Abstract
Background
Although blood pressure elevation and lower nocturnal dipping both increase vascular risk, it is not known if either or both are also associated with brain atrophy, cerebral perfusion, and functional status.
Methods
We investigated the association of elevated blood pressure and nocturnal dipping based on 24-hour ambulatory recordings with brain atrophy and perfusion and functional status in 80 older adults with and without stroke (age 66.4±0.8 years, 51% women, 16% non-white, 46% prior ischemic stroke, 55% hypertension). Anatomical and 3-D continuous arterial spin labeling brain MRI measuring volumes and perfusion and 24-h ambulatory blood pressure readings were completed.
Results
Nocturnal dipping of lesser magnitude in systolic (non-stroke: p=0.03; stroke: p=0.005) and pulse pressure (non-stroke: p=0.002; stroke: p=0.01) was associated with greater brain atrophy, affecting preferentially the fronto-parietal regions. Dipping of lesser magnitude in systolic blood pressure (non-stroke: p=0.01; stroke: p=0.03) and greater brain atrophy (non-stroke: p=0.04; stroke: p= 0.05) were also associated with slower gait speed and worse functional outcome post stroke. Higher 24-hour blood pressure averages were associated with lower cerebral perfusion but not atrophy in those with and without stroke.
Conclusions
In those with and without stroke, dipping of lesser magnitude in systolic and pulse pressure is associated with brain atrophy and worse functional status. Nocturnal dipping, in addition to elevated blood pressure, should be considered as an additional important target in the clinical evaluation of those at risk for cerebrovascular disease or functional loss.
Keywords: Ambulatory blood pressure, MRI brain imaging, gait speed, stroke, hypertension
Introduction
Elevated blood pressure is associated with lower cerebral blood flow and brain atrophy.1–3 In addition, less nighttime dipping in blood pressure, measured by ambulatory blood pressure monitoring (ABPM), is associated with greater risk of stroke4 and worse prognosis after a stroke5. However, it is not clear if dipping of lesser magnitude in blood pressure is also associated with brain atrophy and lower cerebral blood flow.
Elevated blood pressure may also affect cognitive and physical function. However, the evidence for this effect is conflicted.6–9 Limitations of early studies are partially related to their lack of consideration for circadian variability in blood pressure. Therefore, we hypothesized that nocturnal blood pressure changes can affect cerebral blood flow dynamics and possibly cerebral atrophy, independent of age and other vascular factors. We further hypothesized that these changes are related to cognitive, especially executive function, and physical function.
Therefore, we investigated the relationship of both elevated blood pressure and nocturnal blood pressure dipping measured by ABPM with brain volumes and perfusion using high resolution anatomical and 3-D continuous arterial spin labeling (CASL) perfusion magnetic resonance imaging (MRI) in older adults with and without history of stroke. In addition, we investigated the relationship between elevated blood pressure and nocturnal blood pressure dipping and these MRI measures with gait speed, cognitive function and instrumental activities of daily living.
Methods
Subjects
Potential subjects were invited to the study using local advertisement in the greater Boston area. All evaluations were conducted at the Beth Israel Deaconess Medical Center. Since stroke is related to both our predictors and our outcomes, we included two groups, a stroke and a non-stroke group, and performed stratified analysis by the stroke group. Inclusion criteria for the non-stroke group included: 50 years or older, able to perform study procedures including ambulatory blood pressure monitoring, brain MRI, and cognitive testing. The inclusion for the stroke group criteria were the same as the non-stroke group plus (a) having a large-vessel stroke by the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria10 (b) at least 6 months or more from the stroke event (c) documented on MRI or CT scan (d) affecting < 1/3 of MCA territory and (e) had a modified Rankin Scale score < 4. The average time post stroke in our sample was 6.1 years. Due to the effect of antihypertensives on cerebral perfusion measurements, these medications were gradually tapered over 3 days and discontinued prior to the study for at least 2 days. Anticoagulation and antihyperlipidemic medications were allowed. Exclusion criteria included: diabetes mellitus (history or hemoglobin A1C levels >7.0 mg/dl), dementia or Alzheimer’s disease, congestive heart failure (identified by history, medications or clinical examination), post- traumatic brain injury, severe active infections, chronic steroid use, HIV-related brain complications or AIDS, homelessness, chronic renal and liver disease, transplantation, active cancer treatment or prior exposure to chemotherapy or radiation, major life threatening illness or clinically significant cardiac disease, arrhythmias or cancer. All subjects provided written informed consent and the protocol was approved by the Beth Israel Deaconess Medical Center IRB.
We screened potential subjects with detailed medical history and physical and neurological examinations, electrocardiogram, and routine laboratory tests. A trained research nurse performed manual blood pressure measurements according to the American Heart Association guidelines. We measured blood pressure during the screening visit (pre-taper measurement). If the participant was receiving antihypertensive medications, then he or she was given instructions to taper off the medication according to a standard protocol. Participants were then admitted for 2 days to the General Clinical Research Center. Two Manual blood pressure and heart rate measurements were performed three times per day for 2 days. Cognitive assessment (the Trail Making Test Part B10, mini mental status examination (MMSE)11 ), gait speed12 (a 12 minute hallway walking at usual speed) , and assessment of the instrumental activity of daily living (IADL)13 were also collected. In the stroke group, we used the National Institute of Health Stroke Scale14 (NIHSS) to assess post stroke functional status with lower values indicating better functional status.
Ambulatory Blood Pressure Monitoring
ABPM was recorded from 8:00 am the first admission day to 8:00 am the next day using a portable automatic monitor Dynapulse (Pulse Metric Inc., Vista CA). This monitor has been previously validated against intra-arterial blood pressure measurement with a correlation of 98% (p<0.001).15 Systolic (SBP) and diastolic (DBP) blood pressure and heart rate (HR) were measured at 20-minute intervals during the day and at 30-minute intervals during the night. The subjects were asked to lie down at 10 p.m. and get up at 7:00 am and to follow a regime resembling usual daily activities at home documented in a personal diary. They were asked to avoid strenuous exercise and to follow the usual routine. All activities performed during the daytime and the sleep and wake times were confirmed using the diary and direct observation. Obtained blood pressure measurements were averaged over the 24-hour period, the daytime (7:00 am to 10:00 pm) and nighttime period (10:00 pm to 7:00 am). Pulse pressure (PP) calculated as the difference between SBP and DBP was also calculated.
Dipping and hypertension definition
Dipping was calculated according to the standard formula: (daytime blood pressure-nighttime blood pressure)/daytime blood pressure × 100 (Extreme dippers if they drop>20% in SBP or DBP, dippers 10–20% in SBP or DBP, risers if SBP or DBP increases at night).16–19 Participants were considered hypertensive if the average blood pressure was 135/85 mm Hg or greater.19, 20 Those on antihypertensive treatment were also considered hypertensive independent of their ABPM.
Magnetic resonance imaging protocol and data analysis
Brain imaging was performed 3 hours after completing the ABPM recording at the 3-Tesla MRI scanner. High-resolution anatomical image 3D magnetization prepared rapid gradient echo (MP-RAGE) were acquired to quantify volume of white matter, gray matter, and cerebrospinal fluid. A template of anatomical regions was applied to measure regional brain volume in frontal, occipital, parietal, and temporal lobes. Gray and white matter volumes in cm3 were then divided by intracranial cavity in cm3 and multiplied by 100 to account for variation due to intracranial size. In the stroke group, brain volumes were measured on the stroke and the opposite sides. Infarct areas were manually outlined and co-registered on a standard template. Infarcts were excluded from volume calculations for each lobe. Blood pressure was also measured during the MRI procedure. Perfusion was measured by CASL-MRI technology in (ml·100g−1·min−1) 21–23 CASL images were acquired using a custom 3D stack of interleaved spirals fast spin echo sequence. Labeled and unlabeled images were collected over 2 minute periods during normal breathing (4 scans). Quantitative cerebral perfusion data were reconstructed off-line4, 6. Due to the concern that the antihypertensive effect on perfusion may have persisted due to the short taper period, we tested if antihypertensive use was related to perfusion in our sample. (An expanded protocol is described in the online supplement, Item1)
Statistical analysis
We conducted our analyses stratified by stroke group. Our predictor variables were nocturnal dipping (used as a continuous measure) and overall mean SBP, DBP and PP (also used as continuous variables). We performed separate analyses for each. Our outcome variables were global and regional (frontal, occipital, parietal and temporal lobes) gray and white matter volumes normalized to intracranial cavity volume then multiplied by 100. Global and regional cerebral perfusion were obtained from the CASL-MRI images and normalized to regional volumes. For the analysis of the functional outcomes, both MRI and ABPM variables were predictors and Trail Making test part B in seconds, MMSE, gait speed, IADL, and NIHSS were the outcome variables. Regression analyses were used for testing the association between the predictors and the outcomes. For the regional analyses, we used mixed models for correlated data. We used the best-fit test to assess the degree by which the model is robust to fit the observed data.24 All models were adjusted for age, gender, race, body mass index, and prior use of antihypertensives. In the stroke group, we also adjusted for the infarct volume and performed separate analyses for the stroke and the non-stroke sides. To adjust for multiple comparisons, we used the method of Sidak adjusted for the high correlation between the various MRI-based measures and blood pressure measures (Alpha=0.03) 25, 26.
Results
We assessed 80 participants (43 non-stroke and 37 with past stroke, mean age 66.4±0.8 years, 51% women and 16% non-white). Table 1 describes main clinical and ABPM characteristics. After tapering antihypertensives, blood pressure didn’t change in those on antihypertensives in either group. There were no other differences in perfusion, gray matter or white matter volumes between those receiving vs those not receiving antihypertensives except marginally for perfusion in the non-stroke group (34.3±1.8 ml·100g−1·min−1 in those on no antihypertensives vs 37.9±3.8 ml·100g−1·min−1 in those on antihypertensives, p=0.05). Using the 24h ABPM criterion (independent of treatment), hypertension prevalence was similar in both groups. However, use of antihypertensive therapy was higher in the stroke group (p<0.01) and hence hypertension using the ABPM or treatment criterion was also higher (p=0.02). The majority (>98%) of our participants did not dip by more than 20% in SBP or DBP but 49% of those in the non-stroke group and 35% in the stroke group dipped by 10% or more in SBP or DBP.
Table 1.
Clinical and ABPM characteristics of the stroke and non-stroke participants (numbers are mean±standard error unless noted)
| Non-stroke | Stroke | p-value | |
|---|---|---|---|
| N | 43 | 37 | |
| Age, years | 68±1 | 64.5±1.4 | 0.04 |
| Women, % | 56% | 46% | 0.38 |
| Non-white participants, % | 19% | 14% | 0.47 |
| Body Mass Index, Kg/m2 | 25±0.63 | 27.4±0.77 | 0.11 |
| Stroke side ( Right), % | 41% | Na | |
| Current smoking,% | 2% | 24% | <0.01 |
| Past smokers,% | 32% | 79% | <0.001 |
| Alcohol, weekly number of drinks | 2.2±0.56 | 8.01±2.66 | 0.02 |
| Hypertension by ABPM,% | 37% | 46% | 0.55 |
| Hypertension (by ABPM or treatment),% | 51% | 77% | 0.02 |
| HR, beats per min | 64±1 | 69±1 | <0.01 |
| ABPM measurements: | 0 | ||
| SBP, mm Hg | 129±2 | 133±2 | 0.11 |
| DBP, mm Hg | 66±1 | 67±1 | 0.59 |
| PP, mm Hg | 63±1 | 66±1 | 0.1 |
| Day time SBP mean, mm Hg | 130±2 | 134±2 | 0.14 |
| Day time DBP mean, mm Hg | 68±1 | 68±1 | 0.73 |
| Day time PP mean, mm Hg | 63±1 | 66±2 | 0.12 |
| Night time SBP mean, mm Hg | 124±2 | 132±3 | 0.04 |
| Night time DBP mean, mm Hg | 61±1 | 64±1 | 0.18 |
| Night time PP mean, mm Hg | 63±2 | 678±2 | 0.07 |
| Extreme dippers,% | 5% | 3% | 0.64 |
| Dippers,% | 44% | 32% | 0.28 |
| Non-dippers,% | 37% | 46% | 0.43 |
| Reverse dippers (risers),% | 14% | 19% | 0.54 |
| Medications | |||
| Antihypertensive use,% | 40% | 70% | <0.01 |
| Diuretics,% | 21% | 24% | 0.13 |
| Angiotensin converting enzyme inhibitors,% | 16% | 32% | 0.09 |
| Beta Blockers,% | 12% | 24% | 0.14 |
| Calcium Channel blockers,% | 12% | 22% | 0.23 |
| Statin use,% | 23% | 68% | <0.0001 |
| Anticoagulation,% | 0% | 8% | 0.11 |
| Glucose. Mg/dl | 78.2±1.8 | 85.7±2.8 | 0.02 |
| Cognitive function | |||
| Mini-Mental-Status-exam | 27.6±0.3 | 26.6±0.4 | 0.08 |
| Trail Making test-Part B, seconds | 88±7 | 165±22 | <0.01 |
| Functional measures | ± | ± | 0 |
| Gait Speed, m/sec | 1.16±0.02 | 0.92±0.05 | <0.001 |
| IADL | 1±0.1 | 2±0.4 | <0.01 |
| NIHSS | 2.4±0.4 | NA | |
| MRI measures | |||
| Small vessel disease or White matter hyperintensities | 0.005±0.001 | 0.01±0.002 | <0.01 |
| Global Cerebral perfusion, ml/100g/min−1 | 35.6±1.9 | 30.8±1.7 | 0.07 |
| Gray Matter Volume, cm3 | 630.5±13.6 | 632.2±11.7 | 0.9 |
| White Matter Volume, cm3 | 417.3±9.1 | 455.9±13.4 | 0.48 |
SBP: systolic blood pressure; DBP: diastolic blood pressure; PP: pulse pressure; Hypertension by ABPM: if blood pressure average is >=135/85 mm Hg; Hypertension by ABPM or treatment: if blood pressure average >=135/85 mm Hg or receiving antihypertensive medication. Extreme dippers if they drop>20% in SBP or DBP, dippers 10–20% in SBP or DBP, risers if SBP or DBP increases at night
Nocturnal dipping and MRI measures
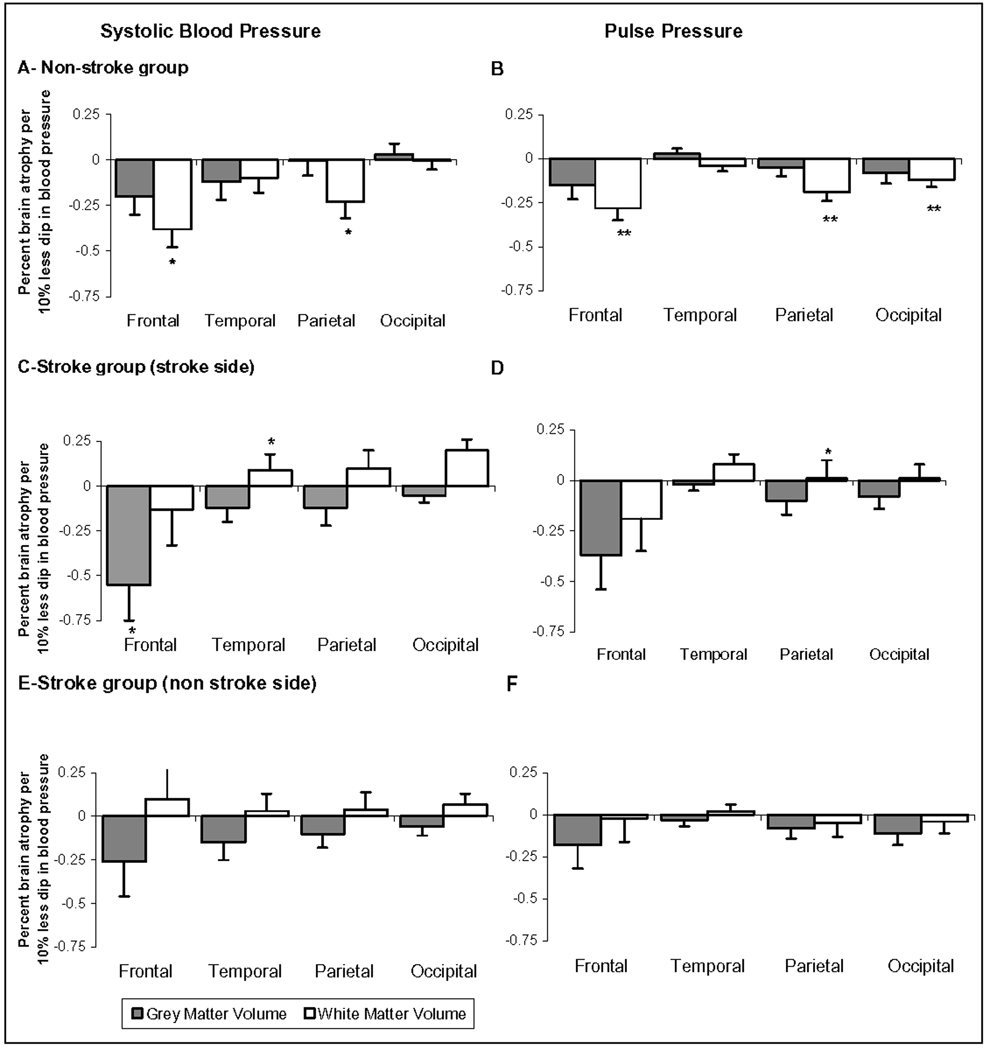
In the non-stroke group, dipping of lesser magnitude in SBP and PP was associated with greater brain atrophy after adjusting for age, gender, race, BMI, and use of antihypertensives. This was predominantly related to loss in white matter volume (SBP: R2=21%, p=0.03 and PP: R2=33%, p<0.01). Regionally, it was predominantly related to frontal and parietal white matter volume loss. Figures 1-A and 1-B demonstrate the relation between nocturnal dipping gray and white matter brain atrophy.
Figure-1.
Typical CASL-MRI perfusion imaging, co-registration and segmentation of high resolution anatomical images and baseline perfusion images in anatomical lobes for a non-stroke subject (A) and a patient with right middle cerebral artery infarct (B).
Associations between regional (percent of ICC) brain volumes and dipping in systolic and pulse pressure in the non-stroke (A, B) and stroke (C, D, E, F) groups.
Similarly in the stroke group, dipping of lesser magnitude in SBP and PP was associated with greater brain atrophy after adjusting for age, gender, race, BMI, infarct size and use of antihypertensives. This was predominantly related to loss in gray matter volume (SBP: R2=52%, p<0.01 and PP: R2=50%, p<0.01). Regionally, this was related to frontal region gray matter atrophy on the stroke side. Figures 1-C and 1-D show the association between nocturnal dipping and gray and white matter atrophy in the four brain regions on the stroke side and figures 1-E and 1-F on the contralateral side.
Dipping in DBP was not associated with brain atrophy except for the gray matter on the stroke side. (Online Table S1) There was no relation between any blood pressure dipping and cerebral perfusion. (Online, Table S2)
Average blood pressure and MRI measures
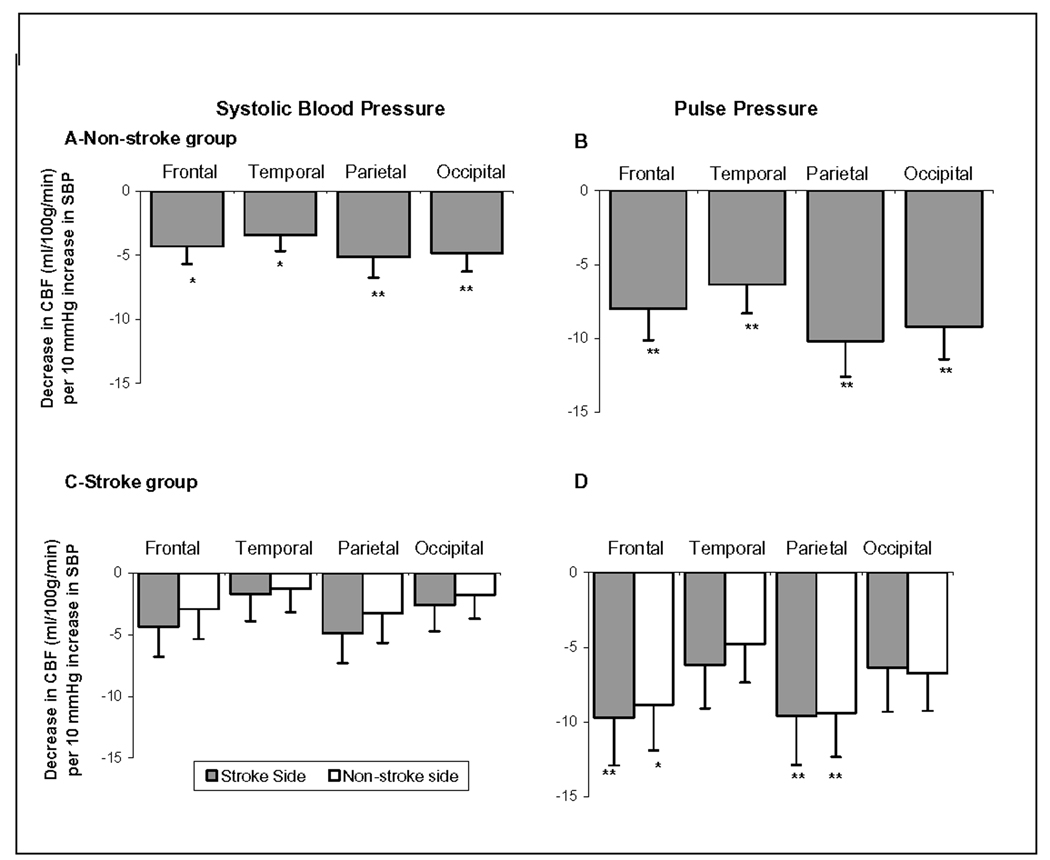
Higher overall 24-h averages of SBP and PP were associated with lower cerebral perfusion in both the stroke and non-stroke groups. Figure 2-A and 2-B shows the decline in regional perfusion per each 10 mm Hg increase in SBP or PP in the non-stroke group and Figures 2-C and 2-D in the stroke group. Although this association was similar in all four brain regions, in the stroke and non-stroke groups and with SBP and PP, it was only consistently significant in the frontal and parietal lobes. When analyzed separately, average SBP and PP during the day but not during the night were associated with cerebral perfusion. In addition, those with hypertension based on ABPM measurements without stroke had 10.4±4.5 ml·100g−1·min−1 lower perfusion compared to normotensives (p=0.02).
Figure-2.
Associations between regional brain volumes and percent dipping in systolic and pulse pressure in the non-stroke (A, B), and the stroke side (C, D) and the opposite side (E, F) in the stroke group.
Footnote: *: p<0.05; **: p<0.01. Values are the slope of the multivariate regression between dipping and brain volume (cm3 brain volume/intracranial cavity cm3 per 10% greater dip in blood pressure*100). P-values are obtained from the multivariate models adjusted for age, gender, race, BMI and antihypertensives
There was no relation between average, daytime or nighttime DBP, SBP or PP and global or regional brain atrophy. (Online Table S3)
Functional measures
Lower dipping magnitude in SBP and greater brain atrophy were both associated with slower gait speed. Dipping of lesser magnitude PP was associated with higher IADL score. In contrast, higher SBP average was associated with worse Trail Making Test-Part B. Table 2 Provides the associations of ABPM and MRI with the functional measures. Dipping of lesser magnitude in SBP was associated with worse NIHSS scores post stroke (SBP dipping p=0.04). No association was detected between MRI or ABPM measures and MMSE.
Table 2.
Association between ABPM and MRI measures with functional measures in the stroke and non-stroke groups.
| Gait (m/sec) | Trail making test, Part B (sec) |
IADL | ||||
|---|---|---|---|---|---|---|
| Non-stroke | SLOPE | P-value | SLOPE | P-value | SLOPE | P-value |
| Average SBP (per each 10 mm Hg) | 0.02±0.04 | 0.53 | 16.00±7.00 | 0.03 | −0.28±0.12 | 0.03 |
| Average PP (per each 10 mm Hg) | 0.02±0.05 | 0.62 | 7.22±9.90 | 0.48 | −0.25±0.17 | 0.16 |
| SBP dipping (per each 10% increase in dipping) | 0.10±0.10 | 0.003 | −14.79±11.39 | 0.21 | −0.07±0.21 | 0.75 |
| PP dipping (per each 10% increase in dipping) | 0.04±0.04 | 0.37 | −9.24±9.27 | 0.33 | −0.03±0.01 | 0.04 |
| Cerebral perfusion (per each ml·100g−1·min−1) | 0.10±0.10 | 0.36 | −0.93±0.77 | 0.25 | 0.10±0.02 | 0.96 |
| Gray Matter (per each cc/intra cranial cavity) | 0.27±1.47 | 0.85 | −129±306 | 0.68 | 4±5 | 0.42 |
| White Matter (per each cc/intra cranial cavity) | 1.30±0.60 | 0.03 | −143±313 | 0.65 | −5 ±5 | 0.36 |
| Stroke | ||||||
| Average SBP (per each 10 mm Hg) | −0.01±0.05 | 0.92 | 7.07±21.52 | 0.75 | −0.44±0.32 | 0.18 |
| Average PP (per each 10 mm Hg) | −0.03±0.07 | 0.63 | 26.25±26.98 | 0.34 | −0.36±0.42 | 0.40 |
| SBP dipping (per each 10% increase in dipping) | 0.10±0.40 | 0.03 | 40.93±28.37 | 0.16 | −0.30±0.45 | 0.52 |
| PP dipping (per each 10% increase in dipping) | 0.08±0.05 | 0.11 | 30.19±19.54 | 0.14 | −0.23±0.31 | 0.48 |
| Cerebral perfusion (per each ml·100g−1·min−1) | 0.01±0.01 | 0.30 | −2.97±3.37 | 0.39 | −0.04±0.05 | 0.39 |
| Gray Matter (per each cc/intra cranial cavity) | 3.20±1.30 | 0.02 | −142±690 | 0.84 | −15±11 | 0.20 |
| White Matter (per each cc/intra cranial cavity) | 2.63±1.77 | 0.15 | −301±689 | 0.67 | −19±11 | 0.10 |
SBP: systolic blood pressure; DBP: diastolic blood pressure; PP: pulse pressure; IADL: Instrumental Activities of Daily Living; Slopes are coefficients obtained from the multiple regression models adjusted for age, gender, race, body mass index and antihypertensive use.
Discussion
This study demonstrates that a decrease in the magnitude of nighttime dipping in systolic and pulse pressures is associated with greater brain atrophy. This atrophy preferentially affects the fronto-parietal region and is independent of stroke. Both, dipping of lesser magnitude and brain atrophy are associated with slower gait speed, worse IADL, and worse functional outcome post stroke. In contrast, higher blood pressure is associated with lower brain perfusion without affecting brain atrophy and with executive cognitive function.
Our study suggests that nighttime dipping in blood pressure rather than the overall blood pressure averages is associated with brain atrophy. Similar to our finding, Nagai et al has also shown that nighttime dipping is correlated with overall brain atrophy in elderly hypertensives27. Goldstein et al demonstrated that greater 24-hour SBP variability is associated with greater brain atrophy independent of age. 3 Although we have found that this association is significant in stroke and non-stroke individuals, prior stroke modulated this relationship in the affected hemisphere. In those with prior hemispheric stroke, the association was related to gray matter loss rather than white matter.
Prior evidence has suggested that casual elevation in blood pressure is associated with lower cerebral blood flow28. This study adds evidence that higher 24-hour SBP and PP, but not DBP, averages are associated with lower brain perfusion measured by CASL-MRI. Since no association was noted with brain volume, the observed association is more likely to be related to vascular changes in the cerebral circulation that lead to decreased brain perfusion.
Both hypertension and brain atrophy are associated with overall loss of physical and cognitive function29–31. Our study suggests that blood pressure dipping of lesser magnitude is also associated with slower gait speed. Conceptually, nighttime dipping which is commonly seen in hypertensives32 is associated with brain tissue atrophy which leads to poor physical function. In contrast, the elevated blood pressure-poor executive function noted in our study and others31, 33 could be related to lower cerebral perfusion. Because of the cross sectional nature of our study, these pathways cannot be fully confirmed and warrant further exploration.
PP is closely correlated with arterial stiffness34 and nighttime PP is associated with increased stroke risk.35 In this study, we provide new evidence that dipping of lesser magnitude in PP is associated with greater atrophy and elevated PP is associated with reduced perfusion. These PP associations are particularly significant in those with prior stroke. PP hence may be a particularly important factor to address in managing those with stroke.
The possible mechanisms of these associations are not clear. It is possible that, lack of adequate nocturnal dipping leads to pathological vascular changes which eventually lead to neuronal death and hence white matter atrophy. It is also possible that in those with stroke, hormonal or structural changes predispose the gray matter to the lower nocturnal dipping in blood pressure.
The clinical implication of this study is that nocturnal dipping may be as important if not more important than the absolute elevation of blood pressure in the evaluation of high risk individuals. Circadian blood pressure changes may provide additional risk stratification for this population.
The advantage of this study is the simultaneous measurements of brain volumes and perfusion using CASL-MRI. Including both stroke and non-stroke participants allowed us to explore the role of stroke in the blood pressure-brain relation. One limitation of our study is its cross sectional design. We cannot describe the temporal relationship between ABPM, brain atrophy and perfusion, and functional measures. The temporal lag between the 24h ABPM and performing the MRI (3 hours) may have affected our results. There were no significant differences between ABPM measurements and blood pressures during the MRI procedures. Another concern in this study, as in most studies that involve hypertensives, is the short and longterm effects of hypertension treatment on the outcome measures. This may have also lead to a misclassification of participants with respect to their hypertension status. In our study, we attempted at addressing the short-term effect by stopping antihypertensives, albeit for a short time for safety reasons. We also did not see any association between use of antihypertensives and brain volume or perfusion, suggesting that this effect is not critical for our brain outcomes. Finally, our sample size might have precluded us from identifying existing associations between ABPM, MRI and function measures.
Conclusion
Dipping of lesser magnitude in SBP and PP is associated with greater brain atrophy independent of stroke whereas blood pressure elevation is associated with lower cerebral perfusion without being related to brain volumes. These associations preferentially affect the fronto-parietal regions. Both nocturnal dipping and brain atrophy are linked to physical and cognitive function. These findings provide further insight into the brain-blood pressure relation: nighttime dipping is related to brain atrophy and physical function whereas elevated blood pressure is related to perfusion and executive function. Dipping, in addition to elevated blood pressure, is an important target in the clinical evaluation of individuals at risk of physical and cognitive limitations.
Supplementary Material
Figure-3.
Associations between cerebral blood flow (and 24-hour average systolic and pulse pressure mm Hg) in the brain regions in those with and without stroke.
Footnote: *: p<0.05; **: p<0.01. Values are the slope of the multivariate regression between dipping and brain volume (ml·100g−1·min−1cm3 per 10 mm increase in blood pressure). P-values are obtained from the multivariate models adjusted for age, gender, race, BMI and antihypertensives
Acknowledgement
We would like to acknowledge contributions of Sarah LaRose, Laura DesRochers, Rob Marquise, Fontini Kourtelidis, and Susan LaRuche from Beth Israel Deaconess Medical Center for their help with data acquisition
Sources of Funding: This analysis was supported by an NIA grant (K23AG30057) to Dr. Hajjar. This study was supported by NIH-NINDS R01-NS045745, NIH-NINDS STTR 1R41NS053128-01A2, ADA1-06-CR-25 and UL1 RR025758 and M01-RR-01032 Grants to Dr. V. Novak.
Footnotes
Conflict of Interest: NONE for all authors
Contributor Information
Ihab Hajjar, Assistant Professor of Medicine, Harvard Medical School, Associate Scientist, Institute for Aging Research/Hebrew SeniorLife, Division of Gerontology, Beth Israel Deaconess Medical Center, 1200 Centre Street, Boston MA, Tel: 617-3638179, Fax: 617-3638936, ihabhajjar@hrca.harvard.edu.
Peng Zhao, Beth Israel Deaconess Medical Center.
David Alsop, Beth Israel Deaconess Medical Center, Harvard Medical School.
Amir Abduljalil, Beth Israel Deaconess Medical Center, Harvard Medical School.
Magdy Selim, Beth Israel Deaconess Medical Center, Harvard Medical School.
Peter Novak, University of Massachusetts.
Vera Novak, Beth Israel Deaconess Medical Center, Harvard Medical School.
References
- 1.Lipsitz LA, Mukai S, Hamner J, Gagnon M, Babikian V. Dynamic regulation of middle cerebral artery blood flow velocity in aging and hypertension. Stroke. 2000 Aug;31(8):1897–1903. doi: 10.1161/01.str.31.8.1897. [DOI] [PubMed] [Google Scholar]
- 2.Heijer T, Skoog I, Oudkerk M, de Leeuw FE, de Groot JC, Hofman A, Breteler MM. Association between blood pressure levels over time and brain atrophy in the elderly. Neurobiol Aging. 2003 Mar–Apr;24(2):307–313. doi: 10.1016/s0197-4580(02)00088-x. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein IB, Bartzokis G, Guthrie D, Shapiro D. Ambulatory blood pressure and brain atrophy in the healthy elderly. Neurology. 2002 Sep 10;59(5):713–719. doi: 10.1212/wnl.59.5.713. [DOI] [PubMed] [Google Scholar]
- 4.Ohkubo T, Hozawa A, Imai Y. Prognostic impact of 24-hour mean blood pressure and pulse pressure on stroke. Circulation. 2001 Dec 18;104(25):E160. [PubMed] [Google Scholar]
- 5.Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001 Oct;38(4):852–857. doi: 10.1161/hy1001.092640. [DOI] [PubMed] [Google Scholar]
- 6.Reitz C, Tang MX, Manly J, Mayeux R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Arch Neurol. 2007 Dec;64(12):1734–1740. doi: 10.1001/archneur.64.12.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinprecht F, Elmstahl S, Janzon L, Andre-Petersson L. Hypertension and changes of cognitive function in 81-year-old men: a 13-year follow-up of the population study "Men born in 1914", Sweden. J Hypertens. 2003 Jan;21(1):57–66. doi: 10.1097/00004872-200301000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Hajjar I, Lackland DT, Cupples LA, Lipsitz LA. Association between concurrent and remote blood pressure and disability in older adults. Hypertension. 2007 Dec;50(6):1026–1032. doi: 10.1161/HYPERTENSIONAHA.107.097667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hebert LE, Scherr PA, Bennett DA, Bienias JL, Wilson RS, Morris MC, Evans DA. Blood pressure and late-life cognitive function change: A biracial longitudinal population study. Neurology. 2004 June 8;62(11):2021–2024. doi: 10.1212/01.wnl.0000129258.93137.4b. 2004. [DOI] [PubMed] [Google Scholar]
- 10.Adams HP, Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EEd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993 January 1;24(1):35–41. doi: 10.1161/01.str.24.1.35. 1993. [DOI] [PubMed] [Google Scholar]
- 11.Cockrell JR, Folstein MF. Mini-Mental State Examination (MMSE) Psychopharmacol Bull. 1988;24(4):689–692. [PubMed] [Google Scholar]
- 12.Cesari M, Kritchevsky SB, Penninx BWHJ, Nicklas BJ, Simonsick EM, Newman AB, Tylavsky FA, Brach JS, Satterfield S, Bauer DC, Visser M, Rubin SM, Harris TB, Pahor M. Prognostic Value of Usual Gait Speed in Well-Functioning Older People Results from the Health, Aging and Body Composition Study. Journal of the American Geriatrics Society. 2005;53(10):1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 13.Cahn-Weiner DA, Malloy PF, Boyle PA, Marran M, Salloway S. Prediction of functional status from neuropsychological tests in community-dwelling elderly individuals. Clin Neuropsychol. 2000 May;14(2):187–195. doi: 10.1076/1385-4046(200005)14:2;1-Z;FT187. [DOI] [PubMed] [Google Scholar]
- 14.Brott T, Adams HP, Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989 July 1;20(7):864–870. doi: 10.1161/01.str.20.7.864. 1989. [DOI] [PubMed] [Google Scholar]
- 15.Brinton TJ, Cotter B, Kailasam MT, Brown DL, Chio SS, O'Connor DT, DeMaria AN. Development and validation of a noninvasive method to determine arterial pressure and vascular compliance. Am J Cardiol. 1997 Aug 1;80(3):323–330. doi: 10.1016/s0002-9149(97)00353-6. [DOI] [PubMed] [Google Scholar]
- 16.Statement on ambulatory blood pressure monitoring by the German Hypertension League. Blood pressure measurement section of the Deutsche Liga zur Bekampfung des hohen Blutdruckes e.V. (German Hypertension League) J Hum Hypertens. 1995 Sep;9(9):777–779. [PubMed] [Google Scholar]
- 17.American Society of Hypertension releases guidelines on home and ambulatory blood pressure monitoring. Am Fam Physician. 1996 Sep 15;54(4):1390. [PubMed] [Google Scholar]
- 18.Myers MG, Haynes RB, Rabkin SW. Canadian hypertension society guidelines for ambulatory blood pressure monitoring. Am J Hypertens. 1999 Nov;12(11 Pt 1):1149–1157. doi: 10.1016/s0895-7061(99)00199-5. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien E, Asmar R, Beilin L, Imai Y, Mallion JM, Mancia G, Mengden T, Myers M, Padfield P, Palatini P, Parati G, Pickering T, Redon J, Staessen J, Stergiou G, Verdecchia P. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003 May;21(5):821–848. doi: 10.1097/00004872-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Lenfant C, Chobanian AV, Jones DW, Roccella EJ. Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): resetting the hypertension sails. Hypertension. 2003 Jun;41(6):1178–1179. doi: 10.1161/01.HYP.0000075790.33892.AE. [DOI] [PubMed] [Google Scholar]
- 21.Floyd TF, Ratcliffe SJ, Wang J, Resch B, Detre JA. Precision of the CASL-perfusion MRI technique for the measurement of cerebral blood flow in whole brain and vascular territories. J Magn Reson Imaging. 2003 Dec;18(6):649–655. doi: 10.1002/jmri.10416. [DOI] [PubMed] [Google Scholar]
- 22.Last D, Alsop DC, Abduljalil AM, Marquis RP, de Bazelaire C, Hu K, Cavallerano J, Novak V. Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care. 2007 May;30(5):1193–1199. doi: 10.2337/dc06-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Novak V, Last D, Alsop DC, Abduljalil AM, Hu K, Lepicovsky L, Cavallerano J, Lipsitz LA. Cerebral blood flow velocity and periventricular white matter hyperintensities in type 2 diabetes. Diabetes Care. 2006 Jul;29(7):1529–1534. doi: 10.2337/dc06-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996 Feb 28;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Leon AC, Heo M. A comparison of multiplicity adjustment strategies for correlated binary endpoints. J Biopharm Stat. 2005;15(5):839–855. doi: 10.1081/BIP-200067922. [DOI] [PubMed] [Google Scholar]
- 26.Shaffer JP. Multiplicity, directional (type III) errors, and the null hypothesis. Psychol Methods. 2002 Sep;7(3):356–369. doi: 10.1037/1082-989x.7.3.356. [DOI] [PubMed] [Google Scholar]
- 27.Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Ambulatory blood pressure as an independent determinant of brain atrophy and cognitive function in elderly hypertension. J Hypertens. 2008 Aug;26(8):1636–1641. doi: 10.1097/HJH.0b013e3283018333. [DOI] [PubMed] [Google Scholar]
- 28.Strandgaard S, Paulson OB. Cerebral blood flow in untreated and treated hypertension. Neth J Med. 1995 Oct;47(4):180–184. doi: 10.1016/0300-2977(95)00065-u. [DOI] [PubMed] [Google Scholar]
- 29.Rosano C, Aizenstein H, Brach J, Longenberger A, Studenski S, Newman AB. Special article: gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J Gerontol A Biol Sci Med Sci. 2008 Dec;63(12):1380–1388. doi: 10.1093/gerona/63.12.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sluimer JD, van der Flier WM, Karas GB, Fox NC, Scheltens P, Barkhof F, Vrenken H. Whole-brain atrophy rate and cognitive decline: longitudinal MR study of memory clinic patients. Radiology. 2008 Aug;248(2):590–598. doi: 10.1148/radiol.2482070938. [DOI] [PubMed] [Google Scholar]
- 31.Waldstein SR, Brown JR, Maier KJ, Katzel LI. Diagnosis of hypertension and high blood pressure levels negatively affect cognitive function in older adults. Ann Behav Med. 2005 Jun;29(3):174–180. doi: 10.1207/s15324796abm2903_3. [DOI] [PubMed] [Google Scholar]
- 32.Kario K, Shimada K, Pickering TG. Abnormal nocturnal blood pressure falls in elderly hypertension: clinical significance and determinants. J Cardiovasc Pharmacol. 2003 Jan;41 Suppl 1:S61–S66. [PubMed] [Google Scholar]
- 33.Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav Neurosci. 2003 Dec;117(6):1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- 34.Safar ME. Pulse pressure, arterial stiffness, and cardiovascular risk. Curr Opin Cardiol. 2000 Jul;15(4):258–263. doi: 10.1097/00001573-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Kario K, Ishikawa J, Eguchi K, Morinari M, Hoshide S, Ishikawa S, Shimada K. Sleep pulse pressure and awake mean pressure as independent predictors for stroke in older hypertensive patients. Am J Hypertens. 2004 May;17(5 Pt 1):439–445. doi: 10.1016/j.amjhyper.2004.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.