease: Correlation of a noninvasive index of lipid peroxidation with disease severity. Ann. Neurol. 2000;48:809–12. [PubMed] [Google Scholar]
- 54.Montine TJ, et al. No difference in plasma or urinary F2-isoprostanes among patients with Huntington’s disease or Alzheimer’s disease and controls. Ann Neurol. 2000;48:950. [PubMed] [Google Scholar]
- 55.Abe T, Tohgi H, Isobe C, Murata T, Sato C. Remarkable increase in the concentration of 8-hydroxyguanosine in cerebrospinal fluid from patients with Alzheimer’s disease. J Neurosci Res. 2002;70:447–50. doi: 10.1002/jnr.10349. [DOI] [PubMed] [Google Scholar]
- 56.Abraham C, Selkoe D, Potter H. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer’s disease. Cell. 1988;52:487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- 57.Thal DR, Schober R, Birkenmeier G. The subunits of alpha2-macroglobulin receptor/low density lipoprotein receptor-related protein, native and transformed alpha2-macroglobulin and interleukin 6 in Alzheimer’s disease. Brain Res. 1997;777:223–7. doi: 10.1016/s0006-8993(97)01021-4. [DOI] [PubMed] [Google Scholar]
- 58.Zanjani H, et al. Complement activation in very early Alzheimer disease. Alzheimer Dis Assoc Disord. 2005;19:55–66. doi: 10.1097/01.wad.0000165506.60370.94. [DOI] [PubMed] [Google Scholar]
- 59.DeKosky S, et al. Plasma and cerebrospinal fluid α1-antichymotrypsin levels in Alzheimer’s disease: Correlation with cognitive impairment. Ann. Neurol. 2003;53:81–90. doi: 10.1002/ana.10414. [DOI] [PubMed] [Google Scholar]
- 60.Hye A, et al. Proteome-based plasma biomarkers for Alzheimer’s disease. Brain. 2006;129:3042–50. doi: 10.1093/brain/awl279. [DOI] [PubMed] [Google Scholar]
- 61.Hu Y, et al. Identification and validation of novel CSF biomarkers for early stages of Alzheimer’s disease. Proteomics - Clin. Appl. 2007;1:1373–1384. doi: 10.1002/prca.200600999. [DOI] [PubMed] [Google Scholar]
- 62.Thambisetty M, et al. Proteome-based identification of plasma proteins associated with hippocampal metabolism in early Alzheimer’s disease. J Neurol. 2008;255:1712–20. doi: 10.1007/s00415-008-0006-8. [DOI] [PubMed] [Google Scholar]
- 63.Smyth MD, et al. Decreased levels of C1q in cerebrospinal fluid of living Alzheimer patients correlate with disease state. Neurobiol Aging. 1994;15:609–14. doi: 10.1016/0197-4580(94)00055-7. [DOI] [PubMed] [Google Scholar]
- 64.Loeffler DA, et al. Cerebrospinal fluid C3a increases with age, but does not increase further in Alzheimer’s disease. Neurobiol Aging. 1997;18:555–7. doi: 10.1016/s0197-4580(97)00110-3. [DOI] [PubMed] [Google Scholar]
- 65.Tan ZS, et al. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology. 2007;68:1902–8. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 66.Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 67.Guerreiro RJ, et al. Peripheral inflammatory cytokines as biomarkers in Alzheimer’s disease and mild cognitive impairment. Neurodegener Dis. 2007;4:406–12. doi: 10.1159/000107700. [DOI] [PubMed] [Google Scholar]
- 68.Ray S, et al. Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nat Med. 2007;13:1359–62. doi: 10.1038/nm1653.Illustrating the potential of ‘unbiased’ screening of multiple analytes in biological fluids to identify early diagnostic and prognostic biomarkers for AD, this work identified a panel of 18 signaling proteins in blood plasma that might be used to distinguish individuals with early AD from those with other causes of dementia, and to predict progression from MCI to dementia attributed to AD.
- 69.Davidsson P, et al. Proteome analysis of cerebrospinal fluid proteins in Alzheimer patients. NeuroReport. 2002;13:611–615. doi: 10.1097/00001756-200204160-00015. [DOI] [PubMed] [Google Scholar]
- 70.Zhang J, et al. Quantitative proteomics of cerebrospinal fluid from patients with Alzheimer disease. J Alzheimers Dis. 2005;7:125–33. doi: 10.3233/jad-2005-7205. discussion 173-80. [DOI] [PubMed] [Google Scholar]
- 71.Finehout EJ, Franck Z, Choe LH, Relkin N, Lee KH. Cerebrospinal fluid proteomic biomarkers for Alzheimer’s disease. Ann Neurol. 2007;61:120–9. doi: 10.1002/ana.21038. [DOI] [PubMed] [Google Scholar]
- 72.Bateman RJ, et al. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med. 2006;12:856–61. doi: 10.1038/nm1438.Using Aβ peptide species as an example, this report describes a novel technique that will greatly facilitate the evaluation of disease-modifying treatments for AD by measuring the production and clearance rates of proteins in the human central nervous system, following administration of a non-radioactive isotopically-labeled amino acid (in this case, leucine).
- 73.Bateman RJ, et al. A gamma-secretase inhibitor decreases amyloid-beta production in the central nervous system. Ann Neurol. 2009 doi: 10.1002/ana.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kauwe JS, et al. Alzheimer’s disease risk variants show association with cerebrospinal fluid amyloid beta. Neurogenetics. 2009;10:13–7. doi: 10.1007/s10048-008-0150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Welsh-Bohmer KA, J S, G J. In: Handbook of Dementing Illnesses. Second Edition Morris JC, Holtzman DM, editors. Taylor & Francis; New York: 2006. [Google Scholar]