Introduction
Research on the regulatory actions of the renin angiotensin system (RAS) continues to provide a wealth of information on how cells maintain their internal homeostatic environment, regulate metabolic processes, and adapt or contribute to disease. Not that long ago, the active product of the system, angiotensin II (Ang II), was considered the single critical hormone product of an endocrine system involved in regulating blood volume and vascular tone. A revised concept emerged following the demonstration that renin and angiotensinogen are present in tissues. These findings suggested that the RAS is comprised of a dual, independently regulated, blood-borne and tissue systems. Today, a broader and complex system is being revealed by advanced genetic and molecular tools, as well as the outcome of clinical studies using medications selective for one or more of the proteins contributing to the generation of angiotensin peptides.
Recognition that the RAS contains both a pressor and depressor arm in exerting regulatory functions on vascular tone and cellular signaling paved the way for the generation of an alternate hypothesis as to how an imbalance of their function contributes to cardiovascular disease.(1) This review summarizes the data supporting the hypothesis of a counter regulatory arm that within the RAS opposes the actions of Ang II. We build upon these earlier discoveries to provide a new insight into an additional pathway in which an extended form of angiotensin I (Ang I), proangiotensin 12 [Ang-(1–12)], may be an alternate substrate for the production of the biological active angiotensins. A comprehensive evaluation of this topic cannot be achieved within the assigned space; therefore, only the key elements of the topic will be addressed, asking for indulgence in not providing a detailed listing of all published studies.
Angiotensin-(1–7): The Paradigm Shift
The period from approximately 1970 to 1980 represented the beginning of a shift in the concept of how the RAS was involved in cardiovascular pathology. Renewed enthusiasm for its study was stimulated by the demonstration that the administration of the angiotensin converting enzyme (ACE) teprotide had a dramatic effect in reducing the blood pressure of hypertensive subjects. (2) These results prompted many laboratories to undertake newer approaches to the investigation of the physiology of Ang II, isolate its receptor, and undertake the eventual synthesis of orally active Ang II receptor antagonists. (3)
The progress made throughout these exciting discoveries, nevertheless continued to posit Ang II as the biologically relevant product of the biochemical cascade that initiated by renin culminated in the production of Ang II. Alternate processes were assumed to have no major relevance in terms of biological function. The publication of the first description that the N-terminal derived heptapeptide, angiotensin-(1–7) [Ang-(1–7)], stimulated the release of vasopressin from rat hypothalamic explants (4) would over time decisively alter the former view.
Although initial studies found that Ang-(1–7) acted as a vasodilator, (5) further research showed that this effect could be best demonstrated in isolated vessels, (6;7) in animals in which the baroreceptors are eliminated, (5) or in conditions in which endogenous levels of Ang II are increased by maneuvers such as sodium depletion (8;9) or renovascular hypertension. (10) These findings underscored the concept that Ang-(1–7) acts as a paracrine hormone when formed in close proximity to the vascular smooth muscle or that its actions depend upon a change in the signaling effector mechanisms associated with increased expression or activity of AT1 receptors. This is not an unreasonable possibility since it has been documented that the antihypertensive action of ACE inhibitors and Ang II receptor antagonists is amplified by concomitant use of thiazide diuretics.
Over the following decades, research would demonstrate that Ang-(1–7) contributes to the cardio-renal control of blood pressure via actions that within the heart, kidney, and the blood vessels opposed the activity of Ang II. (11–13) Ang-(1–7) was shown to reverse the hypertrophic and profibrotic effects of Ang II in the heart and the vasculature, (14–17) oppose Ang II-mediated cardiac arrhythmogenic activity, (18) possess antiatherogenic and antithrombotic actions, (19–24) inhibit oxidative stress and the generation of radical oxygen species, (25;26), and modulate hematopoietic function. (27;28)
Identification of the mas receptor as the conveyor for the cellular signaling responsible for Ang-(1–7) actions (29) and the demonstration that genetic deletion of this receptor abrogates the protective actions of the heptapeptide (30–36) has affirmed its participation in the regulation of cardiovascular function. Second messenger mechanisms responsible for the cellular response mediated by the binding of Ang-(1–7) to the mas receptor include inhibition of the mitogen activated protein (MAP) kinase kinase pathway, stimulation of cellular phosphatases, inhibition of cyclooxygenase 2 (COX2) and facilitation of nitric oxide release. (28;37–44)
ACE2 and Ang-(1–7)
The pace of research on the counter lever role of Ang-(1–7) on Ang II expanded with the identification of an ACE homologue, ACE2, that cleaved Ang II into Ang-(1–7). (45;46) As reviewed elsewhere,(47–50) ACE2 differs from ACE in acting as a mono-carboxypeptidase to cleave the Pro7-Phe8 bond of Ang II to form Ang-(1–7) and not been inhibitable by exposure to ACE inhibitors. A stepping stone in the evolving understanding of the role of ACE2 in cardiac function followed the demonstration that deletion of the ACE2 gene is accompanied by severe cardiac contractility defects and that ACE2 mRNA protein and expression were reduced in three different models of experimental hypertension. (51) Selective overexpression of cardiac ACE2 in rats is associated with reversal of cardiac hypertrophy (52–54) and progression of atherosclerosis (55) while chemical inhibition of ACE2 worsened the progression of renovascular hypertension. (56) While ACE2 has been shown to convert Ang II into Ang-(1–7) in both animals (57) and humans, (58;59) further work is necessary to affirm whether the predominant effects of ACE2 inhibition are primarily due to prevention of Ang II metabolism. Nevertheless, this hypothesis is in keeping with the findings of reduced ACE2 expression or activity in experimental models of hypertension, (51;56;60–64) human prehypertension, (65) heart failure, (65–68) renal disease and type 2 diabetes. (69–72) Following the first demonstration that blockade of AT1 receptors in rats with myocardial infarction were associated with upregulation of cardiac ACE2 mRNA (73), newer studies suggest that ACE2 gene transcription is negatively regulated by Ang II while Ang-(1–7) counteracts the inhibitory effect of Ang II on ACE2 gene expression. (74–76)
Altogether, the proposal that Ang-(1–7) opposes the actions of Ang II became the underpinning for the recognition that the RAS is biochemically constituted by alternate enzymatic pathways leading to the generation of separate peptides acting at receptors of which the Ang II AT1 receptor subtype is only a part of the system. In accepting a more complex view of the system and its function in the regulation of blood pressure and vascular structure, the concept that the arm of the RAS comprised by the ACE2/Ang-(1–7)/mas receptor axis counterbalances the activity of the other arm (ACE/Ang II/AT1 receptor axis) has gained acceptance. The new knowledge is stimulating further research into its possible role as, at the very least, a permissive contributor of the cardio-renal remodeling accompanying the pathogenesis of hypertensive vascular disease and target-organ damage.
Proangiotensin 12 (a.k.a., Angiotensin-(1–12)
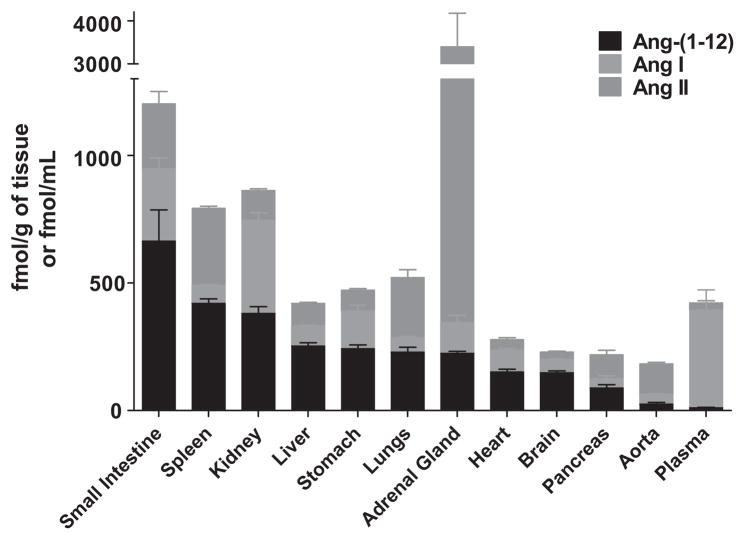
As the science dissecting the contribution of the RAS to cellular function continues to evolve, a study from Japan challenged in 2006 the idea that renin-dependent hydrolysis of angiotensinogen (Aogen) constitutes the rate-limiting step for the formation of angiotensin peptides. (77) Their observations opened a new window toward exploring how angiotensin peptides may be formed within the interior milieu of cells or their immediate surrounding environment. The isolation of a new Aogen-derived peptide by Nagata et al. (77) from the rat’s small intestine contains a shorter form of the synthetic tetradecapeptide synthesized by Skeggs et al. (78) in 1958. The Aogen-derived substrate was termed by them proangiotensin-12 based on its role as an Ang II precursor. In following the terminology approved by the Nomenclature Committee of the Council for High Blood Pressure Research in 1991, (79) we will use the term angiotensin-(1–12) [Ang-(1–12)] throughout this review, as it best follows the appropriate convention in defining the amino acid sequence of the polypeptide within the family of angiotensins (Table 1). Critically important, their study showed the endogenous presence of the peptide in Wistar rats and its ability to serve as a substrate for the in vitro and in vivo generation of Ang II, a finding that strongly differentiates Ang-(1–12) from the tetradecapeptide isolated previously. As illustrated in Figure 1, Ang-(1–12) levels in the small intestine, liver, lungs, adrenal gland, heart, brain, and pancreas are higher than corresponding concentrations of Ang I. The ability of Ang-(1–12) to act as an endogenous substrate for Ang II production followed the observation that Ang-(1–12) vasoconstrictor effects were prevented by prior blockade with either captopril or the AT1 receptor antagonist candesartan in both the isolated aorta and the systemic circulation. (77) Their findings attracted the interest of our laboratory because this was the first time that an extended form of Ang I had been shown to exist endogenously.
Table 1.
Amino Acid Sequences of Main Angiotensin Peptides
| Peptides | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ang-(1–14) | NH2 | Asp | Arg | Val | Tyr | Ile | His | Pro | Phe | His | Leu | Leu | Val | Tyr | Ser | COOH |
| Human Ang-(1–12) | NH2 | Asp | Arg | Val | Tyr | Ile | His | Pro | Phe | His | Leu | Leu | Val | Ile | COOH | |
| Rat Ang-(1–12) | NH2 | Asp | Arg | Val | Tyr | Ile | His | Pro | Phe | His | Leu | Leu | Tyr | Tyr | COOH | |
| OvineAng-(1–12) | NH2 | Asp | Arg | Val | Tyr | Ile | His | Pro | Phe | His | Leu | Leu | Val | His | COOH | |
| Canine Ang-(1–12) | NH2 | Asp | Arg | Val | Tyr | Ile | His | Pro | Phe | His | Leu | Leu | Val | Tyr | COOH | |
| Equine Ang-(1–12) | NH2 | Asp | Arg | Val | Tyr | Ile | His | Pro | Phe | His | Leu | Leu | Val | Tyr | COOH | |
| Ang-(1–10) | NH2 | Asp | Arg | Val | Tyr | Ile | His | Pro | Phe | His | Leu | COOH | ||||
| Ang-(1–8) | NH2 | Asp | Arg | Val | Tyr | Ile | His | Pro | Phe | COOH | ||||||
| Ang-(1–7) | NH2 | Asp | Arg | Val | Tyr | Ile | His | Pro | COOH | |||||||
| Ang-(2–8) | NH2 | Arg | Val | Tyr | Ile | His | Pro | Phe | COOH | |||||||
| Ang-(3–8) | NH2 | Val | Tyr | Ile | His | Pro | Phe | COOH | ||||||||
Figure 1.
Data (Means ± SEM), redrawn from Nagata et al (77), illustrate the relative concentrations of angiotensin-(1–12) [Ang-(1–12)], angiotensin I [Ang I], and angiotensin II [Ang II] in tissues and plasma from the Japanese-derived strain of normotensive Wistar rats.
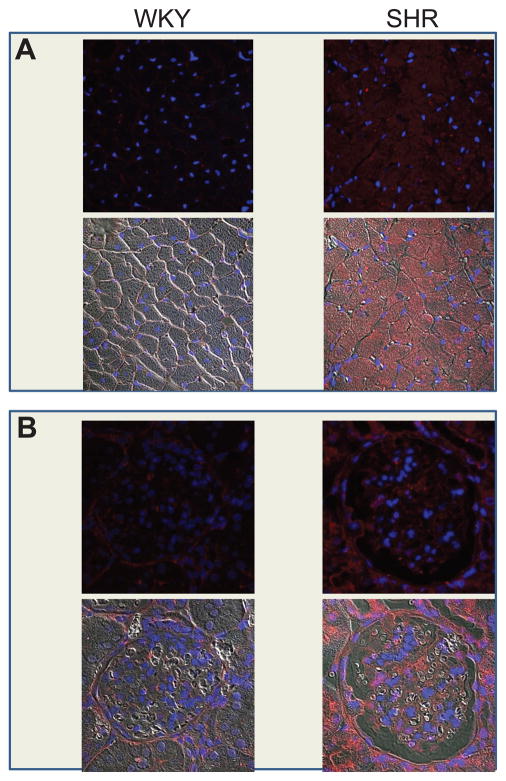
Intrigued by the potential importance of Ang-(1–12) as an alternate substrate for the formation of angiotensin peptides, we explored the location of Ang-(1–12) in cardiac and renal tissues of both Wistar-Kyoto (WKY) and spontaneously hypertensive (SHR) rats. Polyclonal antibodies targeting the specific sequence of rat Ang-(1–12) revealed intense and selective staining of cardiac myocytes and renal tubular cells of both strains (Figure 2). The patchy staining observed in cardiac myocytes of the left ventricle of WKY rats was markedly altered in the SHR, since almost all the cardiac tissue displayed intense staining. (80) To more precisely determine whether the visual display of increased Ang-(1–12) immunoreactive staining reflected a greater endogenous expression of the peptide, we measured Ang-(1–12) tissue concentrations by radioimmunoassay. These experiments showed that the endogenous content of Ang-(1–12) in the cardiac tissue of SHR was 47% higher than those found in WKY. (80) Rat kidney also displayed selective expression of Ang-(1–12) in the proximal, distal, and collecting renal tubules within the deep cortical and outer medullary zones in both strains (Figure 2); however, Ang-(1–12) concentrations in renal tissue were slightly reduced in SHR. (80)
Figure 2.
Representative micrographs of angiotensin-(1–12) immunostaining from cardiac (A) and renal (B) tissue of WKY and SHR (upper images in each panel). In panel A, faint immunostaining for Ang-(1–12) is observed in the myocardium of WKY while intense punctuate staining is expressed in left ventricular myocytes of SHR. Panel B illustrates the expression of Ang-(1–12) immunostaining in sections of kidney cortex from WKY and SHR. Fluorescent staining for Ang-(1–12) found in renal tubules is increased in sections obtained from SHR. Nuclei are indicated by 4′,6-diamidino-2-phenylindole (DAPI) blue fluorescence staining. Lower images in each panel illustrate merged confocal and phase contrast immunostaining for each organ. From studies reported in our reference. (80)
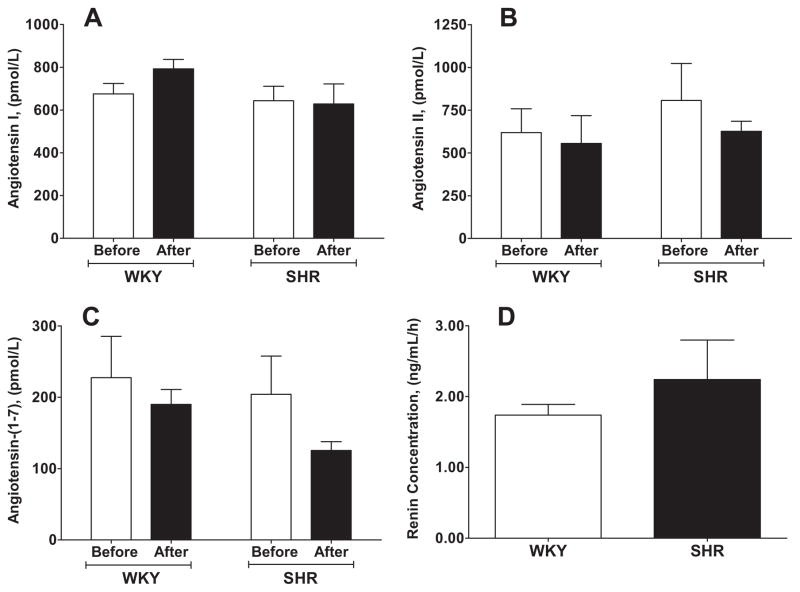
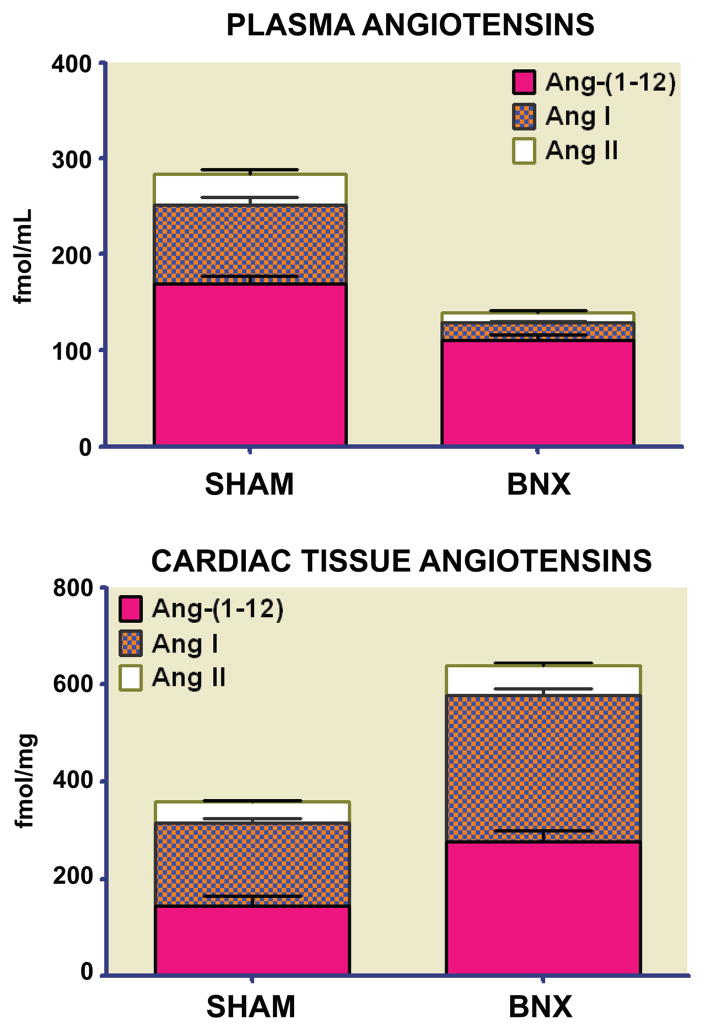
Differential tissue expression of Ang-(1–12) led to a second study focusing on whether renin accounted for the cleavage of Ang-(1–12) into Ang II, Ang-(1–7), or both in isolated perfused hearts from three normotensive and two hypertensive rat strains. (81) As illustrated in Figure 3, Ang-(1–12) caused the rapid appearance of both Ang I and Ang II in the perfusate of WKY and SHR, with highest levels occurring between 30 and 60 min of recirculation (Figure 3). Although renin was present both in the cardiac tissue and in the effluent of isolated perfused WKY and SHR hearts (Figure 3), addition of a selective rat renin inhibitor (WFML-1) did not prevent conversion of Ang-(1–12) into angiotensin peptides. (81) These data thus excluded renin from acting on Ang-(1–12). Recent studies in anephric rats are in agreement with this interpretation. (82) In the anephric state the loss of renal-derived renin resulted in the expected fall in the circulating concentrations of Ang I and Ang II to barely detectable levels while it had only a small reducing effect on plasma Ang-(1–12) levels (Figure 4). In contrast, parallel measures of the cardiac content of angiotensin peptides documented a 91% increase in cardiac Ang-(1–12) tissue concentrations 48 h after bilateral nephrectomy. (82) Furthermore, absence of renin transcripts in cardiac tissue of both sham and nephrectomized rats suggests that both the processing of Aogen into Ang-(1–12) and its secondary conversion to Ang I occurs via a non-renin pathway. Therefore, the biochemical processes associated with the expression and cleavage of Ang-(1–12) through a non-renin pathway suggest an additional level of complexity within the enzymatic pathways accounting for the expression of biologically active peptides.
Figure 3.
Panels A to C are peak concentrations of angiotensin peptides resulting from the addition of 10 nmol Ang-(1–12) to the perfusate of isolated hearts from Wistar Kyoto (WKY) and spontaneously hypertensive (SHR) adult rats. Panel D illustrated renin concentration in the effluent of isolated perfused hearts from WKY and SHR. Before, peak angiotensin values obtained 60 min after administration of Ang-(1–12); After, shows the absence of a change in the peak values of Ang-(1–12) following administration of 1 μmol/L of the rat specific renin inhibitor WFML-1. Data (Means ± SEM) redrawn from our reference. (81)
Figure 4.
Plasma and cardiac tissue concentrations of angiotensin peptides in sham and 48 h bilateral nephrectomized (BNX) rats reveals differential expression of angiotensin-(1–12) [Ang-(1–12)], angiotensin I (Ang I) and angiotensin II (Ang II) in adult Wistar Kyoto rats. Data redrawn from our reference (82)
Insights into the enzymatic pathway (s) responsible for the conversion of Ang-(1–12) into Ang II have been recently expanded by studies of Prosser et al. (83) in the isolated heart of Sprague Dawley rats. In this preparation, ex-vivo and in-vivo conversion of Ang-(1–12) into Ang II was prevented by the administration of chymostatin using combined high performance liquid chromatography and tandem mass spectrometry analysis (83). Urata et al. (84) first implicated a functional role for chymase, a member of the serine protease family, as a tissue enzyme involved in the conversion of Ang I into Ang II. The location of chymase in secretory granules and its ubiquitous existence in mast, vascular endothelial and mesenchymal cells provides a mechanism for the intracellular formation of angiotensin peptides and their functioning as intracrine and paracrine regulators. (85) A potential limitation in terms of ascribing a primary role for chymase in the metabolism of Ang-(1–12) is the previous report by Nagata et al. (77) who found that captopril abolished the constrictor response of aortic strips to Ang-(1–12) exposure, as well as preventing the pressor effect of intravenous Ang-(1–12). Differences in the findings between both studies may have been influenced by employed methodology and the use by Prosser et al. (83) of human recombinant chymase. In this connection, rat, but not human chymase cleaves the Tyr4-Ile5 bond of Ang II. (86) In addition, tissue damage and edema associated with saline perfusion of the preparation may have increased cell permeability exposing the peptide to intracellular chymase. (83)
The tantalizing evidence for the existence of an alternate renin-independent substrate for extra- or intracellular processing of angiotensin peptides awaits further studies as to whether its biological activity is expressed through the formation of Ang II or may act independently of its processing into the known biological peptides of the renin angiotensin system. Further studies should be undertaken to unravel the potential importance of Ang-(1–12) as an alternate substrate for the formation of angiotensin peptides as well as determining the enzyme(s) accounting for the cleavage of Ang-(1–12) from Aogen and its subsequent conversion into Ang II, Ang-(1–7) or both. As research delves further into the potential role of Ang-(1–12) as a source of tissues angiotensins formation the question of whether increased formation of Ang-(1–12) may contribute to pathology comes to the forefront. A preliminary answer to this question may be derived from the demonstration of increased expression and cardiac content of Ang-(1–12) in the myocytes of SHR, (80) increased expression of cardiac Ang-(1–12) and Ang II in both anephric rats, (82) and in those in which hypertension was abated by administration of a mineralocorticoid receptor antagonist. (87) In addition, the recent demonstration that endogenous neutralization of Ang-(1–12) via infusion of a selective Ang-(1–12) antibody into the cerebrospinal fluid of transgenic hypertensive rats lowers blood pressure provides further evidence for its role as a precursor for the formation of Ang II. (88)
Summary
Even from this limited overview of the intricate internal mechanisms regulating the pathways determining the production of angiotensin peptides, it is obvious that the RAS is embodied with a great capacity to use alternate mechanisms in bypassing blockade of primary pathways. In unraveling the complexity of the biochemical physiology of the system it is also apparent that formation of angiotensin peptides within the cellular environment may not follow what has been characterized in the circulation or even the extracellular compartment.
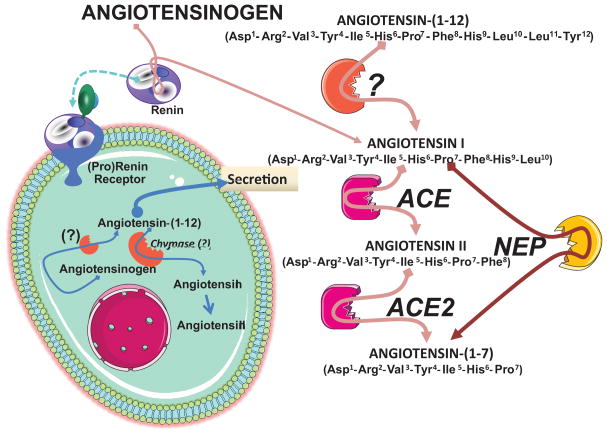
A new level of regulatory complexity is added with the demonstration of Ang-(1–12) as an alternate substrate contributing to forming angiotensin peptides by a non renin-dependent mechanism. The tissue formation and processing of the biological active products of the renin angiotensin system may follow those outlined in Figure 5, whereby synthesis of Ang II and Ang-(1–7) may be determined by availability of Ang-(1–12) either formed through intracellular processing of Aogen, uptake from the extracellular compartment, or both. While there are still many fundamental gaps in uncovering the conditions and processes that determine the enzyme(s) accounting for either the cleavage of Ang-(1–12) from Aogen or the processing of Ang-(1–12) to Ang II and Ang-(1–7), the fact remains that this substrate occurs endogenously, is altered in a genetic model of hypertension, and can clearly produce angiotensin peptides by a non renin dependent mechanism. As the ubiquitous functions of tissue RAS extends outside the cardiovascular system, the detection of high concentrations of Ang-(1–12) in the rat gut needs to be explored since Ang II contributes to jejunal motility and fluid transport. (89;90) Indeed, the finding that high doses of the direct renin inhibitor aliskiren are associated in humans with diarrhea (91;92) posits the question as to whether this side-effect of the drug might be related to increases in the gut content of Ang-(1–12).
Figure 5.
Diagram of the biochemical pathways leading to the formation of angiotensin peptides in tissues. Abbreviations are: ACE, angiotensin converting enzyme; ACE2, angiotensin converting enzyme 2; NEP, neutral endopeptidase 24.11 and related endopeptidases contributing to formation of Ang-(1–7) from Ang I. (12)
Perspectives
Understanding the structure and the dynamics of the complex intercellular web of interactions that contribute to the function of a living cell requires acceptance that a discrete biological action can rarely be attributed to an individual molecule. The intertwined relationship between the ACE/Ang II/AT1 receptor axis and its counter lever ACE2/Ang-(1–7)/mas receptor axis is now buttressed on a firm literature. A further investigation of the potential clinical impact of pursuing the hypothesis of whether genetic or acquired deficiency in one or more of the components of the Ang-(1–7)/ACE2/mas-R axis may explain the progression of hypertensive vascular disease ought to be pursued with greater vigor as research in this field is extending knowledge of disease processes both within and outside the cardiovascular system.
Acknowledgments
Sources of Support: NHLBI of the NIH grant PO1 HL051952
Footnotes
Conflict of Interest
There are no conflicts of interest.
References
- 1.Ferrario CM, Chappell MC, Tallant EA, Brosnihan KB, Diz DI. Counterregulatory actions of angiotensin-(1–7) Hypertension. 1997;30:535–541. doi: 10.1161/01.hyp.30.3.535. [DOI] [PubMed] [Google Scholar]
- 2.Gavras H, Brunner HR, Laragh JH, Gavras I, Vukovich RA. The use of angiotensin-converting enzyme inhibitor in the diagnosis and treatment of hypertension. Clin Sci Mol Med Suppl. 1975;2:57s–60s. doi: 10.1042/cs048057s. [DOI] [PubMed] [Google Scholar]
- 3.Timmermans PB, Duncia JV, Carini DJ, Chiu AT, Wong PC, Wexler RR, Smith RD. Discovery of losartan, the first angiotensin II receptor antagonist. J Hum Hypertens. 1995;9 (Suppl 5):S3–18. [PubMed] [Google Scholar]
- 4.Schiavone MT, Santos RA, Brosnihan KB, Khosla MC, Ferrario CM. Release of vasopressin from the rat hypothalamo-neurohypophysial system by angiotensin-(1–7) heptapeptide. Proc Natl Acad Sci U S A. 1988;85(11):4095–4098. doi: 10.1073/pnas.85.11.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benter IF, Diz DI, Ferrario CM. Cardiovascular actions of angiotensin(1–7) Peptides. 1993;14:679–684. doi: 10.1016/0196-9781(93)90097-z. [DOI] [PubMed] [Google Scholar]
- 6.Brosnihan KB, Li P, Ferrario CM. Angiotensin-(1–7) dilates canine coronary arteries through kinins and nitric oxide. Hypertension. 1996;27:523–528. doi: 10.1161/01.hyp.27.3.523. [DOI] [PubMed] [Google Scholar]
- 7.Neves LA, Averill DB, Ferrario CM, Chappell MC, Aschner JL, Walkup MP, Brosnihan KB. Characterization of angiotensin-(1–7) receptor subtype in mesenteric arteries. Peptides. 2003;24:455–462. doi: 10.1016/s0196-9781(03)00062-7. [DOI] [PubMed] [Google Scholar]
- 8.Iyer SN, Averill DB, Chappell MC, Yamada K, Allred AJ, Ferrario CM. Contribution of angiotensin-(1–7) to blood pressure regulation in salt-depleted hypertensive rats. Hypertension. 2000;36:417–422. doi: 10.1161/01.hyp.36.3.417. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura S, Averill DB, Chappell MC, Diz DI, Brosnihan KB, Ferrario CM. Angiotensin receptors contribute to blood pressure homeostasis in salt-depleted SHR. Am J Physiol Regul Integr Comp Physiol. 2003;284:R164–R173. doi: 10.1152/ajpregu.00210.2002. [DOI] [PubMed] [Google Scholar]
- 10.Nakamoto H, Ferrario CM, Fuller SB, Robaczewski DL, Winicov E, Dean RH. Angiotensin-(1–7) and nitric oxide interaction in renovascular hypertension. Hypertension. 1995;25:796–802. doi: 10.1161/01.hyp.25.4.796. [DOI] [PubMed] [Google Scholar]
- 11.Chappell MC. Emerging evidence for a functional angiotensin-converting enzyme 2-angiotensin-(1–7)-MAS receptor axis: more than regulation of blood pressure? Hypertension. 2007;50:596–599. doi: 10.1161/HYPERTENSIONAHA.106.076216. [DOI] [PubMed] [Google Scholar]
- 12.Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1–7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005;289:H2281–H2290. doi: 10.1152/ajpheart.00618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos RA, Ferreira AJ. Angiotensin-(1–7) and the renin-angiotensin system. Curr Opin Nephrol Hypertens. 2007;16:122–128. doi: 10.1097/MNH.0b013e328031f362. [DOI] [PubMed] [Google Scholar]
- 14.Langeveld B, van Gilst WH, Tio RA, Zijlstra F, Roks AJ. Angiotensin-(1–7) attenuates neointimal formation after stent implantation in the rat. Hypertension. 2005;45:138–141. doi: 10.1161/01.HYP.0000149382.83973.c2. [DOI] [PubMed] [Google Scholar]
- 15.Machado RD, Santos RA, Andrade SP. Opposing actions of angiotensins on angiogenesis. Life Sci. 2000;66:67–76. doi: 10.1016/s0024-3205(99)00562-7. [DOI] [PubMed] [Google Scholar]
- 16.Ren Y, Garvin JL, Carretero OA. Vasodilator action of angiotensin-(1–7) on isolated rabbit afferent arterioles. Hypertension. 2002;39:799–802. doi: 10.1161/hy0302.104673. [DOI] [PubMed] [Google Scholar]
- 17.Santos RA, Ferreira AJ, Nadu AP, Braga AN, de Almeida AP, Campagnole-Santos MJ, Baltatu O, Iliescu R, Reudelhuber TL, Bader M. Expression of an angiotensin-(1–7)-producing fusion protein produces cardioprotective effects in rats. Physiol Genomics. 2004;17:292–299. doi: 10.1152/physiolgenomics.00227.2003. [DOI] [PubMed] [Google Scholar]
- 18.De Mello WC, Ferrario CM, Jessup JA. Beneficial versus harmful effects of Angiotensin (1–7) on impulse propagation and cardiac arrhythmias in the failing heart. J Renin Angiotensin Aldosterone Syst. 2007;8:74–80. doi: 10.3317/jraas.2007.015. [DOI] [PubMed] [Google Scholar]
- 19.Fraga-Silva RA, Pinheiro SV, Goncalves AC, Alenina N, Bader M, Santos RA. The antithrombotic effect of angiotensin-(1–7) involves mas-mediated NO release from platelets. Mol Med. 2008;14:28–35. doi: 10.2119/2007-00073.Fraga-Silva. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gminski J, Stajszczyk M. Anti-atherosclerotic action of hypotensive drugs. Wiad Lek. 1996;49:182–198. [PubMed] [Google Scholar]
- 21.Igase M, Strawn WB, Gallagher PE, Geary RL, Ferrario CM. Angiotensin II AT1 receptors regulate ACE2 and angiotensin-(1–7) expression in the aorta of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2005;289:H1013–H1019. doi: 10.1152/ajpheart.00068.2005. [DOI] [PubMed] [Google Scholar]
- 22.Igase M, Kohara K, Nagai T, Miki T, Ferrario CM. Increased expression of angiotensin converting enzyme 2 in conjunction with reduction of neointima by angiotensin II type 1 receptor blockade. Hypertens Res. 2008;31:553–559. doi: 10.1291/hypres.31.553. [DOI] [PubMed] [Google Scholar]
- 23.Kucharewicz I, Pawlak R, Matys T, Pawlak D, Buczko W. Antithrombotic effect of captopril and losartan is mediated by angiotensin-(1–7) Hypertension. 2002;40(5):774–779. doi: 10.1161/01.hyp.0000035396.27909.40. [DOI] [PubMed] [Google Scholar]
- 24.Lovren F, Pan Y, Quan A, Teoh H, Wang G, Shukla PC, Levitt KS, Oudit GY, Al-Omran M, Stewart DJ, Slutsky AS, Peterson MD, Backx PH, Penninger JM, Verma S. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am J Physiol Heart Circ Physiol. 2008;295:H1377–H1384. doi: 10.1152/ajpheart.00331.2008. [DOI] [PubMed] [Google Scholar]
- 25.Benter IF, Yousif MH, Dhaunsi GS, Kaur J, Chappell MC, Diz DI. Angiotensin-(1–7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am J Nephrol. 2008;28:25–33. doi: 10.1159/000108758. [DOI] [PubMed] [Google Scholar]
- 26.Liao XX, Guo RX, Ma H, Wang LC, Chen ZH, Yang CT, Feng JQ. Effects of angiotensin-(1–7) on oxidative stress and functional changes of isolated rat hearts induced by ischemia-reperfusion. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:1345–1348. [PubMed] [Google Scholar]
- 27.Heringer-Walther S, Eckert K, Schumacher SM, Uharek L, Wulf-Goldenberg A, Gembardt F, Fichtner I, Schultheiss HP, Rodgers K, Walther T. Angiotensin-(1–7) stimulates hematopoietic progenitor cells in vitro and in vivo. Haematologica. 2009;94:857–860. doi: 10.3324/haematol.2008.000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nie W, Yan H, Li S, Zhang Y, Yu F, Zhu W, Fan F, Zhu J. Angiotensin-(1–7) enhances angiotensin II induced phosphorylation of ERK1/2 in mouse bone marrow-derived dendritic cells. Mol Immunol. 2009;46:355–361. doi: 10.1016/j.molimm.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 29.Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de BI, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alenina N, Xu P, Rentzsch B, Patkin EL, Bader M. Genetically altered animal models for Mas and angiotensin-(1–7) Exp Physiol. 2008;93:528–537. doi: 10.1113/expphysiol.2007.040345. [DOI] [PubMed] [Google Scholar]
- 31.Carvalho MB, Duarte FV, Faria-Silva R, Fauler B, da Mata Machado LT, de Paula RD, Campagnole-Santos MJ, Santos RA. Evidence for Mas-mediated bradykinin potentiation by the angiotensin-(1–7) nonpeptide mimic AVE 0991 in normotensive rats. Hypertension. 2007;50:762–767. doi: 10.1161/HYPERTENSIONAHA.107.094987. [DOI] [PubMed] [Google Scholar]
- 32.Castro CH, Santos RA, Ferreira AJ, Bader M, Alenina N, Almeida AP. Evidence for a functional interaction of the angiotensin-(1–7) receptor Mas with AT1 and AT2 receptors in the mouse heart. Hypertension. 2005;46:937–942. doi: 10.1161/01.HYP.0000175813.04375.8a. [DOI] [PubMed] [Google Scholar]
- 33.Gembardt F, Grajewski S, Vahl M, Schultheiss HP, Walther T. Angiotensin metabolites can stimulate receptors of the Mas-related genes family. Mol Cell Biochem. 2008;319:115–123. doi: 10.1007/s11010-008-9884-4. [DOI] [PubMed] [Google Scholar]
- 34.Kostenis E, Milligan G, Christopoulos A, Sanchez-Ferrer CF, Heringer-Walther S, Sexton PM, Gembardt F, Kellett E, Martini L, Vanderheyden P, Schultheiss HP, Walther T. G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation. 2005;111:1806–1813. doi: 10.1161/01.CIR.0000160867.23556.7D. [DOI] [PubMed] [Google Scholar]
- 35.Xu P, Santos RA, Bader M, Alenina N. Alterations in gene expression in the testis of angiotensin-(1–7)-receptor Mas-deficient mice. Regul Pept. 2007;138:51–55. doi: 10.1016/j.regpep.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 36.Xu P, Costa-Goncalves AC, Todiras M, Rabelo LA, Sampaio WO, Moura MM, Santos SS, Luft FC, Bader M, Gross V, Alenina N, Santos RA. Endothelial dysfunction and elevated blood pressure in MAS gene-deleted mice. Hypertension. 2008;51:574–580. doi: 10.1161/HYPERTENSIONAHA.107.102764. [DOI] [PubMed] [Google Scholar]
- 37.Clark MA, Diz DI, Tallant EA. Angiotensin-(1–7) downregulates the angiotensin II type 1 receptor in vascular smooth muscle cells. Hypertension. 2001;37:1141–1146. doi: 10.1161/01.hyp.37.4.1141. [DOI] [PubMed] [Google Scholar]
- 38.Clark MA, Tallant EA, Tommasi E, Bosch S, Diz DI. Angiotensin-(1–7) reduces renal angiotensin II receptors through a cyclooxygenase-dependent mechanism. J Cardiovasc Pharmacol. 2003;41:276–283. doi: 10.1097/00005344-200302000-00017. [DOI] [PubMed] [Google Scholar]
- 39.Giani JF, Gironacci MM, Munoz MC, Pena C, Turyn D, Dominici FP. Angiotensin-(1 7) stimulates the phosphorylation of JAK2, IRS-1 and Akt in rat heart in vivo: role of the AT1 and Mas receptors. Am J Physiol Heart Circ Physiol. 2007;293:H1154–H1163. doi: 10.1152/ajpheart.01395.2006. [DOI] [PubMed] [Google Scholar]
- 40.Giani JF, Gironacci MM, Munoz MC, Turyn D, Dominici FP. Angiotensin-(1–7) has a dual role on growth-promoting signalling pathways in rat heart in vivo by stimulating STAT3 and STAT5a/b phosphorylation and inhibiting angiotensin II-stimulated ERK1/2 and Rho kinase activity. Exp Physiol. 2008;93:570–578. doi: 10.1113/expphysiol.2007.014269. [DOI] [PubMed] [Google Scholar]
- 41.Sampaio WO, Henrique de CC, Santos RA, Schiffrin EL, Touyz RM. Angiotensin-(1–7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension. 2007;50:1093–1098. doi: 10.1161/HYPERTENSIONAHA.106.084848. [DOI] [PubMed] [Google Scholar]
- 42.Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1–7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension. 2007;49:185–192. doi: 10.1161/01.HYP.0000251865.35728.2f. [DOI] [PubMed] [Google Scholar]
- 43.Tallant EA, Clark MA. Molecular mechanisms of inhibition of vascular growth by angiotensin-(1–7) Hypertension. 2003;42:574–579. doi: 10.1161/01.HYP.0000090322.55782.30. [DOI] [PubMed] [Google Scholar]
- 44.Tallant EA, Ferrario CM, Gallagher PE. Angiotensin-(1–7) inhibits growth of cardiac myocytes through activation of the mas receptor. Am J Physiol Heart Circ Physiol. 2005;289:H1560–H1566. doi: 10.1152/ajpheart.00941.2004. [DOI] [PubMed] [Google Scholar]
- 45.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 46.Turner AJ, Tipnis SR, Guy JL, Rice G, Hooper NM. ACEH/ACE2 is a novel mammalian metallocarboxypeptidase and a homologue of angiotensin-converting enzyme insensitive to ACE inhibitors. Can J Physiol Pharmacol. 2002;80:346–353. doi: 10.1139/y02-021. [DOI] [PubMed] [Google Scholar]
- 47.Batlle D, Soler MJ, Wysocki J. New aspects of the renin-angiotensin system: angiotensin-converting enzyme 2 - a potential target for treatment of hypertension and diabetic nephropathy. Curr Opin Nephrol Hypertens. 2008;17:250–257. doi: 10.1097/MNH.0b013e3282f945c2. [DOI] [PubMed] [Google Scholar]
- 48.Raizada MK, Ferreira AJ. ACE2: a new target for cardiovascular disease therapeutics. J Cardiovasc Pharmacol. 2007;50:112–119. doi: 10.1097/FJC.0b013e3180986219. [DOI] [PubMed] [Google Scholar]
- 49.Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2004;25:291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Acton S, Patane M, Nichols A, Tummino P. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J Biol Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 51.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da CJ, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 52.Der SS, Grobe JL, Yuan L, Narielwala DR, Walter GA, Katovich MJ, Raizada MK. Cardiac overexpression of angiotensin converting enzyme 2 protects the heart from ischemia-induced pathophysiology. Hypertension. 2008;51:712–718. doi: 10.1161/HYPERTENSIONAHA.107.100693. [DOI] [PubMed] [Google Scholar]
- 53.Diez-Freire C, Vazquez J, Correa de Adjounian MF, Ferrari MF, Yuan L, Silver X, Torres R, Raizada MK. ACE2 gene transfer attenuates hypertension-linked pathophysiological changes in the SHR. Physiol Genomics. 2006;27:12–19. doi: 10.1152/physiolgenomics.00312.2005. [DOI] [PubMed] [Google Scholar]
- 54.Huentelman MJ, Grobe JL, Vazquez J, Stewart JM, Mecca AP, Katovich MJ, Ferrario CM, Raizada MK. Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol. 2005;90:783–790. doi: 10.1113/expphysiol.2005.031096. [DOI] [PubMed] [Google Scholar]
- 55.Dong B, Zhang C, Feng JB, Zhao YX, Li SY, Yang YP, Dong QL, Deng BP, Zhu L, Yu QT, Liu CX, Liu B, Pan CM, Song HD, Zhang MX, Zhang Y. Overexpression of ACE2 enhances plaque stability in a rabbit model of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:1270–1276. doi: 10.1161/ATVBAHA.108.164715. [DOI] [PubMed] [Google Scholar]
- 56.Burgelova M, Vanourkova Z, Thumova M, Dvorak P, Opocensky M, Kramer HJ, Zelizko M, Maly J, Bader M, Cervenka L. Impairment of the angiotensin-converting enzyme 2-angiotensin-(1–7)-Mas axis contributes to the acceleration of two-kidney, one-clip Goldblatt hypertension. J Hypertens. 2009 doi: 10.1097/HJH.0b013e32832f0d06. (e-PUB July 9) [DOI] [PubMed] [Google Scholar]
- 57.Trask AJ, Averill DB, Ganten D, Chappell MC, Ferrario CM. Primary role of angiotensin-converting enzyme-2 in cardiac production of angiotensin-(1–7) in transgenic Ren-2 hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;292:H3019–H3024. doi: 10.1152/ajpheart.01198.2006. [DOI] [PubMed] [Google Scholar]
- 58.Zisman LS, Keller RS, Weaver B, Lin Q, Speth R, Bristow MR, Canver CC. Increased angiotensin-(1–7)-forming activity in failing human heart ventricles: evidence for upregulation of the angiotensin-converting enzyme Homologue ACE2. Circulation. 2003;108:1707–1712. doi: 10.1161/01.CIR.0000094734.67990.99. [DOI] [PubMed] [Google Scholar]
- 59.Zisman LS, Meixell GE, Bristow MR, Canver CC. Angiotensin-(1–7) formation in the intact human heart: in vivo dependence on angiotensin II as substrate. Circulation. 2003;108:1679–1681. doi: 10.1161/01.CIR.0000094733.61689.D4. [DOI] [PubMed] [Google Scholar]
- 60.Burchill L, Velkoska E, Dean RG, Lew RA, Smith AI, Levidiotis V, Burrell LM. Acute kidney injury in the rat causes cardiac remodelling and increases angiotensin-converting enzyme 2 expression. Exp Physiol. 2008;93:622–630. doi: 10.1113/expphysiol.2007.040386. [DOI] [PubMed] [Google Scholar]
- 61.Burrell LM, Risvanis J, Kubota E, Dean RG, MacDonald PS, Lu S, Tikellis C, Grant SL, Lew RA, Smith AI, Cooper ME, Johnston CI. Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J. 2005;26:369–375. doi: 10.1093/eurheartj/ehi114. [DOI] [PubMed] [Google Scholar]
- 62.Jessup JA, Gallagher PE, Averill DB, Brosnihan KB, Tallant EA, Chappell MC, Ferrario CM. Effect of angiotensin II blockade on a new congenic model of hypertension derived from transgenic Ren-2 rats. Am J Physiol Heart Circ Physiol. 2006;291:H2166–H2172. doi: 10.1152/ajpheart.00061.2006. [DOI] [PubMed] [Google Scholar]
- 63.Oudit GY, Herzenberg AM, Kassiri Z, Wong D, Reich H, Khokha R, Crackower MA, Backx PH, Penninger JM, Scholey JW. Loss of angiotensin-converting enzyme-2 leads to the late development of angiotensin II-dependent glomerulosclerosis. Am J Pathol. 2006;168:1808–1820. doi: 10.2353/ajpath.2006.051091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tikellis C, Bialkowski K, Pete J, Sheehy K, Su Q, Johnston C, Cooper ME, Thomas MC. ACE2 deficiency modifies renoprotection afforded by ACE inhibition in experimental diabetes. Diabetes. 2008;57:1018–1025. doi: 10.2337/db07-1212. [DOI] [PubMed] [Google Scholar]
- 65.Keidar S, Strizevsky A, Raz A, Gamliel-Lazarovich A. ACE2 activity is increased in monocyte-derived macrophages from prehypertensive subjects. Nephrol Dial Transplant. 2007;22:597–601. doi: 10.1093/ndt/gfl632. [DOI] [PubMed] [Google Scholar]
- 66.Epelman S, Shrestha K, Troughton RW, Francis GS, Sen S, Klein AL, Tang WH. Soluble angiotensin-converting enzyme 2 in human heart failure: relation with myocardial function and clinical outcomes. J Card Fail. 2009;15:565–571. doi: 10.1016/j.cardfail.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goulter AB, Goddard MJ, Allen JC, Clark KL. ACE2 gene expression is up-regulated in the human failing heart. BMC Med. 2004;2:19. doi: 10.1186/1741-7015-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keidar S, Gamliel-Lazarovich A, Kaplan M, Pavlotzky E, Hamoud S, Hayek T, Karry R, Abassi Z. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circ Res. 2005;97:946–953. doi: 10.1161/01.RES.0000187500.24964.7A. [DOI] [PubMed] [Google Scholar]
- 69.Leehey DJ, Singh AK, Bast JP, Sethupathi P, Singh R. Glomerular renin angiotensin system in streptozotocin diabetic and Zucker diabetic fatty rats. Transl Res. 2008;151:208–216. doi: 10.1016/j.trsl.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 70.Mizuiri S, Hemmi H, Arita M, Ohashi Y, Tanaka Y, Miyagi M, Sakai K, Ishikawa Y, Shibuya K, Hase H, Aikawa A. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am J Kidney Dis. 2008;51:613–623. doi: 10.1053/j.ajkd.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 71.Reich HN, Oudit GY, Penninger JM, Scholey JW, Herzenberg AM. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int. 2008;74:1610–1616. doi: 10.1038/ki.2008.497. [DOI] [PubMed] [Google Scholar]
- 72.Wong DW, Oudit GY, Reich H, Kassiri Z, Zhou J, Liu QC, Backx PH, Penninger JM, Herzenberg AM, Scholey JW. Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol. 2007;171:438–451. doi: 10.2353/ajpath.2007.060977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 74.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 75.Gallagher PE, Ferrario CM, Tallant EA. Regulation of ACE2 in cardiac myocytes and fibroblasts. Am J Physiol Heart Circ Physiol. 2008;295:H2373–H2379. doi: 10.1152/ajpheart.00426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gallagher PE, Ferrario CM, Tallant EA. MAP kinase/phosphatase pathway mediates the regulation of ACE2 by angiotensin peptides. Am J Physiol Cell Physiol. 2008;295:C1169–C1174. doi: 10.1152/ajpcell.00145.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun. 2006;350:1026–1031. doi: 10.1016/j.bbrc.2006.09.146. [DOI] [PubMed] [Google Scholar]
- 78.Skeggs LT, Jr, Lentz KE, Kahn JR, Shumway NP. The synthesis of a tetradecapeptide renin substrate. J Exp Med. 1958;108:283–297. doi: 10.1084/jem.108.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bumpus FM, Catt KJ, Chiu AT, DeGasparo M, Goodfriend T, Husain A, Peach MJ, Taylor DG, Jr, Timmermans PB. Nomenclature for angiotensin receptors. A report of the Nomenclature Committee of the Council for High Blood Pressure Research. Hypertension. 1991;17:720–721. doi: 10.1161/01.hyp.17.5.720. [DOI] [PubMed] [Google Scholar]
- 80.Jessup JA, Trask AJ, Chappell MC, Nagata S, Kato J, Kitamura K, Ferrario CM. Localization of the novel angiotensin peptide, angiotensin-(1–12), in heart and kidney of hypertensive and normotensive rats. Am J Physiol Heart Circ Physiol. 2008;294:H2614–H2618. doi: 10.1152/ajpheart.91521.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trask AJ, Jessup JA, Chappell MC, Ferrario CM. Angiotensin-(1–12) is an alternate substrate for angiotensin peptide production in the heart. Am J Physiol Heart Circ Physiol. 2008;294:H2242–H2247. doi: 10.1152/ajpheart.00175.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferrario CM, Varagic J, Habibi J, Nagata S, Kato J, Chappell MC, Trask AJ, Kitamura K, Whaley-Connell A, Sowers JR. Differential regulation of angiotensin-(1–12) in plasma and cardiac tissue in response to bilateral nephrectomy. Am J Physiol Heart Circ Physiol. 2009;296:H1184–H1192. doi: 10.1152/ajpheart.01114.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prosser HC, Forster ME, Richards AM, Pemberton CJ. Cardiac chymase converts rat proAngiotensin-12 (PA12) to angiotensin II: effects of PA12 upon cardiac haemodynamics. Cardiovasc Res. 2009;82:40–50. doi: 10.1093/cvr/cvp003. [DOI] [PubMed] [Google Scholar]
- 84.Urata H, Kinoshita A, Misono KS, Bumpus FM, Husain A. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem. 1990;265:22348–22357. [PubMed] [Google Scholar]
- 85.Re RN, Cook JL. The basis of an intracrine pharmacology. J Clin Pharmacol. 2008;48:344–350. doi: 10.1177/0091270007312155. [DOI] [PubMed] [Google Scholar]
- 86.Le TH, Neurath H, Woodbury RG. Substrate specificity of the chymotrypsin-like protease in secretory granules isolated from rat mast cells. Proc Natl Acad Sci U S A. 1987;84:364–367. doi: 10.1073/pnas.84.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Whaley-Connell A, Habibi J, Wei Y, Gutweiler A, Jellison J, Wiedmeyer CE, Ferrario CM, Sowers JR. Mineralcorticoid Receptor Antagonism Attenuates Glomerular Filtration Barrier Remodeling in the Transgenic Ren2 Rat. Am J Physiol Renal Physiol. 2009;296:F1013–F1022. doi: 10.1152/ajprenal.90646.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Isa K, Garcia-Espinosa MA, Arnold AC, Pirro NT, Tommasi EN, Ganten D, Chappell MC, Ferrario CM, Diz DI. Chronic immunoneutralization of brain angiotensin-(1–12) lowers blood pressure in transgenic (mRen2)27 hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2009;297:R111–R115. doi: 10.1152/ajpregu.90588.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duggan KA, Ye VZ, Jones DM, Macdonald GJ. Angiotensin II: a humoral mediator for the gastric sodium monitor. Am J Physiol. 1996;270:F406–F410. doi: 10.1152/ajprenal.1996.270.3.F406. [DOI] [PubMed] [Google Scholar]
- 90.Spak E, Casselbrant A, Olbers T, Lonroth H, Fandriks L. Angiotensin II-induced contractions in human jejunal wall musculature in vitro. Acta Physiol (Oxf) 2008;193:181–190. doi: 10.1111/j.1748-1716.2007.01826.x. [DOI] [PubMed] [Google Scholar]
- 91.Gradman AH, Schmieder RE, Lins RL, Nussberger J, Chiang Y, Bedigian MP. Aliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patients. Circulation. 2005;111:1012–1018. doi: 10.1161/01.CIR.0000156466.02908.ED. [DOI] [PubMed] [Google Scholar]
- 92.Hussar DA. New drugs: aliskiren hemifumarate, lisdexamfetamine dimesylate, and lapatinib. J Am Pharm Assoc. 2007;47:425–430. doi: 10.1331/JAPhA.2007.07504. [DOI] [PubMed] [Google Scholar]