Summary
Endocytosis marks the entry of internalized receptors into the complex network of endocytic trafficking pathways. Endocytic vesicles are rapidly targeted to a distinct membrane-bound endocytic organelle referred to as the early endosome. Despite the existence of numerous internalization routes, early endosomes (EE) serve as a focal point of the endocytic pathway. Sorting events initiated at this compartment determine the subsequent fate of internalized proteins and lipids, destining them either for recycling to the plasma membrane, degradation in lysosomes or delivery to the trans-Golgi network. Sorting of endocytic cargo to the latter compartments is accomplished through the formation of distinct microdomains within early endosomes, through the coordinate recruitment and assembly of the sorting machinery. An elaborate network of interactions between endocytic regulatory proteins ensures synchronized sorting of cargo to microdomains followed by morphological changes at the early endosomal membranes. Consequently, the cargo targeted either for recycling back to the plasma membrane, or for retrograde transport to the trans-Golgi network, localizes to newly-formed tubular membranes. With a high ratio of membrane surface to lumenal volume, these tubules effectively concentrate the recycling cargo, ensuring efficient transport out of the EE. Conversely, receptors sorted for degradation cluster at the flat clathrin lattices involved in invaginations of the limiting membrane, associating with newly formed intralumenal vesicles. In this review we will discuss the characteristics of early endosomes, their role in the regulation of endocytic transport, and their aberrant function in a variety of diseases.
Keywords: Endocytosis, Golgi, Traffic, Disease
Introduction
The internalization of receptors, lipid membranes, and extracellular fluid at the plasma membrane (PM) of higher eukaryotes is carried out by various endocytic trafficking pathways. These include the well-characterized clathrin-dependent pathway as well as clathrin-independent pathways. Among the latter pathways is the phagocytic uptake of large particles, pinocytosis, raft-mediated endocytosis and Arf6-dependent internalization (Mellman, 1996; Mukherjee et al., 1997; Conner and Schmid, 2003; Maxfield and McGraw, 2004). Many of the cargo molecules internalized through these diverse pathways are either recycled back to the PM, or undergo degradation in late endosomes and lysosomes. Accordingly, it is not surprising to find that eukaryotic cells have evolved mechanisms to converge these differentially internalized proteins to a common sorting station from where the cargo destined for degradation and recycling are separated. This common sorting station is known as the early endosome (EE) or the sorting endosome (Mayor et al., 1993).
The EE is defined as the first endocytic compartment to accept incoming cargo internalized from the PM and is a highly dynamic structure with a high propensity to undergo homotypic fusion (Gruenberg et al., 1989). The EE appears as an elaborately complex pleomorphic compartment in most cell types. It is composed of regions of thin tubular extensions (∼60 nm diameter) and large vesicles (∼400 nm diameter) that have membrane invaginations and give rise to a multi-vesicular appearance (Gruenberg, 2001). These morphologically distinct EE sub-domains are thought to be functionally important. For example, proteins targeted for recycling may cluster within primarily tubular membranes, whereas the multi-vesicular elements are usually involved in sorting to the degradative pathway (Mellman, 1996). Vesicles generated from these two distinct membrane regions exhibit differences in their acidification properties. The pH decreases from 6.2 to ∼5.5 in the lumen of multivesicular bodies, whereas it increases in the tubular recycling endosomes to ∼6.5 (Mayor et al., 1993; Mellman, 1996). It is interesting to note that such morphologically, chemically and functionally distinct compartments undergo biogenesis from the same EE.
As mentioned above, the primary function of the EE is the sorting of internalized cargo to different intracellular destinations. Within minutes of internalization, the separation of various endocytic cargo molecules occurs within the EE. Although most of the internalized ligands are degraded, their receptors are often recycled back to the cell surface for additional rounds of ligand-binding and internalization (Dunn et al., 1989). For instance, proteins such as transferrin receptor or low density lipoprotein receptor are slated for recycling from the EE, whereas low density lipoprotein itself and the ligand-bound epidermal growth factor receptor (EGFR) are transported to late endosomes/lysosomes for degradation (Dautry-Varsat et al., 1983; Herbst et al., 1994). The moderately acidic pH of the EE lumen (∼6.3-6.8) allows for the dissociation of ligand from its receptor. Due to the greater membrane surface area of the tubular domain of the EE, receptors (and other membrane proteins) are concentrated in this region. On the other hand, soluble ligands accumulate in the vesicular regions that ultimately fuse with late endocytic organelles. The tubular endosomes return most of the membrane lipids and proteins back to the cell surface and some of the fluid occupied by the membrane lumen (Mellman, 1996). The critical functional role of the EE necessitates that any sorting occurring in this organelle is a precise and highly controlled process.
The protein and lipid content of EE has been extensively analyzed through a variety of biochemical and fluorescence-based techniques. Below we discuss some of the key protein and lipid components of EE that facilitate the sorting of internalized cargo from this compartment to different intracellular destinations.
Proteins at the early endosome
A critical group of endocytic regulators are the Ras-associated binding (Rab) proteins. Rabs are small GTP-binding proteins that cycle between a GTP-bound active state and a GDP-bound inactive state. In their active state they localize to intracellular membranes where they can interact with and recruit a variety of proteins known as Rab effectors (Grosshans et al., 2006). Indeed, it is through binding to specialized effectors that Rab proteins carry out multiple roles in endocytic trafficking events, including vesicle tethering, fusion, budding and motility (Pfeffer, 2001; Zerial and McBride, 2001; Deneka and van der Sluijs, 2002; Grosshans et al., 2006). The Rab proteins primarily localized to the EE include Rab5 and Rab4, which regulate distinct early endocytic events (Gorvel et al., 1991; Bucci et al., 1992; van der Sluijs et al., 1992a; Daro et al., 1996). In addition to these two Rab proteins, some of the other less well-characterized Rabs at the EE include Rab10 (Babbey et al., 2006), Rab14 (Junutula et al., 2004; Proikas-Cezanne et al., 2006), Rab21 (Simpson et al., 2004) and Rab22 (Mesa et al., 2001, 2005; Kauppi et al., 2002; Magadan et al., 2006).
Rab5 is the most extensively analyzed Rab of the early endocytic pathway (Gorvel et al., 1991; Bucci et al., 1992; Barbieri et al., 1996; Zerial and McBride, 2001; Grosshans et al., 2006). It regulates entry of the cargo from the plasma membrane to the early endosomes, generation of phosphotidylinositol-3-phosphate (PtdIns(3)P) lipid which is enriched on EE (Christoforidis et al., 1999; Murray et al., 2002), homotypic fusion (Gorvel et al., 1991) and the motility of EE on actin and microtubules tracks (Nielsen et al., 1999; Pal et al., 2006). In addition, it also functions in activating signaling pathways from EE (Benmerah, 2004; Miaczynska et al., 2004; Schenck et al., 2008). The active or GTP-bound Rab5 is generated at the EE by the action of Rabex-5 which acts as a guanine exchange factor (GEF) for Rab5 (Horiuchi et al., 1997). This in turn recruits Rabaptin-5, which binds to the GTP-bound Rab5 and recruits Rabex-5 in a positive feedback loop to maintain and stabilize GTP-bound Rab5 on EE (Stenmark et al., 1995; Lippe et al., 2001). These transient but high levels of active Rab5 are sufficient to recruit effector proteins to the EE where they can carry out their specialized functions in trafficking and sorting (Grosshans et al., 2006). Several key Rab5 effectors and their proposed functions at the EE are outlined below:
PtdIns(3)P-kinase/hVPS34/p150 (VPS34) is thought to be one of the first Rab5 effector proteins to be recruited to the EE (Christoforidis et al., 1999). As suggested by its name, its primary role is to generate PtdIns(3)P, which is the most abundant phosphoinositide in the EE membrane. The concomitant presence of GTP-Rab5 and PtdIns(3)P acts as a signal to recruit effector proteins such as EEA1 (Lawe et al., 2000, 2002; Pfeffer, 2001), Rabenosyn-5 (Nielsen et al., 2000) and/or Sorting nexins (SNXs) (Cozier et al., 2002) that bind to both active Rab5 and PtdIns(3)P simultaneously. Treatment of cells with inhibitors of PtdIns(3)P-kinase such as Wortmannin (Hazeki, 1995) and LY294002 (Vlahos et al., 1994) prevents localization of PtdIns(3)P-binding proteins (such as EEA1) to the EE (Patki et al., 1997) and impairs trafficking from this compartment (van Dam et al., 2002), thus highlighting a crucial role for PtdIns(3)P-kinase in proper functioning of this organelle.
Early endosomal antigen-1 (EEA1) is a well-characterized effector of Rab5 and one of the most widely used markers for EE due to its specific localization to this compartment (Simonsen et al., 1998). EEA1, in coordination with members of the SNARE family, is essential for EE fusion in vivo (Mills et al., 1998, 1999). EEA1 can directly interact with syntaxin6 and syntaxin13, two target-SNAREs (t-SNAREs) that mediate homotypic fusion of EE (McBride et al., 1999; Simonsen et al., 1999; Mills et al., 2001). EEA1 also contains a FYVE domain that specifically binds to PtdIns(3)P (Raiborg et al., 2001). Thus dual binding of EEA1 to GTP-Rab5 and PtdIns(3)P modulates its localization to EE membranes (Lawe et al., 2002).
Rabenosyn-5 is another FYVE-domain-containing Rab5 effector that localizes to EE (Nielsen et al., 2000). Rabenosyn-5 is regarded as a dual Rab5 and Rab4 effector as it can bind to both of these Rabs in their active states, thus linking these Rab domains at the EE where it can coordinate sorting activity with the fast recycling of cargo directly from EE (de Renzis et al., 2002). In addition, Rabenosyn-5 also interacts with EHD1, a member of the recently discovered C-terminal EHD family of proteins which regulates recycling of cargo from the perinuclear endocytic recycling compartment (ERC) back to the plasma membrane (Naslavsky et al., 2004). Thus Rabenosyn-5 might serve as the Rab5 effector linking sorting events at the EE to recycling of cargo back to the plasma membrane either directly from EE or via the ERC.
APPL1 and APPL2 (Adaptor protein containing PH domain, PTB domain and Leucine zipper motif) are two recently identified homologous Rab5 effectors that are associated with a subset of Rab5-positive EE (Miaczynska et al., 2004). APPL proteins are specialized Rab5 effectors as their primary role appears to be in signaling rather than trafficking. APPL proteins translocate from EE to the nucleus upon stimulation with EGF. Once inside the nucleus, these proteins can interact with the nucleosome remodeling and histone deacetylase multiprotein complex NuRD/MeCP1 which regulates chromatin structure and gene expression (Miaczynska et al., 2004). Accordingly, APPL proteins are essential for cell proliferation and more importantly their binding to Rab5 is crucial for their function in mitogenesis (Miaczynska et al., 2004). A recent study by Zerial and colleagues suggests that APPL proteins also regulate the activity of protein kinase B (Akt) and influence its substrate specificity in a Zebra fish model system (Schenck et al., 2008). Moreover, the endosomal localization of APPL is essential for the activation of Akt. These studies suggest that the EE is not only a sorting station, but a subset of these organelles actively plays a role in signal transduction events initiated at the plasma membrane.
In addition to Rab5 and its effectors, Rab4 is also localized to EE (Van Der Sluijs et al., 1991), as well as to Rab11-positive recycling endosomes (Trischler et al., 1999). Unlike Rab5, which potentiates fusion events of primary endocytic vesicles with EE and homotypic fusion of EE (Gorvel et al., 1991), Rab4 regulates the exit of constitutive recycling cargo from EE directly back to the plasma membrane as well as directing the sorting of cargo to the ERC (van der Sluijs et al., 1992a; Sheff et al., 1999a). Rab4 also regulates specialized trafficking events such as transport of the glucose transporter GLUT4 upon insulin stimulation from an intracellular storage compartment to the plasma membrane in adipocytes and muscle cells (Aledo et al., 1995; Cormont et al., 1996; Vollenweider et al., 1997). Consistent with this role, Rab4 is also involved in the budding of synaptic-like microvesicles (SLMVs) from EE in PC12 neuroendocrine cells (de Wit et al., 2001). These SLMVs are similar to synaptic vesicles (SVs) of neurons and are involved in endo-and exocytosis of neurotransmitters, suggesting a potential role for Rab4 in SV trafficking as well. Rab4 has also been implicated in the regulation of an essential step in receptor-mediated antigen processing in B lymphocytes (Lazzarino et al., 1998), in regulated exocytosis of dense granules in platelets (Shirakawa et al., 2000), and α-amylase exocytosis in pancreatic cells (Ohnishi et al., 1999). Thus Rab4 is involved in regulating constitutive as well as specialized trafficking/recycling pathways.
SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) are highly conserved membrane proteins that mediate fusion events in membrane trafficking pathways (Bennett, 1995; Sollner, 1995). The hallmark of SNARE proteins is the presence of a SNARE motif: an evolutionarily conserved domain of 60-70 amino acids arranged in heptad repeats (Fasshauer, 2003). Monomeric SNAREs are unstructured; however, when appropriate sets of SNAREs interact with one another, their SNARE motifs spontaneously associate to form helical core complexes of extraordinary stability (Chen and Scheller, 2001). The center of the helical core is highly hydrophobic except for a central ‘0’ layer that contains three highly conserved glutamine (Q) residues and one highly conserved arginine (R) residue. Consequently, the SNARE motifs contributing these residues are classified as Qa-, Qb-, Qc- and R-SNAREs. A functional SNARE complex that drives membrane fusion is formed by four SNAREs, with one member each of Qa-, Qb-, Qc- and R-SNARE subfamilies (Fasshauer et al., 1998; Bock et al., 2001). According to the ‘zipper’ model of SNARE function, during vesicle fusion, the SNARE motifs of interacting SNAREs assemble into a parallel four-helix bundle. This is initiated by ‘zipping’ of the membrane-distal N-termini to the membrane-proximal carboxyl-termini, forming a stable complex that can bring the membranes in close apposition and thus overcome the energy barrier to drive fusion of the lipid bilayers (Hanson et al., 1997; Lin and Scheller, 1997).
The key SNARE machinery known to be involved in homotypic fusion of EE are syntaxin13 (Qa), vti1a (Vps10p tail interactor 1, Qb), syntaxin6 (Qc), and VAMP4 (R) (Brandhorst et al., 2006; Zwilling et al., 2007). The specificity of SNARE fusion is likely determined by upstream targeting proteins that interact and recruit these SNAREs onto specific membranes (Brandhorst et al., 2006). In the case of homotypic EE fusion, the Rab5 effector and tethering protein EEA1 can directly bind and thus recruit Synatxin13 and Syntaxin6 to the EE (McBride et al., 1999; Simonsen et al., 1999). Interestingly it has been observed that the PtdIns(3)P-kinase inhibitor, Wortmannin blocks endosome fusion (Patki et al., 1997). This observation conforms to a model whereby EEA1 fails to be recruited to EE upon treatment with Wortmanin, inhibiting targeting of the SNARE machinery to the EE and thus preventing fusion. Subsequent to membrane fusion, SNARE recycling occurs upon dissociation of the helical bundle, which is mediated by the AAA+ (ATPases Associated with diverse cellular Activities) protein, NSF (N-ethylmaleimide-sensitive factor) (Mayer et al., 1996; Hanson and Whiteheart, 2005).
Motor proteins at the early endosome
The movement of intracellular membrane-bound structures in the cell is powered by motor proteins. For EE in particular, the activity of Rab5 and its effector protein PtdIns(3)P-kinase is required for motility on microtubule tracks (Nielsen et al., 1999). One of the motor protein complexes involved in the movement of vesicles budding from EE to the ERC or towards lysosomes is the dynein-dynactin complex which moves along microtubule tracks from the cell periphery towards the microtubule-organizing center (MTOC) (Valetti et al., 1999). A recent study by Buss and colleagues has also shown that the actin-based motor Myosin VI and its interacting partner LMTK2 (lemur tyrosine kinase 2) are involved in cargo delivery from EE to ERC (Chibalina et al., 2007). The movement of EE in the opposite direction (from center of the cell to the periphery) is mediated by the plus-end directed kinesin-3 motor KIF16B, which transports vesicles budding from EE in a process regulated by the small GTPase Rab5 and its effector, the PtdIns(3)P-kinase/hVPS34 (Hoepfner et al., 2005). KIF16B localizes to EE in vivo owing to the presence of a PX domain which mediates its recruitment to PtdIns(3)P-enriched EE membranes. Overexpression of KIF16B causes the relocation of EE to the cell periphery, while expression of dominant negative KIF16B or RNAi-mediated knockdown causes EE to cluster in the perinuclear region (Hoepfner et al., 2005). A recent study by Zerial and colleagues has identified a novel effector of Rab5, Huntingtin-associated protein-40 (HAP-40) that regulates the dynamics of EE (Pal et al., 2006). HAP-40 can interact with Huntingtin (Htt) protein and recruit it to EE in a GTP-Rab5 dependent manner. Htt binds to microtubules and is implicated in clathrin-mediated endocytosis. Overexpression of HAP-40 sequesters Htt, causing detachment of EE from microtubules and a preferential association with actin filaments, thus reducing long-range movement on microtubule tracks to short-range movements along actin microfilaments (Pal et al., 2006). Surprisingly, HAP-40 is highly up-regulated in fibroblasts and brain tissue from human patients affected by Huntington's disease (HD) (see Table I). In cells from diseased patients, the shift of EE from microtubules to actin filaments could be observed, causing a severe decrease in mobility in vivo (Pal et al., 2008).
Table 1.
Aberrant EE function contributes to multiple diseases.
| Disease name | Clinical features | Mutated protein that causes the disease | Effect on early endosome | References |
|---|---|---|---|---|
| Alzheimer's disease (AD) | Personality changes, gradual decrease in memory function and intellectual ability | ? | Increased endocytic uptake and fusion leading to enlargement of EE and increase of Amyloid Precursor Protein (APP) and Apolipoprotein E (ApoE) processing | (Cataldo et al., 1997; Tate and Mathews, 2006) |
| Down syndrome | Mental retardation, congenital malformations of heart and gastrointestinal tract, etc. | Amyloid Precursor Protein (APP) | Increased APP gene dosage leads to increased endocytic uptake, and enlargement of both EE and late endosomes as well as increased soluble beta-Amyloid peptides in neurons and fibroblasts | (Cataldo et al., 2003, 2008) |
| Huntington's disease | Dementia (commonly associated with seizures), rigidity, and progressive chorea | Huntingtin (Htt) | Expansion of CAG repeat in the Htt gene leads to upregulation of Htt-associated protein 40 (HAP40), decrease in transferrin uptake and impaired EE motility | (Pal et al., 2006) |
| Lowe's syndrome | Renal Fanconi Syndrome, mental retardation, congenital cataracts | Oculocerebrorenal syndrome of Lowe (OCRL1) | Mutations in OCRL impair its co-localization with APPL1 (adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain, and leucine zipper motif 1) and binding with Rab5 on early endosomes. Depletion of OCRL1 changes the rate of transport between early endosomes and Trans-Golgi Network (TGN) | (Choudhury et al., 2005; Erdmann et al., 2007) |
| Myotubular myopathy | Facial and eye muscle weakness, low muscle tone, and respiratory failure | Myotubularin | Impaired vesicular transport from EE | (Dowling et al., 2008) |
| Hermansky-Pudlak syndrome types 2, 3, 5, 6, 7 and 8 | Albinism, bleeding, and innate immune defects | BLOC-(Biogenesis of lysosomal-related complex)1, BLOC-2, and Adaptor Protein (AP)-3 | Surface accumulations of CD63 and tyrosinase-related protein 1 (Tyrp1) due to missorting of Tyrp1 from EE to the plasma membrane | (Di Pietro et al., 2006) |
Defective early endosomal proteins can lead to a variety of diseases. Most of these diseases are neurological-related illnesses, likely resulting from impaired neuron function in rapid signal transmission from one neuron to another, which requires intact synaptic vesicle transport. Among the additional diseases related to genes encoding early endosomal proteins are schizophrenia and bipolar disorder; the dysbindin subunit of BLOC1 (encoded by gene DTNBP1) is a candidate gene for schizophrenia (Di Pietro et al., 2006), whereas a rare variant in the promoter region of hVps34 gene is related to bipolar disorder and schizophrenia (Di Pietro et al., 2006; Nicot and Laporte, 2008). Early endosomal proteins also play an important role in the replication of bacteria and viruses. Perskvist and co-workers reported that Rab5 binds tightly to phagosomes containing Mycobacterium tuberculosis, which causes delayed phagolysosome maturation (Perskvist et al., 2002). The SpoE (Streptococcus pneumoniae) protein of Salmonella recruits non-prenylated Rab5 on Salmonella-containing phagosomes to induce fusion of early endosomes, thus preventing phagolysosome maturation by acting as a Rab5-specific guanine exchange factor (Mukherjee et al., 2001). Rab5 and other early endosomal proteins have also been reported to associate with Hepatitis C virus (HCV) NS4B (one of the non-structural proteins) (Stone et al., 2007).
C-terminal EHD proteins at the early endosome
In addition to the well-characterized Rab proteins, another family of proteins recently implicated in endocytic trafficking is the C-terminal Eps15 Homology Domain (EHD) proteins (Naslavsky and Caplan, 2005; Grant and Caplan, 2008). Four highly homologous C-terminal EHD proteins (EHD1-EHD4) have been identified in mammals (Mintz et al., 1999; Pohl et al., 2000). EHD1 is the best characterized of these EHD proteins, and has been implicated in regulating the recycling of various receptors including transferrin (Tf) (Lin et al., 2001; Naslavsky et al., 2004), major histocompatibility complex class I (Caplan et al., 2002) and β1 integrin receptors (Jovic et al., 2007). Recent studies suggest that both EHD3 and EHD4 are involved in trafficking from EE to the ERC (Naslavsky et al., 2006; Sharma et al., 2008). Depletion of EHD3 or EHD4 prevents the delivery of TfR and other cargo to the ERC, and it accumulates in enlarged EE structures (Fig. 1). In addition, EHD4-depletion also induces enhanced activation of Rab5 (Sharma et al., 2008). EHD proteins also interact with Rab effectors such as Rabenosyn-5 at the EE (Naslavsky et al., 2004) which provides insight as to the mode by which EHD3 and EHD4 can coordinate their function with Rab4 and Rab5 in EE trafficking.
Fig. 1.
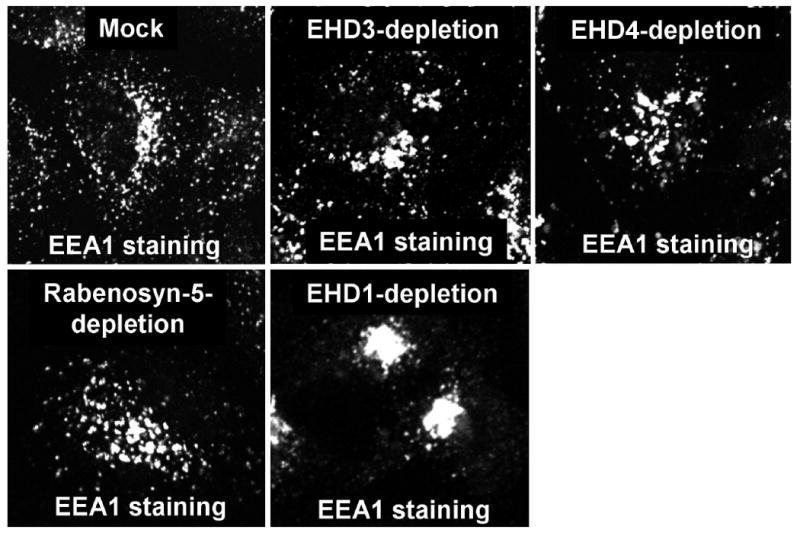
HeLa cells on coverslips were either mock-treated or treated with specific siRNA oligonucleotides against EHD3 (Naslavsky et al., 2006; Sharma et al., 2008), EHD4 (Sharma et al., 2008), Rabenosyn-5 and EHD1 (Naslavsky et al., 2004) as described previously. After treatment with siRNA, cells were fixed and stained for endogenous EEA1 followed by the goat anti-mouse Alexa-488 secondary antibodies. Depletion of EHD3, EHD4 and Rabenosyn-5 induced formation of enlarged early endosomes while depletion of EHD1 caused accumulation of EEA1 in the ERC.
Lipids at the early endosome
Phospholipids play a crucial role in membrane trafficking pathways by recruiting proteins that contain specific domains capable of binding to them (Simonsen et al., 2001; Gruenberg, 2003). Localized synthesis of lipids at distinct cellular locations ensures selective recruitment of endocytic proteins at these sites. The EE compartment and multivesicular bodies in the cell are enriched in PtdIns(3)P (Gillooly et al., 2000). In its active form, the small GTPase Rab5 recruits PtdIns(3)P-kinase/Vps34 to EE membranes where it mediates localized synthesis of PtdIns(3)P (Christoforidis et al., 1999). This in turn can recruit PtdIns(3)P-binding proteins containing a FYVE domain (such as EEA1, Rabenosyn-5 or Hrs), or a PX domain (such as SNX1) (Nielsen et al., 2000; Raiborg et al., 2001; Cozier et al., 2002). Recruitment of EEA1 can assemble the SNAREs Syntaxin13 and Syntaxin6 at EE membranes thus enabling EE fusion (Mills et al., 2001). On the other hand, recruitment of Hrs to EE is essential for the sorting of EGFR into multivesicular bodies, which fuse with lysosomes and leads to EGFR degradation (Morino et al., 2004). In addition, recruitment of sorting nexins to the EE is essential for transport of cargo to the Golgi (Carlton et al., 2004). Generation of PtdIns(3)P is thus essential for regulating almost all of the EE sorting events. Interestingly, a study by Petiot et al., has shown that the process of bulk transport (fluid-phase) from EE to late endosomes does not require the activity of PtdIns(3)P-kinase and consequently is insensitive to Wortmanin treatment (Petiot et al., 2003).
One of the proteins recruited by PtdIns(3)P at the EE is PIKFyve, the only known enzyme that synthesizes PtdIns(3,5)P2 from PtdIns(3)P in mammalian cells (Cabezas et al., 2006). PIKFyve is recruited to EE via its FYVE domain and is sensitive to Wortmanin treatment (Sbrissa et al., 2002). Once localized to the EE, this kinase can phosphorylate PtdIns(3)P to PtdIns(3,5)P2, a phosphoinositide that is enriched on late endosomal membranes (Ikonomov et al., 2002). Knockdown of PIKfyve or expression of a kinase-dead mutant induces EE enlargement and profound cytoplasmic vacuolation (Ikonomov et al., 2001; Rutherford et al., 2006). This is most likely caused due to a lack of PtdIns(3,5)P2 synthesis which promotes endosomal fission and an increase in the cellular pool of PtdIns(3)P which promotes homotypic EE fusion (Shisheva, 2008). PIKFyve dysfunction causes a delay in fluid-phase transport to late endosomes and also impairs EE-to-TGN retrograde traffic (Ikonomov et al., 2003; Rutherford et al., 2006). However, receptor sorting to lysosomes or recycling to the PM remains unaffected (Ikonomov et al., 2003). These data suggest a role for PIKFyve in maintaining EE morphology by regulating PI3P levels and the exit of cargo from early endosomes to late endosomes.
In addition to PtdIns(3)P conversion to PtdIns(3,5)P2, a second mechanism to downregulate PtdIns(3)P at the EE is by the action of the lipid phosphatase MTM1, a member of the myotubularin family of proteins (Blondeau et al., 2000; Begley and Dixon, 2005). MTM1 primarily localizes to Rab5 and EEA1 positive EE (Cao et al., 2007). In this recent study, it was demonstrated that MTM1 is the major phosphatase regulating PtdIns(3)P levels, since overexpression of MTM1 causes depletion of PtdIns(3)P from EE and multivesicular bodies (Cao et al., 2007). Conversely, depletion of MTM1 leads to increased PtdIns(3)P levels in the cell (Cao et al., 2008). Interestingly, in either scenario EGFR sorting to the late endocytic pathway is impaired (Cao et al., 2008). This highlights the importance of maintaining the overall balance of PtdIns(3)P levels in the cell for proper sorting to occur and suggests that a highly coordinated mechanism of PtdIns(3)P-kinases and phosphatases must exist at the EE. Indeed, MTM1 directly interacts with theVps15/Vps34 PtdIns(3)P-kinase complex and this interaction might regulate the activity of the phosphatase at the EE (Cao et al., 2007).
Regulation of endocytic trafficking at the early endosome
Sorting for degradation
The sorting of receptors from EE to the degradation pathway depends upon the presence of sorting signals. Signaling receptors en route to lysosomes, including the epidermal growth factor receptor (EGFR), contain cytosolic domains necessary for recruitment of sorting machinery. Mono-ubiquitination of one or more lysine residues in receptor tyrosine kinases is critical for this process (Haglund et al., 2003; Mosesson et al., 2003). Initial recognition of the mono-ubiquitinated cargo on the EE is carried out by the hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) via its ubiquitin interacting motif (UIM) (Raiborg et al., 2002). Hrs also associates with clathrin found in flat clathrin lattices through its C-terminal clathrin box motif (Raiborg et al., 2002). Flat clathrin lattices are present only on select EE membranes involved in the sorting of cargo to the degradation pathway (Raiborg et al., 2002). Binding of Hrs to ubiquitin and clathrin thus results in formation of a unique sorting microdomain that actively concentrates and sorts cargo into intralumenal vesicles and away from the tubule-forming, actively recycling membranes. However, the weak binding of Hrs to ubiquitin necessitates the presence of additional ubiquitin-binding proteins. Two additional UIM-containing proteins, Eps15 and signal transducing adaptor molecule 2 (STAM2), stabilize the association with ubiquitinated cargo and in concert with Hrs form a functional sorting complex (Bache et al., 2003b). Furthermore, Hrs interacts with the Tsg101 subunit of the endosomal sorting complex required for transport (ESCRT-I), thus recruiting it to late endosomal membranes (Bache et al., 2003a). Subsequently ESCRT-II complex binds to ESCRT-I, followed by recruitment of ESCRT-III, leading to the onset of membrane invagination and formation of multivesicular bodies (MVBs) in a yet poorly understood process (Babst et al., 2002a,b; Bonifacino and Hurley, 2008). Ultimately, fusion of such MVBs with the lysosomal vesicles carrying proteolytic enzymes results in degradation of EGFR and other sorted receptors.
Sorting for Recycling
Upon delivery to the EE, internalized receptors can be sorted into one of at least two distinct recycling pathways (Fig. 2). Efficient sorting of receptors undergoing recycling is achieved through extensive tubulation of the EE membranes in a process of “geometry-based sorting” (Maxfield and McGraw, 2004), whereby receptors that are sorted into the newly-formed tubular membranes recycle back to the PM. In parallel with tubulation, Rab4 targets receptors to the ERC where they proceed via a ‘slow-recycling’ route back to the plasma membrane (Deneka et al., 2003). However, Rab4 can also sort receptors directly back to the plasma membrane in a “fast recycling” route (van der Sluijs et al., 1992b; Sheff et al., 1999b). Classical kinetic studies measuring the recycling rates of the canonical recycling receptor, the transferrin receptor (TfR), confirmed the existence of two routes with differing kinetics; the ‘fast’ recycling pathway (t1/2=5 min) and the ‘slow’ recycling pathway (t1/2=15-30 min) (Hopkins and Trowbridge, 1983).
Fig. 2.
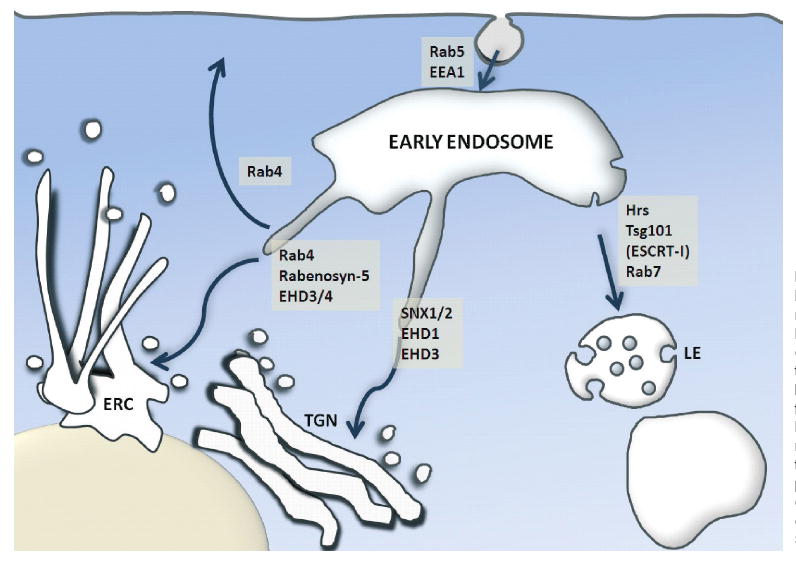
Endocytic sorting at the EE. Delivery of internalized cargo molecules to EE is mediated by GTP-Rab5 and its effector EEA1 in a series of homotypic fusion events. Cargo can then either be sorted for recycling back to the plasma membrane in a fast (Rab4-mediated) or slow (Rab4, Rabenosyn-5, EHD3 and/or EHD4-mediated) pathway. Alternatively, in the case of mono-ubiquitinated cargo, proteins are sorted for lysosomal degradation (via Hrs, Tsg101, ESCRT complex, Rab7) or to the Golgi (via SNX1/2 and EHD1/3).
For transferrin to undergo fast recycling, it needs to be quickly sorted away from an early endosomal Rab5-containing microdomain into a Rab4 microdomain on the same vesicle. Alternatively, transferrin is delivered to a Rab4/Rab11-containing ERC in a trafficking step that exhibits significantly slower kinetic rates (Sonnichsen et al., 2000). Perturbation of the Rab4 GTPase activity in GDP-locked dominant-negative mutants results in reduced exit of TfR from the EEs and subsequent accumulation of TfR (McCaffrey et al., 2001). However, although the constitutively-active Rab4 mutant induces a very modest increase in TfR recycling, the overexpression of this mutant led to a dramatic increase in TfR localization to the tubular membranes. An identical effect has been observed upon wild-type Rab4 overexpression, implying the role of Rab4 in budding and formation of recycling tubules at the level of EE.
Rab4 exerts its endocytic regulatory role through interaction with various effector proteins, including the previously discussed Rabenosyn-5 and Rabaptin-5/Rabex-5 complex (Vitale et al., 1998; de Renzis et al., 2002; Mattera and Bonifacino, 2008). Immunodepletion of Rabaptin-5/Rabex-5 in permeabilized, cytosol-free cells resulted in stimulation of the recycling vesicle formation in vitro, while the addition of purified Rabaptin-5/Rabex-5 had the opposite effect (Pagano et al., 2004). Although both proteins also serve as Rab5 effectors, the process of recycling vesicle formation appears to be only Rab4-mediated since the immunodepletion of Rab5 itself had no effect. Interestingly, a study by Deneka et al. previously identified Rabaptin-5 as a linker protein between Rab4 and the γ1-adaptin subunit of AP-1 (Deneka et al., 2003). Therefore the binding of Rabaptin-5/Rabex-5 to AP-1 could potentially have a regulatory role in preventing the association of clathrin with AP-1 γ1-adaptin, resulting in inhibition of coat formation and budding at the EE membranes. Subsequent formation of the recycling vesicles, however, likely requires the presence of a nexus protein linking Rab4 on EE to proteins at the ERC. Rabenosyn-5, a multivalent Rab4/Rab5 effector, may act as such a linker between Rab4 and EHD1, an endocytic regulator found primarily on the tubulo-vesicular ERC membranes (Naslavsky et al., 2004). Depletion of Rabenosyn-5 leads to accumulation of transferrin in an EE compartment containing EEA1 and Rab5, indicating its role in mediating the delivery of recycling cargo to the ERC. On the other hand, depletion of EHD1 results in cargo accumulation at the ERC (Naslavsky et al., 2004).
A fork in the road
Until recently, most models proposed that receptors lacking a degradation or biosynthetic targeting signal would by default undergo recycling due to lipid-based sorting and subsequent tubulation (reviewed by (Gruenberg, 2001; Maxfield and McGraw, 2004)). Accordingly, truncation of the TfR cytosolic tail did not affect its recycling rate (Johnson et al., 1993), while a more recent study showed that the fusion of a mono-ubiquitin moiety to the TfR cytosolic tail induced its sorting into the degradative pathway, further supporting the precedence of degradative sorting signals over the signal-independent recycling events (Raiborg et al., 2002). However, a recent finding has challenged this model. The Arf6 GTPase-activating protein, ACAP1, serves as a sorting molecule involved in direct binding to two phenylalanine-containing clusters on the TfR (Dai et al., 2004). Mutation of these critical residues abolishes the ACAP1 association and delays TfR recycling, although the lack of a complete block in recycling indicates the existence of an alternative recycling route(s) (Dai et al., 2004). Other receptors also contain sorting motifs that can be transplanted to a non-recycling receptor and induce recycling (Pattni and Stenmark, 2006). Although there is no single consensus motif, phosphorylation of serine 411 of the β2-adrenergic receptor recruits the EBP50 (ezrin-radixin-moesin (ERM)-binding phosphoprotein-50) to regulate recycling of this receptor (Cao et al., 1999). Hrs also binds to the cytosolic tail of both the β2-adrenergic and μ-opioid receptors and directs them to a recycling pathway, thus exerting a critical role in receptor recycling independent of its function as a lysosomal sorting determinant (Hanyaloglu et al., 2005).
Sorting to the TGN
In addition to its role as a diverging point between the degradative and recycling pathways, the EE also serves as a junction between the endocytic and biosynthetic routes. Such a diverse role of the EE as a critical sorting organelle is reflected in its complex morphology. Distinct membrane subdomains mediate sorting of cargo into various pathways. While the formation of endosomal tubular structures facilitates efficient recycling of receptors back to the plasma membrane, retromer-mediated tubulation is essential for retrograde transport of cargo from EE to the trans-Golgi network (TGN) (Bonifacino and Hurley, 2008). A defining property that differentiates these two tubulation events is the maturation state of the EE. Sorting of the recycling cargo into endosomal tubular structures precedes the retromer-mediated formation of the tubules recently described as SNX1/sortilin-enriched endosome-to-TGN transport carriers (ETCs) (Mari et al., 2008). Retromer machinery is preferentially recruited to maturing EEs containing increasing concentrations of PtdIns(3,5)P2 generated by PIKfyve kinase (Rutherford et al., 2006) and an increased number of intralumenal vesicles (Arighi et al., 2004), both hallmarks of maturation into late endosomes.
Instrumental to the understanding of retrograde transport were the trafficking studies conducted using lysosomal/vacuolar acid-hydrolase receptors as prototypical retromer cargos. S. cerevisiae hydrolase receptor Vps10p and mammalian cation-independent mannose 6-phosphate receptor (CI-MPR) directly associated with the sorting subunit of the retromer complex, Vps35p (Nothwehr et al., 2000) and mammalian hVps35 (Arighi et al., 2004), respectively. This interaction in S. cerevisiae is followed by the direct binding of Vps29p to Vps35p/Vps10p that gives rise to a stable retromer subcomplex responsible for cargo sorting (Seaman et al., 1998). In addition, a second retromer subcomplex involved in membrane association is assembled through dimerization of the two members of the sorting nexin family of proteins, Vps5p and Vps17p (Horazdovsky et al., 1997; Seaman et al., 1998). Both proteins contain a PtdIns(3)P-binding Phox (PX) domain, essential for association with EE membranes (Burda et al., 2002), as well as a curvature-sensing Bin Amphiphysin Rvs (BAR) domain, essential for retromer-regulated formation of tubular membrane structures necessary for efficient retrograde transport (Carlton et al., 2004; Bonifacino and Rojas, 2006). Final assembly of the two subcomplexes into a functional retromer requires the interaction of Vps5p/Vps17p with Vps35p and is facilitated by an additional retromer subunit, Vps29p (Reddy and Seaman, 2001). Depletion of Vps26p by SiRNA and knock-out approaches rendered the retromer inactive, resulting in significant defects in the retrograde transport of cargo, including the CI-MPR (Seaman, 2004).
Recent studies have identified two additional sorting nexins as critical components of the mammalian retromer. An RNAi-mediated loss-of-function screen of the entire SNX family of proteins has yielded a retrograde trafficking role for SNX5 and SNX6 (Wassmer et al., 2007). Interestingly, endogenous SNX6 associates with endogenous SNX1, while both SNX5 and SNX6 co-localize with SNX1 on tubular and vesicular EE structures. Furthermore, knockdown of either SNX5 or SNX6 results in destabilization and dramatic loss of endogenous SNX1, most likely through degradation (Wassmer et al., 2007). Collectively these recent findings have attributed a more complex composition of mammalian retromer than that observed in yeast. The current model for the assembly of the membrane-bound subcomplex proposes that it consists of SNX1 and SNX2 as the interchangeable Vps5p orthologues, and SNX5 and SNX6 as the functional orthologues of Vps17p (Bonifacino and Hurley, 2008; Collins, 2008; Cullen, 2008). However, the exact modality of the SNX subcomplex assembly as well as existence of additional components remains to be determined.
A recent study by Rojas et al. has shed a new light on the mechanism of retromer recruitment to the EE through the coordinate action of Rab5 and Rab7, with the VPS26/29/35 subcomplex acting as a Rab7 effector (Rojas et al., 2008). Once the GTP-bound active Rab5 associates with EE, numerous effectors are recruited, including a class III PtdIns(3)-kinase that generates PtdIns(3)P at these membranes. Subsequently the enrichment of EE membranes with PtdIns(3)P results in the increased recruitment of the SNX subcomplex. Nevertheless, due to the weak interaction of two retromer subcomplexes, the association of SNX subcomplex with the EE is insufficient for the recruitment of the cargo-sorting subunits (Rojas et al., 2008). Instead, Rab5 binds to a known Rab7 GEF, the VPS C-homotypic fusion and vacuole protein sorting (HOPS) complex, resulting in Rab7 activation and association with the EE membranes (Rink et al., 2005). Rab7-GTP can then serve as a nexus between the two subcomplexes through direct association with the cargo-sorting subcomplex. This interaction is then sufficient to bring the two subcomplexes in close proximity to generate a more stable association, leading to retromer assembly (Rojas et al., 2008).
In addition to its central role in retromer assembly, VPS35 also mediates cargo recognition by low affinity binding to a conserved cargo sorting motif Trp/Phe-Leu-Met/Val at the cytosolic tail of CI-MPR (Arighi et al., 2004; Gokool et al., 2007). Furthermore, studies aimed at identifying retromer-binding proteins have proven instrumental to the efforts of dissecting the cargo selection. Yeast sorting nexin Grd19 associates with retromer and mediates its binding to the C-terminal sorting sequence GHLPFTKNLQ of the Ftr1/Fet3 reductive iron transporter (Voos and Stevens, 1998; Strochlic et al., 2007). Other endocytic proteins, such as the C-terminal EHD protein, EHD1, can also bind the retromer (Gokool et al., 2007). Although it remains to be determined whether EHD1 can bind retromer directly, it plays a role in facilitating efficient retrograde transport and tubulation of the retromer-associated membranes. Moreover, it has been recently demonstrated that EHD3, the closest mammalian EHD1 paralog, regulates endosome-to-Golgi transport and retromer subunit localization (Naslavsky et al., 2009).
Proper sorting of endocytic cargo at the EE depends on the coordinate action of numerous endocytic regulatory molecules. Loss of regulation of sorting machinery can therefore result in aberrant protein localization and potentially lead to the onset of various pathological conditions (Table 1). However, in spite the apparent significance of this process, our understanding of EE sorting is still evolving as we further dissect the protein and lipid interactions occurring at the EE.
References
- Aledo JC, Darakhshan F, Hundal HS. Rab4, but not the transferrin receptor, is colocalized with GLUT4 in an insulin-sensitive intracellular compartment in rat skeletal muscle. Biochem Biophys Res Commun. 1995;215:321–328. doi: 10.1006/bbrc.1995.2469. [DOI] [PubMed] [Google Scholar]
- Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J Cell Biol. 2004;165:123–33. doi: 10.1083/jcb.200312055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbey CM, Ahktar N, Wang E, Chen CC, Grant BD, Dunn KW. Rab10 regulates membrane transport through early endosomes of polarized Madin-Darby canine kidney cells. Mol Biol Cell. 2006;17:3156–3175. doi: 10.1091/mbc.E05-08-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babst M, Katzmann DJ, Estepa-Sabal EJ, Meerloo T, Emr SD. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev Cell. 2002a;3:271–282. doi: 10.1016/s1534-5807(02)00220-4. [DOI] [PubMed] [Google Scholar]
- Babst M, Katzmann DJ, Snyder WB, Wendland B, Emr SD. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev Cell. 2002b;3:283–289. doi: 10.1016/s1534-5807(02)00219-8. [DOI] [PubMed] [Google Scholar]
- Bache KG, Brech A, Mehlum A, Stenmark H. Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes. J Cell Biol. 2003a;162:435–442. doi: 10.1083/jcb.200302131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bache KG, Raiborg C, Mehlum A, Stenmark H. STAM and Hrs are subunits of a multivalent ubiquitin-binding complex on early endosomes. J Biol Chem. 2003b;278:12513–12521. doi: 10.1074/jbc.M210843200. [DOI] [PubMed] [Google Scholar]
- Barbieri MA, Roberts RL, Mukhopadhyay A, Stahl PD. Rab5 regulates the dynamics of early endosome fusion. Biocell. 1996;20:331–338. [PubMed] [Google Scholar]
- Begley MJ, Dixon JE. The structure and regulation of myotubularin phosphatases. Curr Opin Struct Biol. 2005;15:614–620. doi: 10.1016/j.sbi.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Benmerah A. Endocytosis: signaling from endocytic membranes to the nucleus. Curr Biol. 2004;14:R314–316. doi: 10.1016/j.cub.2004.03.053. [DOI] [PubMed] [Google Scholar]
- Bennett MK. SNAREs and the specificity of transport vesicle targeting. Curr Opin Cell Biol. 1995;7:581–586. doi: 10.1016/0955-0674(95)80016-6. [DOI] [PubMed] [Google Scholar]
- Blondeau F, Laporte J, Bodin S, Superti-Furga G, Payrastre B, Mandel JL. Myotubularin, a phosphatase deficient in myotubular myopathy, acts on phosphatidylinositol 3-kinase and phosphatidylinositol 3-phosphate pathway. Hum Mol Genet. 2000;9:2223–2229. doi: 10.1093/oxfordjournals.hmg.a018913. [DOI] [PubMed] [Google Scholar]
- Bock JB, Matern HT, Peden AA, Scheller RH. A genomic perspective on membrane compartment organization. Nature. 2001;409:839–841. doi: 10.1038/35057024. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Hurley JH. Retromer. Curr Opin Cell Biol. 2008;20:427–436. doi: 10.1016/j.ceb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol. 2006;7:568–579. doi: 10.1038/nrm1985. [DOI] [PubMed] [Google Scholar]
- Brandhorst D, Zwilling D, Rizzoli SO, Lippert U, Lang T, Jahn R. Homotypic fusion of early endosomes: SNAREs do not determine fusion specificity. Proc Natl Acad Sci USA. 2006;103:2701–2706. doi: 10.1073/pnas.0511138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci C, Parton RG, Mather IH, Stunnenberg H, Simons K, Hoflack B, Zerial M. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell. 1992;70:715–728. doi: 10.1016/0092-8674(92)90306-w. [DOI] [PubMed] [Google Scholar]
- Burda P, Padilla SM, Sarkar S, Emr SD. Retromer function in endosome-to-Golgi retrograde transport is regulated by the yeast Vps34 PtdIns 3-kinase. J Cell Sci. 2002;115:3889–3900. doi: 10.1242/jcs.00090. [DOI] [PubMed] [Google Scholar]
- Cabezas A, Pattni K, Stenmark H. Cloning and subcellular localization of a human phosphatidylinositol 3-phosphate 5-kinase, PIKfyve/Fab1. Gene. 2006;371:34–41. doi: 10.1016/j.gene.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the beta2-adrenergic receptor. Nature. 1999;401:286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- Cao C, Laporte J, Backer JM, Wandinger-Ness A, Stein MP. Myotubularin lipid phosphatase binds the hVPS15/hVPS34 lipid kinase complex on endosomes. Traffic. 2007;8:1052–1067. doi: 10.1111/j.1600-0854.2007.00586.x. [DOI] [PubMed] [Google Scholar]
- Cao C, Backer JM, Laporte J, Bedrick EJ, Wandinger-Ness A. Sequential actions of myotubularin lipid phosphatases regulate endosomal PI(3)P and growth factor receptor trafficking. Mol Biol Cell. 2008;19:3334–3346. doi: 10.1091/mbc.E08-04-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan S, Naslavsky N, Hartnell LM, Lodge R, Polishchuk RS, Donaldson JG, Bonifacino JS. A tubular EHD1-containing compartment involved in the recycling of major histocompatibility complex class I molecules to the plasma membrane. EMBO J. 2002;21:2557–2567. doi: 10.1093/emboj/21.11.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton J, Bujny M, Peter BJ, Oorschot VM, Rutherford A, Mellor H, Klumperman J, McMahon HT, Cullen PJ. Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high- curvature membranes and 3-phosphoinositides. Curr Biol. 2004;14:1791–1800. doi: 10.1016/j.cub.2004.09.077. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, Barnett JL, Pieroni C, Nixon RA. Increased neuronal endocytosis and protease delivery to early endosomes in sporadic Alzheimer's disease: neuropathologic evidence for a mechanism of increased beta-amyloidogenesis. J Neurosci. 1997;17:6142–6151. doi: 10.1523/JNEUROSCI.17-16-06142.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo AM, Mathews PM, Boiteau AB, Hassinger LC, Peterhoff CM, Jiang Y, Mullaney K, Neve RL, Gruenberg J, Nixon RA. Down syndrome fibroblast model of Alzheimer-related endosome pathology: accelerated endocytosis promotes late endocytic defects. Am J Pathol. 2008;173:370–384. doi: 10.2353/ajpath.2008.071053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo AM, Petanceska S, Peterhoff CM, Terio NB, Epstein CJ, Villar A, Carlson EJ, Staufenbiel M, Nixon RA. App gene dosage modulates endosomal abnormalities of Alzheimer's disease in a segmental trisomy 16 mouse model of down syndrome. J Neurosci. 2003;23:6788–6792. doi: 10.1523/JNEUROSCI.23-17-06788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol. 2001;2:98–106. doi: 10.1038/35052017. [DOI] [PubMed] [Google Scholar]
- Chibalina MV, Seaman MN, Miller CC, Kendrick-Jones J, Buss F. Myosin VI and its interacting protein LMTK2 regulate tubule formation and transport to the endocytic recycling compartment. J Cell Sci. 2007;120:4278–4288. doi: 10.1242/jcs.014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury R, Diao A, Zhang F, Eisenberg E, Saint-Pol A, Williams C, Konstantakopoulos A, Lucocq J, Johannes L, Rabouille C, Greene LE, Lowe M. Lowe syndrome protein OCRL1 interacts with clathrin and regulates protein trafficking between endosomes and the trans-Golgi network. Mol Biol Cell. 2005;16:3467–3479. doi: 10.1091/mbc.E05-02-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip SC, Waterfield MD, Backer JM, Zerial M. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat Cell Biol. 1999;1:249–252. doi: 10.1038/12075. [DOI] [PubMed] [Google Scholar]
- Collins BM. The structure and function of the retromer protein complex. Traffic. 2008;9:1811–1822. doi: 10.1111/j.1600-0854.2008.00777.x. [DOI] [PubMed] [Google Scholar]
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- Cormont M, Bortoluzzi MN, Gautier N, Mari M, van Obberghen E, Le Marchand-Brustel Y. Potential role of Rab4 in the regulation of subcellular localization of Glut4 in adipocytes. Mol Cell Biol. 1996;16:6879–6886. doi: 10.1128/mcb.16.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozier GE, Carlton J, McGregor AH, Gleeson PA, Teasdale RD, Mellor H, Cullen PJ. The phox homology (PX) domain-dependent, 3-phosphoinositide-mediated association of sorting nexin-1 with an early sorting endosomal compartment is required for its ability to regulate epidermal growth factor receptor degradation. J Biol Chem. 2002;277:48730–48736. doi: 10.1074/jbc.M206986200. [DOI] [PubMed] [Google Scholar]
- Cullen PJ. Endosomal sorting and signalling: an emerging role for sorting nexins. Nat Rev Mol Cell Biol. 2008;9:574–582. doi: 10.1038/nrm2427. [DOI] [PubMed] [Google Scholar]
- Dai J, Li J, Bos E, Porcionatto M, Premont RT, Bourgoin S, Peters PJ, Hsu VW. ACAP1 promotes endocytic recycling by recognizing recycling sorting signals. Dev Cell. 2004;7:771–776. doi: 10.1016/j.devcel.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Daro E, van der Sluijs P, Galli T, Mellman I. Rab4 and cellubrevin define different early endosome populations on the pathway of transferrin receptor recycling. Proc Natl Acad Sci USA. 1996;93:9559–9564. doi: 10.1073/pnas.93.18.9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautry-Varsat A, Ciechanover A, Lodish HF. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci USA. 1983;80:2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Renzis S, Sonnichsen B, Zerial M. Divalent Rab effectors regulate the sub-compartmental organization and sorting of early endosomes. Nat Cell Biol. 2002;4:124–133. doi: 10.1038/ncb744. [DOI] [PubMed] [Google Scholar]
- de Wit H, Lichtenstein Y, Kelly RB, Geuze HJ, Klumperman J, van der Sluijs P. Rab4 regulates formation of synaptic-like microvesicles from early endosomes in PC12 cells. Mol Biol Cell. 2001;12:3703–3715. doi: 10.1091/mbc.12.11.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneka M, van der Sluijs P. ‘Rab’ing up endosomal membrane transport. Nat Cell Biol. 2002;4:E33–35. doi: 10.1038/ncb0202-e33. [DOI] [PubMed] [Google Scholar]
- Deneka M, Neeft M, Popa I, van Oort M, Sprong H, Oorschot V, Klumperman J, Schu P, van der Sluijs P. Rabaptin-5alpha/rabaptin-4 serves as a linker between rab4 and gamma(1)-adaptin in membrane recycling from endosomes. EMBO J. 2003;22:2645–2657. doi: 10.1093/emboj/cdg257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro SM, Falcon-Perez JM, Tenza D, Setty SR, Marks MS, Raposo G, Dell'Angelica EC. BLOC-1 interacts with BLOC-2 and the AP-3 complex to facilitate protein trafficking on endosomes. Mol Biol Cell. 2006;17:4027–4038. doi: 10.1091/mbc.E06-05-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JJ, Gibbs EM, Feldman EL. Membrane traffic and muscle: lessons from human disease. Traffic. 2008;9:1035–1043. doi: 10.1111/j.1600-0854.2008.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KW, McGraw TE, Maxfield FR. Iterative fractionation of recycling receptors from lysosomally destined ligands in an early sorting endosome. J Cell Biol. 1989;109:3303–3314. doi: 10.1083/jcb.109.6.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann KS, Mao Y, McCrea HJ, Zoncu R, Lee S, Paradise S, Modregger J, Biemesderfer D, Toomre D, De Camilli P. A role of the Lowe syndrome protein OCRL in early steps of the endocytic pathway. Dev Cell. 2007;13:377–390. doi: 10.1016/j.devcel.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer D. Structural insights into the SNARE mechanism. Biochim Biophys Acta. 2003;1641:87–97. doi: 10.1016/s0167-4889(03)00090-9. [DOI] [PubMed] [Google Scholar]
- Fasshauer D, Sutton RB, Brunger AT, Jahn R. Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc Natl Acad Sci USA. 1998;95:15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly DJ, Morrow IC, Lindsay M, Gould R, Bryant NJ, Gaullier JM, Parton RG, Stenmark H. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 2000;19:4577–4588. doi: 10.1093/emboj/19.17.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokool S, Tattersall D, Seaman MN. EHD1 interacts with retromer to stabilize SNX1 tubules and facilitate endosome-to-Golgi retrieval. Traffic. 2007;8:1873–1886. doi: 10.1111/j.1600-0854.2007.00652.x. [DOI] [PubMed] [Google Scholar]
- Gorvel JP, Chavrier P, Zerial M, Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Grant BD, Caplan S. Mechanisms of EHD/RME-1 protein function in endocytic transport. Traffic. 2008;9:2043–2052. doi: 10.1111/j.1600-0854.2008.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg J. The endocytic pathway: a mosaic of domains. Nat Rev Mol Cell Biol. 2001;2:721–730. doi: 10.1038/35096054. [DOI] [PubMed] [Google Scholar]
- Gruenberg J. Lipids in endocytic membrane transport and sorting. Curr Opin Cell Biol. 2003;15:382–388. doi: 10.1016/s0955-0674(03)00078-4. [DOI] [PubMed] [Google Scholar]
- Gruenberg J, Griffiths G, Howell KE. Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J Cell Biol. 1989;108:1301–1316. doi: 10.1083/jcb.108.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol. 2003;5:461–466. doi: 10.1038/ncb983. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Roth R, Morisaki H, Jahn R, Heuser JE. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell. 1997;90:523–535. doi: 10.1016/s0092-8674(00)80512-7. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Whiteheart SW. AAA+ proteins: have engine, will work. Nat Rev Mol Cell Biol. 2005;6:519–529. doi: 10.1038/nrm1684. [DOI] [PubMed] [Google Scholar]
- Hanyaloglu AC, McCullagh E, von Zastrow M. Essential role of Hrs in a recycling mechanism mediating functional resensitization of cell signaling. EMBO J. 2005;24:2265–2283. doi: 10.1038/sj.emboj.7600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeki O. Wortmannin, an inhibitor of phosphatidylinositol 3-kinase. Seikagaku. 1995;67:33–36. In Japanese. [PubMed] [Google Scholar]
- Herbst JJ, Opresko LK, Walsh BJ, Lauffenburger DA, Wiley HS. Regulation of postendocytic trafficking of the epidermal growth factor receptor through endosomal retention. J Biol Chem. 1994;269:12865–12873. [PubMed] [Google Scholar]
- Hoepfner S, Severin F, Cabezas A, Habermann B, Runge A, Gillooly D, Stenmark H, Zerial M. Modulation of receptor recycling and degradation by the endosomal kinesin KIF16B. Cell. 2005;121:437–450. doi: 10.1016/j.cell.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Hopkins CR, Trowbridge IS. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983;97:508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horazdovsky BF, Davies BA, Seaman MN, McLaughlin SA, Yoon S, Emr SD. A sorting nexin-1 homologue, Vps5p, forms a complex with Vps17p and is required for recycling the vacuolar protein-sorting receptor. Mol Biol Cell. 1997;8:1529–1541. doi: 10.1091/mbc.8.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi H, Lippe R, McBride HM, Rubino M, Woodman P, Stenmark H, Rybin V, Wilm M, Ashman K, Mann M, et al. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell. 1997;90:1149–1159. doi: 10.1016/s0092-8674(00)80380-3. [DOI] [PubMed] [Google Scholar]
- Ikonomov OC, Sbrissa D, Foti M, Carpentier JL, Shisheva A. PIKfyve controls fluid phase endocytosis but not recycling/degradation of endocytosed receptors or sorting of procathepsin D by regulating multivesicular body morphogenesis. Mol Biol Cell. 2003;14:4581–4591. doi: 10.1091/mbc.E03-04-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomo OC, Sbrissa D, Mlak K, Kanzaki M, Pessin J, Shisheva A. Functional dissection of lipid and protein kinase signals of PIKfyve reveals the role of PtdIns 3,5-P2 production for endomembrane integrity. J Biol Chem. 2002;277:9206–9211. doi: 10.1074/jbc.M108750200. [DOI] [PubMed] [Google Scholar]
- Ikonomov OC, Sbrissa D, Shisheva A. Mammalian cell morphology and endocytic membrane homeostasis require enzymatically active phosphoinositide 5-kinase PIKfyve. J Biol Chem. 2001;276:26141–26147. doi: 10.1074/jbc.M101722200. [DOI] [PubMed] [Google Scholar]
- Johnson LS, Dunn KW, Pytowski B, McGraw TE. Endosome acidification and receptor trafficking: bafilomycin A1 slows receptor externalization by a mechanism involving the receptor's internalization motif. Mol Biol Cell. 1993;4:1251–1266. doi: 10.1091/mbc.4.12.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovic M, Naslavsky N, Rapaport D, Horowitz M, Caplan S. EHD1 regulates beta1 integrin endosomal transport: effects on focal adhesions, cell spreading and migration. J Cell Sci. 2007;120:802–814. doi: 10.1242/jcs.03383. [DOI] [PubMed] [Google Scholar]
- Junutula JR, De Maziere AM, Peden AA, Ervin KE, Advani RJ, van Dijk SM, Klumperman J, Scheller RH. Rab14 is involved in membrane trafficking between the Golgi complex and endosomes. Mol Biol Cell. 2004;15:2218–2229. doi: 10.1091/mbc.E03-10-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppi M, Simonsen A, Bremnes B, Vieira A, Callaghan J, Stenmark H, Olkkonen VM. The small GTPase Rab22 interacts with EEA1 and controls endosomal membrane trafficking. J Cell Sci. 2002;115:899–911. doi: 10.1242/jcs.115.5.899. [DOI] [PubMed] [Google Scholar]
- Lawe DC, Chawla A, Merithew E, Dumas J, Carrington W, Fogarty K, Lifshitz L, Tuft R, Lambright D, Corvera S. Sequential roles for phosphatidylinositol 3-phosphate and Rab5 in tethering and fusion of early endosomes via their interaction with EEA1. J Biol Chem. 2002;277:8611–8617. doi: 10.1074/jbc.M109239200. [DOI] [PubMed] [Google Scholar]
- Lawe DC, Patki V, Heller-Harrison R, Lambright D, Corvera S. The FYVE domain of early endosome antigen 1 is required for both phosphatidylinositol 3-phosphate and Rab5 binding. Critical role of this dual interaction for endosomal localization. J Biol Chem. 2000;275:3699–3705. doi: 10.1074/jbc.275.5.3699. [DOI] [PubMed] [Google Scholar]
- Lazzarino DA, Blier P, Mellman I. The monomeric guanosine triphosphatase rab4 controls an essential step on the pathway of receptor-mediated antigen processing in B cells. J Exp Med. 1998;188:1769–1774. doi: 10.1084/jem.188.10.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RC, Scheller RH. Structural organization of the synaptic exocytosis core complex. Neuron. 1997;19:1087–1094. doi: 10.1016/s0896-6273(00)80399-2. [DOI] [PubMed] [Google Scholar]
- Lin SX, Grant B, Hirsh D, Maxfield FR. Rme-1 regulates the distribution and function of the endocytic recycling compartment in mammalian cells. Nat Cell Biol. 2001;3:567–572. doi: 10.1038/35078543. [DOI] [PubMed] [Google Scholar]
- Lippe R, Miaczynska M, Rybin V, Runge A, Zerial M. Functional synergy between Rab5 effector Rabaptin-5 and exchange factor Rabex-5 when physically associated in a complex. Mol Biol Cell. 2001;12:2219–2228. doi: 10.1091/mbc.12.7.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magadan JG, Barbieri MA, Mesa R, Stahl PD, Mayorga LS. Rab22a regulates the sorting of transferrin to recycling endosomes. Mol Cell Biol. 2006;26:2595–2614. doi: 10.1128/MCB.26.7.2595-2614.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari M, Bujny MV, Zeuschner D, Geerts WJ, Griffith J, Petersen CM, Cullen PJ, Klumperman J, Geuze HJ. SNX1 Defines an Early Endosomal Recycling Exit for Sortilin and Mannose 6-Phosphate Receptors. Traffic. 2008;9:380–393. doi: 10.1111/j.1600-0854.2007.00686.x. [DOI] [PubMed] [Google Scholar]
- Mattera R, Bonifacino JS. Ubiquitin binding and conjugation regulate the recruitment of Rabex-5 to early endosomes. EMBO J. 2008;27:2484–2494. doi: 10.1038/emboj.2008.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of Sec17p (alpha-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Mayor S, Presley JF, Maxfield FR. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol. 1993;121:1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride HM, Rybin V, Murphy C, Giner A, Teasdale R, Zerial M. Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell. 1999;98:377–386. doi: 10.1016/s0092-8674(00)81966-2. [DOI] [PubMed] [Google Scholar]
- McCaffrey MW, Bielli A, Cantalupo G, Mora S, Roberti V, Santillo M, Drummond F, Bucci C. Rab4 affects both recycling and degradative endosomal trafficking. FEBS Lett. 2001;495:21–30. doi: 10.1016/s0014-5793(01)02359-6. [DOI] [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Mesa R, Salomon C, Roggero M, Stahl PD, Mayorga LS. Rab22a affects the morphology and function of the endocytic pathway. J Cell Sci. 2001;114:4041–4049. doi: 10.1242/jcs.114.22.4041. [DOI] [PubMed] [Google Scholar]
- Mesa R, Magadan J, Barbieri A, Lopez C, Stahl PD, Mayorga LS. Overexpression of Rab22a hampers the transport between endosomes and the Golgi apparatus. Exp Cell Res. 2005;304:339–353. doi: 10.1016/j.yexcr.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Miaczynska M, Christoforidis S, Giner A, Shevchenko A, Uttenweiler-Joseph S, Habermann B, Wilm M, Parton RG, Zerial M. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell. 2004;116:445–456. doi: 10.1016/s0092-8674(04)00117-5. [DOI] [PubMed] [Google Scholar]
- Mills IG, Jones AT, Clague MJ. Involvement of the endosomal autoantigen EEA1 in homotypic fusion of early endosomes. Curr Biol. 1998;8:881–884. doi: 10.1016/s0960-9822(07)00351-x. [DOI] [PubMed] [Google Scholar]
- Mills IG, Jones AT, Clague MJ. Regulation of endosome fusion. Mol Membr Biol. 1999;16:73–79. doi: 10.1080/096876899294788. [DOI] [PubMed] [Google Scholar]
- Mills IG, Urbe S, Clague MJ. Relationships between EEA1 binding partners and their role in endosome fusion. J Cell Sci. 2001;114:1959–1965. doi: 10.1242/jcs.114.10.1959. [DOI] [PubMed] [Google Scholar]
- Mintz L, Galperin E, Pasmanik-Chor M, Tulzinsky S, Bromberg Y, Kozak CA, Joyner A, Fein A, Horowitz M. EHD1--an EH-domain-containing protein with a specific expression pattern. Genomics. 1999;59:66–76. doi: 10.1006/geno.1999.5800. [DOI] [PubMed] [Google Scholar]
- Morino C, Kato M, Yamamoto A, Mizuno E, Hayakawa A, Komada M, Kitamura N. A role for Hrs in endosomal sorting of ligand-stimulated and unstimulated epidermal growth factor receptor. Exp Cell Res. 2004;297:380–391. doi: 10.1016/j.yexcr.2004.03.038. [DOI] [PubMed] [Google Scholar]
- Mosesson Y, Shtiegman K, Katz M, Zwang Y, Vereb G, Szollosi J, Yarden Y. Endocytosis of receptor tyrosine kinases is driven by monoubiquitylation, not polyubiquitylation. J Biol Chem. 2003;278:21323–21326. doi: 10.1074/jbc.C300096200. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Mukherjee K, Parashuraman S, Raje M, Mukhopadhyay A. SopE acts as an Rab5-specific nucleotide exchange factor and recruits non-prenylated Rab5 on Salmonella-containing phagosomes to promote fusion with early endosomes. J Biol Chem. 2001;276:23607–23615. doi: 10.1074/jbc.M101034200. [DOI] [PubMed] [Google Scholar]
- Murray JT, Panaretou C, Stenmark H, Miaczynska M, Backer JM. Role of Rab5 in the recruitment of hVps34/p150 to the early endosome. Traffic. 2002;3:416–427. doi: 10.1034/j.1600-0854.2002.30605.x. [DOI] [PubMed] [Google Scholar]
- Naslavsky N, Caplan S. C-terminal EH-domain-containing proteins: consensus for a role in endocytic trafficking, EH? J Cell Sci. 2005;118:4093–4101. doi: 10.1242/jcs.02595. [DOI] [PubMed] [Google Scholar]
- Naslavsky N, Boehm M, Backlund PS, Jr, Caplan S. Rabenosyn-5 and EHD1 interact and sequentially regulate protein recycling to the plasma membrane. Mol Biol Cell. 2004;15:2410–2422. doi: 10.1091/mbc.E03-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky N, McKenzie J, Altan-Bonnet N, Sheff D, Caplan S. EHD3 regulates early-endosome-to-Golgi transport and preserves Golgi morphology. J Cell Sci. 2009;122:389–400. doi: 10.1242/jcs.037051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky N, Rahajeng J, Sharma M, Jovic M, Caplan S. Interactions between EHD proteins and Rab11-FIP2: a role for EHD3 in early endosomal transport. Mol Biol Cell. 2006;17:163–177. doi: 10.1091/mbc.E05-05-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicot AS, Laporte J. Endosomal phosphoinositides and human diseases. Traffic. 2008;9:1240–1249. doi: 10.1111/j.1600-0854.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen E, Severin F, Backer JM, Hyman AA, Zerial M. Rab5 regulates motility of early endosomes on microtubules. Nat Cell Biol. 1999;1:376–382. doi: 10.1038/14075. [DOI] [PubMed] [Google Scholar]
- Nielsen E, Christoforidis S, Uttenweiler-Joseph S, Miaczynska M, Dewitte F, Wilm M, Hoflack B, Zerial M. Rabenosyn-5, a novel Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain. J Cell Biol. 2000;151:601–612. doi: 10.1083/jcb.151.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr SF, Ha SA, Bruinsma P. Sorting of yeast membrane proteins into an endosome-to-Golgi pathway involves direct interaction of their cytosolic domains with Vps35p. J Cell Biol. 2000;151:297–310. doi: 10.1083/jcb.151.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi H, Mine T, Shibata H, Ueda N, Tsuchida T, Fujita T. Involvement of Rab4 in regulated exocytosis of rat pancreatic acini. Gastroenterology. 1999;116:943–952. doi: 10.1016/s0016-5085(99)70078-8. [DOI] [PubMed] [Google Scholar]
- Pagano A, Crottet P, Prescianotto-Baschong C, Spiess M. In vitro formation of recycling vesicles from endosomes requires adaptor protein-1/clathrin and is regulated by rab4 and the connector rabaptin-5. Mol Biol Cell. 2004;15:4990–5000. doi: 10.1091/mbc.E04-04-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A, Severin F, Hopfner S, Zerial M. Regulation of endosome dynamics by Rab5 and Huntingtin-HAP40 effector complex in physiological versus pathological conditions. Methods Enzymol. 2008;438:239–257. doi: 10.1016/S0076-6879(07)38017-8. [DOI] [PubMed] [Google Scholar]
- Pal A, Severin F, Lommer B, Shevchenko A, Zerial M. Huntingtin-HAP40 complex is a novel Rab5 effector that regulates early endosome motility and is up-regulated in Huntington's disease. J Cell Biol. 2006;172:605–618. doi: 10.1083/jcb.200509091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patki V, Virbasius J, Lane WS, Toh BH, Shpetner HS, Corvera S. Identification of an early endosomal protein regulated by phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1997;94:7326–7330. doi: 10.1073/pnas.94.14.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattni K, Stenmark H. Protein sorting in endosomes. In: Dikic I, editor. Endosomes. Landes Bioscience and Springer; Austin, TX: 2006. pp. 76–88. [Google Scholar]
- Perskvist N, Roberg K, Kulyte A, Stendahl O. Rab5a GTPase regulates fusion between pathogen-containing phagosomes and cytoplasmic organelles in human neutrophils. J Cell Sci. 2002;115:1321–1330. doi: 10.1242/jcs.115.6.1321. [DOI] [PubMed] [Google Scholar]
- Petiot A, Faure J, Stenmark H, Gruenberg J. PI3P signaling regulates receptor sorting but not transport in the endosomal pathway. J Cell Biol. 2003;162:971–979. doi: 10.1083/jcb.200303018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 2001;11:487–491. doi: 10.1016/s0962-8924(01)02147-x. [DOI] [PubMed] [Google Scholar]
- Pohl U, Smith JS, Tachibana I, Ueki K, Lee HK, Ramaswamy S, Wu Q, Mohrenweiser HW, Jenkins RB, Louis DN. EHD2, EHD3, and EHD4 encode novel members of a highly conserved family of EH domain-containing proteins. Genomics. 2000;63:255–262. doi: 10.1006/geno.1999.6087. [DOI] [PubMed] [Google Scholar]
- Proikas-Cezanne T, Gaugel A, Frickey T, Nordheim A. Rab14 is part of the early endosomal clathrin-coated TGN microdomain. FEBS Lett. 2006;580:5241–5246. doi: 10.1016/j.febslet.2006.08.053. [DOI] [PubMed] [Google Scholar]
- Raiborg C, Bache KG, Gillooly DJ, Madshus IH, Stang E, Stenmark H. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat Cell Biol. 2002;4:394–398. doi: 10.1038/ncb791. [DOI] [PubMed] [Google Scholar]
- Raiborg C, Bremnes B, Mehlum A, Gillooly DJ, D'Arrigo A, Stang E, Stenmark H. FYVE and coiled-coil domains determine the specific localisation of Hrs to early endosomes. J Cell Sci. 2001;114:2255–2263. doi: 10.1242/jcs.114.12.2255. [DOI] [PubMed] [Google Scholar]
- Reddy JV, Seaman MN. Vps26p, a component of retromer, directs the interactions of Vps35p in endosome-to-Golgi retrieval. Mol Biol Cell. 2001;12:3242–3256. doi: 10.1091/mbc.12.10.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Rojas R, van Vlijmen T, Mardones GA, Prabhu Y, Rojas AL, Mohammed S, Heck AJ, Raposo G, van der Sluijs P, Bonifacino JS. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol. 2008;183:513–526. doi: 10.1083/jcb.200804048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford AC, Traer C, Wassmer T, Pattni K, Bujny MV, Carlton JG, Stenmark H, Cullen PJ. The mammalian phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) regulates endosome-to-TGN retrograde transport. J Cell Sci. 2006;119:3944–3957. doi: 10.1242/jcs.03153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbrissa D, Ikonomov OC, Shisheva A. Phosphatidylinositol 3-phosphate-interacting domains in PIKfyve. Binding specificity and role in PIKfyve Endomenbrane localization. J Biol Chem. 2002;277:6073–6079. doi: 10.1074/jbc.M110194200. [DOI] [PubMed] [Google Scholar]
- Schenck A, Goto-Silva L, Collinet C, Rhinn M, Giner A, Habermann B, Brand M, Zerial M. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell. 2008;133:486–497. doi: 10.1016/j.cell.2008.02.044. [DOI] [PubMed] [Google Scholar]
- Seaman MN. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol. 2004;165:111–122. doi: 10.1083/jcb.200312034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MN, McCaffery JM, Emr SD. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol. 1998;142:665–681. doi: 10.1083/jcb.142.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Naslavsky N, Caplan S. A role for EHD4 in the regulation of early endosomal transport. Traffic. 2008;9:995–1018. doi: 10.1111/j.1600-0854.2008.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheff DR, Daro EA, Hull M, Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J Cell Biol. 1999a;145:123–139. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheff DR, Daro EA, Hull M, Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J Cell Biol. 1999b;145:123–139. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa R, Yoshioka A, Horiuchi H, Nishioka H, Tabuchi A, Kita T. Small GTPase Rab4 regulates Ca2+-induced alpha-granule secretion in platelets. J Biol Chem. 2000;275:33844–33849. doi: 10.1074/jbc.M002834200. [DOI] [PubMed] [Google Scholar]
- Shisheva A. PIKfyve: Partners, significance, debates and paradoxes. Cell Biol Int. 2008;32:591–604. doi: 10.1016/j.cellbi.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A, Gaullier JM, D'Arrigo A, Stenmark H. The Rab5 effector EEA1 interacts directly with syntaxin-6. J Biol Chem. 1999;274:28857–28960. doi: 10.1074/jbc.274.41.28857. [DOI] [PubMed] [Google Scholar]
- Simonsen A, Lippe R, Christoforidis S, Gaullier JM, Brech A, Callaghan J, Toh BH, Murphy C, Zerial M, Stenmark H. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 1998;394:494–498. doi: 10.1038/28879. [DOI] [PubMed] [Google Scholar]
- Simonsen A, Wurmser AE, Emr SD, Stenmark H. The role of phosphoinositides in membrane transport. Curr Opin Cell Biol. 2001;13:485–492. doi: 10.1016/s0955-0674(00)00240-4. [DOI] [PubMed] [Google Scholar]
- Simpson JC, Griffiths G, Wessling-Resnick M, Fransen JA, Bennett H, Jones AT. A role for the small GTPase Rab21 in the early endocytic pathway. J Cell Sci. 2004;117:6297–6311. doi: 10.1242/jcs.01560. [DOI] [PubMed] [Google Scholar]
- Sollner T. SNAREs and targeted membrane fusion. FEBS Lett. 1995;369:80–93. doi: 10.1016/0014-5793(95)00594-y. [DOI] [PubMed] [Google Scholar]
- Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol. 2000;149:901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H, Vitale G, Ullrich O, Zerial M. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell. 1995;83:423–432. doi: 10.1016/0092-8674(95)90120-5. [DOI] [PubMed] [Google Scholar]
- Stone M, Jia S, Heo WD, Meyer T, Konan KV. Participation of rab5, an early endosome protein, in hepatitis C virus RNA replication machinery. J Virol. 2007;81:4551–4563. doi: 10.1128/JVI.01366-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strochlic TI, Setty TG, Sitaram A, Burd CG. Grd19/Snx3p functions as a cargo-specific adapter for retromer-dependent endocytic recycling. J Cell Biol. 2007;177:115–125. doi: 10.1083/jcb.200609161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate BA, Mathews PM. Targeting the role of the endosome in the pathophysiology of Alzheimer's disease: a strategy for treatment. Sci Aging Knowledge Environ. 2006;10:re2. doi: 10.1126/sageke.2006.10.re2. [DOI] [PubMed] [Google Scholar]
- Trischler M, Stoorvogel W, Ullrich O. Biochemical analysis of distinct Rab5- and Rab11-positive endosomes along the transferrin pathway. J Cell Sci. 1999;112:4773–4783. doi: 10.1242/jcs.112.24.4773. [DOI] [PubMed] [Google Scholar]
- Valetti C, Wetzel DM, Schrader M, Hasbani MJ, Gill SR, Kreis TE, Schroer TA. Role of dynactin in endocytic traffic: effects of dynamitin overexpression and colocalization with CLIP-170. Mol Biol Cell. 1999;10:4107–4120. doi: 10.1091/mbc.10.12.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam EM, Ten Broeke T, Jansen K, Spijkers P, Stoorvogel W. Endocytosed transferrin receptors recycle via distinct dynamin and phosphatidylinositol 3-kinase-dependent pathways. J Biol Chem. 2002;277:48876–48883. doi: 10.1074/jbc.M206271200. [DOI] [PubMed] [Google Scholar]
- van der Sluijs P, Hull M, Webster P, Male P, Goud B, Mellman I. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell. 1992;70:729–740. doi: 10.1016/0092-8674(92)90307-x. [DOI] [PubMed] [Google Scholar]
- Van Der Sluijs P, Hull M, Zahraoui A, Tavitian A, Goud B, Mellman I. The small GTP-binding protein rab4 is associated with early endosomes. Proc Natl Acad Sci USA. 1991;88:6313–6317. doi: 10.1073/pnas.88.14.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale G, Rybin V, Christoforidis S, Thornqvist P, McCaffrey M, Stenmark H, Zerial M. Distinct Rab-binding domains mediate the interaction of Rabaptin-5 with GTP-bound Rab4 and Rab5. EMBO J. 1998;17:1941–1951. doi: 10.1093/emboj/17.7.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Vollenweider P, Martin SS, Haruta T, Morris AJ, Nelson JG, Cormont M, Le Marchand-Brustel Y, Rose DW, Olefsky JM. The small guanosine triphosphate-binding protein Rab4 is involved in insulin-induced GLUT4 translocation and actin filament rearrangement in 3T3-L1 cells. Endocrinology. 1997;138:4941–4949. doi: 10.1210/endo.138.11.5493. [DOI] [PubMed] [Google Scholar]
- Voos W, Stevens TH. Retrieval of resident late-Golgi membrane proteins from the prevacuolar compartment of Saccharomyces cerevisiae is dependent on the function of Grd19p. J Cell Biol. 1998;140:577–590. doi: 10.1083/jcb.140.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassmer T, Attar N, Bujny MV, Oakley J, Traer CJ, Cullen PJ. A loss-of-function screen reveals SNX5 and SNX6 as potential components of the mammalian retromer. J Cell Sci. 2007;120:45–54. doi: 10.1242/jcs.03302. [DOI] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- Zwilling D, Cypionka A, Pohl WH, Fasshauer D, Walla PJ, Wahl MC, Jahn R. Early endosomal SNAREs form a structurally conserved SNARE complex and fuse liposomes with multiple topologies. EMBO J. 2007;26:9–18. doi: 10.1038/sj.emboj.7601467. [DOI] [PMC free article] [PubMed] [Google Scholar]


