Abstract
β-Catenin-mediated Wnt signaling is essential in embryonic development and in adult tissues. Recent studies have demonstrated that Axin not only plays an important inhibitory role in coordinating β-catenin degradation, but is itself degraded by the low-density-lipoprotein receptor-related protein (LRP)5/6 Wnt co-receptor. Here, we demonstrate that the endocytic adaptor molecule Disabled-2 (Dab2), which we have previously demonstrated to act as an inhibitor of β-catenin signaling, interacts with Axin and prevents its interaction with and degradation by the LRP5 co-receptor, thereby increasing its half-life and stabilization. Dab2 levels induced during retinoic acid-induced differentiation of F9, or during transforming growth factor-β-induced epithelial–mesenchymal transdifferentiation of mouse mammary epithelial cells result in the stabilization of Axin and concomitant inhibition of β-catenin signaling. Ectopic expression of Dab2 in F9 cells as well as in transformed cell lines results in increased Axin expression and attenuation of Wnt-mediated signaling. We conclude that Dab2 may play an important role in the maintenance of the differentiated state and restrain Wnt-mediated proliferation through its association with and modulation of Axin.
Keywords: Disabled-2 (Dab2), Axin, Wnt, β-catenin, differentiation
Introduction
Canonical Wnt signaling is mediated through the regulated phosphorylation and degradation of the transcriptional coactivator β-catenin (Tolwinski and Wieschaus, 2004). In the absence of Wnt, β-catenin is assembled in a multimeric β-catenin destruction complex that contains, amongst others, Axin, adenomatous polyposis coli (APC) tumor suppressor protein, casein kinase 1 and glycogen synthase kinase 3 (GSK3). In this complex, β-catenin is sequentially phosphorylated at a cluster of Ser and Thr residues near its N terminus by casein kinase 1 and GSK3, and earmarked for degradation through the SCFβTrCP-mediated ubiquitin–proteasome pathway. In the presence of Wnt, activation of the frizzled and low-density-lipoprotein receptor-related protein (LRP)5/6/arrow co-receptors results in destabilization of the β-catenin destruction complex and inhibition of β-catenin phosphorylation and degradation. Stabilized β-catenin then enters the nucleus and interacts with transcriptional regulators, including leukocyte enhance factor-1 and T cell factor, and leads to Wnt responsive gene expression (He et al., 2004; Cadigan and Liu, 2005).
The mechanism by which Wnt promotes destabilization of the β-catenin destruction complex remains unclear, although this process is likely to involve the Dishevelled proteins (Dvl). Dvls are scaffolding proteins that act upstream in the Wnt pathway and are recruited to the plasma membrane by frizzled (Cliffe et al., 2003; Wong et al., 2003). Early studies focused on the role of Dvl in mediating the effects on GSK3 kinase (Liu et al., 2002). Recently, however, GSK3-independent regulation of Wnt signaling has been suggested (Tolwinski and Wieschaus, 2004) involving Dvl and the scaffolding protein Axin that binds APC, β-catenin, GSK3, casein kinase 1 and Dvl (Polakis, 2002). Axin facilitates the formation of the β-catenin destruction complex, resulting in β-catenin phosphorylation and degradation. Axin levels are very low, approximately 1000 times lower than those of the other destruction complex components, and thus Axin might be the limiting component in the pathway (Lee et al., 2003). Axin levels are regulated in a Wnt-dependent manner. Wnt stimulation leads to dephosphorylation and destabilization of Axin, and this decrease in Axin prevents the formation of the destruction complex and to a stabilization of β-catenin (Willert et al., 1999; Yamamoto et al., 1999; Jho et al., 2002). Axin turnover, therefore, modulates the level of β-catenin transcriptional activity and the output of Wnt signaling.
The intracellular domain of the LRP5/6 and Arrow co-receptors can bind and recruit Axin to the plasma membrane, providing a direct link between the Wnt co-receptor and a Wnt intracellular signaling mediator (reviewed in He et al., 2004). Wnt stimulation induces the sequential phosphorylation of LRP5/6 co-receptor by a ‘dual kinase’ mechanism involving GSK3 and casein kinase 1 at multiple PPPSP sites, reiterated five times in the cytoplasmic domain of LRP5/6 (Tamai et al., 2004; Zeng et al., 2005). This phosphorylation of LRP5/6 promotes the recruitment and engagement of LRP5/6 with Axin (Zeng et al., 2005). Dvl aids in the Wnt-induced recruitment of Axin to the membrane and to its interaction with the phosphorylated LRP5/6 co-receptor, and this interaction leads to Axin dephosphorylation and degradation (Cliffe et al., 2003). As the levels of Axin are decreased, the destruction complex is destabilized and β-catenin levels accumulate.
Disabled-2 (Dab2) is a widely expressed adaptor protein shown to be involved in several receptor-mediated signaling pathways (Xu et al., 1995; Hocevar et al., 2001; Prunier et al., 2004). Like other adaptors, Dab2 has no catalytic activity but elicits its function through interaction and modulation of other proteins. Dab2 contains a conserved N-terminal phosphotyrosine-binding domain (Yun et al., 2003) that binds to members of the low-density lipoprotein receptor (LDLR) family and with phosphoinositides (Morris and Cooper, 2001). Its linker and C-terminal regions bind clathrin, the clathrin adaptor protein AP2 and myosin VI, facilitating clathrin-coated pit assembly and receptor-mediated endocytosis (Morris and Cooper, 2001; Mishra et al., 2002; Morris et al., 2002). The endocytic and vesicular trafficking functions of Dab2 are postulated to mediate its effects on cellular signaling (Morris et al., 2002).
Previously we reported that Dab2 functions as a negative regulator of canonical Wnt signaling by stabilizing the β-catenin destruction complex, and postulated that this may contribute to its proposed role as a tumor suppressor (Hocevar et al., 2003). Dab2 overexpression inhibits Wnt-3A-induced accumulation of β-catenin, and Dab2 ablation leads to increased nuclear β-catenin and elevated β-catenin/T cell factor/leukocyte enhance factor-1-dependent gene induction. Dab2 associates with Dvl-3 and its overexpression was shown to decrease Dvl3/Axin interactions and maintain Axin/β-catenin/GSK3 interactions(Hocevar et al., 2003). In this report, we demonstrate that undifferentiated and low-Dab2-expressing cells are Wnt-signaling competent, where as differentiated and high-Dab2-expressing cells are Wnt-signaling incompetent. Induced endogenous Dab2 associates with Axin and prevents Axin association, and subsequent degradation, with the LRP5 co-receptor, resulting in a stabilization and an increase in the half-life of Axin. We further demonstrate that in low-Dab2-expressing transformed cell lines that ectopically expressed Dab2 results in increased Axin expression levels and modulation of β-catenin signaling only in cells with wild-type (WT) APC.
Results
RA-induced Dab2 inhibits Wnt signaling
The mouse F9 cell line is a model for RA-induced visceral endoderm differentiation. Since Dab2 expression in these cells is induced during differentiation (Smith et al., 2001; Prunier and Howe, 2005) and Dab2 is a negative regulator of Wnt signaling (Hocevar et al., 2003), we used this model to examine the role of Dab2 in Wnt-mediated signaling. Figure 1a demonstrates that RA (100 nM) significantly increases Dab2, with a concomitant increase in the expression of the visceral endoderm marker GATA binding protein 4 (GATA-4) (Smith et al., 2001). We next investigated whether F9 cells displayed differentiation state-dependent responsiveness to Wnt and whether Dab2 played any regulatory role in the transitional response of these cells to Wnt. We took advantage of an F9 cell line, which we have established in which Dab2 expression is ablated by stable expression of small interfering RNA (si-RNA) (siDab2/F9 cells) (Prunier and Howe, 2005). As shown in Figures 1b and c, Wnt stimulation for either short term (0–3 h) or long term (0–72 h) has no effect either on Dab2 or on the differentiation marker Sparc (Wiles, 1988). RA-induced Dab2 and Sparc levels were unaffected by Wnt treatment and in siDab2/F9 cells, Dab2 and Sparc are not induced and Wnt treatment has no effect. The differentiation marker GATA-4 is not induced in Dab2-ablated cells (Prunier and Howe, 2005) and by cDNA array analysis villin and collagen type IV are also not induced (data not shown).
Figure 1.
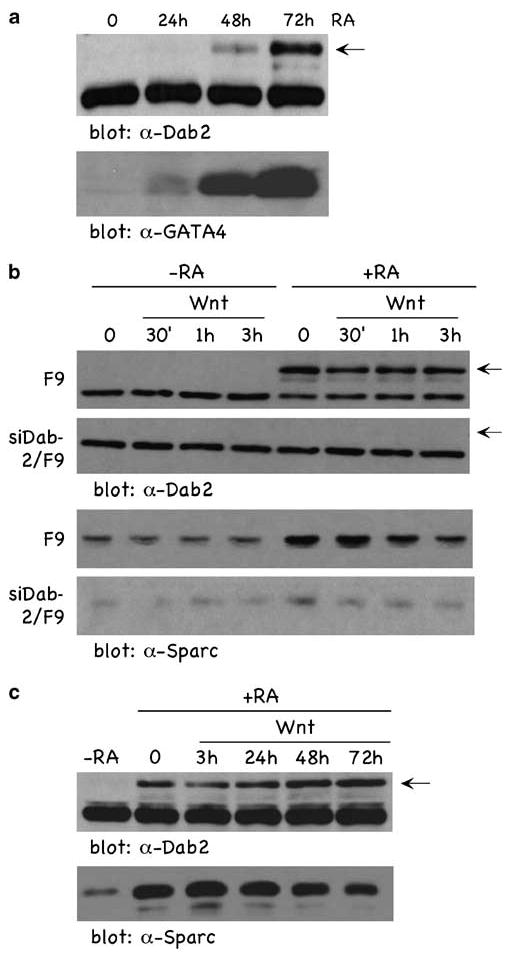
RA induces the upregulation of Dab2 concomitant with visceral endoderm differentiation in F9 cells. (a) F9 cells were treated with 100 nm RA for the times indicated, and cellular lysates were analysed by immunoblotting. The arrow in the figure points to the Dab2 band; the band migrating below Dab2 is a nonspecific band detected by the antibody. (b and c) F9 and siDab2/F9 cells treated (+ RA) and not treated (−RA) with RA (100 nM) for 72 h were stimulated ± Wnt-3A conditioned media for the indicated times, and lysates were analysed by immunoblotting using α-Dab2 (b) and α-Sparc (c) antibody. Dab2, Disabled-2; RA, retinoic acid.
We next investigated the Wnt signaling status of these cells by analysis of nuclear β-catenin (Figure 2a), cyclin D1 (Figure 2b) and two well-characterized Wnt-responsive luciferase reporters, TOPFLASH (Figure 2c) and cyclin D1 (Figure 2d). In undifferentiated cells (−RA), Wnt induced a time-dependent increase in nuclear β-catenin (Figure 2a, upper panel) and cyclin D1 (Figure 2b, upper panel), with maximal effects observed following a 3-h treatment. Further, Wnt induced the transactivation of TOPFLASH (Figure 2c) and cyclin D1 (Figure 2d) reporters ∼6-fold. However, in RA-differentiated cells (+RA), Wnt stimulation failed to induce nuclear β-catenin (Figure 2a, upper panel), cyclin D1 expression (Figure 2b, upper panel) or transactivate TOPFLASH (Figure 2c) and cyclin D1 (Figure 2d) reporters. In contrast, in the Dab2-deficient cells(siDab2/F9), Wnt-induced nuclear β-catenin (Figure 2a, lower panel) and cyclin D1 (Figure 2b, lower panel) were not attenuated by RA treatment. TOPFLASH (Figure 2c) and cyclin D1 (Figure 2d) promoters were similarly transactivated in Dab2-deficient siDab2/F9 cells treated (+ RA) or not treated (−RA) with RA. These results demonstrate that in the undifferentiated state, F9 cells are Wnt signaling competent, whereas once differentiated, become Wnt signaling incompetent. Further, the data demonstrate that the differentiation state (Figure 1) and the Wnt-signaling status (Figure 2) of these cells is dependent on Dab2 expression levels.
Figure 2.
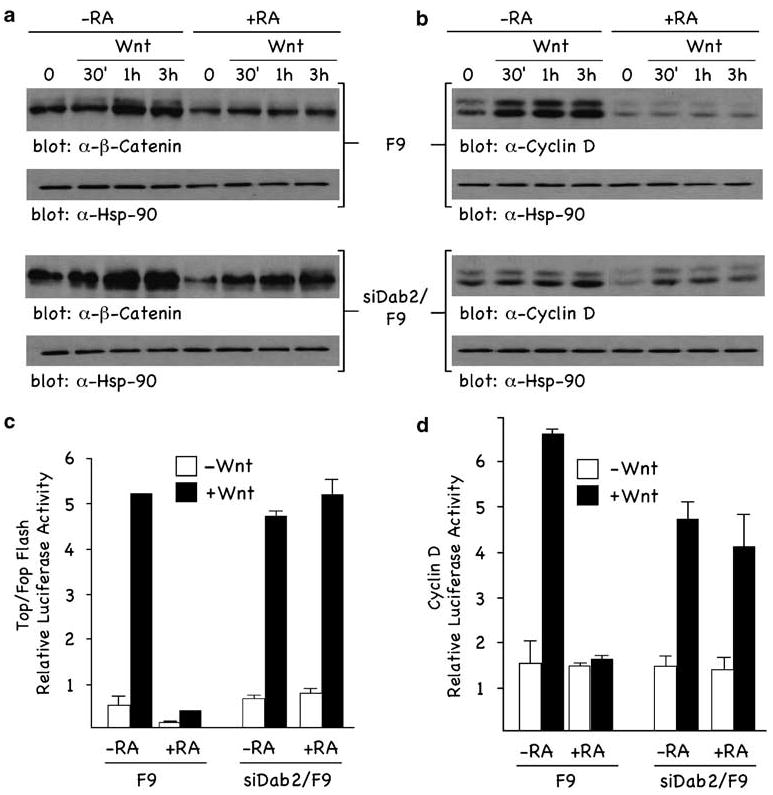
Dab2 inhibits Wnt-stimulated responses. (a and b) F9 and siDab2/F9 cells treated (+ RA) and not treated (−RA) with RA (100 nM) for 72 h were stimulated ± Wnt-3A for the indicated times. Nuclear fractions (a) and total cellular lysates (b) were analysed by immunoblotting using α-β-catenin (a) and α-cyclin D1 (b) antibodies. α-Hsp-90 immunoblot analysis of lysates was used as loading controls. (c and d) F9 and siDab2/F9 cells treated (+ RA) and not treated (−RA) with RA (100 nM) for 72 h and cells were transfected with either the TOPFLASH (c) or cyclin D1 (d) luciferase reporters. Luciferase activity assays were performed and quantitated as described in Materials and methods. Dab2, Disabled-2; RA, retinoic acid.
RA-induced Dab2 stabilizes Axin
Since Axin has been implicated as a primary regulator of canonical Wnt signaling (Lee et al., 2003), we wanted to determine whether the inhibitory effect of Dab2 was mediated through effects on Axin. Figure 3a demonstrates that Wnt-induced transactivation of TOPFLASH activity is decreased in a concentration-dependent manner with increasing concentrations of cotransfected Axin. Next we examined whether RA-induced differentiation of F9 cells might have any effect on Axin expression levels. Figure 3b (left panels) demonstrates that RA treatment of cells induces a time-dependent increase in Axin, concomitant with RA-induced Dab2 levels. RA treatment also increases the level of the Wnt inhibitor Dickkopf-1, as previously demonstrated in F9 cells (Shibamoto et al., 2004). Further, increased Axin levels are associated with a decrease in β-catenin, confirming its inhibitory role on β-catenin signaling. In Dab2-ablated cells (siDab2/F9; Figure 3b, right panels), RA treatment failed to induce Dab2, Axin and Dickkopf-1 expression levels, or attenuate β-catenin levels. In Figure 3c, we confirm that Wnt treatment of undifferentiated (−RA) cells leads to a time-dependent decrease in Axin expression, whereas in RA-differentiated (+ RA) cells, basal Axin is elevated compared with undifferentiated levels, and Wnt stimulation does not decrease Axin expression. The experiment was repeated three times and the quantitation of the results are depicted in Figure 3d. These results suggest that Axin levels are stabilized during RA-induced differentiation of F9 cells, concomitant with increased expression of Dab2, and that once the cells are differentiated, Axin is not regulated in a Wnt-dependent manner.
Figure 3.
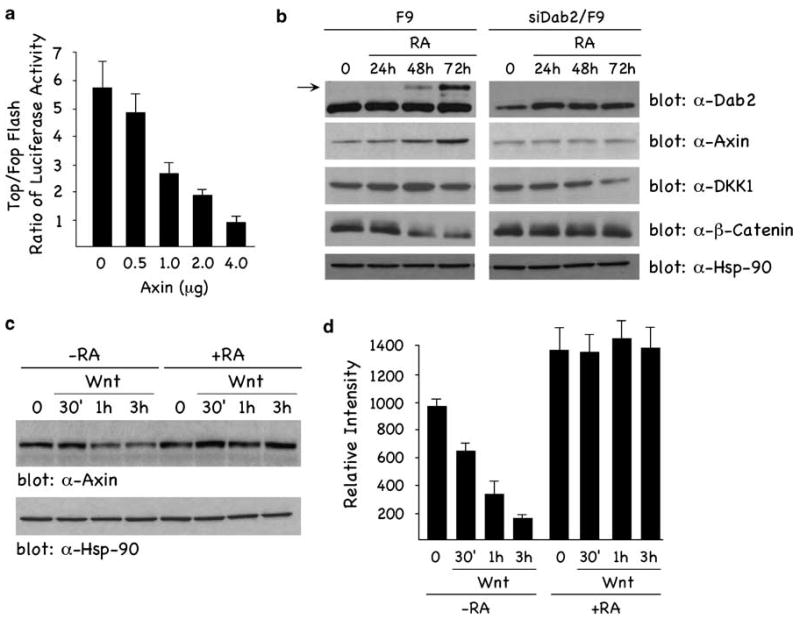
Axin inhibits Wnt signaling and is upregulated by RA treatment of F9 cells. (a) F9 cells were cotransfected with 1 μg of TOP/FOPFLASH reporter constructs and increasing concentrations of Myc-tagged Axin expression construct. TOP/FOPFLASH luciferase activity assays were performed and quantitated as described in Materials and methods. (b) RA induces Axin expression levels concomitant with increased Dab2 expression. F9 and siDab2/F9 cells were treated with 100 nM RA for times indicated, and lysates were analysed by immunoblotting (α-Hsp-90 antibody, loading control). Nuclear lysates were analysed by western blot using α-β-catenin. (c) Wnt-induced degradation of Axin is blocked in RA-differentiated cells. F9 cells treated (+ RA) and not treated (−RA) with RA (100 nM) for 72h were stimulated ± Wnt-3A conditioned media for the indicated times, and lysates were analysed by immunoblotting. (d) Levels of Axin in panel (c) from three separate experiments were determined by densitometric scanning and quantified using a STORM scanner and ImageQuant software from Molecular Dynamics (Fairfield, CT, USA). Levels are expressed as relative intensities at the various time points. The data are indicated as means ± s.d. Dab2, Disabled-2; RA, retinoic acid.
To directly determine Dab2'srole in regulating Axin expression, we overexpressed Flag-tagged Dab2 and examined endogenous and ectopically overexpressed Axin levels. Figure 4a demonstrates that ectopically expressed Dab2 results in an increase in endogenous Axin. Figure 4b demonstrates that Dab2, in a concentration-dependent manner, leads to an increase expression of exogenous Myc-tagged Axin. In Figure 4c, the effects of Dab2 on Wnt-induced Axin regulation are shown. In the absence of Dab2 expression (−Flag-Dab2), Myc-tagged Axin levels are downregulated in a time-dependent manner in response to Wnt treatment. Within 1 h of Wnt treatment, ectopically expressed Axin is undetectable. Dab2 expression however not only results in higher expression of Myc-tagged Axin, but also renders Axin insensitive to downregulation by Wnt. The data presented in Figure 4d demonstrate that increasing Dab2 has little, if any, effect on the transcription levels of Axin, and suggest that Dab2 expression, whether endogenously induced in RA-differentiated F9 cells (Figure 3) or ectopically overexpressed (Figure 4), results in stabilization of Axin expression. Pulse-chase analysis in Supplementary Figure S1 confirms that Dab2 increases the half-life of Axin.
Figure 4.
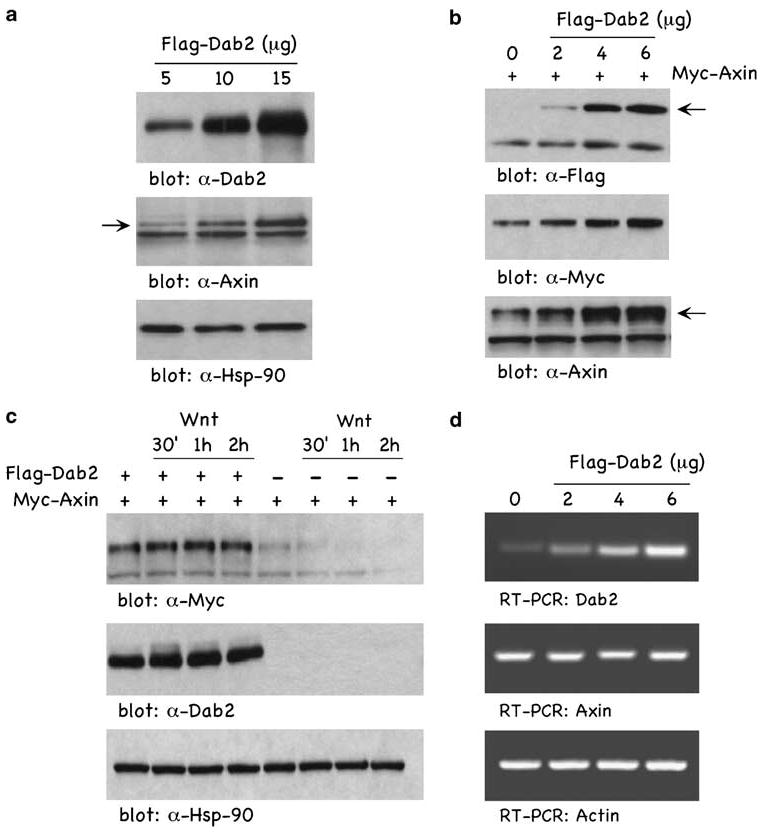
Dab2 overexpression stabilizes Axin expression. (a) F9 cells were transfected with increasing concentrations of Flag-tagged Dab2, and lysates were analysed by western blot. (b) F9 cells were cotransfected with Myc-tagged Axin and increasing concentrations of Flag-tagged Dab2 and lysates were analysed by immunoblotting. (c) F9 cells were transfected with Myc-tagged Axin in the presence or absence of Flag-tagged Dab2, and stimulated with Wnt-3A for the times indicated. Lysates were prepared and analysed by western blot. (d) F9 cells were transfected with increasing concentrations of Flag-tagged Dab2 and RNA was isolated from cellular lysates and subjected to reverse transcription–polymerase chain reaction analysis with primers for Dab2, Axin and actin. Dab2, Disabled-2.
Dab2 inhibits Axin/LRP5 interactions
Axin has been shown to be destabilized by Wnt following its translocation and interaction with the LRP5/6 Wnt co-receptors. To investigate the effects of Dab2 on LRP5/6/Axin interactions, cells were transfected with Flag-tagged LRP5 and Myc-Axin in the presence of increasing concentrations of Flag-tagged Dab2 expression plasmid, and anti-Myc-Axin immunoprecipitates (IPs) were analysed for co-immunoprecipitating proteins. Figure 5a (α-Flag panel) demonstrates that in the absence of Dab2, LRP5 is co-precipitated in an Axin IP, indicating an interaction between LRP5/Axin. Increasing Dab2 expression, however, significantly reduces the level of co-immunoprecipitated LRP5 in the Axin IP. The results also demonstrate an interaction between Dab2/Axin, in that there is a dose-dependent correlation between transfected Dab2 levels and co-immunoprecipitated Dab2 in the Axin IP (α-Dab2 panel). Immunoprecipitations using control IgG antisera (IP: IgG) show no precipitated LRP5 or Dab2 proteins. Subsequent panels in Figure 5a are controls demonstrating that equal Axin was immunoprecipitated in the α-Myc IP (third panel ‘α-Myc blot), and demonstrating the relative expression levels of transfected Dab2, LRP5 and Axin expression plasmids, respectively.
Figure 5.
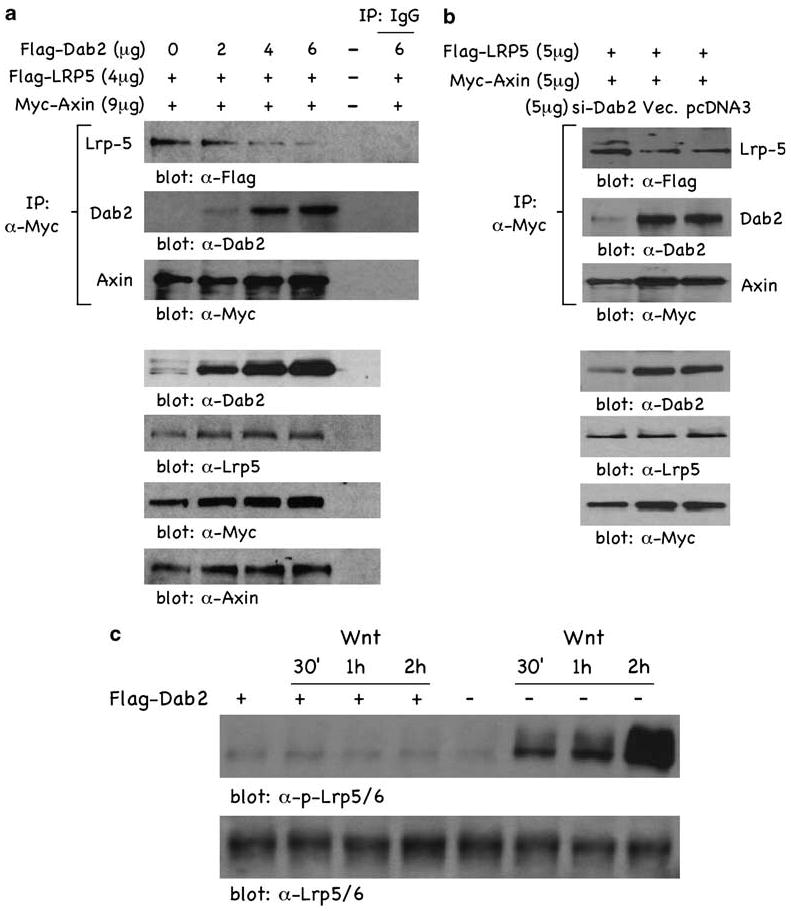
Dab2 expression blocks LRP5/Axin interactions. (a) F9 cells were transfected with Flag-tagged LRP5 (4 μg), Myc-tagged Axin (9 μg) and increasing concentrations of Flag-tagged Dab2. Following transfection, lysates were subjected to immunoprecipitation with control IgG or α-Myc antisera. Immunocomplexes were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and subjected to western blot analysis. Cellular expression levels of Dab2, LRP5 and Axin in the transfected F9 cells were determined by western blot analysis (lower four panels). (b) F9 cells treated with 100 nM RA for 72h were transfected with Flag-tagged LRP5 (5 μg), Myc-tagged Axin (5 μg) and either si-Dab2, si-empty vector or pcDNA3 (5 μg). Following transfection, lysates were subjected to immunoprecipitation with α-myc antisera. Immunocomplexes were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and subjected to immunoblotting. Cellular expression levels of Dab2, LRP5 and Axin in the transfected F9 cells were also determined (lower three panels). (c) F9 cells (transfected with Flag-tagged Dab2 and not transfected) were stimulated with Wnt-3A for the times indicated. Lysates were prepared and analysed by immunoblotting. Dab2, Disabled-2; RA, retinoic acid.
To provide further support for Dab2 preventing LRP5/Axin interaction, we cotransfected Flag-tagged LRP5 and Myc–Axin in the presence or absence of the si-Dab2 expression vector in RA-differentiated F9 cells and Axin IPs were analysed for co-precipitating proteins (Figure 5b). Attenuating Dab2 expression in RA-differentiated cells with the si-Dab2 vector, as compared with the si-RNA empty vector or an irrelevant pcDNA3 vector, results in increased co-immunoprecipitation of LRP5 (α-Flag panel). si-Dab2 expression in differentiated cells successfully lowered Dab2 levels compared with si-RNA empty vector and pcDNA3 (Figure 5b, α-Dab2 panel), and supporting Dab2's role in stabilizing Axin, there is less Axin expression in the si-Dab2-attenuated, differentiated cells (α-Myc panel). The lower panels in Figure 5b demonstrate the relative expression levels of transfected LRP5 and Axin expression plasmids. Since Wnt-induced LRP5/Axin interactions result in the phosphorylation of LRP5/6 (Zeng et al., 2005), we reasoned that if Dab2 was inhibiting LRP5/Axin interactions, it might have effects on Wnt-mediated LRP5 phosphorylation. Figure 5c demonstrates that when cells are stimulated with Wnt, LRP5 is phosphorylated in a time-dependent manner, but that in cells overexpressing Dab2 (+ Flag-Dab2), Wnt-induced LRP5/6 phosphorylation is not observed (blot: α-p-LRP5/6). Steady-state levels of LRP5/6 do not change upon Dab2 overexpression or during the course of Wnt treatment (blot: α-LRP5/6). The data of Figure 5 establish interactions between LRP5/Axin and Dab2/Axin, but, more importantly, indicate that Dab2 expression levels modulate the interaction between LRP5/Axin and the Axin-dependent phosphorylation of LRP5/6. Thus, the ability of Dab2 to stabilize and increase the half-life of Axin may be due to its ability to inhibit LRP5/Axin interactions at the plasma membrane.
We next examined whether the effects of Dab2 on Axin expression and on β-catenin signaling were specific to F9 cells. We previously demonstrated in a TGFβ-induced epithelial to mesenchymal transdifferentiation model in mouse mammary epithelial cells (NMuMG) that Dab2 expression is induced and required for transdifferentiation (Prunier and Howe, 2005). As previously demonstrated, TGFβ induces Dab2 expression levels in these cells following a 24-h treatment (Figure 6a, α-Dab2 panel). Confirming the results in F9 cells, Dab2 induction in TGFβ-treated NMuMG cells (+ TGFβ) is accompanied by an increase in Axin (Figure 6a, α-Axin panel) and a decrease in β-catenin expression (Figure 6a, α-β-catenin panel). In NMuMG cells stably expressing si-Dab2 (siDab2/NMuMG), TGFβ-induced Dab2 expression is not observed and Axin and β-catenin levels are not modulated (Figure 6a). Corroborating these results, Wnt-induced TOPFLASH transactivation is greatly attenuated in TGFβ-treated NMuMG cells compared with si-Dab2/NMuMG cells (Figure 6c).
Figure 6.
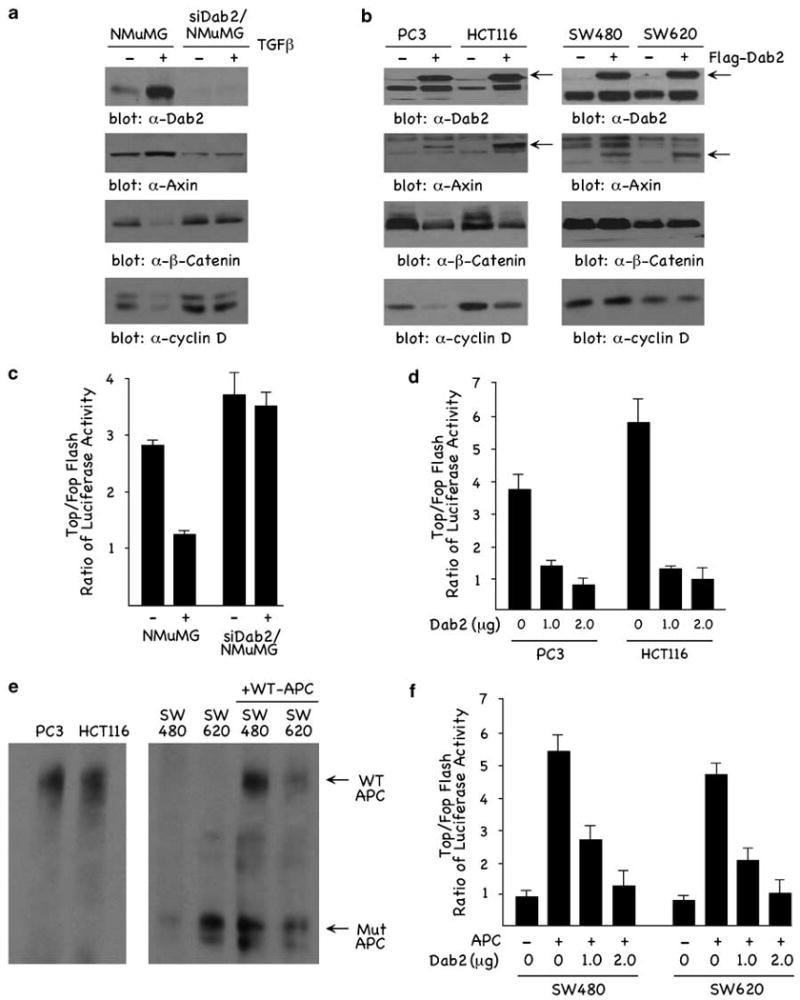
Dab2 expression modulates Axin and β-catenin levels. (a) NMuMG and siDab2/NMuMG cells were treated ± TGFβ (5 μg/ml) for 24 h andlysates or nuclear fractions (β-catenin) were analysed by western blot. (b) PC3, HCT116, SW480 and SW620 cells were transfected with 6 μg of control pcDNA3 vector (−) or Flag-tagged Dab2 (+), and following transfection lysates or nuclear fractions (β-catenin) were analysed by immunoblotting. (c) NMuMG and siDab2/NMuMG cells treated ± TGFβ were transfected with the TOPFLASH luciferase reporters. Luciferase activity assays were performed and quantitated as described in Materials and methods. (d) PC3 and (e) HCT116 cells were transfected with the TOPFLASH luciferase reporter in the presence of 1 and 2 μg of Flag-tagged Dab2, and luciferase activity was determined as described above. (e) PC3, HCT116, SW480 and SW620 cell lysates together with lysates from SW480 and SW620 transfected with WT APC were prepared and analysed by western blot using α-APC antibody. Following transfection, WT APC was induced with 50 μM ZnCl2 for 24 h as described (Morin et al., 1996). (f) SW480 and SW620 cells transfected with WT APC were transfected with the TOPFLASH luciferase reporter in the presence of 1 and 2 μg of Flag-tagged Dab2, and luciferase activity was determined as described above. APC, adenomatous polyposis coli; Dab2, Disabled-2; TGFβ, transforming growth factor-β; WT, wild type.
We further examined Dab2, Axin, β-catenin and TOPFLASH transactivation in four transformed epithelial cell lines. As shown in Figure 6b, PC3 (prostate), HCT116 (colon), SW480 and SW620 (colon) have low to non-existing levels of endogenous (−) Dab2 and Axin, but have endogenous levels of β-catenin and cyclin D1. Transient ectopic expression of Flag-tagged Dab2 in these cells (+Flag-Dab2) results in increased Dab2, with a concomitant increase in Axin. Interestingly, only PC3 and HCT116 cells showed decreased β-catenin and cyclin D1 expression in response to Dab2 overexpression. β-Catenin and cyclin D1 are not attenuated in SW480 and SW620 colon lines (Figure 6b). This is further confirmed by the results in Figure 6d, where Dab2 is shown to attenuate Wnt-mediated TOPFLASH transactivation in PC3 and HCT116 cells, but not in SW480 and SW620 cells (data not shown). While the SW480 and SW620 cell lines showed Dab2 modulation of Axin, β-catenin in these cells was not decreased (Figure 6b), nor wasDab2 and Axin overexpression able to modulate Wnt-mediated TOPFLASH transactivation (data not shown). PC3 and HCT116 cells express WT APC, whereas both the SW480 and SW620 cells express truncated, mutant forms of APC (Daniel and Reynolds, 1995; Ilyas et al., 1997). To determine whether WT APC could rescue the effects of Dab2 on TOPFLASH transactivation in SW480 and SW620 cells, we cotransfected cells with TOPFLASH and an inducible WT APC expression plasmid (Morin et al., 1996), in the presence or absence of Dab2 (1 and 2 μg). The APC status of the cells is demonstrated in Figure 6e. PC3 and HCT116 express full-length WT APC, whereas SW480 and SW620 express mutant APC. Induction with 50 μM ZnCl2 for 24h resulted in the expression of WT APC in SW480 and SW620 cells (Figure 6e). SW480 and SW620 cells have elevated basal TOPFLASH activity, and Wnt treatment does not result in enhanced transactivation (Figure 6f). Induction and expression of WT APC in both SW480 and SW620 cells results in Wnt-induced TOPFLASH transactivation, which is attenuated by cotransfection with increasing concentrations of Dab2 (Figure 6f). These results demonstrate that Dab2 regulation of β-catenin and TOPFLASH activity requires WT APC, suggesting that Dab2 and Axin act upstream of APC to negatively regulate β-catenin signaling.
Discussion
Mouse embryonal F9 cells are an in vitro model for visceral endoderm differentiation (Smith et al., 2001; Prunier and Howe, 2005), and have been demonstrated to resemble absorptive epithelial cells (Shibamoto et al., 2004). Herein, we demonstrate that in undifferentiated F9 cells, the expression of cytosolic adaptor Dab2 is low and cells are responsive to Wnt. In RA-differentiated F9 cells, Dab2 expression is induced and Wnt signaling is attenuated. In siDab2/F9 cells, in which Dab2 expression is abrogated by stable si-RNA-mediated expression, RA-induced differentiation and attenuation of the Wnt pathway are not observed. These results were confirmed in the TGFβ-induced epithelial to mesenchymal transdifferentiation model in NMuMG cells, where we demonstrate that in the absence of Dab2 the cells are responsive to β-catenin signaling and upon differentiation and induction of Dab2, cells become refractory to Wnt signaling. Thus, in differentiated cells, we speculate that Dab2 maintains the differentiated state and restrains proliferation through its negative regulation of Wnt signaling, whereas in the proliferative state, Wnt attenuates expression of its inhibitor to maintain proliferation and block induction of the differentiation state. These results are consistent with a recent report (Lickert et al., 2005) demonstrating that Dab2 gene expression is upregulated in conditional β-catenin mutant mouse embryos, suggesting that not only is Dab2 a Wnt/β-catenin target gene, but that β-catenin signaling leads to its attenuation.
In many intestinal neoplasias, constitutive Wnt signaling (Gregorieff and Clevers, 2005) and aberrantly low levels of Dab2 protein (Kleeff et al., 2002; Prunier et al., 2004) are often observed. In fact, Dab2 has been designated as a putative tumor suppressor gene because its expression is often found to be downregulated in many transformed cell lines. In these tumors, therefore, constitutive expression of Wnt target genes such as cyclin D1 and c-myc may not only lead to excessive proliferation, but may also block the induction of differentiation markers and Wnt inhibitors such as Dab2. We show in transformed epithelial cell lines that Dab2 and Axin levels are low and that the cells have elevated β-catenin and cyclin D1 levels. Transient ectopic expression of Dab2 in these lines is able to restore Axin expression, and, in the two lines that have previously been demonstrated to express WT APC (Daniel and Reynolds, 1995; Ilyas et al., 1997), increased Dab2 and Axin leads to attenuation of β-catenin and cyclin D1. Overexpression of Dab2 and stabilized Axin does not modulate β-catenin signaling in the lines containing mutant forms of APC. However, WT APC can rescue the inhibitory effects of Dab2 on Wnt signaling in cell lines with mutated APC. This suggests that the inhibitory effects of Dab2 and Axin on β-catenin signaling are upstream of and require APC function.
Recent studies demonstrate that Axin levels play a key regulatory function in β-catenin/Wnt signaling (Cliffe et al., 2003; Lee et al., 2003). We demonstrate that Dab2 not only interacts with Axin, but also stabilizes its expression. Dab2 expression, whether endogenously induced during differentiation or ectopically overexpressed, results in the stabilization of Axin. Since Wnt stimulation promotes Axin/LRP5/6 interactions (Tamai et al., 2004), leading to Axin destabilization, and the phosphotyrosine-binding domain of Dab2 is known to bind to other LDL receptor family members (Morris and Cooper, 2001), we reasoned that Dab2 may interact with LRP5/6 and thereby block LRP5/6/Axin interactions. While we could not demonstrate an interaction between Dab2 and LRP5 (data not shown), we did observe that Dab2 blocked the interaction of LRP5/Axin and the phosphorylation of LRP5/6 in response to Wnt. We postulate that the ability of Dab2 to stabilize and increase the half-life of Axin may be due to its ability to interfere with LRP5/Axin interactions at the plasma membrane.
How Dab2 interferes with Axin/LRP5/6 at the plasma membrane is unknown. Axin and Dvl are colocalized in punctate intracellular ‘dots’ thought to represent intracellular vesicles (Fagotto et al., 1999; Cliffe et al., 2003). Wnt also induces co-clustering of LRP5/6 and Dvl into plasma membrane-associated aggregates that contain Wnt-pathway components(Bilic et al., 2007). Both Axin and Dvl contain phospholipid-binding motifs that are conserved in their DIX domains, and this domain in the Dvl protein is essential for its recruitment to membranes and its function in controlling the degradation of β-catenin (Capelluto et al., 2002). On the basis of these findings, a ‘vesicle transport’ model has been proposed whereby Axin and Dvl are associated with the same vesicle, and Dvl recruits or shuttles Axin to the plasma membrane, where it interacts with, and is subsequently regulated by, the LRP5/6 co-receptors (Cliffe et al., 2003; He et al., 2004). Thus, endocytic vesicles are postulated to provide a platform for assembly and transport of the Axin complex to the plasma membrane for inactivation by Wnt receptors (Cliffe et al., 2003). Consistent with this model, several recent reports have demonstrated that endocytosis and endosomal transport have been shown to facilitate and maximize Wnt signaling in Drosophila (Chen et al., 2003; Yamamoto et al., 2006).
Dab2 is an adaptor involved in endocytosis and protein trafficking (Morris and Cooper, 2001; Mishra et al., 2002). It contains a phosphotyrosine-binding domain that binds to both LDLR family members and directly to phospholipids. In addition, Dab2 binds to clathrin and the clathrin adaptor AP-2 and displays a punctate ‘dot-like’ staining pattern in cells, suggestive of its association with intracellular vesicles, endocytic and secretory vesicles (Morris and Cooper, 2001; Kakimura and Cooper, 2006). Overexpressed Dab2 results in a major redistribution of the AP-2 clathrin adaptor into large clusters or aggregates near the cell periphery that are devoid of clathrin (Morris and Cooper, 2001). It is postulated that high Dab2 levels saturate AP-2-binding sites, sequestering and preventing AP-2 recycling to the cell surface. In the absence of AP-2 at the membrane, receptor complexes are not stabilized and clathrin-coated pits are not formed. On the basis of these data, we postulate that in cells with low Dab2, such as undifferentiated F9, Wnt signaling is operative because endocytosis and endosomal transport of the Wnt signalosome are maintained and allowed to recycle to the plasma membrane. In cells that have induced and high levels of Dab2, such as RA-differentiated F9 cells, the Wnt signalosome is either not properly endocytosed and/or not properly assembled at the plasma membrane due to AP-2 sequestration. Interestingly, AP-2 and Dvl associations have recently be shown to be required for frizzled endocytosis (Yu et al., 2007). We have previously demonstrated that Dab2 can interact with Dvl and that Dab2 overexpression can disrupt Axin/Dvl interactions(Hocevar et al., 2003). Herein we demonstrate that Dab2 stabilizes Axin expression by preventing its interaction with the LRP5 co-receptor. Since Dvl proteins are required for the recruitment of Axin to LRP5 (Cliffe et al., 2003; Zeng et al., 2005) and LRP5 phosphorylation (Bilic et al., 2007), we postulate that disruption of Dvl/Axin interactions by Dab2 results in Axin not being recruited to the membrane, resulting in its stabilization and attenuation of Wnt signaling.
Materials and methods
Cell culture, transient transfection and luciferase assay
F9 mouse embryonic carcinoma cells and the siDab2F9 clone were cultured and differentiated with RA (100 nM) as described (Prunier and Howe, 2005). NMuMG cells were cultured and differentiated with TGFβ as described (Prunier and Howe, 2005). PC3, HCT116, SW480 and SW620 were obtained from American Type Culture Collection and cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics (penicillin and streptomycin). For luciferase reporter and transient expression assays, cells were transiently transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) as previously described (Hocevar et al., 2003). Relative luciferase activity is expressed as the ratio of TOP/FOPFLASH and cyclin D1 activity in control and Wnt-3A-treated cells. All assays were performed in triplicate and the data are presented as means ± s.d. Wnt-3A-conditioned media was prepared as described previously from mouse L-cells overexpressing Wnt-3A (Shibamoto et al., 1998).
DNA constructs and antibodies
TOPFLASH and FOPFLASH luciferase reporter constructs were purchased from Upstate (Lake Placid, NY, USA). The cyclin D1 promoter luciferase construct has been described previously (Herber et al., 1994). Expression constructs for Myc–Axin (Furuhashi et al., 2001), Flag-tagged Dab2 (Hocevar et al., 2001) and Flag-tagged LRP5 (kindly provided by Dr Dianqing Wu, University of Connecticut) have been previously described. The monoclonal antibody to Dab2/p96 was purchased from Transduction Laboratories (San Jose, CA, USA). Anti-Axin antibody was purchased from Upstate, anti-Myc antibody from Cell Signaling Technology (Danvers, MA, USA), anti-Flag antibody from Sigma (St Louis, MO, USA), anti-cyclin D1 and anti GATA-4 antibodies from Santa Cruz Biotechnologies(Santa Cruz, CA, USA), anti-LRP5 antibody from ZyMed (South San Francisco, CA, USA), and anti-Sparc antibody from R&D Systems (Minneapolis, MN, USA). Anti-APC antibody was purchased from Oncogene (San Diego, CA, USA), anti-Dickkopf-1 was from Novus (Littleton, CO, USA) and anti-phospho-LRP5/6 was kindly provided by Dr Zeng and Dr He.
Preparation of cell lysates, immunoprecipitation and western blot analysis
For immunoprecipitation and western blot analysis, cells were lysed in buffer D and immunoprecipitation carried out as previously described (Hocevar et al., 2003). For APC western blot assay, APC expression was induced with ZnCl2 (50 mM) for 24 h asdescribed (Morin et al., 1996), and lysates were run on 4.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels and transferred to polyvinylidene difluoride membrane overnight at 300 mA. Preparation of cytosolic and nuclear fractions was carried out as described (Hocevar et al., 2003).
Reverse transcription–PCR analysis
RNA was isolated using the RNeasy Mini kit of Qiagen (Valencia, VA, USA). RNA from each sample (4 μg) was reverse transcribed using the Invitrogen SuperScript III first-strand synthesis system kit. Reverse transcription products were subjected to polymerase chain reaction for 25 cycles. The polymerase chain reaction products were stained with ethidium bromide and analysed on 1.5% agarose gels. Primers utilized were as follows: Axin, top 5′ -CACAAGCTGCCTTCTGTCCCAGC-3′, bottom 5′-GAATTATGGTGAACATGGTGGTGGC-3′; DAB2, top 5′-GGAGCATGTAGACCATGATG-3′, bottom 5′-AAAGGATTTCCGAAAGGGCT-3′; actin, top 5′-AGCTGTGCTATGTTGCTCTAGACTT-3′, bottom 5′-CACTTCATGATGGAATTGAATGTAG-3′.
Supplementary Material
Acknowledgments
We thank Dr S Takada for the generous provision of control Wnt-3A-producing mouse L-cells and Drs Furuhashi, Wu, Zeng and He for generous provision of the Myc–Axin, Flag-tagged LRP5 plasmids and LRP5/6 antibody, respectively. We also thank Dr Gary Wildey for helpful discussion and also thank Dr S Ledbetter at Genzyme Inc. for generous provision of TGFβ This work was supported by Grants CA55536 and CA80095 from the National Cancer Institute to PHH.
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
References
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, et al. Wnt inducesLRP6 signalosomesand promotes Dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J Cell Sci. 2005;119:395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- Capelluto DG, Kutateladze TG, Habas R, Finkielstein CV, He X, Overduin M. The DIX domain targets dishevelled to actin stress fibres and vesicular membranes. Nature. 2002;419:726–729. doi: 10.1038/nature01056. [DOI] [PubMed] [Google Scholar]
- Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, et al. Dishevelled 2 recruits β-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301:1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- Cliffe A, Hamada F, Bienz M. A role of dishevelled in relocating Axin to the plasma membrane during wingless signaling. Curr Biol. 2003;13:960–966. doi: 10.1016/s0960-9822(03)00370-1. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Reynolds AB. The tyrosine kinase substrate p120cas binds directly to E-cadherin but not to the adenomatous polyposis coli protein or alpha-catenin. Mol Cell Biol. 1995;15:4819–4824. doi: 10.1128/mcb.15.9.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F, Jho E, Zeng L, Kurth T, Joos T, Kaufmann C, et al. Domains of Axin involved in protein-protein interactions, Wnt pathway inhibition, and intracellular localization. J Cell Biol. 1999;145:741–756. doi: 10.1083/jcb.145.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi M, Yagi K, Yamamoto H, Furukawa Y, Shimada S, Nakamura Y, et al. Axin facilitates Smad3 activation in the transforming growth factor beta signaling pathway. Mol Cell Biol. 2001;21:5132–5141. doi: 10.1128/MCB.21.15.5132-5141.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorieff A, Clevers H. Wnt signaling in the intestinal epithelium: from endoderm to cancer. Genes Dev. 2005;19:877–890. doi: 10.1101/gad.1295405. [DOI] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins5 and 6 in Wnt/β-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- Herber B, Truss M, Beato M, Muller R. Inducible regulatory elementsin the human cyclin D1 promoter. Oncogene. 1994;9:1295–1304. [PubMed] [Google Scholar]
- Hocevar BA, Mou F, Rennolds JL, Morris SM, Cooper JA, Howe PH. Interactionsbetween disabled-2 (Dab2) and the Wnt signaling pathway. EMBO J. 2003;22:3084–3094. doi: 10.1093/emboj/cdg286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocevar BA, Smine A, Xu XX, Howe PH. The adaptor molecule Disabled-2 links the transforming growth factor β receptors to the Smad pathway. EMBO J. 2001;20:2789–2801. doi: 10.1093/emboj/20.11.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyas M, Tomlinson IP, Rowan A, Pignatelli M, Bodmer WF. catenin mutations in cell lines established from colorectal cancers. Proc Natl Acad Sci USA. 1997;94:10330–10334. doi: 10.1073/pnas.94.19.10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimura DM, Cooper JA. Clathrin interaction and subcellular localization of Ce-Dab-1, an adaptor for protein secretion in Caenorhabditis elegans. Traffic. 2006;7:324–336. doi: 10.1111/j.1600-0854.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- Kleeff J, Huang Y, Mok SC, Zimmermann A, Friess H, Buchler MW. Down-regulation of DOC-2 in colorectal cancer points to its role as a tumor suppressor in this malignancy. Dis Colon Rectum. 2002;45:1242–1245. doi: 10.1007/s10350-004-6399-2. [DOI] [PubMed] [Google Scholar]
- Lee E, Salic A, Kruger R, Heinrich R, Kirchner MW. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1:116–132. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickert H, Cox B, Wehrle C, Taketo MM, Kemler R, Rossant J. Dissecting Wnt/β-catenin signaling during gastrulation using RNA interference in mouse embryos. Development. 2005;132:2599–2609. doi: 10.1242/dev.01842. [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, et al. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Keyel PA, Hawryluk J, Agostinelli NR, Watkins SC, Traub LM. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J. 2002;21:4915–4926. doi: 10.1093/emboj/cdf487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin PJ, Vogelstein B, Kinzler KW. Apoptosis and APC in colorectal tumorigenesis. Proc Natl Acad Sci USA. 1996;93:7950–7954. doi: 10.1073/pnas.93.15.7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SM, Arden SD, Roberts RC, Kendrick-Jones J, Cooper JA, Luzio JP, et al. Myosin VI binds to and localises with Dab2, potentially linking receptor-mediated endocytosis and the actin cytoskeleton. Traffic. 2002;3:331–341. doi: 10.1034/j.1600-0854.2002.30503.x. [DOI] [PubMed] [Google Scholar]
- Morris SM, Cooper JA. Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic. 2001;2:111–123. doi: 10.1034/j.1600-0854.2001.020206.x. [DOI] [PubMed] [Google Scholar]
- Polakis P. Casein kinase I: a Wnt'er of disconnect. Curr Biol. 2002;12:R499–R501. doi: 10.1016/s0960-9822(02)00969-7. [DOI] [PubMed] [Google Scholar]
- Prunier C, Hocevar BH, Howe PH. Wnt signaling: physiology and pathology. Growth Factors. 2004;22:141–150. doi: 10.1080/08977190410001720860. [DOI] [PubMed] [Google Scholar]
- Prunier C, Howe PH. Disabled-2 (Dab2) is required for transforming growth factor β-induced epithelial to mesenchymal transition (EMT) J Biol Chem. 2005;280:17540–17548. doi: 10.1074/jbc.M500974200. [DOI] [PubMed] [Google Scholar]
- Shibamoto S, Higano K, Takada R, Ito F, Takeichi M, Takada S. Cytoskeletal reorganization by soluble Wnt-3a protein signaling. Genes Cells. 1998;3:659–670. doi: 10.1046/j.1365-2443.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Shibamoto S, Winer J, Williams M, Polakis P. A blockade in Wnt signaling is activated following the differentiation of F9 teratocarcinoma cells. Exp Cell Res. 2004;292:11–20. doi: 10.1016/j.yexcr.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Smith ER, Capochichi CD, He J, Smedberg JL, Yang DH, Prowse AH, et al. Disabled-2 mediates c-Fos suppression and the cell growth regulatory activity of retinoic acid in embryonic carcinoma cells. J Biol Chem. 2001;276:47303–47310. doi: 10.1074/jbc.M106158200. [DOI] [PubMed] [Google Scholar]
- Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, et al. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- Tolwinski NS, Wieschaus E. Rethinking Wnt signaling. Trends Genet. 2004;20:177–181. doi: 10.1016/j.tig.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Wiles MV. Isolation of differentially expressed human cDNA clones: similarities between mouse and human embryonal carcinoma cell differentiation. Development. 1988;104:403–413. doi: 10.1242/dev.104.3.403. [DOI] [PubMed] [Google Scholar]
- Willert K, Shibamoto S, Nusse R. Wnt-induced dephosphorylation of Axin releases beta-catenin from the Axin complex. Genes Dev. 1999;13:1768–1773. doi: 10.1101/gad.13.14.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D, et al. Direct binding of the PDX domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol Cell. 2003;12:1251–1260. doi: 10.1016/s1097-2765(03)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XX, Yang W, Jackowski S, Rock CO. Cloning of a novel phosphoprotein regulated by colony-stimulating factor 1 shares a domain with the Drosophila disabled gene product. J Biol Chem. 1995;270:14184–14191. doi: 10.1074/jbc.270.23.14184. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A. Phosphorylation of Axin, a Wnt signal negative regulator, by glycogen synthase kinase-3β regulates its stability. J Biol Chem. 1999;274:10681–10684. doi: 10.1074/jbc.274.16.10681. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Komekado H, Kikuchi A. Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of β-catenin. Dev Cell. 2006;11:213–223. doi: 10.1016/j.devcel.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Yu A, Rual JF, Tamai K, Harada Y, Vidal M, He X, et al. Association of dishevelled with the clathrin AP-2 adaptor is required for Frizzled endocytosis and planar cell polarity signaling. Dev Cell. 2007;12:129–141. doi: 10.1016/j.devcel.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun M, Keshvara L, Park CG, Zhang YM, Dickerson JB, Zheng J, et al. Crystal structures of the Dab homology domains of mouse disabled 1 and 2. J Biol Chem. 2003;278:36572–36581. doi: 10.1074/jbc.M304384200. [DOI] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B, Shita L, Huang H, Habas R, et al. A dual-mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


