Abstract
Purpose
The aim of this study was to elucidate the effects of the Rho-kinase inhibitor, H-1152, on cultured human trabecular meshwork (HTM) cells, TM morphology, and intraocular pressure (IOP) in rats.
Methods
Cultured HTM cells were treated with H-1152. Changes in cell morphology and the organization of the actin cytoskeleton and focal adhesions were evaluated by microscopy and immunofluorescence. H-1152 was administered topically to the eyes of conscious rats, and IOP was measured with a commercially available tonometer before and after treatment. The eyes were enucleated 1 h after treatment, fixed, and processed for morphologic analysis by light and electron microscopy.
Results
Exposure of the cultured HTM cells to 20 μM of H-1152 induced elongation and separation of cells, deterioration, and loss of actin stress fibers and focal adhesions within 2 h. Topical administration of H-1152 resulted in a significant decrease in IOP from 0.5 to 6 h, with the maximum IOP reduction of 28.1% at 1 h post-treatment (P < 0.001; n = 10). H-1152 caused an expansion of the intercellular spaces and loss of extracellular material in the juxtacanalicular region of the TM in rat eyes.
Conclusions
The IOP-lowering effect of H-1152 in rat eyes is likely due to changes in TM-cell morphology, the actin cytoskeleton, and cellular adhesions in the conventional outflow pathway. H-1152 has potential as a new antiglaucoma medication.
Introduction
Primary open-angle glaucoma (POAG), a disease characterized by optic neuropathy, affects 1–2% of the population worldwide and elevated intraocular pressure (IOP) is considered as a major risk factor.1,2 Lowering IOP is currently the only therapeutic approach for glaucoma. It is generally accepted that the main resistance to outflow resides in the juxtacanalicular (JXT) region of the trabecular meshwork (TM) and inner-wall cells of Schlemm's canal, and that changes in the actin cytoskeleton and associated cell-cell junctions and cell-extracellular matrix (ECM) interactions in these areas will alter outflow facility.3 There are currently a number of therapies designed to lower IOP for glaucoma treatment, but most of them do not target the actin cytoskeleton and associated cellular adhesions. In addition, side effects, especially those involved in the cardiovascular and pulmonary systems, remain problems associated with their use.4
The actin cytoskeleton and associated cellular-adhesion proteins are attractive targets for novel therapeutic approaches for glaucoma. Compounds and proteins capable of disrupting actin and associated cellular adhesions were able to lower IOP and increase outflow facility in organ-cultured anterior segments in vitro and in rats, rabbits, and primates in vivo.5–12 Certain inhibitors of Rho and Rho-associated coiled-coil kinase (Rho kinase/ROCK) seem to be in this new category of IOP-lowering agents. The RhoA protein from the Rho family of small GTPases plays a central role in the organization and distribution of the actin cytoskeleton and cellular adhesions. RhoA coordinates these events through its downstream effectors, such as the ROCK.13 Topical administration of the ROCK inhibitors, Y-27632 or HA-1077, and the myosin light-chain kinase (MLCK) inhibitor, ML-7, increased outflow facility or decreased IOP in rabbit eyes,14 organ-cultured porcine,11 and bovine eyes,15 as well as live monkey eyes.12 The mechanisms for the IOP-lowering and outflow-facility-increasing effects are not entirely clear. Rao et al. showed that Y-27632 increased outflow facility mostly likely by expanding the spaces in the JXT region of the porcine eye.11
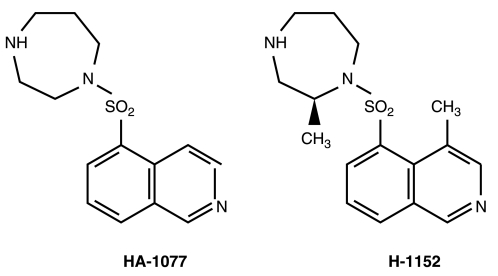
A newly synthesized compound, (S)-(+)-2-methyl-1-[(4-methyl-5-isoquinolinyl) sulfonyl]-homopiperazine (H-1152; Fig. 1), is an isoquinoline sulfonamide derivative of 1-(5-isoquinolinesulfonyl)-homopiperazine (HA-1077). It more selectively inhibits ROCK, with a Ki value of 0.0016 μM for Rho-kinase, as compared to HA-1077 and Y-27632, with Ki values of 0.33 and 0.44 μM, respectively.16 It was reported that H-1152 is 8–20 times more potent than Y-27632 and HA-1077 in inhibiting cellular contraction.17 In this study, we evaluated the effects of H-1152 on IOP and on the ultra-structure of the TM.
FIG. 1.
Chemical structure of HA-1077 and H-1152.
Methods
Culture of human TM (HTM) cells
HTM cells (from the University of Wisconsin–Madison) were maintained in Dulbecco's modified Eagle's medium (DMEM; Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (FBS; Mediatech), 25 μg/mL of gentamycin, and 2.5 μg/mL of amphotericin B at 37°C in an atmosphere of 8% CO2.18
H-1152 treatment
H-1152 was supplied by SiChem GmbH (Sirius Fine Chemicals SiChem GmbH., Bremen, Germany). For topical administration, four 1-μL drops of either H-1152 at a concentration of 10 mM or phosphate-buffered saline (PBS) vehicle were administered to the central cornea of opposite eyes of normal rats, at 30-sec intervals. Lids were retracted to prevent blinking between drops. The dose was chosen based on our experiments with H-1152 on rabbits.
The changes in cell morphology were observed and photographed by phase-contrast microscopy before and 0.5, 1, 2, 3, 4, 5, 6, and 24 h after treatment with H-1152 at 20 μM. The H-1152 containing medium was removed after the 24-h time point and replaced with fresh H-1152-free DMEM. Recovery of cell morphology was examined 2 and 24 h later. At each time interval, the identical field of cells was located and photographed.
Immunofluorescence
For immunohistochemistry, HTM cells were plated on glass coverslips precoated with poly-l-lysine, incubated with or without H-1152 for the time as indicated, and then fixed and fluorescently labeled with probes specific for actin and vinculin. The cells were washed with 50 mM MES [2-(N-morpholine) ethanesulfonic acid] buffer, permeabilized with 0.5% Triton X-100, and then fixed with 3% paraformaldehyde. The cells were blocked in 5% normal goat serum for 30 min. Alexa 488-conjugated phalloidin (Sigma, St. Louis, MO) was used for the fluorescent labeling of actin. The primary antibody used to detect vinculin was the monoclonal clone, hVin-1 (Sigma). The secondary antibody was Cy3-conjugated goat antimouse IgG H+L (116-165-062; Jackson ImmunoResearch Laboratories, West Grove, PA). Fluorescence was observed with a Zeiss Axioplan 2 microscope equipped with an Axiocam HRm camera, together with Axiovision 3.1 software (Carl Zeiss Inc., Oberkochen, Germany).18
Experimental animals
Adult male Wistar rats used in this study, weighing 200–250 g, were obtained from the Experimental Animal Center of Sichuan University (Chengdu, People's Republic of China). All studies were conducted in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
IOP measurement and slit-lamp biomicroscopy in rats
IOP was measured by using a TonoLab (Colonial Medical Supply, Franconia, NH), which is a noninvasive tonometer specially designed for measuring IOP in mice and rats. No topical or systemic anesthesia was required.19 IOP was measured before and 0.5, 1, 3, 6, 9, and 24 h after the administration of H-1152. Rats were kept in room light for the duration of the 0–9 h interval.
In order to determine the presence of abnormalities in the anterior segment (including corneal edema, lens opacities, and anterior-chamber cells and flare), slit-lamp biomicroscopy was performed before and at 3 and 24 h after the topical drug administration.
Light and electron microscopy
Rats were killed at 1 h after H-1152 treatment. The eyes were enucleated, bisected at the equator, and the corneoscleral angle was dissected into approximately 3 × 1 mm cubes and fixed by immersion in 2.5% glutaraldehyde in 0.1 M of sodium cacodylate buffer at 4°C for 4 h. Following fixation, the tissues were further treated in 1.0% osmium tetra oxide in 0.1 M of sodium cacodylate buffer and then stained with 1% uranyl acetate. Finally, for each eye, 70-nm sections from one cube, taken from the upper part of the eye, were stained sequentially with uranyl acetate and lead citrate for an examination by a CM10 transmission electron microscope (Philips, Eindhoven, The Netherlands) at an accelerating voltage of 40 kV.
Statistical analysis
Data are presented as the mean ± standard error of the mean for 10 eyes or animals as indicated: pre- or post-H-1152-treated eyes versus contralateral control eyes, post-H-1152-, or postvehicle-treated, eyes versus ipsilateral baseline, and baseline-corrected post-H-1152-treated eyes versus control eyes were statistically analyzed by the two-tailed paired Student t-test for differences compared to 0.0. Results were considered statistically significant if P < 0.05.
Results
Effects of H-1152 on morphology of cultured HTM cells
Cultured HTM cells form a confluent, endothelial-like monolayer of closely attached cells. Cells were photographed before and during exposure to 20 μM of H-1152, a concentration 500 times less than that used in vivo. This dose was chosen based on screening experiments conducted with 10-and 50-μM concentrations. In order to track morphologic changes for 24 h with treatment and then 24 h without treatment, 10 μM was found to be too low of a concentration (morphologic changes were not obvious), whereas 50 μM was judged to be too high (drug-induced changes were too strong and cell detachment occurred). Therefore, 20 μM was chosen for this study. After 24 h of exposure to 20 μM of H-1152, recovery was evaluated by culturing the cells in H-1152-free medium for another 24 h. HTM cell morphology changed in a time-dependent manner during the exposure period. Cells appeared elongated, thickened toward the center of the cells, and separated from each other. After longer treatment, the cells became more markedly separated from each other but still remained attached (Fig. 2). Recovery appeared almost complete after 24 h in H-1152-free medium. The full complement of cells appeared flattened and packed, similar to 0-h cells (Figs. 2 and 3).
FIG. 2.
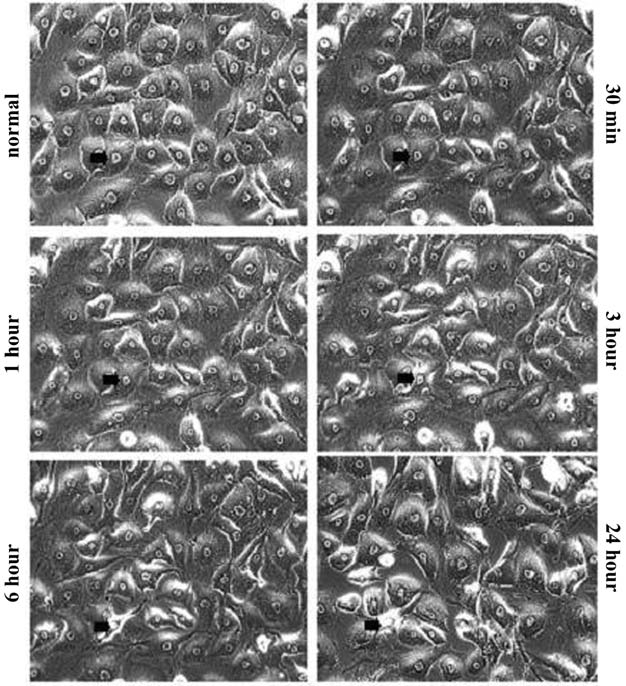
Effect of H-1152 on human trabecular meshwork cell morphology. Cells were treated with 20 μM of H-1152 and photographed at 0.5, 1, 3, 6, and 24 h (Fig. 2). For each time point, the same field of cells was located and photographed, and no obvious cell loss was noted. One cell (the same cell) is indicated by an arrow at each time point. Data from 2, 4, and 5 h of treatment are not shown. Bar = 80 μm.
FIG. 3.
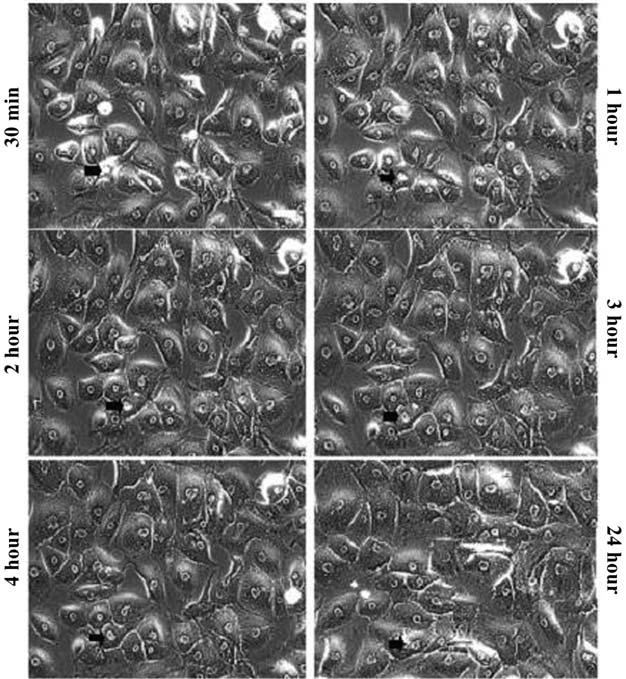
Recovery of human trabecular meshwork cell morphology after the withdrawal of H-1152. Cells were treated with 20 μM of H-1152 for 24 h, then incubated with H-1152-free medium and photographed for another 0.5, 1, 2, 3, 4, and 24 h. For each time point, the same field of cells was located and photographed, and no obvious cell loss was noted. The same cell is indicated by an arrow at each time point (Figs. 2 and 3). Bar = 80 μm.
Effects of H-1152 on cytoskeleton of cultured HTM cells
Fluorescent staining for F-actin and vinculin in untreated cells showed numerous thick stress fibers and vinculin containing focal adhesions, with the latter being clearly located at the termini of the actin bundles. H-1152 induced disruption of the stress fibers and focal adhesions. Actin-containing bundles deteriorated markedly after 2 h of exposure to 20 μM of H-1152. With longer exposures (24 h), the stress-fiber network was obliterated, leaving only shortened, curved actin filaments, mostly at the cell periphery (Fig. 4).
FIG. 4.
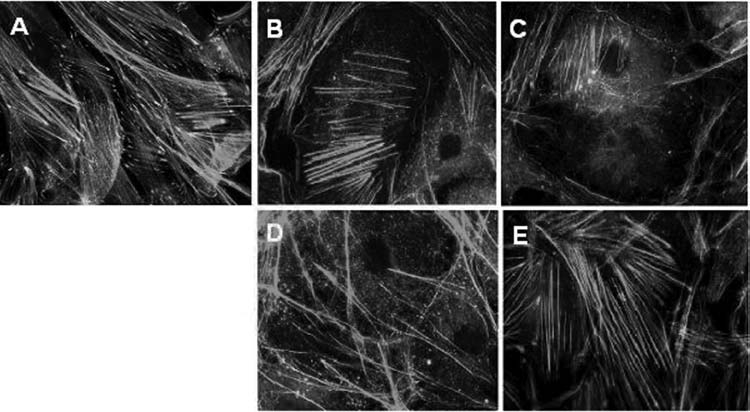
Distribution of actin and vinculin containing focal adhesions in human trabecular meshwork cells treated with or without H-1152. (A) Distribution of actin and vinculin in untreated cells. Cells were treated with 20 μM of H-1152 for 2 h (B) and 24 h (C). Then, the cells treated for 24 h were allowed to recover in H-1152-free medium for 2 h (D) and 24 h (E).
For the assessment of recovery of the actin cytoskeleton and focal adhesion, cells were treated with 20 μM of H-1152 for 24 h, after which the medium was changed to H-1152-free medium for 2 and 24 h. Partial recovery of stress fibers was seen at the 2-h recovery time point and was more complete after 24 h in the absence of H-1152, redistributing into both radial and circumferential bundles consistent with the recovery of cell morphology (Fig. 4). In contrast, it seems that vinculin recovery lagged behind that of actin microfilaments, since there is much less vinculin seen at the termini of the stress fibers, when compared to that in untreated cells (Fig. 4, 4A compared to 4E).
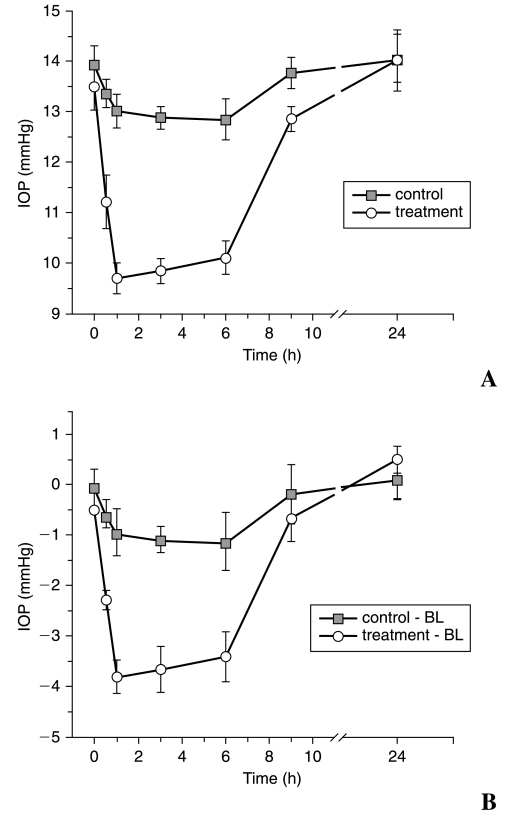
Effect of H-1152 on IOP and slit-lamp biomicroscopy
Results of the effect of H-1152 on IOP of rats are shown in Figure 5. The pretreatment IOP was 13.50 ± 0.47 and 13.94 ± 0.38 mmHg in treated and control eyes, respectively (n = 10 for each group), and there was no significant difference in pretreatment IOPs between the two groups (P = 0.154). H-1152 at 10 mM (4 μL containing 14 μg) significantly lowered IOP in rats from 0.5 to 6 h after topical application, when compared to the ipsilateral pretreatment baseline (P < 0.001), with the maximal decrease of 3.80 ± 0.33 mmHg at 1 h. IOP gradually returned to control-eye values by 9 h posttreatment. For vehicle-treated eyes, the difference in IOP between pretreatment baseline and 3 h after treatment was also significant (P < 0.005; Fig. 5).
FIG. 5.
Effect of H-1152 on the intraocular pressure (IOP) of rats. H-1152 (10 mM) was administered topically to rat eyes. The contralateral eyes were treated with phosphate-buffered saline. The results are presented as the mean ± standard error of the mean (n = 10 for each group). IOP decreased significantly 0.5–6 h after H-1152 treatment (A). (B) Average IOP for H-1152- and contralateral vehicle-treated eyes corrected for baseline. Differences were significantly different from 0.0 by the two-tailed paired Student t-test.
When compared to the contralateral vehicle-treated eyes, before baseline adjustment, there was a significant decrease in IOP in the H-1152 treated eyes at 0.5, 1, 3, and 6 h (P < 0.001), with the maximal decrease of 3.32 ± 0.45 mmHg at 1 h (Fig. 5A). When corrected for baseline and compared to the contralateral control eye, H-1152, at the same dose, also significantly lowered IOP in rats from 0.5 to 6 h after topical application (P < 0.001), with the maximal difference of 2.88 ± 0.52 mmHg at 1 h (Figure 5B). Slit-lamp biomicroscopy showed no abnormality of the corneal epithelium, anterior chamber, or lens.
H-1152-induced changes in TM ultrastructure of rats
H-1152-induced ultrastructural changes in rat TM were assessed by transmission electron microscopy. Three control specimens exhibited compact trabecular beams and few open spaces in the JXT area, whereas all H-1152-treated eyes (5 eyes) demonstrated a widening of the extracellular spaces, particularly the optically empty areas in the JXT and TM (Fig. 6). Additionally, the entire TM appeared distended in H-1152-treated specimens, compared with controls. Quantitative analysis was not done. No obvious cell loss or accumulation of cell debris was observed in the TM of treated eyes, and the integrity of the inner wall aqueous plexi was observed to be intact, indicating no cytotoxic effects of H-1152 (Fig. 6).
FIG. 6.
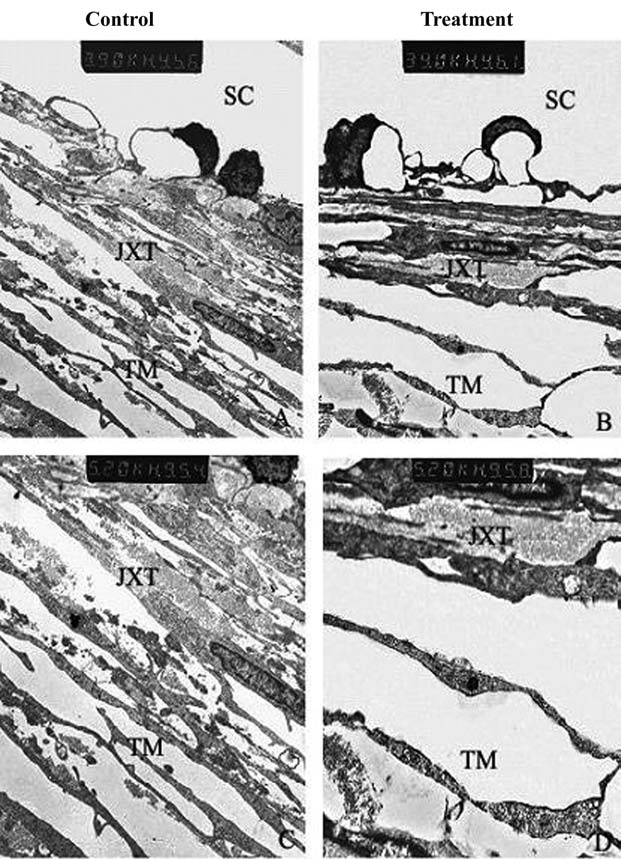
H-1152-induced ultrastructural changes in rat TM. H-1152-treated specimens showed an expansion of the subcellular spaces in the inner wall of Schlemm canal and intercellular spaces in the juxtacanalicular meshwork, accompanied by a loss of extracellular material, compared to controls. SC, Schlemm canal; JXT, juxtacanalicular tissue; TM, trabecular meshwork.
Discussion
ROCK is a serine-threonine protein kinase that inhibits myosin light-chain phosphatase and phosphorylates myosin light chain, resulting in an upregulation of myosin II activity. The increased myosin II activity, in turn, favors the assembly of actin-myosin filaments that generate the tensile strength underlying the RhoA-ROCK–associated dynamic events.20 The inactivation of either Rho GTPase or Rho-kinase induced changes in cell morphology, intercellular, and cell-ECM interactions in various cell types, including TM cells, by suppressing myosin light-chain phosphorylation.10–12,14,20 Thus, inhibitors of both proteins, when administrated to the eye, could affect flow dynamics and facilitate aqueous-humor outflow through a common mechanism: loss of cellular contractility of cells in the conventional pathway. Rho-kinase inhibitors, such as HA-1077 and Y-27632, are able to lower IOP and increase outflow facility in vivo.10,12,14 The present study demonstrates that the newly synthesized ROCK inhibitor, H-1152, also induces a significant decrease in IOP in rat eyes.
H-1152 is membrane permeable and a strong inhibitor of Rho-kinase activity, and its inhibitory mechanism is the same as that of other kinase inhibitors, such as HA-1077 and Y-27632.16,21 However, it is much more specific than these compounds, based on its Ki values for Rho-kinase and protein kinase C (PKC), cAMP-dependent protein kinase (PKA) or MLCK.16 Teixeira et al. showed that H-1152 is 8–20 times more potent than Y-27632 and HA-1077 in its ability to inhibit cellular contraction.17 Though no conformational data are available, Sasaki et al. speculated that the presence of two additional methyl groups in the HA-1077 molecule (Fig. 1) will facilitate a conformation more suitable for interacting with the ATP-binding site of Rho-kinase.16
H-1152, Y-27632, and HA-1077 were all effective at lowering IOP, as reported by this and previous studies,10,14,15 when applied topically to animal eyes at a concentration of 10 mM. It seems that the IOP-lowering effects of all three compounds lasted approximately 6 h, with the maximum IOP lowering response occurring at 1 h after the treatment.10,14 Comparison of the percent change from baseline for the various groups indicates that the IOP reduction for Y-27632 and HA-1077 is about 36% in rabbits,10,14 whereas that for H-1152 in the current study was 28% in rats. When compared to the effect of topical H-7 on the IOP of live monkey eyes, H-1152 (14 μg) appeared to be more effective in IOP lowering but less effective than H-7 at the same doses on glaucomatous monkey eyes.22 Tian et al. showed that 1 mg of H-7 maximally decreased IOP by 2.5 (16.7%) and 5.8 mmHg (16.9%) in normotensive and glaucomatous eyes, respectively.22
Although the magnitude of the IOP response to Y-27632 and HA-1077 in rabbits appears greater than that of H-1152 in rats, it should not be concluded that Y-27632 and HA-1077 are more effective. In addition to different species and tonometers and the conditions under which the measurements were taken, differences in baseline IOPs may account for the differences in the magnitude of the IOP responses. The baseline IOP in rats used in this study was about 10–11 mmHg lower than that in rabbits.10,14 This is also supported by the studies of the effects of H-7 on the IOP of normotensive and hypertensive monkey eyes, as described above, indicating that the greater IOP lowering at the higher starting IOP does not mean greater effectiveness.
Whereas the monkey is the animal model most closely resembling the human for studies of IOP-lowering agents,23 the development and morphology of the anterior ocular structures and, especially, the aqueous-humor filtration tissues in the rat are also analogous to that in man.24,25 Hence, the rat eye is also a suitable model for testing new antiglaucoma medication and, at least, is a predictor for primates.
Conclusions
In this study, we reported the significant IOP-lowering effect of H-1152 in rat eyes, most likely due to the H-1152-induced changes in the inner wall of Schlemm's canal and the juxtacanalicular TM. Our ultrastructural studies indicated that H-1152 induced generalized relaxation and an apparent expansion of the subendothelial region of the Schlemm's canal IW, and of the juxtacanalicular area accompanied by loss of extracellular material. H-1152 treatment also induced changes in the morphology of human TM cells in culture, and disrupted actin filaments and vinculin containing focal adhesions. This suggests that H-1152, like some other ROCK inhibitors or actin-disrupting reagents,11,26 may lower IOP by inhibiting cell contractility, leading to a “relaxation” of the trabecular-outflow pathway and an expansion of the draining surface, thus allowing an easier, more extensive flow through the meshwork. Further studies to define the effects of H-1152 on glaucomatous eyes are needed.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (NNSF 30772379) and U.S. National Institutes of Health Grants EY016665 and EY02698. The authors thank Carol Rasmussen, B'Ann Gabelt, and Baohe Tian for reviewing and editing the original manuscript for this paper.
References
- 1.Quigley H.A. Open-angle glaucoma. N. Engl. J. Med. 1993;328:1097–1106. doi: 10.1056/NEJM199304153281507. [DOI] [PubMed] [Google Scholar]
- 2.Hart W.M. Intraocular pressure. In: Hart W.M., editor. Adler Physiology of the Eye: Clinical Application. St. Louis: Mosby; 1992. pp. 248–267. [Google Scholar]
- 3.Polansky J.R. Alvarado J.A. Cellular mechanisms influencing the aqueous humor outflow pathway. In: Albert D.M., editor; Jakobiec F.A., editor. Principles and Practice of Ophthalmology: Basic Science. Philadelphia: WB Saunders; 1994. pp. 226–251. [Google Scholar]
- 4.Lee D.A. Higginbotham E.J. Glaucoma and its treatment: A review. Am. J. Health Syst. Pharm. 2005;62:691–699. doi: 10.1093/ajhp/62.7.691. [DOI] [PubMed] [Google Scholar]
- 5.Tian B. Kaufman P.L. Volberg T., et al. H-7 disrupts the actin cytoskeleton and increases outflow facility. Arch. Ophthalmol. 1998;116:633–643. doi: 10.1001/archopht.116.5.633. [DOI] [PubMed] [Google Scholar]
- 6.Wang H. Liu X. Guo L., et al. Effects of MISA A on actin cytoskeleton of cultured HTM cells and intraocular pressure of rats and glaucomatous monkeys. Curr. Eye Res. 2007;32:843–850. doi: 10.1080/02713680701585880. [DOI] [PubMed] [Google Scholar]
- 7.Peterson J.A. Tian B. McLaren J.W., et al. Latrunculins' effects on intraocular pressure, aqueous humor flow, and corneal endothelium. Invest. Ophthalmol. Vis. Sci. 2001;41:1749–1758. [PubMed] [Google Scholar]
- 8.Tian B. Brumback L.C. Kaufman P.L. ML-7, chelerythrine, and phorbol ester increase outflow facility in the monkey eye. Exp. Eye Res. 2000;71:551–566. doi: 10.1006/exer.2000.0919. [DOI] [PubMed] [Google Scholar]
- 9.Honjo M. Inatani M. Kido N., et al. A myosin light-chain kinase inhibitor, ML-9, lowers the intraocular pressure in rabbit eyes. Exp. Eye Res. 2002;75:135–142. doi: 10.1006/exer.2002.2009. [DOI] [PubMed] [Google Scholar]
- 10.Honjo M. Tankhara H. Inatani M., et al. Effects of Rho-associated protein kinase inhibitor, Y-27632, on intraocular pressure and outflow facility. Invest. Ophthalmol. Vis. Sci. 2001;42:137–144. [PubMed] [Google Scholar]
- 11.Rao P.V. Deng P.F. Kumar J., et al. Modulation of aqueous-humor outflow facility by the Rho-kinase-specific inhibitor, Y-27632. Invest. Ophthalmol. Vis. Sci. 2001;42:1029–1037. [PubMed] [Google Scholar]
- 12.Tian B. Kaufman P.L. Effects of the Rho-kinase inhibitor, Y-27632, and the phosphatase inhibitor, calyculin A, on outflow facility in monkeys. Exp. Eye Res. 2005;80:215–225. doi: 10.1016/j.exer.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 14.Honjo M. Inatani M. Kido N., et al. Effects of protein kinase inhibitor, HA1077, on intraocular pressure and outflow facility in rabbit eyes. Arch. Ophthalmol. 2001;119:1171–1178. doi: 10.1001/archopht.119.8.1171. [DOI] [PubMed] [Google Scholar]
- 15.Rosenthal R. Choritz L. Schlott S., et al. Effects of ML-7 and Y-27632 on carbachol- and endothelin-1-induced contraction of bovine trabecular meshwork. Exp. Eye Res. 2005;80:837–845. doi: 10.1016/j.exer.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki Y. Suzuki M. Hidaka H. The novel and specific Rho-kinase inhibitor(S)-(+)-2-methyl-1-[(4-methyl-5-isoquinoline) sulfonyl]-homopiperazine as a probing molecule for Rho-kinase-involved pathway. Pharmacol. Ther. 2002;93:225–232. doi: 10.1016/s0163-7258(02)00191-2. [DOI] [PubMed] [Google Scholar]
- 17.Teixeira C.E. Jin L. Ying Z., et al. Ca2+ sensitization and the regulation of contractility in rat anococcygeus and retractor penis muscle. Biochem. Pharmacol. 2005;69:1483–1492. doi: 10.1016/j.bcp.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Liu X. Hu Y. Filla M., et al. The effect of C3 transgene expression on actin and cellular adhesions in cultured human trabecular meshwork cells and on outflow facility in organ cultured monkey eyes. Mol. Vis. 2005;11:1112–1121. [PubMed] [Google Scholar]
- 19.Pease M.E. Hammond J.C. Quigley H.A. Manometric calibration and comparison of TonoLab and TonoPen tonometers in rats with experimental glaucoma and in normal mice. J. Glaucoma. 2006;15:512–519. doi: 10.1097/01.ijg.0000212276.57853.19. [DOI] [PubMed] [Google Scholar]
- 20.Wettschureck N. Offermanns S. Rho/Rho-kinase-mediated signaling in physiology and pathophysiology. J. Mol. Med. 2002;80:629–638. doi: 10.1007/s00109-002-0370-2. [DOI] [PubMed] [Google Scholar]
- 21.Ikenoya M. Hidaka H. Hosoya T., et al. Inhibition of Rho-kinase-induced myristoylated alanine-rich C kinase substrate (MARCKS) phosphorylation in human neuronal cells by H-1152, a novel and specific Rho-kinase inhibitor. J Neurochem. 2002;81:9–16. doi: 10.1046/j.1471-4159.2002.00801.x. [DOI] [PubMed] [Google Scholar]
- 22.Tian B. Wang R.F. Podos S.M., et al. Effects of topical H-7 on outflow facility, intraocular pressure, and corneal thickness in monkeys. Arch. Ophthalmol. 2004;122:1171–1177. doi: 10.1001/archopht.122.8.1171. [DOI] [PubMed] [Google Scholar]
- 23.Ryder M.I. Weinreb R.N. The cytoskeleton of the cynomolgus monkey trabecular cell. I. General considerations. Invest. Ophthalmol. Vis. Sci. 1986;27:1305–1311. [PubMed] [Google Scholar]
- 24.Nucci P. Tredici G. Manitto M.P., et al. Neuron-specific enolase and embryology of the trabecular meshwork of the rat eye: An immunohistochemical study. Int. J. Biol. Markers. 1992;7:253–255. doi: 10.1177/172460089200700410. [DOI] [PubMed] [Google Scholar]
- 25.McMenamin P.G. al-Shakarchi M.J. The effect of various levels of intraocular pressure on the rat aqueous outflow system. J. Anat. 1989;162:67–82. [PMC free article] [PubMed] [Google Scholar]
- 26.Sabanay I. Gabelt B.T. Tian B., et al. H-7 effects on the structure and fluid conductance of monkey trabecular meshwork. Arch. Ophthalmol. 2000;118:955–962. [PubMed] [Google Scholar]