Abstract
Rationale
Exposure to smoking-related cues can trigger relapse in smokers attempting to maintain abstinence.
Objectives
In the present study we evaluated the effect of 24-hr smoking abstinence on brain responses to smoking-related cues using functional magnetic resonance imaging (fMRI).
Methods
Eighteen adult smokers underwent fMRI scanning following smoking as usual (satiated condition) and following 24-hr abstinence (abstinent condition). During scanning they viewed blocks of photographic smoking and control cues.
Results
Following abstinence, greater activation was found in response to smoking cues compared to control cues in parietal (BA 7/31), frontal (BA 8/9), occipital (BA 19) and central (BA 4) cortical regions and in dorsal striatum (putamen) and thalamus. In contrast, no smoking cue > control cue activations were observed following smoking as usual. Direct comparisons between conditions (satiated vs. abstinent) showed greater brain reactivity in response to smoking cues following abstinence. In addition, positive correlations between pre-scan craving in the abstinent condition and smoking cue activation were observed in right dorsomedial prefrontal cortex (dmPFC) including superior frontal gyrus (BA 6/10), anterior cingulate gyrus (BA 32) and supplementary motor area (BA 6).
Conclusions
The present findings indicate smoking abstinence significantly potentiates neural responses to smoking-related cues in brain regions subserving visual sensory processing, attention and action planning. Moreover, greater abstinence-induced craving was significantly correlated with increased smoking cue activation in dmPFC areas involved in action planning and decision making. These findings suggest that drug abstinence can increase the salience of conditioned cues which is consistent with incentive-motivation models of addiction.
Keywords: cue-reactivity, craving, nicotine dependence, fMRI, smoking, dorsal striatum
Introduction
Nicotine dependence is a chronic relapsing disorder. As many as 70% of smokers report a desire to quit but less than 5% of unaided quit attempts result in abstinence for more than three months (CDC 2002). One trigger of smoking relapse is exposure to cues previously associated with cigarette smoking (e.g. sight of a pack of cigarettes or another smoker). Smokers show robust self-reported craving in the face of such cues (Carter and Tiffany 1999) and exposure to them has been shown to precipitate relapse (Shiffman et al. 1996).
Consistent with the notion that smoking-related cues are of increased significance to smokers, the presentation of these cues (compared to neutral cues) results in activation of a corticomesolimbic network of regions subserving attention, reward and goal directed behavior. Specific regions commonly shown to be active in response to cue exposure include extrastriate areas including precuneus (Brody et al. 2007; McBride et al. 2006), the anterior cingulate (Brody et al. 2002; McBride et al. 2006; McClernon et al. 2005b; Wilson et al. 2005), superior frontal gyrus (McClernon et al. 2005b; McClernon et al. 2007; Wilson et al. 2005), amygdala (Due et al. 2002; Franklin et al. 2007; McClernon et al. 2007) and ventral striatum/nucleus accumbens (David et al. 2005; Franklin et al. 2007). These findings have been interpreted as suggesting that exposure to smoking cues increases attentional resources focused on processing external, smoking-related information and triggers the planning of behaviors aimed at obtaining cigarettes.
Abstinence from smoking results in increased motivation to smoke as evidenced by increased self-reported craving for cigarettes and increased smoking reinforcement (Epstein et al. 1991; Gilbert et al. 2002). In conjunction with these increases in craving and reinforcement, incentive motivation theories of drug use (Robinson and Berridge 1993; Stewart et al. 1984) propose that abstinence from a drug should also increase the perceived reward value (incentive salience) of cues previously associated with drug use. Evidence for abstinence-induced amplification of incentive salience should come in the form of greater cue-provoked reactions to smoking cues following periods of abstinence as compared to periods of smoking satiety. Consistent with this, previous studies have observed greater interference with task performance by smoking-related cues in abstinent as compared to satiated smokers (Gross et al. 1993; Waters and Feyerabend 2000). Moreover, support for incentive-motivation theory comes from research with animals showing that heroin withdrawal enhances the incentive salience of the drug as measured by increased drug seeking behavior (Hutcheson et al. 2001).
In contrast with these behavioral laboratory studies, two previous fMRI studies have not shown that smoking abstinence increases brain reactivity to smoking cues (McBride et al. 2006; McClernon et al. 2005b), while one study found abstinence to decrease reactivity in ventral striatum in an all female sample of smokers (David et al. 2007). These findings of no or decreased activation in response to smoking cues by overnight or 12-hr abstinence have been interpreted as possibly reflecting smoking abstinence-induced hypoactivation of the dopamine reward system (McBride et al. 2006).
Though previous neuroimaging studies have not observed potentiation of brain cue-reactivity by smoking abstinence, this may be accounted in large part by the relatively short durations of abstinence required prior to cue-exposure (overnight or 12-hrs). Such durations, particularly when including overnight hours, may require a smoker to forego few if any cigarettes and thus, may not be sufficiently long enough to increase the incentive salience of cues. Therefore, the primary goal of the present study was to evaluate the effects of longer term (24-hr) smoking abstinence on neural responses to smoking-related visual cues in a sample of dependent smokers. Twenty-four hour abstinence requires a smoker to forego as many cigarettes as typically smoked in a day which putatively should increase the incentive salience of smoking-related cues. We hypothesized that, consistent with incentive motivation models of drug use, 24-hr smoking abstinence would increase brain activation in response to smoking cues in regions subserving reward, motivation and affective processes.
Materials and Methods
Participants
Twenty adult smokers, recruited from the community, completed all aspects of the study. To be enrolled, participants had to report smoking ≥ 10 cigarettes per day for at least two years, have an afternoon expired-air carbon monoxide (CO) level greater than 10 ppm (in order to establish smoking status), be right-handed, free of serious health problems (e.g., hypertension), not currently undergoing treatment for a psychiatric illness, free of medications altering CNS functioning, test negative for illicit drug use, not have any conditions making MRI research unsafe, and among females, have a negative serum pregnancy test. Data for one participant were unusable due to reported blurred vision and excessive head motion during scanning. Another's data were unusable due to scanner error. Thus, the final sample consisted of 18 smokers.
Procedures
All participants first completed a 1.5-hr screening session in which they read and signed an Institutional Review Board approved informed consent form, completed questionnaires regarding smoking history and suitability for fMRI research and provided a breath sample. They were also placed in a mock scanner in order to habituate them to the scanning environment.
Participants who passed screening then completed one to five, 1-hr training sessions. The main purpose of these sessions was to train participants on a working memory task to stable performance (to be presented elsewhere). During one of the training sessions, participants viewed a version of the cue-reactivity task (described below).
Following the training period, participants completed two fMRI sessions that were identical except that prior to one session they were allowed to smoke their usual amount of cigarettes up until entering the scanning facility (satiated condition) while in the other session they were required to be 24-hrs abstinent from smoking prior to, and following, scanning (abstinent condition). Order of condition was randomly assigned and counterbalanced. As part of the informed consent process, participants were informed that participation would require 48-hr abstinence and signed an honor statement promising to maintain abstinence for that period of time. Compliance with instructions to smoke or not was tested with breath CO levels at the beginning of the session and confirmed with saliva samples also collected at the beginning of the scanning session. Continued abstinence was also verified 24 hrs following the abstinent condition scanning session during a .5-hr quit check in which breath and saliva samples were collected.
Cue-Viewing Task
Photographic smoking and control cues were presented in a boxcar design with 4 blocks per category, as represented in Figure 1. Smoking cues (n = 40) consisted of full-color pictures of people smoking. Control cues (n = 40) consisted of pictures of people engaged in everyday activities (e.g. talking on phone, writing). Equal numbers of females and males were represented in each category. The set of cues included pictures acquired from a variety of sources (Gilbert and Rabinovich 1999; Lang et al. 1995; in-house) and has been used in previous imaging studies (McClernon et al. 2005b; McClernon et al. 2007). Each block was 1 minute in length during which 10 cues were presented. Before and after each block a crosshair was presented for 5 s. Participants were then asked to rate their current craving level on a 4-point scale (‘not at all’ to ‘extreme’). The scale was presented for 10 s followed by presentation of a crosshair for another 15 s. Thus, the total interblock-interval was 30 s. Total task time was 12.5 minutes.
Figure 1.
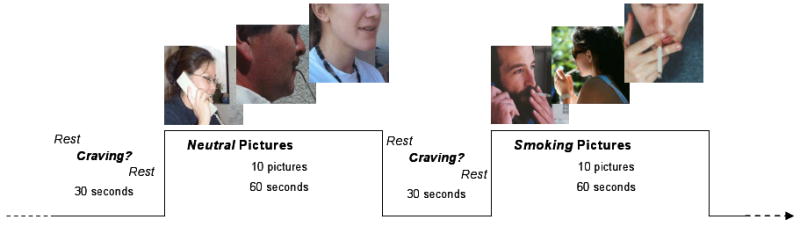
Cue-Viewing Task
fMRI Acquisition
A 4.0-T GE LX NVi scanner with 41 mT/m gradients was used for image acquisition. Blood-oxygen-level dependent (BOLD) functional images were collected for 34 contiguous slices (4 mm thick) parallel to the horizontal plane connecting the anterior and posterior commissures. An inverse spiral pulse sequence sensitive to BOLD contrast was used, with TR = 1.5 s, TE = 6 ms, FOV = 24 cm, matrix = 64 × 64, flip angle = 60°, and in-plane resolution = 3.75 mm2. After completion of the functional data collection, a T1-weighted 3D fast spoiled gradient recalled (FSPGR) structural image was collected for 68 slices (1.9 mm thick) with TR = 12.3 s, TE = 5.4 ms, FOV = 24 cm, matrix = 256 × 256, flip angle = 20°, and in-plane resolution = 0.9375 mm2.
fMRI Analysis
Analyses focused on evaluating brain activation during viewing of smoking and control cues under smoking as usual (satiated) and 24-hr abstinent conditions. Preprocessing was conducted using statistical parametric mapping software (SPM5; Wellcome Department of Imaging Neuroscience, London) to remove noise and artifacts. The first four volumes of each run were discarded to allow for T1 stabilization. All functional images underwent correction for acquisition timing and for head motion using rigid-body rotation and translation (Friston et al. 1994). Each participant's data was then subsequently warped into a standard stereotaxic space (Montreal Neurological Institute) with an isotropic 2 mm voxel size and smoothed with an 8 mm FWHM Gaussian filter.
Each participant's data from each session was entered into a first-level voxel-by-voxel analysis using the General Linear Model (Friston et al. 1994). Each cue block (smoke, control) was modeled as a boxcar function convolved with a canonical hemodynamic response function that began at the onset of the first cue in the block and ended at the end of the block (duration = 60 sec). A high-pass filter was applied to remove slow signal drift. A smoking cue > control cue contrast image was then created and input into a random effects analysis. One sample t-tests were performed to examine smoking cue-reactivity (smoking > control) separately for each condition (abstinent, satiated). A paired t-test was also conducted to examine the differences in brain responses between conditions. Resulting activations were considered significant at a p ≤ 0.001 (uncorrected) with a minimum cluster extent threshold of 20 contiguous voxels. Smoking cue > control cue contrast images for each participant were also input into two separate random effects regression analyses. One analysis explored correlations between brain responses to cues and pre-scan craving, whereas the other explored correlations between brain responses to cues and cue-provoked craving.
Withdrawal, Craving and Biochemical Smoking Measures
A 32-item version of the Shiffman-Jarvik Withdrawal Questionnaire (SJWQ; Shiffman and Jarvik 1976) modified by Rose et al. (1990) was used to measure pre-scan craving and withdrawal symptoms (negative affect, hunger, arousal, somatic symptoms and habit withdrawal). Participants completed the questionnaire at the beginning of each scanning session.
Cue-provoked craving was measured during scanning using a 1-item, 4-point scale. Mean craving scores following smoking and control blocks were entered into a 2 (Cue: smoking, control) × 2 (Condition: abstinent, satiated) ANOVA. For the purpose of regression analyses, cue-provoked craving in each smoking condition was calculated by taking the average craving level following smoking cue blocks minus the average following control cue blocks.
Nicotine dependence was measured using the 6-item Fagerström Test for Nicotine Dependence (FTND; Heatherton et al. 1991).
Expired air CO concentrations were measured using a handheld CO monitor (Vitalograph Inc., Lenexa, KS) and was calculated by subtracting the background (ambient) CO from the peak CO reading. Saliva samples were stored in an on site -80° freezer. Assays for cotinine (screening session) and nicotine (scanning sessions) were performed on saliva using gas chromatography modified for use of a capillary column (Jacob et al. 1981).
Results
Sample characteristics
The final sample (n = 18) was 61.1% female (n = 11). Reported racial/ethnic group membership was 77.8% Caucasian (n = 14), 16.7% African-American (n = 3), and 5.6% Asian-American (n = 1). Mean age was 28.6 years (SD = 7.5). The sample reported smoking a mean of 17.8 cigarettes/day (SD = 2.8) for 11.6 years (SD = 6.7). Mean FTND score was 4.4 (SD = 1.4) suggesting the sample was moderately nicotine dependent. At screening, mean expired breath CO concentration was 26.9 (SD = 13.4) whereas mean salivary cotinine was 225.7 ng/ml (SD = 160.6).
Abstinence verification
Expired breath CO concentrations indicated compliance with study requirements. In the satiated condition, mean CO level (ppm) was 24.6 (SD = 10.7) whereas in the 24-hr abstinent condition mean CO was 2.4 (SD = 1.4). Mean CO 24 hrs after the abstinent fMRI session was 1.9 (SD = 2.3).
The results of salivary nicotine analyses were consistent with CO results and also indicative of compliance. In the satiated and abstinent conditions, mean salivary nicotine (ng/ml) was 443.5 (SD = 351.5) and 20.7 (SD = 21.8), respectively. Mean salivary nicotine 24 hrs after the abstinent fMRI session was 22.0 (SD = 23.8), again indicating compliance with the instruction to maintain abstinence for 24 hrs following the abstinent condition scanning session.
Self-report results
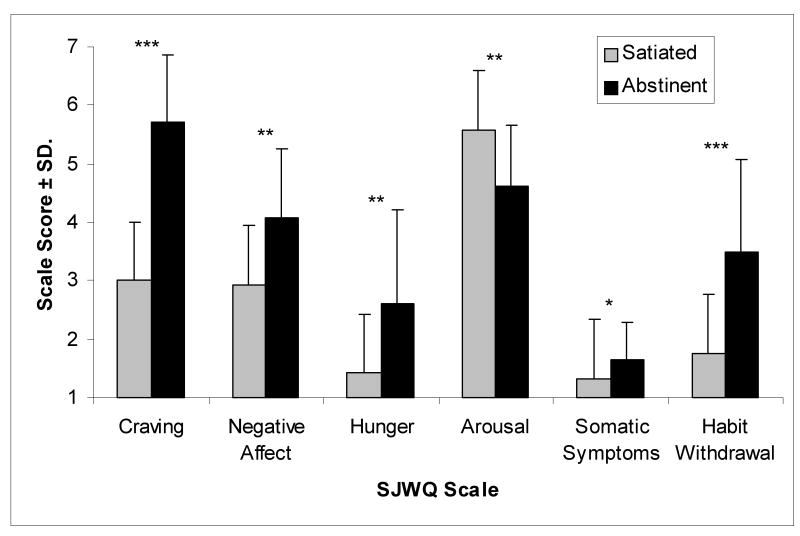
Twenty-four hour abstinence from smoking resulted in significant withdrawal symptoms (see Figure 2). Compared to the satiated condition, abstinence significantly increased prescan craving, negative affect, hunger, somatic symptoms and habit withdrawal, but significantly decreased arousal.
Figure 2.
Pre-scan measures of withdrawal symptoms as reported on the Shiffman-Jarvik Withdrawal Questionnaire (SJWQ). Compared to the satiated condition, abstinence increased pre-scan craving [F(1,17) = 102.67, p < .001], negative affect [F(1,17) = 11.65, p = .003], hunger [F(1,17) = 11.56, p = .003], somatic symptoms [F(1,17) = 6.42, p = .021], and habit withdrawal [F(1,17) = 27.86, < .001]; but decreased arousal [F(1,17) = 13.54, p = .002]. *p<0.05; **p<0.01; ***p<0.001.
Craving scores measured during the cue-viewing task were not available for one participant due to technical problems. Across conditions, craving for cigarettes was greater following blocks of smoking cues as compared to control cues [F(1,16) = 6.95, p = .018]. Moreover, across cue types, abstinence increased ratings of craving during the cue viewing task [F(1,16) = 5.42, p = .033]. The Cue × Condition interaction was not significant.
fMRI Results
Across satiated and abstinent conditions, smoking cues elicited greater activation than control cues in right paracentral lobule (BA 5), right cuneus (BA 19), bilateral hypothalamus, left middle occipital gyrus (BA 19), right superior and medial frontal gyri (BA 6) and left insula (see Table S1 and Figure S1). Control cues did not elicit greater activation than smoking cues in any brain regions.
The effect of smoking cues (compared to control cues) on brain activation was evaluated separately for each smoking condition (see Table 1 and Figure 3). Following 24-hr abstinence, greater activation was found in response to smoking cues compared to control cues in parietal regions including right superior parietal lobule and bilateral precuneus (BA 7); bilateral posterior cingulate (BA 31); striatum including bilateral putamen and internal capsule; left superior frontal gyrus (BA 8/9); left occipital cortex (BA 19); right dorsal thalamus; bilateral cerebellum; and central cortical regions including right pre/postcentral gyrus and bilateral paracentral lobule (BA 4). In contrast, no smoking cue > control cue activations were observed in the satiated condition.
Table 1.
Brain areas of significant activation for smoking cues > control cues as measured by BOLD-fMRI in abstinent and satiated conditions
| Side | Brain Area | BA | Cluster Size (mm3) |
x,y,z | Tmax |
|---|---|---|---|---|---|
| Smoke > Control - Abstinent Condition | |||||
| Parietal Cortex/Posterior Cingulate | |||||
| L | Precuneus | 7 | 424 | -10, -60, 18 | 5.64 |
| L/R | Posterior Cingulate | 31 | 1600 | -6, -34, 34 | 5.46 |
| L | Precuneus | 7 | 192 | -14, -56, 70 | 4.67 |
| R | Superior Parietal Lobule | 7 | 464 | 20, -62, 46 | 4.48 |
| L/R | Precuneus | 7 | 464 | 0, -46, 56 | 4.31 |
| Striatum | |||||
| L | Internal Capsule | 424 | -28, -26, 14 | 5.28 | |
| L | Putamen | 224 | -32, 0, -12 | 5.07 | |
| R | Putamen | 240 | 32, -4, -4 | 4.31 | |
| Frontal Cortex | |||||
| L | Superior Frontal Gyrus | 9 | 216 | -14, 58, 20 | 4.91 |
| L | Superior Frontal Gyrus | 8 | 160 | -26, 40, 44 | 4.26 |
| L | Superior Frontal Gyrus | 9 | 240 | -8, 48, 26 | 4.21 |
| Occipital Cortex | |||||
| L | Medial Inferior Occipital Gyrus | 19 | 208 | -22, -60, 6 | 4.6 |
| L | Middle Occipital Gyrus | 19 | 232 | -30, -80, 36 | 4.27 |
| L | Superior Occipital Gyrus | 19 | 368 | -16, -86, 40 | 4.23 |
| Central Cortex | |||||
| R | Pre/Postcentral Gyrus | 4 | 288 | 32, -24, 48 | 4.43 |
| L/R | Paracentral Lobule | 4 | 240 | 0, -32, 60 | 3.92 |
| Cerebellum | |||||
| R | Cerebellum | 208 | 10, -82, -20 | 4.32 | |
| L | Cerebellum | 256 | -40, -68, -20 | 4.27 | |
| Thalamus | |||||
| R | Dorsal Thalamus | 256 | 12, -18, 22 | 4.57 | |
| Smoke > Control - Satiated Condition | |||||
| no significant areas of activation | |||||
p<0.001(uncorrected), minimum cluster size ≥ 20 voxels
Figure 3.
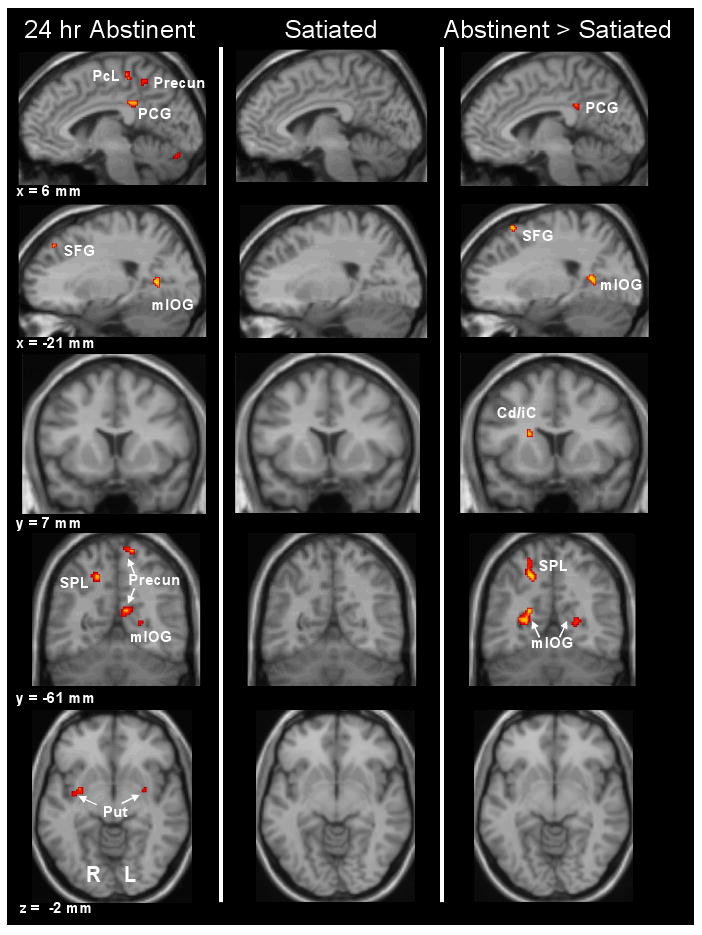
BOLD response to smoking cues versus control cues was greater following 24-hr abstinence in right paracentral lobule (PcL; BA 4), right posterior cingulate gyrus (PCG; BA 31), bilateral precuneus (Precun; BA 7), left superior frontal gyrus (SFG; BA 8), left medial inferior occipital gyrus (mIOG; BA 19), right superior parietal lobule (SPL; BA 7), and bilateral putamen (Put). There were no significant areas of activation for smoking cues > control cues in the satiated condition. Greater cue-reactivity to smoking cues in the abstinent condition when compared to the satiated condition was observed in the right PCG (BA 31), left SFG (BA 6), bilateral mIOG (BA 19/30), right SPL (BA 7) and right caudate/internal capsule (Cd/iC).
When activation was contrasted between the two conditions, 24-hr abstinence resulted in greater activation than satiety (see Table 2 and Figure 3) in left superior frontal gyrus (BA 6), right superior parietal lobule (BA 7), right posterior cingulate gyrus (BA 31), bilateral occipital cortex (BAs 19 and 30), a region on the boundary between right pre- and post-central gyrus (BA 1/2/3/4), and right caudate/internal capsule. There were no regions in which smoking cue activation was greater in the satiated condition compared to the abstinent condition.
Table 2.
Brain areas of significant activation for smoking cues > control cues as measured by BOLD-fMRI when comparing abstinent versus satiated condition
| Side | Brain Area | BA | Cluster Size (mm3) | x,y,z | Tmax |
|---|---|---|---|---|---|
| Abstinent > Satiated | |||||
| Frontal Cortex | |||||
| L | Superior Frontal Gyrus | 6 | 208 | -20, 26, 60 | 4.94 |
| Parietal Cortex/Posterior Cingulate | |||||
| R | Superior Parietal Lobule | 7 | 720 | 18, -54, 48 | 4.93 |
| R | Posterior Cingulate Gyrus | 31 | 168 | 8, -38, 32 | 4.10 |
| Occipital Cortex | |||||
| R | Middle Occipital Gyrus | 19 | 824 | 30,-76, 22 | 4.67 |
| R | Medial Inferior Occipital Gyrus | 19 | 752 | 26, -58, 8 | 4.56 |
| L | Medial Inferior Occipital Gyrus | 30 | 224 | -22, -52, 10 | 4.51 |
| Central Cortex | |||||
| R | Pre/Postcentral Gyrus | 1/2/3/4 | 320 | 26, -34, 72 | 4.53 |
| Striatum | |||||
| R | Caudate/Internal Capsule | 272 | 20, 16, 20 | 4.33 | |
| Satiated > Abstinent | |||||
| no significant areas of activation | |||||
p<0.001(uncorrected), minimum cluster size ≥ 20 voxels
fMRI/Self-Report Craving Correlations
Correlations between pre-scan craving (as measured by the SJWQ) and smoking cue activation (smoking cue > control cue) were examined separately for each smoking condition. In the abstinent condition, significant positive correlations between craving and smoking cue activation were observed in right frontal regions (BA 6/32/10) including a relatively large cluster in the superior frontal gyrus (BA 6). Significant correlations were also observed in right occipital cortex (BA 18/19), right inferior parietal cortex (BA 40), left postcentral gyrus (BA 1/2/3), and cerebellum (see Table 3 and Figure 4). Negative correlations were not observed. Neither positive nor negative correlations between pre-scan craving and smoking cue activation were observed in the satiated condition.
Table 3.
Brain areas for smoking cues > control cues as measured by BOLD-fMRI that are significantly correlated with measures of pre-scan craving
| Side | Brain Area | BA | Cluster Size (mm3) |
x,y,z | Tmax |
|---|---|---|---|---|---|
| Abstinent Condition | |||||
| Pre-Scan Craving - Positive Correlations | |||||
| Frontal Cortex | |||||
| R | Superior Frontal Gyrus | 6 | 5304 | 20, 30, 54 | 5.84 |
| R | Anterior Cingulate Gyrus | 32 | 568 | 4, 44, 8 | 4.87 |
| R | Superior Frontal Gyrus | 10 | 240 | 12, 58, 18 | 4.55 |
| R | Supplementary Motor Area | 6 | 280 | 14, 10, 62 | 4.37 |
| Central Cortex | |||||
| L | Postcentral Gyrus | 1/2/3 | 440 | -44, -28, 42 | 4.96 |
| Parietal Cortex | |||||
| R | Inferior Parietal Gyrus | 40 | 184 | 46, -42, 50 | 4.55 |
| Occipital Cortex | |||||
| R | Lingual Gyrus | 18 | 264 | 20, -76, -12 | 4.54 |
| R | Fusiform | 19 | 200 | 36, -68, -20 | 4.36 |
| Cerebellum | |||||
| R | Cerebellum | 568 | 4, -56, -2 | 5.25 | |
| Pre-Scan Craving - Negative Correlations | |||||
| no significant areas of activation | |||||
| Satiated Condition | |||||
| no significant positive or negative correlations | |||||
p<0.001(uncorrected), minimum cluster size ≥ 20 voxels
Figure 4.
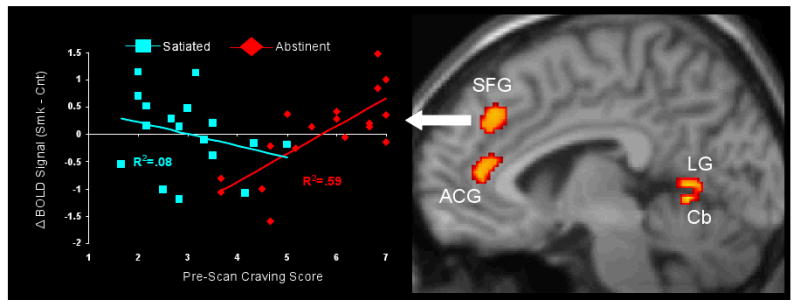
A significant positive correlation between pre-scan craving and smoking cue > control cue activation was observed in right superior frontal gyrus (SFG; BA 6) during the abstinent condition (R2=0.59), but not in the satiated condition (R2=0.08). Similar correlations in the abstinent and satiated conditions were also observed in right anterior cingulated gyrus (ACG; BA 32), right lingual gyrus (LG; BA 18), and right cerebellum. A test of difference between dependent correlations (Steiger 1980) indicated that correlations in the abstinent and satiated conditions were significantly different.
Correlations between cue-provoked craving (craving following smoking cue blocks – control cue blocks) and smoking cue activation were similarly examined but no significant correlations, positive or negative, were observed in either abstinent or satiated conditions.
Discussion
The current investigation sought to evaluate the effects of 24-hr tobacco abstinence on brain responses to visual smoking cues in dependent smokers. Smoking abstinence results in a state of increased motivation to seek and use cigarettes, which according to incentive-motivation models of drug addiction (Robinson and Berridge 1993; Stewart et al. 1984) should increase the perceived incentive value of cues previously associated with smoking (i.e. conditioned drug cues). Evidence in favor of incentive-motivation models comes from research showing that heroin withdrawal augments the incentive-salience of the drug (i.e. and presumably cues associated with the drug), but only among animals that have learned that the drug reverses withdrawal symptoms (Hutcheson et al. 2001). This same process may potentially play a role in human smoking since most dependent smokers have frequent experience using cigarettes in the context of withdrawal (e.g. first cigarette of the day or following periods of smoking restriction). Elucidating the neural mechanisms underlying the effect of smoking abstinence on smoking cue reactivity (and more broadly the effect of drug abstinence on drug cue reactivity), is critical since exposure to these cues can trigger smoking relapse (Shiffman et al. 1996).
Effects of smoking abstinence on brain cue-reactivity
In the present study, greater brain activation in response to smoking cues (as compared to control cues) was observed following 24-hr abstinence in a distributed network of cortical, dorsal striatal and thalamic brain regions. Following abstinence, visual smoking cues elicited activation in brain regions responsible for primary visual sensory processing (left occipital gyri) and for higher level visual attention (bilateral precuneus and right superior parietal lobule)(Coull 1998; Kastner and Ungerleider 2000); similar sites of activation have been observed in previous studies of smoking cue-reactivity (Brody et al. 2007; McBride et al. 2006; Wilson et al. 2005). Greater smoking cue activation was also observed following abstinence in left prefrontal cortex (superior frontal gyrus)—a region shown to be active in previous brain cue-reactivity studies (McClernon et al. 2005a; McClernon et al. 2007) and which subserves sustained attention, memory and decision making processes (Cabeza and Nyberg 2000; Rushworth et al. 2004). Additionally, we observed smoking cue activation following abstinence in posterior cingulate which is involved in visuospatial attention and information processing (Gron et al. 2000; Vogt et al. 1992) and has been shown to be active in response to visual smoking (McBride et al. 2006) and cocaine (Kilts et al. 2004) cues. Moreover, greater cue reactivity in this region was previously shown to be predictive of worse cocaine treatment outcomes (Kosten et al. 2006). In addition to the above cortical areas, smoking cues activated central cortical regions in BA 4 (right pre/postcentral gyrus, bilateral paracentral lobule) involved in motor planning and execution. Finally, putamen and thalamus also exhibited activation during abstinence. The putamen connects motor and somatosenory areas with thalamus and is involved in sensorimotor integration, action selection and movement (Everitt and Robbins 2005; Lawrence et al. 1998).
Collectively, the above findings suggest that following smoking abstinence, smoking cues robustly activate corticostriatal areas possibly involved in the processing of smoking-related sensory information and the planning/selection of relevant actions (i.e. smoking). It has recently been hypothesized that striatal involvement in addiction progresses from ventral (e.g. nucleus accumbens) to dorsal (e.g. putamen/caudate) striatal control as self-administration behavior transitions from voluntary to habitual or compulsive use (Everitt and Robbins 2005). Consistent with this hypothesis and with the present findings, cocaine associated cues have been shown to elicit dorsal striatal dopamine release in rats (Ito et al. 2002) and human neuroimaging studies have observed greater dorsal striatal dopamine release (Volkow et al. 2006) and fMRI-BOLD response (Garavan et al. 2000) following exposure to cocaine cues in cocaine addicts. In a similar vein, a previous study of cigarette smokers using PET also found that an index of metabolic activity in the dorsal striatum was correlated with the self-reported frequency of experiencing extreme craving (Rose et al. 2007). The present study extends these findings by demonstrating for the first time that cue-provoked dorsal striatum activation is modulated by the current drug deprivation state and suggests that drug abstinence possibly increases the level of activation of corticostriatal networks underlying drug stimulus-response associations.
In contrast to the effects following abstinence, activation in response to smoking cues was not greater than control cues following smoking as usual. This finding stands in contrast with previous studies that have observed significant brain cue-reactivity in smokers under conditions of satiety (Franklin et al. 2007; McBride et al. 2006). Interestingly, these previous studies made use of video and/or in vivo smoking cues which by their nature may evoke greater brain responses.
The direct comparison of smoking cue activation in satiated and abstinent conditions revealed findings consistent with effects observed independently in each condition. That is, there were no regions in which brain cue-reactivity was greater in the satiated as compared to abstinent condition whereas cue-reactivity was greater in a number of brain regions on the abstinent as compared to satiated condition. Abstinent > satiated activation was observed in many of the same regions as noted above in the abstinent condition including prefrontal (superior frontal gyrus), parietal (superior parietal lobule), posterior cingulate, occipital and central cortical areas. Additionally, activation in the dorsal striatum (right caudate/internal capsule) was again observed.
The present findings suggest that smoking abstinence potentiates neural responding to smoking-related cues, which may reflect an increase in the incentive salience of these cues resulting from an increase in smoking motivation. Interestingly, the current findings are in contrast with previous fMRI studies which have found no change in smoking cue activation following smoking abstinence (McBride et al. 2006; McClernon et al. 2005b). However, in these previous studies smoking abstinence periods were relatively short (overnight to 12 hrs) while in the current study a full 24 hrs of abstinence was required. The longer duration of abstinence in the present study resulted in each participant forgoing as many cigarettes as smoked in a typical day, whereas overnight/12 hr abstinence might require a participant to forgo only a fraction of typical consumption (in some cases 0-1 cigarette).
The results of the present study should also be considered in light of studies which have observed abstinence-induced decreases in cue-reactivity in ventral striatum (David et al. 2007) or negative correlations between pre-scan craving and cue-reactivity in this region (McClernon et al. 2008). These earlier studies made use of event-related paradigms and this suggests the possibility that transient responses to drug cues may elicit a different pattern of striatal activation (ventral and negatively correlated with baseline craving) than sustained responses (dorsal and positively correlated with baseline craving). Future paradigms that simultaneously model sustained and transient responses can more fully address this issue.
Brain cue-reactivity and craving
In this study we observed significant positive associations between smoking cue activation and pre-scan craving in the abstinent condition. These associations, which were primarily restricted to right hemisphere regions, were observed in frontal, central, parietal and occipital cortical brain areas. Of particular note were correlations in right dorsomedial prefrontal cortex (dmPFC) including the superior frontal gyrus (both medial and lateral), right anterior cingulate gyrus and supplementary motor area. Previous studies have observed correlations between abstinence-induced craving and brain responses to drug cues in superior frontal gyrus (McClernon et al. 2005b) and this and other dmPFC areas including the rostral ACG are involved in decision making, emotional information processing, and goal directed action planning (Bush et al. 2000; Majdandzic et al. 2007; Rushworth et al. 2004). Moreover, activation of dmPFC areas has been found in the context of high versus low conflict decision making (Pochon et al. 2008). Thus, our findings lead to the hypothesis that the magnitude of abstinence-induced craving modulates the degree to which cues provoke decisional conflict. This makes intuitive sense as high craving for a drug should lead to conflict about use whereas no or low craving should not lead to such conflict. Future studies that parametrically manipulate smoking motivation and cue salience can address this hypothesis in a more refined manner.
Unlike previous studies (Franklin et al. 2007; Smolka et al. 2006), we did not observe significant correlations between cue-provoked craving and smoking cue activation. This may have been due to a number of factors including some methodological. The 1-item scale used to assess cue-provoked craving may not have provided the reliability of a multi-item craving scale or the range of a visual analog scale. Previous studies that observed correlations assessed cue-provoked craving outside the scanner and in smokers with a wide range of nicotine dependence levels (Smolka et al. 2006) or using absolute measures of cerebral blood flow (Franklin et al. 2007). The lack of cue-provoked craving correlation findings in the present study suggests the possibility that cue-provoked craving is only related to smoking cue-related brain activation at moderate levels of smoking abstinence, not at the extremes of satiety and 24-hr abstinence. Additional work in which both pre-scan and cue-provoked craving are manipulated at a range of levels might help clarify this issue.
Summary and Implications
The present study was designed to evaluate neural responses to smoking cues in a context similar to early cessation. It yielded two main findings. First, 24-hr smoking abstinence resulted in smoking cue brain activation in distributed network of cortical, dorsal striatal and thalamic regions involved in visual sensory, attention and action execution functions. Second, higher levels of craving following 24-hr abstinence were associated with increased smoking cue activation, predominantly in right dmPFC brain regions which are neural substrates of decision making and motivation. Together, these novel findings are consistent with incentive motivation models of addiction which predict that drug abstinence will increase the incentive salience of cues associated with previous drug use. Future studies can assess whether abstinence-induced amplification of brain reactivity to smoking cues is predictive of smoking cessation outcomes or differentially modulated by smoking cessation treatments (e.g. nicotine replacement therapy).
Acknowledgments
This research was supported by NIDA grant K23DA017261 to Dr. McClernon and by an unrestricted research grant from Philip Morris U.S.A., Inc to Dr. Rose. We thank Natalie Goutkin for her assistance with MRI scanning.
Footnotes
Supplementary information is available at the Psychopharamacology website
References
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Archives of General Psychiatry. 2002;59:1162–72. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, Scheibal D, London ED, Monterosso JR, Tiffany ST, Korb A, Gan JJ, Cohen MS. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62:642–51. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–40. [PubMed] [Google Scholar]
- CDC. Cigarette Smoking Among Adults-- United States, 2000. Morbidity and Mortality Weekly Report. 2002;51:642–645. [PubMed] [Google Scholar]
- Coull JT. Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol. 1998;55:343–61. doi: 10.1016/s0301-0082(98)00011-2. [DOI] [PubMed] [Google Scholar]
- David SP, Munafo MR, Johansen-Berg H, Mackillop J, Sweet LH, Cohen RA, Niaura R, Rogers RD, Matthews PM, Walton RT. Effects of Acute Nicotine Abstinence on Cue-elicited Ventral Striatum/Nucleus Accumbens Activation in Female Cigarette Smokers: A Functional Magnetic Resonance Imaging Study. Brain Imaging Behav. 2007;1:43–57. doi: 10.1007/s11682-007-9004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Munafo MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, Walton RT. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;58:488–94. doi: 10.1016/j.biopsych.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Due DL, Huettel SA, Hall WG, Rubin DC. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. American Journal of Psychiatry. 2002;159:954–60. doi: 10.1176/appi.ajp.159.6.954. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Bulik CM, Perkins KA, Caggiula AR, Rodefer J. Behavioral economic analysis of smoking: money and food as alternatives. Pharmacol Biochem Behav. 1991;38:715–21. doi: 10.1016/0091-3057(91)90232-q. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Wang J, Sciortino N, Harper D, Li Y, Ehrman R, Kampman K, O'Brien CP, Detre JA, Childress AR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–9. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Jezzard P, Turner R. Analysis of functional MRI time-series. Human Brain Mapping. 1994;1:153–171. [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. American Journal of Psychiatry. 2000;157:1789–98. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, Plath LC, Masson CL, Anderson AE, Sly KF. Mood disturbance fails to resolve across 31 days of cigarette abstinence in women. J Consult Clin Psychol. 2002;70:142–52. doi: 10.1037//0022-006x.70.1.142. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Rabinovich NE. International Smoking Image Series, Version 1.2. 1.2. Intergrative Neuroscience Laboratory, Southern Illinois University; Carbondale: Intergrative Neuroscience Laboratory, Southern Illinois University; Carbondale: 1999. [Google Scholar]
- Gron G, Wunderlich AP, Spitzer M, Tomczak R, Riepe MW. Brain activation during human navigation: gender-different neural networks as substrate of performance. Nat Neurosci. 2000;3:404–8. doi: 10.1038/73980. [DOI] [PubMed] [Google Scholar]
- Gross TM, Jarvik ME, Rosenblatt MR. Nicotine abstinence produces content-specific Stroop interference. Psychopharmacology (Berl) 1993;110:333–6. doi: 10.1007/BF02251289. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hutcheson DM, Everitt BJ, Robbins TW, Dickinson A. The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nat Neurosci. 2001;4:943–7. doi: 10.1038/nn0901-943. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22:6247–53. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P, 3rd, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatogr. 1981;222:61–70. doi: 10.1016/s0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–41. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–41. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–50. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. NIMH Center for the Study of Emotion and Attention, University of FL; Gainesville, FL: NIMH Center for the Study of Emotion and Attention, University of FL; Gainesville, FL: 1995. [Google Scholar]
- Lawrence AD, Sahakian BJ, Robbins TW. Cognitive functions and corticostriatal circuits: Insights from Huntington's disease. Trends Cogn Sci. 1998;2 doi: 10.1016/s1364-6613(98)01231-5. [DOI] [PubMed] [Google Scholar]
- Majdandzic J, Grol MJ, van Schie HT, Verhagen L, Toni I, Bekkering H. The role of immediate and final goals in action planning: an fMRI study. Neuroimage. 2007;37:589–98. doi: 10.1016/j.neuroimage.2007.04.071. [DOI] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of Expectancy and Abstinence on the Neural Response to Smoking Cues in Cigarette Smokers: an fMRI Study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Beckham JC, Mozley SL, Feldman ME, Vrana SR, Rose JE. The effects of trauma recall on smoking topography in posttraumatic stress disorder and non-posttraumatic stress disorder trauma survivors. Addict Behav. 2005a;30:247–57. doi: 10.1016/j.addbeh.2004.05.013. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Huettel SA, Rose JE. Abstinence-induced changes in self-report craving correlate with event-related FMRI responses to smoking cues. Neuropsychopharmacology. 2005b;30:1940–7. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Liu J, Salley AN, Behm FM, Rose JE. Selectively reduced responses to smoking cues in amygdala following extinction-based smoking cessation: results of a preliminary functional magnetic resonance imaging study. Addict Biol. 2007;12:503–12. doi: 10.1111/j.1369-1600.2007.00075.x. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33:2148–57. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- Pochon JB, Riis J, Sanfey AG, Nystrom LE, Cohen JD. Functional imaging of decision conflict. J Neurosci. 2008;28:3468–73. doi: 10.1523/JNEUROSCI.4195-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Salley AN, Bates JE, Coleman RE, Hawk TC, Turkington TG. Regional brain activity correlates of nicotine dependence. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301379. [DOI] [PubMed] [Google Scholar]
- Rose JE, Levin ED, Behm FM, Adivi C, Schur C. Transdermal nicotine facilitates smoking cessation. Clinical Pharmacology and Therapeutics. 1990;47:323–30. doi: 10.1038/clpt.1990.35. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–7. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64:366–79. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman SM, Jarvik ME. Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology (Berl) 1976;50:35–9. doi: 10.1007/BF00634151. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Buhler M, Klein S, Zimmermann U, Mann K, Heinz A, Braus DF. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology (Berl) 2006;184:577–88. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- Steiger JH. Tests for Comparing Elements of a Correlation Matrix. Psychological Bulletin. 1980;87:245–251. [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–68. [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–43. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–8. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Feyerabend C. Determinants and effects of attentional bias in smokers. Psychol Addict Behav. 2000;14:111–20. doi: 10.1037//0893-164x.14.2.111. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Instructed smoking expectancy modulates cue-elicited neural activity: A preliminary study. Nicotine & Tobacco Research. 2005;7:637–45. doi: 10.1080/14622200500185520. [DOI] [PMC free article] [PubMed] [Google Scholar]