Abstract
The basic helix-loop-helix (bHLH) gene Hes6 is known to promote neural differentiation in vitro. Here, we report the expression and functional studies of Hes6 in the inner ear. The expression of Hes6 appears to be parallel to that of Math1 (also known as Atoh1), a bHLH gene necessary and sufficient for hair cell differentiation. Hes6 is expressed initially in the presumptive hair cell precursors in the cochlea. Subsequently, the expression of Hes6 is restricted to morphologically differentiated hair cells. Similarly, the expression of Hes6 in the vestibule is in the hair cell lineage. Hes6 is dispensable for hair cell differentiation, and its expression in inner ear hair cells is abolished in the Math1-null animals. Furthermore, the introduction of Hes6 into the cochlea in vitro is not sufficient to promote sensory or neuronal differentiation. Therefore, Hes6 is downstream of Math1 and its expression in the inner ear delineates the sensory lineage. However, the role of Hes6 in the inner ear remains elusive.
Keywords: bHLH, cochlea, hair cells, Hes1, Hes6, Math1, organ of Corti
Introduction
The mammalian auditory sensory organ, the organ of Corti, consists of stereotypically arranged one row of inner and three rows of outer sensory hair cells. The hair cells in the organ of Corti are separated from each other by several types of non-sensory supporting cells, forming a cellular mosaic of alternating hair cells and supporting cells. During development, both the hair cells and supporting cells of the organ of Corti are derived from a precursor domain recognized as a zone of non-proliferating cells around embryonic day 13.5 (E13.5) to E14.5 in mice (Ruben, 1967; Chen and Segil, 1999). This precursor domain is distinctively marked by the expression of p27Kip1 (Chen and Segil, 1999), Islet1 (Radde-Gallwitz et al., 2004), and Sox2 (Kiernan et al., 2005a). Subsequently, within the zone of non-proliferating cells, a wave of hair cell differentiation initiates with the inner hair cells near the base of the cochlear duct (Sher, 1971; Chen and Segil, 1999). The differentiation propagates bi-directionally along the longitudinal axis of the cochlear duct to the apical and the basal-most regions of the cochlea, and from the inner to the outer hair cell regions. In parallel to hair cell differentiation, supporting cells are differentiated during the same period. By E18.5, the characteristic one row of inner and three rows of outer hair cells can be observed along the length of the cochlear duct, except at the extremities of the cochlea. The sensory epithelia in the vestibule of the inner ear share a similar cellular arrangement of alternating hair cells and supporting cells. However, the birth and the differentiation of sensory hair cells in the vestibule progress in a longer period from E11.5 to postnatal day 3 (P3) (Ruben, 1967).
The molecular mechanisms underlying the development of the sensory lineage and the patterning of the organ of Corti have been studied extensively (Adam et al., 1998; Bermingham et al., 1999; Bryant et al., 2002; Chen et al., 2002; Daudet and Lewis, 2005; Eddison et al., 2000; Fekete and Wu, 2002; Haddon et al., 1998; Kiernan et al., 2005a; Kiernan et al., 2005b; Lanford et al., 1999; Lanford et al., 2000; Lewis et al., 1998; Lewis, 1998; Matei et al., 2005; Morrison et al., 1999; Radde-Gallwitz et al., 2004; Riley et al., 1999; Tsai et al., 2001; Woods et al., 2004; Zhang et al., 2000; Zheng et al., 2000; Zine et al., 2000; Zine et al., 2001). In particular, two classes of basic helix-loop-helix (bHLH)-containing factors, namely the positively and negatively acting transcription factors, have been shown to play essential roles in the differentiation of sensory hair cells and the patterning of the sensory mosaic in the organ of Corti (Bermingham et al., 1999; Chen et al., 2002; Woods et al., 2004; Zheng et al., 2000; Zine et al., 2000; Zine et al., 2001). Math1, a mouse homolog of the Drosophila proneural basic helix-loop-helix (bHLH) gene Atonal, also known as Atoh1 (Atonal homolog 1), is upregulated within the sensory precursor domain of the cochlea around E13.5–E14.5 (Chen et al., 2002; Woods et al., 2004; Kiernan et al., 2005a; Matei et al., 2005). The onset of Math1 expression in the cochlea leads the differentiation gradient of hair cells (Chen et al., 2002). Although the precursor domain of the organ of Corti appears to still be specified in the absence of Math1 (Chen et al., 2002; Kiernan et al., 2005a; Matei et al., 2005; Woods et al., 2004), none of the examined hair cell markers is expressed, and Math1 is required for the differentiation of hair cells and the survival of the sensory lineage (Chen et al., 2002; Woods et al., 2004). In addition, introduction of Math1 to the cochlear epithelium leads to the expression of markers specific for hair cells and morphological differentiation of ectopic hair cells (Zheng and Gao, 2000; Shou et al., 2003; Kawamoto et al., 2003; Izumikawa et al., 2005; Woods et al., 2004). These data together indicated that Math1 is necessary and sufficient to activate downstream targets for hair cell differentiation.
bHLH Hes genes are homologous to the Drosophila enhancer of split (Hes) characterized with uniquely conserved amino acid residues in the basic domain and the carboxy-terminal WRPW sequence. In contrast to positive-acting bHLH genes such as Math1, Hes genes are transcriptional repressors (Akazawa et al., 1995; Chen et al., 1997; Ishibashi et al., 1995; Jarriault et al., 1995; Kageyama et al., 2000; Ohtsuka et al., 1999; Sasai et al., 1992; Thomas and Rathjen, 1992). They are capable of repressing directly the transcription of positively acting bHLH genes, and inhibiting the positively acting bHLH heterodimers from binding to their DNA targets (Akazawa et al., 1995; Cau et al., 2000; Tomita et al., 1996; Ohtsuka et al., 1999; Ishibashi et al., 1995; Hatakeyama et al., 2004; Hu et al., 2004). Therefore, Hes genes commonly exhibit an anti-neurogenic effect, and are therefore considered to be negatively acting bHLH genes. In the inner ear, the expression of two members of the Hes family of genes, Hes1 and Hes5, are excluded from hair cells (Zheng et al., 2000a; Zine et al., 2001). Hes1 and Hes5 are expressed in regions adjacent to the organ of Corti and in the supporting cells, respectively (Zheng et al., 2000a; Zine et al., 2001). Mouse mutants of Hes1 and Hes5 show increased inner and outer hair cells (Zheng et al., 2000a; Zine et al., 2001). In the organ cultures, Hes1 can directly antagonize hair cell differentiation induced by Math1 (Zheng et al., 2000). Together, these data suggest that Hes1 and Hes5 play an anti-neurogenic role for hair cell differentiation. It is hypothesized that Hes1 and Hes5 act downstream of activated Notch pathway (Chen et al., 1997; Jarriault et al., 1995; Lewis, 1998; Ohtsuka et al., 1999; Zheng et al., 2000a; Zine et al., 2001) to prevent the cells surrounding the hair cells from differentiating into the same cell type (Adam et al., 1998; Eddison et al., 2000; Haddon et al., 1998; Kiernan et al., 2001; Kiernan et al., 2005b; Lanford et al., 1999; Lewis et al., 1998; Morrison et al., 1999; Riley et al., 1999; Tsai et al., 2001; Zhang et al., 2000; Zine et al., 2000) and to restrict the boundary of the sensory epithelium (Daudet and Lewis, 2005).
Hes6 is a bHLH protein exhibiting structural homology to other Hes genes, including the uniquely conserved amino acid residue in the basic domain and the carboxy-terminal WRPW sequence (Pissarra et al., 2000; Vasiliauskas and Stern, 2000). In contrast to other known Hes genes, however, Hes6 has been shown to be expressed in both precursor and differentiated neuronal cells (Bae et al., 2000; Fior and Henrique, 2005; Gratton et al., 2003; Koyabi-Nakagawa et al., 2000; Pissarra et al., 2000; Vasiliauskas and Stern, 2000). Functional studies further demonstrated that Hes6 promotes neuronal and myogenic differentiation in vitro by antagonizing the action of Hes1 (Bae et al., 2000; Cossins et al., 2002; Fior and Henrique, 2005; Gao et al., 2001; Gratton et al., 2003; Koyabi-Nakagawa et al., 2000). Here, we isolated Hes6 from the cochlear tissue by both differential gene expression and candidate gene approaches. We found that its expression is similar to that of Math1 in presumptive hair cell precursors and in differentiated hair cells in both the cochlea and the vestibule of the inner ear. However, the examination of Hes6 null animals did not reveal any detectable defect in the inner ear, which is similar to observations in other tissues (Koyabi-Nakagawa et al., 2000). In contrast to Math1, introduction of Hes6 into the cochlear epithelium is not sufficient for hair cell differentiation. Therefore, Hes6 is specifically expressed in the sensory lineage of the inner ear, and its function in the inner ear remains undetermined.
Results
Hes6 expression marks the sensory lineage in the vestibule
We detected the presence of Hes6 in the differentiating cochlea using RNA isolated from cochlear epithelia at several developmental stages (Radde-Gallwitz et al., 2004). To determine the expression of Hes6 in the inner ear, we amplified Hes6 cDNA from the inner ear tissue and performed in situ hybridization.
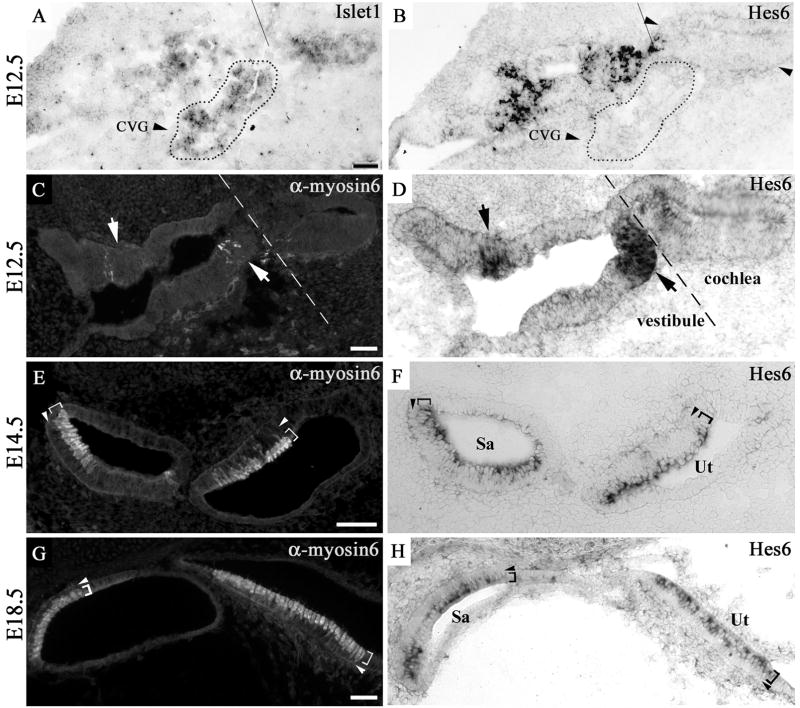
The earliest expression of Hes6 was observed in the vestibule of the inner ear at E12.5 (Fig. 1). At this stage, a transcriptional factor Islet1 is expressed in the entire floor of the cochlear duct and in the cochleovestibular neurons (Fig. 1A, marked by dotted outline), in addition to its expression in the vestibular epithelium (Fig. 1A) (Radde-Gallwitz et al., 2004). However, Hes6 expression is absent or below the detection limit in the cochleovestibular neurons (Fig. 1B, marked by dotted outline) and has yet to begin in the cochlear epithelium (Fig. 1B, marked by arrowheads). The sensory organs in the vestibule undergo differentiation prior to the organ of Corti in the cochlea (Bryant et al., 2002). At E12.5, the developing sensory epithelia in the vestibule can be molecularly recognized by the expression of a hair cell-specific marker myosin 6 in the differentiating hair cells (Fig. 1C, indicated by arrows). High levels of Hes6 mRNA expression were detected in the same region where myosin6 is expressed (Fig. 1D). By E14.5, the sensory epithelia of the vestibule have undergone substantial differentiation. The cells in the luminal layer of the epithelia express myosin6 (Fig. 1E, cytoplasmic localization) and show the typical hair cell morphology of a cylindrical shape and basal location of nuclei (Fig. 1E, indicated by brackets). In adjacent sections probed for Hes6 mRNA (Fig. 1F), Hes6 mRNA was detected exclusively in the luminal hair cell layer in the vestibular sensory epithelia at this stage (Fig. 1F, indicated by brackets). By E18.5, the expression of Hes6 is maintained in the hair cell layer in the vestibular sensory organs (Fig. 1G–H, indicated by brackets).
Fig. 1.
Hes6 expression in the vestibular sensory organs. The adjacent inner ear sections from an E12.5 embryo (A–B and C–D), and the adjacent vestibular sections from E14.5 (E–F) and E18.5 (G–H) embryos were probed for Islet1 message (A) or Hes6 message (B, D, F, H) or stained for myosin6 (C, E, G). Islet1 is expressed in the floor of the cochlear duct and in the cochleovestibular ganglion (A, marked by dotted outline), while Hes6 message is not detected in the corresponding regions (B). The sensory regions of the vestibule at E12.5 were recognized by the expression of the hair cell-specific marker myosin6 (C, indicated by arrows). The expression of Hes6 is detectable in the otocyst by E12.5 in the same region in an adjacent section (D, indicated by arrows). The vestibular and cochlear regions of the inner ear at E12.5 are indicated, and the dashed line approximates where the two regions meet (A–D). By E14.5, the expression of myosin6 (E) and Hes6 (F) is limited to the luminal hair cell layer (E–F, indicated by brackets) of the vestibular sensory organs. The expression of Hes6 in the hair cells in the vestibule persists at E18.5 (G–H). The arrowheads (E–H) mark the supporting cell layer underneath the luminal hair cell layer. CVG: Cochleovestibular ganglion. Sa: Saccule. Ut: Utricle. Scale bars: 50 μm.
Hes6 expression delineates the morphogenesis of the hair cell lineage in the cochlea
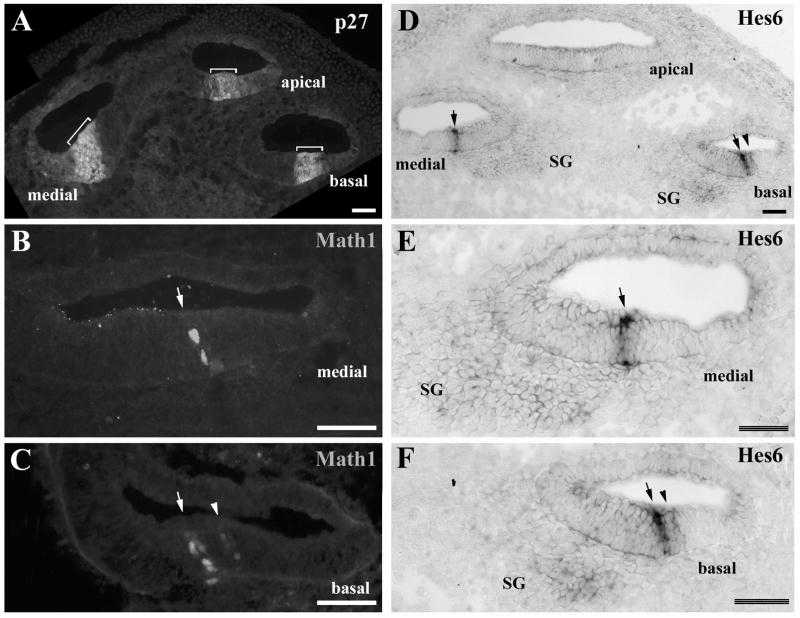
In the cochlea, the expression of Hes6 was also observed in the sensory lineage (Fig. 2 and 3). At E14.5, the precursor domain of the organ of Corti is marked by the expression of a cyclin-dependent kinase inhibitor, p27Kip1 (Cdkn1b) (Chen and Segil, 1999) (Fig. 2A), Sox2 (Kiernan et al., 2005a), and Islet1 (Radde-Gallwitz et al., 2004). We and others have observed the onset of Math1 in columns of cells spanning the depth of the cochlear epithelium within the primordial organ of Corti (Chen et al., 2002; Kiernan et al., 2005a; Wang et al., 2005) (Fig. 2B–C), and we have hypothesized that these columns of cells are the early hair cell precursors in a multi-cell layered primordial organ of Corti at E14.5. Subsequently, cellular intercalation movements, characteristic of convergent extension (Keller, 2002), lead to the extension of the organ of Corti and the formation of a single hair cell layer during terminal differentiation (Chen et al., 2002; Montcouquiol et al., 2003; McKenzie et al., 2004; Wang et al., 2005). At the basal region of the cochlea, the presumptive hair cell precursors marked by the expression of Math1 were seen as two columns of cells, presumably at the inner hair cell and the first row of outer hair cell locations (Fig. 2C, indicated by arrowheads). Toward the medial region, Math1 was only detected in a single column of cells at the inner hair cell region (Fig. 2B). No Math1 expression was detected toward the apical region in the same cochlea (data not shown) (Chen et al., 2002). Strikingly, the expression of Hes6 in adjacent sections at E14.5 exhibited a similar pattern (Fig. 2D–F). At the base of the cochlea, Hes6 was seen in two columns of cells spanning the depth of the cochlear epithelium (Fig. 2D, 2F, indicated by an arrow and an arrowhead). Its expression was limited to a single column of cells toward the medial region (Fig. 2D, 2E, indicated by an arrow), and was not detectable at the apical region (Fig. 2D). Careful comparison of the expression of Math1 and Hes6 suggested that Hes6 is expressed in Math1-expressing presumptive hair cell precursors within the primordial organ of Corti at E14.5 (Fig. 2 and Fig. 3). The gradient of Hes6 and Math1 expression from the basal to the apical region along the longitudinal axis and from the inner to the outer hair cell region mirrored precisely the base-to-apex and inner-to-outer gradient of hair cell differentiation.
Fig. 2.
Expression of Hes6 in the presumptive hair cell precursors. In the cochlea, the primordial organ of Corti at E14.5 is marked by the expression of p27Kip1 (A, indicated by brackets). Adjacent sections were stained for an antibody against Math1 (B–C) or probed with a Hes6 antisense probe (D–F). The basal, medial, and apical regions of the cochlea are indicated. The arrows and arrowheads indicate the expression of Math1 and Hes6 in the presumptive inner and outer hair cell regions, respectively (B–F). SG: spiral ganglion. Scale bars: 50 μm.
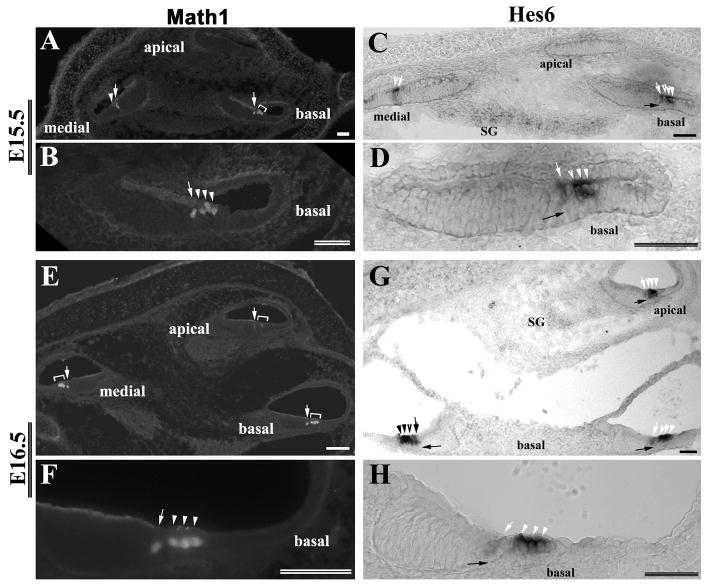
Fig. 3.
The expression of Hes6 marks the differentiation gradient of hair cells. Adjacent cochlear sections from E15.5 (A–D) and E16.5 (E–H) embryos were stained for an antibody against dsRED expressed under the control of Math1 locus (A–B, E–F) or probed for a Hes6 antisense probe (C–D, G–H). The white arrows indicate the inner hair cells. The arrowheads or brackets indicate the outer hair cells. The black arrows indicate the layer of supporting cells underneath the hair cell layer. SG: spiral ganglion. Note that the spiral ganglion (SG) neuron region showed weak signals for Hes6. The signal in the SG neuron region was inconsistent, and was sometimes displayed with sense probes. We do not know whether the signal is specific or not. Scale bars: 50 μm.
Examination of Hes6 expression in the cochlea at later stages confirmed that Hes6 was limited to the hair cell lineage in the cochlea (Fig. 3). By E15.5, cellular intercalation has occurred and the hair cells in the basal region of the cochlea have become morphologically differentiated. The morphogenesis of the hair cell lineage coincides with the expression of Hes6, parallel to that of Math1 (Fig. 3). In the base of the cochlea, the organ of Corti consists of one row of inner and three rows of outer hair cells at the luminal layer of the cochlear epithelium, above the layer of supporting cells (Fig. 3A–B). Toward the medial region that has yet to complete cellular intercalation, Math1-expressing cells were still observed as multi-cell layered columns (Fig. 3A), and no Math1 expression was seen in the apical region (Fig. 3A). Hes6 was expressed in a pattern apparently similar to that of Math1, showing restriction to the inner and outer hair cells in the basal region of the cochlea (Fig. 3C–D). Toward the medial region, Hes6 expression was observed as multi-cell layered columns spanning the depth of cochlear epithelium (Fig. 3C). No Hes6 expression was detected in the apical region. One day later, at E16.5, along the length of the cochlea the organ of Corti was mostly patterned into one row of inner and three rows of outer hair cells (Fig. 3E–F), and Hes6 expression was expanded to the apical region (Fig. 3G). At the base of the cochlea, Hes6 seemed to be down regulated from the earliest differentiated inner hair cells (Fig. 3G–H, indicated by arrows), indicating a tight temporal control of the expression of Hes6 in the hair cell lineage.
Together, our expression study of Hes6 revealed its specific expression in the sensory lineage of the inner ear. The signals for Hes6 mRNA were robust, and thus clearly marked the sensory lineage of the inner ear.
Hes6 expression in sensory hair cells depends on Math1
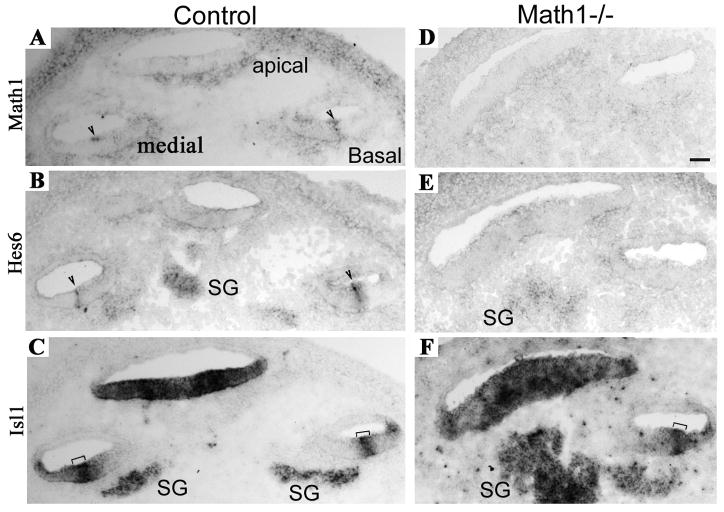
The complete parallel expression of Hes6 to that of Math1 in the earliest detectable cells of the sensory lineage suggested the following two possibilities. First, Hes6 expression might not depend on Math1. Alternatively, Hes6 is an immediate early hair cell gene activated by Math1-initiated hair cell differentiation program. To determine whether Hes6 expression depends on Math1, we examined the expression of Hes6 in Math1 null animals (Bermingham et al., 1999). At E14.5, Hes6 expression was detected in columns of hair cell precursors in wild type littermates (Fig. 4A) as expected (Fig. 4B). In contrast, in Math1 null littermates (Fig. 4D), the expression of Hes6 was abolished (Fig. 4E). Furthermore, examinations of Hes6 in Math1 null embryos at later stages indicated the loss of expression of Hes6 in the absence of Math1 at all the stages examined (data not shown). As we observed previously, the primordial organ of Corti is established in the absence of Math1 (Chen et al., 2002; Kiernan et al., 2005a). Therefore, the loss of Hes6 expression in Math1 null animals was not likely to result from the loss of the primordial organ of Corti. To confirm that the elimination of Hes6 expression in Math1 null animals was not due to the loss of the primordial organ of Corti, we analyzed the expression of a Lim-homeodomain- (LIM-HD) containing transcriptional factor Islet1 (Isl1) in the same wild-type control and Math1 null animals (Fig. 4C and F). Isl1 is expressed in the otocyst as early as E10.5, and is gradually restricted to the floor of the cochlear duct and to the primordial organ of Corti in the cochlear epithelium (Radde-Gallwitz et al., 2004). Isl1 is also expressed in the neuronal lineage in the inner ear (Radde-Gallwitz et al., 2004). The expression of Isl1 revealed by in situ hybridization in the adjacent E14.5 cochlear sections showed an earlier onset of Isl1 than that of Math1 and Hes6 (Fig. 4C). In the less differentiated apical region of the cochlea where the expression of Math1 and Hes6 had not yet started, Isl1 expression was seen in the entire floor of cochlear duct (Fig. 4C). Toward the basal region, Isl1 appeared more restricted to the primordial organ of Corti (Fig. 4C). This Isl1 expression pattern was maintained in Math1 null animals (Fig. 4F and data not shown).
Fig. 4.
The expression of Hes6 is abolished in the absence of Math1. Adjacent cochlear sections from wild-type control (A–C) and Math1 null embryos (D–F) at E14.5 were probed for antisense probes against Math1 (A, D), Hes6 (B, E), or Islet1 (C, F). Arrows (A–B) indicate the columns of cells expressing Math1 (A) or Hes6 (B). Brackets (C, F) indicate the primordial organ of Corti. SG: spiral ganglion. Scale bar: 50 μm.
To confirm that Hes6 expression, but not Isl1 expression, depends on Math1, we further performed RT-PCR of Hes6, Math1, Islet1, and a house-keeping ribosomal gene L19 with cDNA templates from Math1−/− inner ear tissues (Fig. 5). Our results confirmed that the expression of Hes6 in the cochlear epithelium was abolished in Math1−/− animals (Fig. 5, lane 8), while the expression of Islet1 was maintained in the Math1−/− cochlear epithelium (Fig. 5, lane 8). As controls, we performed parallel RT-PCR assays with cDNA templates from wild type inner ear tissues representing different developmental stages (Fig. 5, Fig. 1–7). Consistent with Math1 (Chen et al., 2002) and Hes6 (Fig. 1) in the vestibule at E12.5, and Islet 1 (Radde-Gallwitz et al., 2004) expression in the cochlea and vestibule at E12.5, messages for the three genes were detected in E12.5 inner ear epithelial tissue (Fig. 5, lane 1). For differential expression of Math1, Hes6, and Islet1 in the differentiating cochlea, we took advantage of the basal-to-apical gradient of hair cell differentiation along the longitudinal axis of the cochlear duct. Cochlear epithelia isolated from E14.5 and E16.5 embryos were divided into the basal, medial, and apical portions to represent the more differentiated to less differentiated stages (Fig. 5, lanes 2–7). The semi-quantitative RT-PCR reproduced the basal to apical higher differentiation expression of Math1 and Hes6 (Fig. 5, lanes 2–7), and the apical to basal higher differential expression of Islet1 (Fig. 5, lanes 2–7).
Fig. 5.
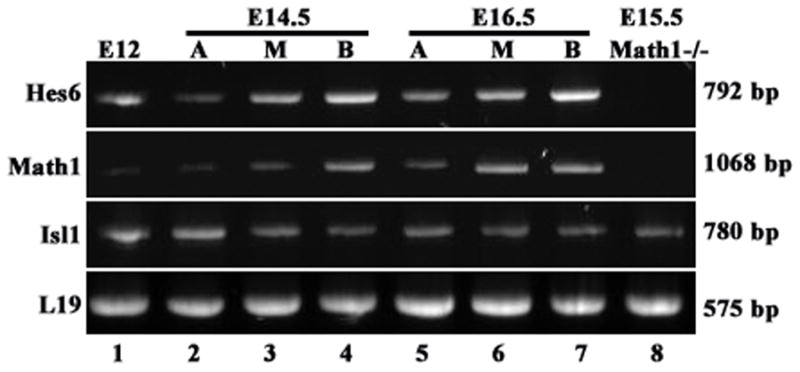
RT-PCR confirmation of Hes6 expression dependent on Math1 in the cochlea. cDNAs from E12.5 epithelial inner ears including both the vestibule and the cochlea (lane1), the apical (A), medial (M), or basal (B) regions of the E14.5 or E16.5 epithelial cochleae were prepared from wild type animals. cDNA was also prepared from pure E15.5 cochlear epithelia isolated from Math1−/− embryos. PCR reactions were carried out using these cDNAs as templates for Hes6, Math1, Islet1 (Isl1), and L19, and run on 1% agarose gel.
Fig. 7.
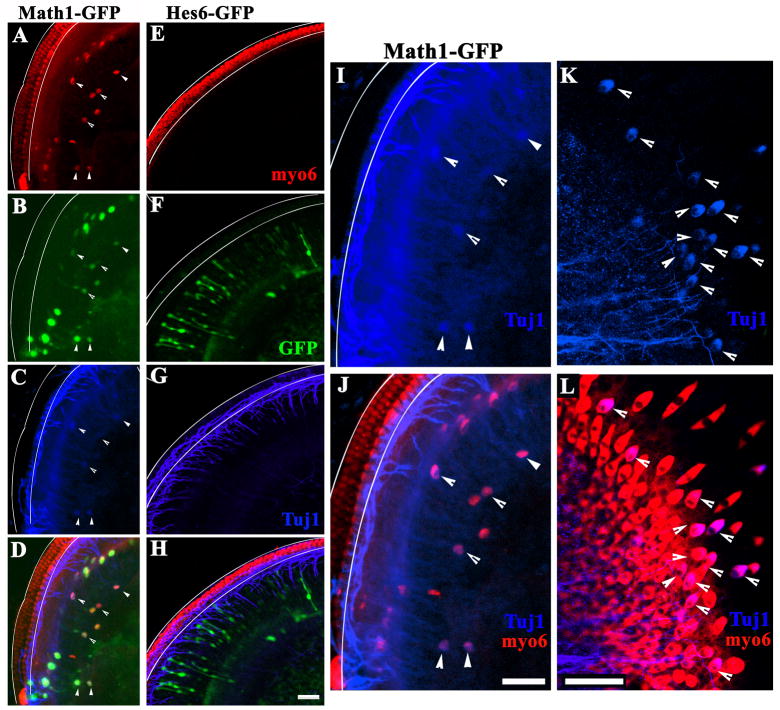
Hes6 is not sufficient to induce sensori- or neuronal marker expression in the cochlear epithelium in vitro. Neonatal rat cochlear epithelia were electroporated with Math1-IRESGFP (A–D) or Hes6-IRESGFP (E–H) expression vectors, and cultured for 6 days in vitro. The cultures were stained for both myosin6 (A, E, myo6), and tubulin β–III (C, G, blue). Transfected cells express GFP (B, F, green) in Math1 (B) or Hes6 (F) transfected cells. The overlays of the myosin6 (red), GFP (green), and tubulin β-III (blue) for Math1-GFP and Hes6-GFP transfected cultures were shown in (D) and (H), respectively. The pair of solid lines (A–J) outline the organ of Corti where endogenous hair cells are stereotypically arrayed. Arrows (A–D) indicate Math1 transfected cells positive for both myosin6 and tubulin β-III. The images in C and D at a higher resolution are shown in I and J, respectively, to better visualize the cells that were positive for both myosin6 (J, red) and tubulin β-III (I–J, blue). Hair cells positive for tubulin β-III were also seen in utricle cultures (K–L, blue). Scale bars (A–H, I–J, K–L): 50 μm.
Together, our data indicate that the onset of Hes6 expression in the hair cell lineage depends specifically on Math1.
Hes6 is not required for the normal development of inner ear
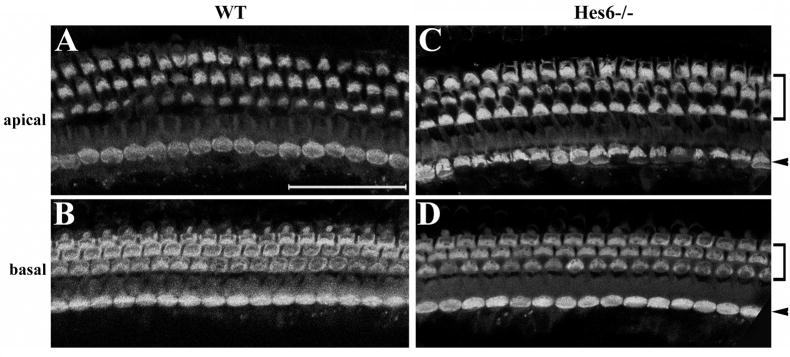
To test whether the expression of Hes6 in the inner ear is required for the normal development, we analyzed Hes6 null animals (Fig. 6). Hes6 null animals were generated by replacement of the three coding exons of the Hes6 locus with LacZ, and the loss of function of Hes6 was confirmed by Southern blot, RT-PCR, and in situ hybridization (Koyabi-Nakagawa et al., 2000, and data not shown). It was reported that no abnormalities were observed in the brain, spinal cord, and muscle tissues where Hes6 is expressed (Koyabi-Nakagawa et al., 2000). We examined the inner ears from adult wild-type control and Hes6 null littermates (Fig. 6). The gross morphology of the inner ears from Hes6 null animals was normal (data not shown). Staining for sensory hair cells with an antibody against myosin6 further revealed the normal appearance (Fig. 6A–B) of one row of inner (Fig. 6C–D, indicated by arrowheads) and three rows of outer hair cells (Fig. 6C–D, brackets) along the entire length of the cochlear duct (Fig. 6C–D). The sensory hair cell differentiation and cellular patterning in the vestibule is also normal as revealed by phalloidin staining of the stereocilia of hair cells (data not shown). Similar to its dispensability in other tissues (Koyabi-Nakagawa et al., 2000), Hes6 is not required for hair cell differentiation.
Fig. 6.
Inner ear sensory organs are normal in Hes6 null animals. The cochleae from wild type control (A–B) and Hes6 null (C–D) littermates at 6 month old were stained for myosin6. Both the apical (A, C) and the basal (B, D) regions are shown. Arrows and brackets indicate the inner and outer hair cells, respectively. The organ of Corti from the Hes6 null animals consists of the normal three rows of outer and one row of inner hair cells. No abnormality was observed in Hes6 null animals. Scale: 50 μm.
Hes6 is not sufficient for hair cell differentiation in cochlear epithelial cells
Although Hes6 was found to be nonessential for the tissues examined (Koyabi-Nakagawa et al., 2000) (Fig. 6), it is capable of promoting expression of neuronal genes in vitro, as well as expanding the myotome in Xenopus embryos (Bae et al., 2000; Cossins et al., 2002; Gao et al., 2001; Gratton et al., 2003; Koyabi-Nakagawa et al., 2000). It was further demonstrated that Hes6 antagonizes and releases the transcriptional repression of Hes1 in both the neuronal and muscle lineages to promote the expression of positively acting bHLH genes, or to promote the action of positively acting bHLH factors (Bae et al., 2000; Cossins et al., 2002; Gao et al., 2001; Gratton et al., 2003; Koyabi-Nakagawa et al., 2000). Interestingly, Hes1 is expressed in the cochlear epithelium adjacent to the organ of Corti (Zheng et al., 2000a; Zine et al., 2001), and the number of hair cells is increased in Hes1 knockout animals (Zheng et al., 2000a; Zine et al., 2001). These studies are consistent with the hypothesis that Hes1 acts as a negatively acting factor for Math1 expression and for hair cell differentiation (Lewis, 1998; Chen et al., 1997; Ishibashi et al., 1995; Jarriault et al., 1995; Zheng et al., 2000a; Zine et al., 2001). Thus, it is possible that Hes6 may antagonize the action of Hes1 for the development of the sensory lineage in the inner ear.
To test the possible antagonist function of Hes6 against Hes1 in the cochlea for hair cell differentiation, we isolated the cochlear epithelia from neonatal rats, and electroporated with Hes6/IRESEGFP-expressing vectors, and control vectors (Fig. 7). As expected (Zheng and Gao, 2000), introduction of Math1-expressing vector to the region adjacent to the inner hair cells in the organ culture resulted in the expression of a hair cell-specific marker myosin6 and the morphological differentiation of transfected cells from a bipolar shape (Zheng and Gao, 2000) to a typical cylindrical hair cell morphology (Fig. 7A–D). This result confirmed that the cells medial to the organ of Corti are competent for hair cell differentiation upon expression of Math1 and that our culture conditions are permissive for hair cell differentiation (Zheng and Gao, 2000; Woods et al., 2004). In contrast, introduction of Hes6 in the same type of cells in the medial region adjacent to the organ of Corti did not lead to the expression of an early hair cell marker myosin6, or the morphological change of the bipolar cells (Fig. 7E–H). Similar results were obtained from vestibular cultures (data not shown). Therefore, Hes6 is not sufficient to activate hair cell differentiation in these cells under our experimental conditions.
In addition, we also performed staining for a neuronal-specific tubulin, tubulin-β III, in the culture to see whether Hes6 is sufficient to activate the expression of neuronal-specific genes as observed in other nervous systems (Fig. 7). The antibody reacted positively with the endogenous neuronal tubulin in cochlear neurons that innervate the sensory hair cells (Fig. 7C, 7G, Tuj1). However, Hes6 transfected cells did not show any signal for the neuronal tubulin staining (Fig. 7G, Tuj1). In conclusion, Hes6 is not sufficient to activate either hair cell differentiation or the expression of neuronal tubulin in cochlear epithelial cells in the culture.
Interestingly, some Math1-transfected cells appeared to co-express myosin6 and tubulin β-III (Fig. 7A–D, 7I–J). Similarly, we observed similar myosin6 and tubulin β-III co-expression in hair cells in vestibular epithelial culture (Fig. 7K–L). This observation suggests that the cochlear and vestibular epithelial cells are competent to express tubulin-β-III.
Discussion
bHLH genes function in cascades for neuronal differentiation. The bHLH gene Math1 stimulates sensory hair cell differentiation, whereas the bHLH genes Hes1 and Hes5 appear to act negatively for sensory hair cell differentiation (Bermingham et al., 1999; Chen et al., 2002; Zheng et al., 2002; Zine et al., 2001). Here, we describe the expression of another bHLH gene, Hes6, in the ear, as well as our analysis of its potential role in inner ear development.
The morphogenesis of the inner ear sensory lineage marked by the expression of Hes6
The entire inner ear is derived from a patch of ectoderm cells, known as the otic placode, near the hindbrain. Once established, around E8.5 in mice, the otic placode invaginates to form the enclosed otocyst by E10.5. By E12.5, the primordia for various structures of the vestibule can be recognized by both morphological landmarks and molecular markers (Bryant et al., 2002; Chen et al., 2002; Lim and Anniko, 1985; Morsli et al., 1998). In particular, the primordial sensory organs of the vestibule, including three cristae and one saccular and one utricular maculae, are established. Math1 and myosin6 are expressed in vestibular hair cells by this stage (Fig. 1). Our study of the expression of Hes6 identified another early hair cell-specific marker for the sensory lineage in the vestibule.
By E12.5, the cochlear duct is formed at the ventral region of the otocyst. However, the cells in the future sensory organ of the cochlea have yet to be distinguished from their neighboring cells molecularly and morphologically. Gene expression studies have identified several early (E13.5–E14.5) molecular markers, including Lunatic Fringe, BMP4, Jagged1, in regions adjacent to the primordial organ of Corti, and Islet 1 and p27Kip1 in the primordial organ of Corti (Chen and Segil, 1999; Morsli et al., 1998; Kiernan et al., 2001; Radde-Gallwitz et al., 2004). There are reports that Math1 is also expressed in the entire primordial organ of Corti at the same stage at low levels (Lanford et al., 2000; Woods et al., 2004). Subsequently, hair cells differentiate within the primordial organ of Corti in a gradient that expands from the basal to apical and from the inner to outer hair cells. Intriguingly, Math1 is expressed in a subpopulation of cells within the primordial organ of Corti as columns of cells spanning the depth of the cochlear epithelium at this stage (Fig. 2) (Chen et al., 2002; Kiernan et al., 2005a; Wang et al., 2005). We and others previously have shown that the longer and thinner mature organ of Corti is derived from a shorter and thicker postmitotic precursor domain (Ruben, 1967; Chen and Segil, 1999; Lowenheim et al., 1999), likely though cellular intercalation movements characteristic of convergent extension (Chen et al., 2002; McKenzie et al., 2004; Wang et al., 2005). Such a morphogenesis process would predict that the precursors for hair cells and supporting cells line up in columns of cells spanning the depth of the epithelium in the primordial organ of Corti, and that cellular intercalation during terminal differentiation leads to the spreading of the cells in the longitudinal direction and the formation of a single layer of hair cells (Chen et al., 2002; Wang et al., 2005). The expression of Math1 at E14.5 (Fig. 2) in columns of cells spanning the depth of the cochlear epithelium suggested that these Math1+ cells are hair cell precursors.
The expression of Hes6 mirrors the expression of Math1 in the developing cochlea. It apparently occurs in the same subpopulation of precursor cells in the primordial organ of Corti at E14.5 and is subsequently restricted to the earliest morphologically recognizable hair cells in the cochlea (Fig. 2 and 3). The identification of a second marker with the same dynamics and in the same subpopulation of precursors within the primordial organ of Corti supports the identity of these Math1+ and Hes6+ cells as presumptive hair cell precursors and delineates the morphogenetic process of the sensory lineage. It is worth noting, however, there is no experimental evidence to directly support the lineage identity of these cells within the primordial organ of Corti at E14.5.
The potential role of Hes6 in inner ear development
The development of the nervous systems involves cascades of positively and negatively acting bHLH genes for neuronal differentiation. The negatively acting bHLH factors commonly consist of unique amino acid residues in the basic domain and in the C-terminal region (Sasai et al., 1992; Thomas et al., 1992). They act by directly repressing transcription of positively acting bHLH genes and/or antagonizing the activity of positively acting bHLH factors (Akazawa et al., 1995; Chen et al., 1997; Hatakeyama et al., 2004; Hu et al., 2004; Ishibashi et al., 1995; Jarriault et al., 1995; Kageyama et al., 2000; Ohtsuka et al., 1999; Sasai et al., 1992; Thomas and Rathjen, 1992; Cau et al., 2000; Tomita et al., 1996). Hes6 is structurally related to negatively acting bHLH factors, and contains characteristic amino acid sequences for this class (Pissarra et al., 2000; Vasiliaudkas et al., 2000). However, previous Hes6 expression studies suggested a role in promoting neurogenesis (Pissarra et al., 2000; Vasiliaudkas et al., 2000). Functional in vitro studies further demonstrated its capability in antagonizing the negatively acting bHLH factor Hes1 to promote the expression of neuronal-specific markers (Bae et al., 2000; Fior et al., 2005; Gratton et al., 2003; Koyabi-Nakagawa et al., 2000). The role that Hes6 plays in vivo, however, remains unknown (Koyabi-Nakagawa et al., 2000). Examination of Hes6 null animals revealed no phenotypical abnormalities in the systems studied (Koyabi-Nakagawa et al., 2000).
In the inner ear, Hes6 is expressed in the sensory lineage downstream of Math1 (Fig. 1–5). The inner ear appears normal in the absence of Hes6 (Fig. 6). These observations are consistent with what has been observed in other regions of the nervous system (Koyabi-Nakagawa et al., 2000). Our in vitro functional study also failed to identify potential targets of Hes6 and a role for Hes6 in promoting sensori-neuronal differentiation in the cochlea (Fig. 7). As reported previously, Hes1 is expressed in the medial region adjacent to the organ of Corti and is capable of inhibiting Math1 for hair cell differentiation (Zheng et al., 2000; Zine et al., 2001). In the absence of Hes1, numerous extra inner hair cells are produced (Zheng et al., 2000; Zine et al., 2001), indicating that the relief of Hes1 action is sufficient to induce hair cell differentiation. Transfection of cells in the medial region adjacent to the organ of Corti with Hes6 expression vectors, however, failed to induce the morphological and molecular differentiation of hair cells (Fig. 7 and data not shown). Under the same conditions, transfection of Math1 induced hair cell differentiation (Fig. 7) as expected (Zheng and Gao, 2000; Woods et al., 2004). The Hes6 expression vectors we constructed are bi-cistronic, including the Hes6 coding sequence followed by IRESGFP (Fig. 7) or IRESRFP (data not shown). The expression of both genes in the bi-cistronic vector overlaps completely in all the studies (Jackson et al., 1990), and we have used RT-PCR and confirmed the expression of Hes6 mRNA by the bi-cistronic expression vector (data not shown). The GPF signals in the cells transfected with Hes6-IRESGFP bi-cistronic vector were at least as strong as the ones in cells transfected with Math1-IRESGFP, indicating that the expression of Hes6 in transfected cells was most likely comparable to Math1. It is possible that the expression levels of Hes6 in the transfected cells are not sufficient to antagonize Hes1 to activate hair cell differentiation. Alternatively, antagonizing Hes1 action might not be sufficient to induce hair cell differentiation, and there are parallel negatively acting factors inhibiting hair cell differentiation in the medial region adjacent to the organ of Corti. The production of extra inner hair cell production in Hes1 null animals is limited (Zheng et al., 2000; Zine et al., 2001; and unpublished data), suggesting parallel pathways in restricting hair cell differentiation in the cochlea. We have observed the expression of other Hes genes in the regions adjacent to the organ of Corti (unpublished data). These additional Hes genes potentially play overlapping roles with Hes1.
Surprisingly, a neuronal marker expressed early in neuronal differentiation, tubulin β-III, was expressed in newly differentiated hair cells induced by Math1 expression in cultures (Fig. 7C, D, I, J). The expression of tubulin β-III was also detected in hair cells that are on the peripheral of the vestibular cultures (Fig. 7K, L). As reported, new hair cell differentiation is observed at the periphery of the vestibular epithelial culture (Zheng et al., 1997; Zheng et al., 1999). Therefore, the tubulin β-III positive hair cells in the vestibular cultures are likely newly differentiated hair cells. We did not detect the expression of tubulin β-III in the sensory lineage in vivo during development (Radde-Gallwitz et al., 2004; Jensen-Smith et al., 2003; and unpublished data). Tubulin β-III has been detected in the avian hair cells (Molea et al., 1999). It is possible that tubulin β-III is expressed in murine hair cells in vivo during development as well, and that we failed to detect its transient expression. Nevertheless, the observation of tubulin β-III positive cells in the inner ear epithelia in the culture suggested that the cochlear epithelial cells in the medial region are competent to express neuronal markers given appropriate signals. No tubulin β-III expression was detected in Hes6 expression vector-transfected cells.
Together, our experiments showed that, in contrast to what has been observed in other regions of the nervous system (Bae et al., 2000; Gratton et al., 2003; Koyabi-Nakagawa et al., 2000), Hes6 is not sufficient to induce the expression of hair cell-specific or neuronal-specific markers in the cochlear tissue culture. The elusive role of Hes6 in the inner ear may indicate the complexity and the redundancy of the regulation of inner ear development necessary to ensure the precise cellular patterning for auditory transduction.
Materials and Methods
Animal maintenance and handling
Inner ears from CD-1 animals were used for immunostaining and in situ hybridization. For Math1 expression, transgenic animals expressing the ds-red fluorescent protein (dsRED) under the control of Math1 locus were used (Wang et al., 2005). For Hes6 null animals (Koyabi-Nakagawa et al., 2000), the heterozygous Hes6+/− animals were mated to generate homozygous Hes6−/− and wild type littermates for phenotypic analyses. The day the vaginal plug was found was scored as E0.5. Animal care and use was in accordance with NIH guidelines and was approved by the Animal Care and Use Committee of Emory University.
Inner ear cryo-section preparation
The preparation of embryonic cochlear sections was performed as previously described (Radde-Gallwitz et al., 2004). Briefly, dissected inner ears were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) for 1 hour to overnight at 4°C, soaked in 20% sucrose in PBS on ice for up to 6 hours, embedded in OCT, sectioned at 10–12 μm, air dried for 1 hour, and stored at −20°C until use.
Immunostaining
For immunostaining, the frozen sections were rehydrated in PBS, then incubated with 0.1% Triton and 10% normal serum in PBS for 30 minutes at room temperature. The sections were then incubated with primary antibodies, washed in PBS, incubated with secondary antibodies, and washed in PBS before cover slipped for observation. The primary antibodies used for this study were: myosin6 (Proteus, rabbit polyclonal, 1:200); dsRED (Clontech, mouse monoclonal, 1:100), p27Kip1 (BD Biosciences, mouse monoclonal, 1:100); Tuj1 (Covance, mouse monoclonal, 1:200). For p27Kip1 staining, citric acid treatment was included for antigen retrieval. The secondary antibodies used for this study were: rhodamine-conjugated goat anti-rabbit (Molecular Probes, 1:500); Cy5-conjugated goat anti-mouse (Molecular Probes, 1:500); Cy2-conjugated goat anti-mouse (Molecular Probes, 1:500).
An Olympus IX71 inverted microscope equipped with epi-fluorescence and a Zeiss LSM510 were used for imaging.
In situ hybridization
In situ hybridizations were performed as described (Radde-Gallwitz et al., 2004). The cDNA for Islet1 antisense probe was amplified by primers: 5′-CAGCAAGAACGACTTCGTGA-3′ and 5′-GGACTGGCTACCATGCTGTT-3′. For Math1 and Hes6 probes, the following primer pairs were used: Math1, 5′-GGTGAGCTGGTAAGGAGAAG-3′ and 5′-CTGGCCTCATCAGAGTCACTGT-3′; Hes6, 5′-CATCGATGCCACTGTCTCAG-3′ and 5′-AGCTTCAGTGACCGCAGAAT-3′. The identities of the cDNAs were verified by sequencing.
Inner ear cDNA preparations and RT-PCR
Pure epithelial tissues from E12.5 to E16.5 inner ears were isolated as previously described (Chen et al., 2002). Briefly, inner ears from E12.5 embryos were dissected and subjected to collagenase (Worthington Biochemical Corporation) and dispase (GibcoBRL) digestion to isolate the epithelial inner ear tissue including both the cochlea and vestibule. For epithelial cochlear tissue preparations, inner ears were dissected from E14.5 to E16.5 wild-type or Math1−/− embryos and subjected similarly to collagenase and dispase digestion. The epithelial cochlear ducts were isolated, and divided into the apical, medial, and basal portions. Total RNAs were prepared by using RNeasy Mini Kit (Qiagen, Valencia, CA) and subjected to reverse transcription with random primers and oligodT using both OminiScriptase (Qiagen) and MMLV (Promega, Madison, WI) reverse transcriptases. Resulted cDNA populations were analyzed by PCR for the housekeeping gene L19, Math1, Hes6, and Islet1. The primers used are as follows: L19, 5′-CTCAGGCTACAGAAGAGGCTT-3′ and 5′-CTTGGTCTCCTCCTCCTTGGA-3′; Math1, 5′-ATGTCCCGCCTGCTGCATGC-3′ and 5′-ACTCTGATGAGGCCAGTTAG-3′; Hes6, 5′-CATCGATGCCACTGTCTCAG-3′ and 5′-AGCTTCAGTGACCGCAGAAT-3′; Islet1, 5′-CAGCAAGAACGACTTCGTGA-3′ and 5′-GGACTGGCTACCATGCTGTT-3′.
Construction of the Hes6-IRES-GFP bi-cistronic expression vector
The cDNA sequences encoding the full-length Hes6 were amplified with a pair of primers: 5′-AGATCTATGGCTCCGTCCCGGGCGCCC-3′, and 5′-GTCGACTCACCAAGGCCTCCACACACT-3′. Hes6 cDNA was first cloned into TA vector (In Vitrogen), then cloned into pIRESEGFP (Clontech). Correct cDNA sequence was verified by complete sequencing of the Hes6 cDNA. Math1-IRESEGFP vector was described previously (Zheng and Gao, 2000).
Organ culture
Electroporation and organ culture were performed as previously described (Zheng and Gao, 2000). Briefly, middle turn cochlear explants were dissected from P1 rats and transfected with the plasmids using an electroporator (Model CUY-21, BEX, Tokyo) with a train of eight pulses: 25V, 50 milliseconds duration and 100 milliseconds interval. After electroporation, the tissues were plated on a collagen-coated slide in serum-free medium. The cultures were fixed 6 days after transfection, and processed for immunostaining and imaging.
Acknowledgments
We would like to thank Dr. Marla Luskin for critically reading the manuscript, Sharayne Mark for animal work and genotyping, Seung-Jong Yoo and Jeff Saek for technical assistance in cochlear section preparation. The Math1 mutant animals were obtained from Dr. Huda Zogbi. This work is supported by the US National Institute of Health (to P.C.) and the Woodruff Foundation (to P.C.).
This research is supported by: NIH/NIDCD RO1 DC005213
References
- Adam J, Myat A, Le Rous I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Cell fate choices and the expression of Notch, Delta and Serrate homologues in the chick inner ear: parallels with Drosophila sensory organ development. Development. 1998;125:4645–4654. doi: 10.1242/dev.125.23.4645. [DOI] [PubMed] [Google Scholar]
- Akazawa C, Ishibashi M, Shimizu C, Nakanishi S, Kayeyama R. A mammalian helix-loop-helix factor structurally related to the product of Drosophila proneural gene atonal is a positive transcriptional regulator expressed in the developing nervous system. J Biol Chem. 1995;270:8730–8738. doi: 10.1074/jbc.270.15.8730. [DOI] [PubMed] [Google Scholar]
- Bae S, Bessho Y, Hojo M, Kageyama R. The bHLH gene Hes6, an inhibitor of Hes1, promotes neuronal differentiation. Development. 2000;127:2933–2943. doi: 10.1242/dev.127.13.2933. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bryant J, Goodyear RJ, Richardson GP. Sensory organ development in the inner ear: molecular and cellular mechanisms. Br Med Bull. 2002;63:39–57. doi: 10.1093/bmb/63.1.39. [DOI] [PubMed] [Google Scholar]
- Cau E, Gradwohl G, Casarosa S, Kageyama R, Guillemot F. Hes genes regulate sequential stages of neurogenesis in the olfactory epithelium. Development. 2000;127:2323–2332. doi: 10.1242/dev.127.11.2323. [DOI] [PubMed] [Google Scholar]
- Chen H, Thiagalingam A, Chopra H, Borges MW, Feder JN, Nelkin BD, Baylin SB, Ball DW. Conservation of the Drosophila lateral inhibition pathway in human lung cancer: a hairy-related protein (HES-1) directly represses achaete-scute homolog-1 expression. Proc Natl Acad Sci U S A. 1997;94:5355–5360. doi: 10.1073/pnas.94.10.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Segil N. p27Kip1 links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, Segil N. The role of Math1 in inner ear development, uncoupling the establishment of the sensory primordium from hair cell fate determination. Development. 2002;129:2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Cossins J, Vernon AE, Zhang Y, Philpott A, Jones PH. Hes6 regulates myogenic differentiation. Development. 2002;129:2195–2207. doi: 10.1242/dev.129.9.2195. [DOI] [PubMed] [Google Scholar]
- Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005;132:541–551. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- Eddison M, Le Roux I, Lewis J. Notch signaling in the development of the inner ear: lessons from Drosophila. Proc Natl Acad Sci U S A. 2000;97:11692–11699. doi: 10.1073/pnas.97.22.11692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete DM, Wu DK. Revisiting cell fate specification in the inner ear. Curr Opin Neurobiol. 2002;12:35–42. doi: 10.1016/s0959-4388(02)00287-8. [DOI] [PubMed] [Google Scholar]
- Fior R, Henrique D. A novel hes5/hes6 circuitry of negative regulation controls Notch activity during neurogenesis. Dev Biol. 2005;281:318–333. doi: 10.1016/j.ydbio.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Gao X, Chandra T, Gratton MO, Quelo I, Prud’homme J, Stifani S, St-Arnaud R. HES6 acts as a transcriptional repressor in myoblasts and can induce the myogenic differentiation program. J Cell Biol. 2001;154:1161–1171. doi: 10.1083/jcb.200104058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton MO, Torban E, Jasmin SB, Theriault FM, German MS, Stifani S. Hes6 promotes cortical neurogenesis and inhibits Hes1 transcription repression activity by multiple mechanisms. Mol Cell Biol. 2003;23:6922–6935. doi: 10.1128/MCB.23.19.6922-6935.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddon C, Jiang YJ, Smithers L, Lewis J. Delta-Notch signaling and the patterning of sensory cell differentiation in the zebrafish ear: evidence from a mindbomb mutant. Development. 1998;125:4637–4644. doi: 10.1242/dev.125.23.4637. [DOI] [PubMed] [Google Scholar]
- Hatakeyama J, Bessho Y, Katoh K, Ookawara S, Fujioka M, Guillemot F, Kageyama R. Hes genes regulate size, shape and histogenesis of the nervous system by control of the timing of neural stem cell differentiation. Development. 2004;131:5539–5550. doi: 10.1242/dev.01436. [DOI] [PubMed] [Google Scholar]
- Hu Y, Wang T, Stormo GD, Gordon JI. RNA interference of achaete-scute homolog 1 in mouse prostate neuroendocrine cells reveals its gene targets and DNA binding sites. Proc Natl Acad Sci U S A. 2004;101:5559–5564. doi: 10.1073/pnas.0306988101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi M, Ang S-L, Shiota K, Nakanishi S, Kageyama R. Targeted disruption of mammalian hairy and enhancer of split homolog-1 (Hes-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes & Development. 1995;9:3136–3148. doi: 10.1101/gad.9.24.3136. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nature Medicine. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Jackson RJ, Howell MT, Kaminski A. The novel mechanism of initiation of picornavirus RNA translation. Trends Biochem Sci. 1990;15:477–483. doi: 10.1016/0968-0004(90)90302-r. [DOI] [PubMed] [Google Scholar]
- Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- Jensen-Smith HC, Eley J, Steyger PS, Luduena RF, Hallworth R. Cell type-specific reduction of beta tubulin isotypes synthesized in the developing gerbil organ of Corti. J Neurocytol. 2003;32:185–197. doi: 10.1023/b:neur.0000005602.18713.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Tomita K. The bHLH gene Hes1 regulates differentiation of multiple cell types. Mol Cells. 2000;10:1–7. doi: 10.1007/s10059-000-0001-0. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neuroscience. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Ahituv N, Fuchs H, Balling R, Avraham KB, Steel KP, Hrabe de Angelis M. The Notch ligand Jagged1 is required for inner ear sensory development. Proc Natl Acad Sci USA. 2001;98:3873–3878. doi: 10.1073/pnas.071496998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005a;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Cordes R, Kopan R, Gossler A, Gridley T. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development. 2005b;132:4353–4362. doi: 10.1242/dev.02002. [DOI] [PubMed] [Google Scholar]
- Koyabi-Nakagawa N, Kim J, Anderson D, Kintner C. Hes6 acts in a positive feedback loop with the neurogenins to promote neuronal differentiation. Development. 2000;127:4203–4216. doi: 10.1242/dev.127.19.4203. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T, Kelley MW. Notch signaling pathway mediates hair cell development in mammalian cochlea. Nature Genetics. 1999;21:289–292. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Shailam R, Norton CR, Gridley T, Kelley MW. Expression of Math1 and Hes5 in the cochleae of wildtype and Jag2 mutant mice. JARO. 2000;1:161–171. doi: 10.1007/s101620010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AK, Frantz GD, Carpenter DA, de Sauvage FJ, Gao WQ. Distinct expression patterns of notch family receptors and ligands during development of the mammalian inner ear. Mech Dev. 1998;78:159–163. doi: 10.1016/s0925-4773(98)00165-8. [DOI] [PubMed] [Google Scholar]
- Lewis J. Notch signalling and the control of cell fate choices in vertebrates. Semin Cell Dev Biol. 1998;9:583–589. doi: 10.1006/scdb.1998.0266. [DOI] [PubMed] [Google Scholar]
- Lim DJ, Aniko M. Developmental morphology of the mouse inner ear. Acta Otolaryngology. 1985;422(Suppl):1–69. [PubMed] [Google Scholar]
- Lowenheim H, Furness DN, Kil J, Zinn C, Gultig K, Fero ML, Frost D, Gummer AW, Roberts JM, Rubel EW, Hackney CM, Zenner HP. Gene disruption of p27 (Kip1) allows cell proliferation in the post-natal and adult organ of Corti. PNAS. 1999;96:4084–4088. doi: 10.1073/pnas.96.7.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B. Smaller inner ear sensory epithelia in Neurog1 null mice are related to earlier hair cell terminal mitosis. Dev Dyn. 2005;234:633–650. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie E, Krupin A, Kelley MW. Cellular growth and rearrangement during the development of the mammalian organ of Corti. Dev Dyn. 2004;229:802–812. doi: 10.1002/dvdy.10500. [DOI] [PubMed] [Google Scholar]
- Molea D, Stone JS, Rubel EW. Class III beta-tubulin expression in sensory and nonsensory regions of the developing avian inner ear. J Comp Neurol. 1999;406:183–198. [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Morrison A, Hodgetts C, Gossler A, Hrabe de Angelis M, Lewis J. Expression of Delta1 and Serrate1 (Jagged1) in the mouse inner ear. Mech Dev. 1999;84:169–172. doi: 10.1016/s0925-4773(99)00066-0. [DOI] [PubMed] [Google Scholar]
- Morsli H, Choo D, Ryan A, Johnson R, Wu DK. Development of the mouse inner ear and origin of its sensory organs. J Neurosci. 1998;18:3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka T, Ishibashi M, Gradwohl G, Nakanishi S, Guillemot F, Kageyama R. Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBO J. 1999;18:2196–2207. doi: 10.1093/emboj/18.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pissarra L, Henrique D, Duarte A. Expression of hes6, a new member of the Hairy/Enhancer-of-split family, in mouse development. Mech Dev. 2000;95:275–278. doi: 10.1016/s0925-4773(00)00348-8. [DOI] [PubMed] [Google Scholar]
- Radde-Gallwitz K, Pan L, Gan L, Lin X, Segil N, Chen P. Expression of Islet1 marks the sensory and neuronal lineages in the mammalian inner ear. J Comp Neuro. 2004;477:412–421. doi: 10.1002/cne.20257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley BB, Chiang M, Farmer L, Heck R. The delta gene of zebrafish mediates lateral inhibition of hair cells in the inner ear and is regulated by pax2.1. Development. 1999;126:5669–5678. doi: 10.1242/dev.126.24.5669. [DOI] [PubMed] [Google Scholar]
- Ruben RJ. Development of the inner ear of mouse: a radioautographic study of terminal mitoses. Acta Oto-laryngologica Supplementum. 1967;220:1–44. [PubMed] [Google Scholar]
- Sasai Y, Kageyama R, Tagawa Y, Shigemoto R, Nakanishi S. Two mammalian helix-loop-helix factors structurally related to Drosophila hairy and Enhancer of split. Genes Dev. 1992;6:2620–2634. doi: 10.1101/gad.6.12b.2620. [DOI] [PubMed] [Google Scholar]
- Sher AE. The embryonic and postnatal development of the inner ear of the mouse. Acta Oto-laryngologica Supplementum. 1971;285:1–77. [PubMed] [Google Scholar]
- Shou J, Zheng JL, Gao WQ. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol Cell Neurosci. 2003;23:169–179. doi: 10.1016/s1044-7431(03)00066-6. [DOI] [PubMed] [Google Scholar]
- Thomas PQ, Rathjen PD. HES-1, a novel homeobox gene expressed by murine embryonic stem cells, identifies a new class of homeobox genes. Nucleic Acids Res. 1992;20:5840. doi: 10.1093/nar/20.21.5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita K, Ishibashi M, Nakahara K, Ang SL, Nakanishi S, Guillemot F, Kageyama R. Mammalian hairy and Enhancer of split homolog 1 regulates differentiation of retinal neurons and is essential for eye morphogenesis. Neuron. 1996;16:723–734. doi: 10.1016/s0896-6273(00)80093-8. [DOI] [PubMed] [Google Scholar]
- Tsai H, Hardisty RE, Rhodes C, Kiernan AE, Roby P, Tymowska-Lalanne Z, Mburu P, Rastan S, Hunter AJ, Brown SD, Steel KP. The mouse slalom mutant demonstrates a role for Jagged1 in neuroepithelial patterning in the organ of Corti. Hum Mol Genet. 2001;10:507–512. doi: 10.1093/hmg/10.5.507. [DOI] [PubMed] [Google Scholar]
- Vasiliauskas D, Stern CD. Expression of mouse HES-6, a new member of the Hairy/Enhancer of split family of bHLH transcription factors. Mech Dev. 2000;98:133–137. doi: 10.1016/s0925-4773(00)00443-3. [DOI] [PubMed] [Google Scholar]
- Wang J, Mark S, Zhang X, Qian D, Yoo S-J, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, Chen P. Regulation of polarized extension and planar cell polarity in the cochlea by vertebrate PCP pathway. Nature Genetics. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nature Neuroscience. 2004;7:1310–1318. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- Zhang N, Martin GV, Kelley MW, Gridley T. A mutation in the Lunatic fringe gene suppresses the effects of a Jagged2 mutation on inner hair cell development in the cochlea. Curr Biol. 2000;10:659–662. doi: 10.1016/s0960-9822(00)00522-4. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Helbig C, Gao WQ. Induction of cell proliferation by fibroblast and insulin-like growth factors in pure rat inner ear epithelial cultures. J Neuroscience. 1997;17:216–226. doi: 10.1523/JNEUROSCI.17-01-00216.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Frantz G, Lewis AK, Sliwkowski M, Gao WQ. Heregulin enhances regenerative proliferation in postnatal rat utricular sensory epithelium after ototoxic damage. J Neurocytol. 1999;28:901–912. doi: 10.1023/a:1007078307638. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Shou J, Guillemot F, Kageyama R, Gao WQ. Hes1 is a negative regulator of inner ear hair cell differentiation. Development. 2000;127:4551–4560. doi: 10.1242/dev.127.21.4551. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nature Neuroscience. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zine A, Van De Water TR, de Ribaupierre F. Notch signaling regulates the pattern of auditory hair cell differentiation in mammals. Development. 2000;127:3373–3383. doi: 10.1242/dev.127.15.3373. [DOI] [PubMed] [Google Scholar]
- Zine A, Aubert A, Qiu J, Therianos S, Guillemot F, Kageyama R, de Ribaupierre F. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J Neuroscience. 2001;21:4712–4720. doi: 10.1523/JNEUROSCI.21-13-04712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]