Abstract
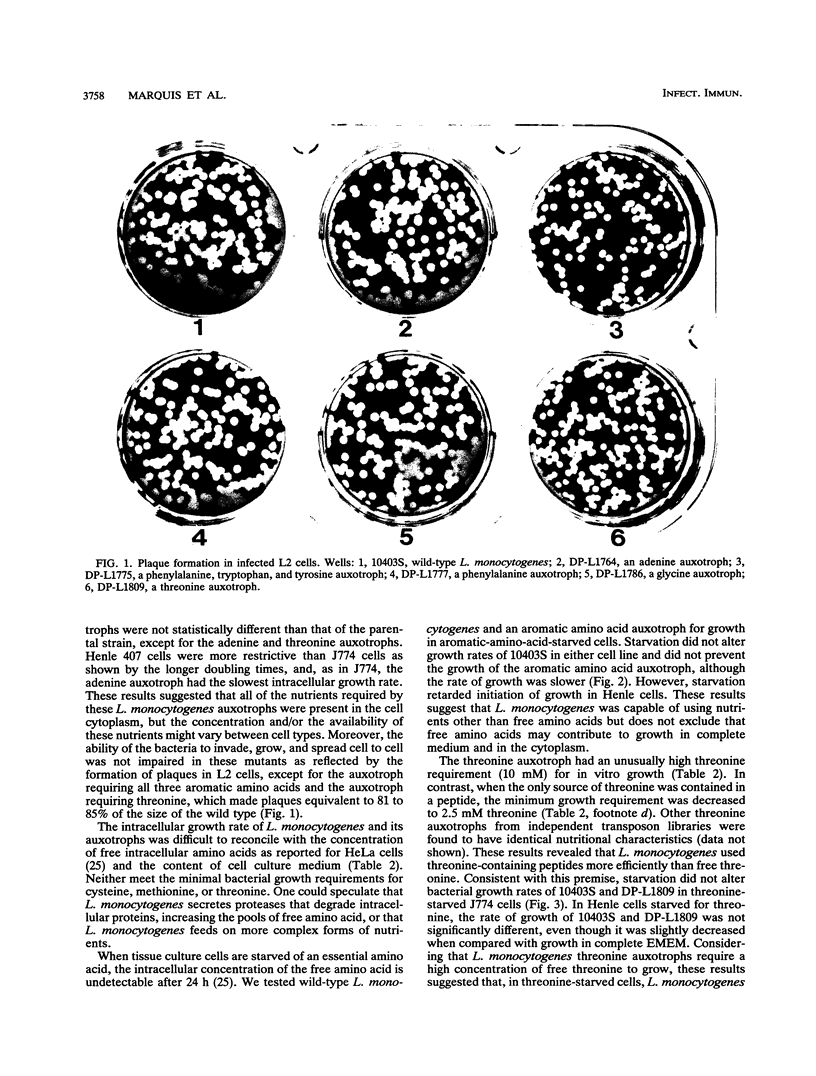
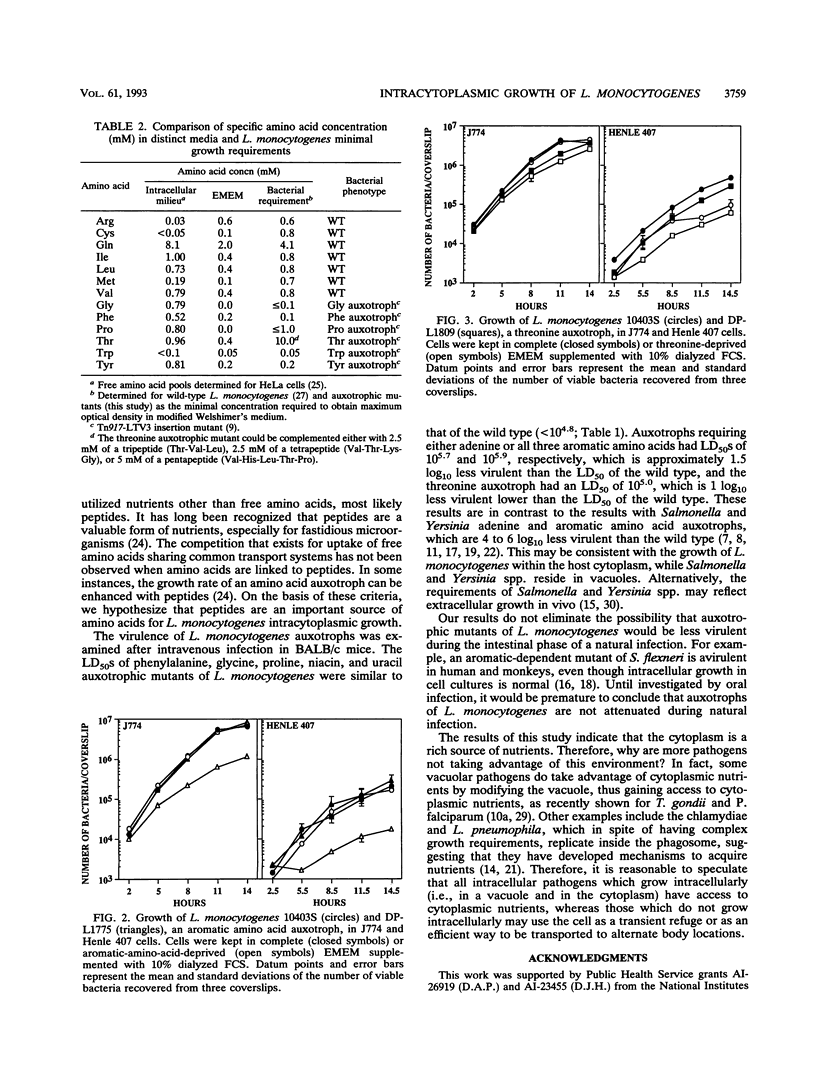
The intracellular growth of several auxotrophic mutants of Listeria monocytogenes was examined in cell culture, and virulence was evaluated in mice by intravenous injection of log-phase bacteria. L. monocytogenes transposon insertion mutants requiring either uracil, phenylalanine, glycine, proline, or nicotinic acid for growth were fully virulent and grew similarly to the parental strain as shown by their growth rates in cell culture. Those requiring all three aromatic amino acids (phenylalanine, tryptophan, and tyrosine) or adenine were 1.5 log10 less virulent than the wild type. A threonine auxotroph, which showed enhanced growth in the presence of threonine-containing peptides as compared with that in the presence of free threonine, was approximately 1 log10 less virulent than the wild type. When host cells were deprived of specific amino acids required by both the host cell and L. monocytogenes, the bacteria continued to grow intracellularly. These studies suggest that the cytoplasm of eucaryotic cells behaves like rich medium, facilitating the growth of an intracellular bacterial pathogen with complex growth requirements. In addition, results related to amino acid deprivation during intracellular growth and specific extracellular growth requirements of a threonine auxotroph suggest that L. monocytogenes may utilize intracellular peptides as a source of amino acids.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed Z. U., Sarker M. R., Sack D. A. Protection of adult rabbits and monkeys from lethal shigellosis by oral immunization with a thymine-requiring and temperature-sensitive mutant of Shigella flexneri Y. Vaccine. 1990 Apr;8(2):153–158. doi: 10.1016/0264-410x(90)90139-d. [DOI] [PubMed] [Google Scholar]
- Austin F. E., Turco J., Winkler H. H. Rickettsia prowazekii requires host cell serine and glycine for growth. Infect Immun. 1987 Jan;55(1):240–244. doi: 10.1128/iai.55.1.240-244.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin F. E., Winkler H. H. Proline incorporation into protein by Rickettsia prowazekii during growth in Chinese hamster ovary (CHO-K1) cells. Infect Immun. 1988 Dec;56(12):3167–3172. doi: 10.1128/iai.56.12.3167-3172.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACON G. A., BURROWS T. W., YATES M. The effects of biochemical mutation on the virulence of Bacterium typhosum; the loss of virulence of certain mutants. Br J Exp Pathol. 1951 Apr;32(2):85–96. [PMC free article] [PubMed] [Google Scholar]
- Bielecki J., Youngman P., Connelly P., Portnoy D. A. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature. 1990 May 10;345(6271):175–176. doi: 10.1038/345175a0. [DOI] [PubMed] [Google Scholar]
- Bishop D. K., Hinrichs D. J. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987 Sep 15;139(6):2005–2009. [PubMed] [Google Scholar]
- Bowe F., O'Gaora P., Maskell D., Cafferkey M., Dougan G. Virulence, persistence, and immunogenicity of Yersinia enterocolitica O:8 aroA mutants. Infect Immun. 1989 Oct;57(10):3234–3236. doi: 10.1128/iai.57.10.3234-3236.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker R. R. The Vwa+ virulence factor of yersiniae: the molecular basis of the attendant nutritional requirement for Ca++. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S748–S758. doi: 10.1093/clinids/5.supplement_4.s748. [DOI] [PubMed] [Google Scholar]
- Camilli A., Portnoy A., Youngman P. Insertional mutagenesis of Listeria monocytogenes with a novel Tn917 derivative that allows direct cloning of DNA flanking transposon insertions. J Bacteriol. 1990 Jul;172(7):3738–3744. doi: 10.1128/jb.172.7.3738-3744.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai S. A., Krogstad D. J., McCleskey E. W. A nutrient-permeable channel on the intraerythrocytic malaria parasite. Nature. 1993 Apr 15;362(6421):643–646. doi: 10.1038/362643a0. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Swanson R. V., Haidaris C. G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Mounier J., Richard S., Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987 Nov;55(11):2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983 Oct 1;158(4):1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. 1980 Sep;66(3):441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H. S. Pathogenesis and immunity in murine salmonellosis. Microbiol Rev. 1989 Dec;53(4):390–409. doi: 10.1128/mr.53.4.390-409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärnell A., Stocker B. A., Katakura S., Sweiha H., Reinholt F. P., Cam P. D., Trach D. D., Lindberg A. A. An auxotrophic live oral Shigella flexneri vaccine: development and testing. Rev Infect Dis. 1991 Mar-Apr;13 (Suppl 4):S357–S361. doi: 10.1093/clinids/13.supplement_4.s357. [DOI] [PubMed] [Google Scholar]
- Leung K. Y., Finlay B. B. Intracellular replication is essential for the virulence of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11470–11474. doi: 10.1073/pnas.88.24.11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. A., Kärnell A., Stocker B. A., Katakura S., Sweiha H., Reinholt F. P. Development of an auxotrophic oral live Shigella flexneri vaccine. Vaccine. 1988 Apr;6(2):146–150. doi: 10.1016/s0264-410x(88)80018-5. [DOI] [PubMed] [Google Scholar]
- McFarland W. C., Stocker B. A. Effect of different purine auxotrophic mutations on mouse-virulence of a Vi-positive strain of Salmonella dublin and of two strains of Salmonella typhimurium. Microb Pathog. 1987 Aug;3(2):129–141. doi: 10.1016/0882-4010(87)90071-4. [DOI] [PubMed] [Google Scholar]
- Mintz C. S., Chen J. X., Shuman H. A. Isolation and characterization of auxotrophic mutants of Legionella pneumophila that fail to multiply in human monocytes. Infect Immun. 1988 Jun;56(6):1449–1455. doi: 10.1128/iai.56.6.1449-1455.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991 Mar;55(1):143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnalue N. A., Stocker B. A. Test of the virulence and live-vaccine efficacy of auxotrophic and galE derivatives of Salmonella choleraesuis. Infect Immun. 1987 Apr;55(4):955–962. doi: 10.1128/iai.55.4.955-962.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada N., Sasakawa C., Tobe T., Yamada M., Nagai S., Talukder K. A., Komatsu K., Kanegasaki S., Yoshikawa M. Virulence-associated chromosomal loci of Shigella flexneri identified by random Tn5 insertion mutagenesis. Mol Microbiol. 1991 Jan;5(1):187–195. doi: 10.1111/j.1365-2958.1991.tb01839.x. [DOI] [PubMed] [Google Scholar]
- PIEZ K. A., EAGLE H. The free amino acid pool of cultured human cells. J Biol Chem. 1958 Mar;231(1):533–545. [PubMed] [Google Scholar]
- Portnoy D. A., Jacks P. S., Hinrichs D. J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988 Apr 1;167(4):1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premaratne R. J., Lin W. J., Johnson E. A. Development of an improved chemically defined minimal medium for Listeria monocytogenes. Appl Environ Microbiol. 1991 Oct;57(10):3046–3048. doi: 10.1128/aem.57.10.3046-3048.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansonetti P. J., Ryter A., Clerc P., Maurelli A. T., Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986 Feb;51(2):461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet M., Richard S., Berche P. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect Immun. 1990 Mar;58(3):841–845. doi: 10.1128/iai.58.3.841-845.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A. N., Camilli A., Portnoy D. A. Isolation of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect Immun. 1990 Nov;58(11):3770–3778. doi: 10.1128/iai.58.11.3770-3778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Rickettsia species (as organisms). Annu Rev Microbiol. 1990;44:131–153. doi: 10.1146/annurev.mi.44.100190.001023. [DOI] [PubMed] [Google Scholar]




