Abstract
Purpose
To search for proprioceptive nerve terminals in human ciliary muscle.
Methods
In 48 human donor eyes, histologic and ultrathin sections cut in different planes and wholemounts of the ciliary muscle were studied. Immunohistochemical staining with antibodies against pan-neuronal antigens and antigens reported as markers for sensory terminals in other organs was performed.
Results
Among the markers for proprioceptive terminals, only calretinin was present in the ciliary body. Calretinin-immunoreactive (IR) nerve terminals surrounded the posterior and reticular ciliary muscle tips and their elastic tendons. Terminals in that region contained mitochondria and neurofilaments. At the anterior tips larger terminals with numerous membrane-filled vesicles were located between the muscle fibers. The most elaborate network of calretinin-IR nerve fibers was present in the ground plate covering the circular muscle portion. Here calretinin-IR neurons with morphologic features of mechanoreception were present. Within the circular muscle portion numerous calretinin-IR ganglion cells were found. Their processes were connected to the calretinin-IR network but also surrounded ciliary muscle cells and NADPH-diaphorase-positive ganglion cells.
Conclusions
These morphologic findings indicate that there are proprioreceptors in the ciliary muscle that morphologically and presumably functionally differ at different locations. At the posterior muscle tips, the receptors could measure stretch of the tendons, whereas the large receptor organs located at the anterior muscle tips morphologically resemble mechanoreceptors measuring shear stress. The presence of the numerous intrinsic nerve cells indicates that contraction of the circular muscle portion can be modulated locally via a self-contained reflex arc.
The complex process of accommodation in primates is mediated by the ciliary muscle that, by configurational changes of its three portions, moves anterior inwardly, thereby influencing the tension of the zonular fibers and thus the shape of the lens. This complicated process is reflected in the structure of the ciliary muscle itself. Unlike smooth muscle seen in vessels or gut wall, the ciliary muscle has tendons for anterior and posterior attachment and the muscle cells exhibit a regular array of dense bands and dense bodies resembling Z-stripes1,2 and histochemical staining qualities, similar to striated muscle.3
The innervation of the ciliary muscle also differs from that of vessel or gut wall smooth muscle. Ciliary muscle cells are extremely densely innervated by cholinergic parasympathetic terminals of the oculomotor nerve1,4–6 and lack gap junctions.2,7–9 Additionally, there are large numbers of intrinsic nerve cells staining for NADPH diaphorase and nitric oxide synthase (NOS).10 These cells are present only in those primate species with a well-developed fovea centralis.11 In human eyes approximately 900 such cells are found in total, localized only in the inner circular and reticular portions of the ciliary muscle, where they are assumed to induce relaxation of the muscle cells.10
In contrast to this prominent efferent innervation, sparse data exist about the muscle's afferent neurons. Large nerve endings are present in the scleral spur of human eyes, where the tendons of the outer longitudinal ciliary muscle portion insert.12,13 Also within the trabecular meshwork, where tendons of the ciliary muscle radiate to form connections between the muscle and the aqueous humor outflow region, afferent terminals staining for calcitonin gene-related peptide (CGRP) and substance P (SP) are often found in contact with elastic fibers.13 Similar results have also been obtained for the monkey eye.14 Morphologically, these nerve endings resemble the afferent terminals of visceral mechanoreceptors measuring stretch or distension.
The presence of afferent nerve endings perceiving tension or shearing forces in smooth muscle is not unusual. In the gastrointestinal tract, abundant endings have been located in the connective tissue between the two muscle layers of the muscular wall (for reviews see Refs. 15–17). In other tissues such as vasculature (for reviews see Refs. 18–20), periodontal tissue (for reviews see Refs. 21, 22), urogenital,23,24 and respiratory tract (for reviews see Refs. 25–28), afferent endings representing proprioreceptors have been described. In most cases the putative muscular mechanoreceptors express the calcium-binding proteins calretinin and calbindin.16,29–34 Within the vagal mechanosensory terminals in the tunica muscularis of the esophagus (the intraganglionic laminar endings) and in vagal bronchopulmonary and skeletomuscular mechanosensors, the presence of vesicular glutamate transporter 2 (VGLUT 2) has been detected.16
The complex process of ciliary muscle contraction and its significance for dynamic, precise, and rapid visual focus on objects at any distance suggests the existence of afferent proprioreception to modulate the rapid and controlled adjustment of the fine muscle movements needed for efficient accommodation and disaccommodation. In the present study, we have, therefore, examined the entire human ciliary muscle and its posterior and anterior tendons with respect to proprioreceptive nerve endings using immunohistochemical staining methods applied to sections cut in different planes and to various wholemount preparations. Additionally, selected specimens fixed in glutaraldehyde were investigated ultrastructurally.
Materials and Methods
Eyes
Forty-eight eyes from 29 human donors (age range, 38–91 years) were investigated. The eyes were obtained from the Institute of Anatomy in Erlangen or from the cornea bank in Amsterdam, after appropriate consent and in accordance with the Declaration of Helsinki and local regulations. Most of the Amsterdam eyes had been trepanized for corneal transplantation; those lacking muscle tips were used only for investigation of the inner and posterior muscle portions. No donor had a known history of ocular disease. After a postmortem time of 5 to 28 hours, the enucleated eyes were fixed at 4°C in neutral buffered 4% paraformaldehyde (24 hours) for immunocytochemistry or (4–8 hours) for NADPH-diaphorase. Specimens used for ultrastructural investigation were immersed in Ito's solution. After fixation, the paraformaldehyde-fixed eyes were washed for 24 hours at 4°C in PBS alone (for demonstration of NADPH-diaphorase) or in PBS using graded saccharose concentrations for cryoprotection of eyes that were to be stained immunohistochemically. Ito-fixed specimens were rinsed in cacodylate buffer. The washed eyes were cut equatorially behind the ora serrata, and the anterior halves were processed further as described.
Immunohistochemistry
Staining of Cryostat Sections
The anterior halves of the eyes were freed from the lens and dissected into four quadrants. From each quadrant 2- to 3-mm-wide and 5- to 6-mm-wide wedge-shaped specimens were cut and frozen in isopentane precooled with liquid nitrogen. From the 2- to 3-mm-wide specimens, cryostat sections were cut from each quadrant at a thickness of 16 μm in a sagittal (meridional) plane. From the 5- to 6-mm-wide specimens, series of 20- to 50-μm-thick sections were cut in frontal (from the iris to the retina) and tangential (parallel to the surface of the sclera and cornea) planes. Sections were mounted on 0.1% poly-l-lysine–coated slides and allowed to dry for several hours. After preincubation in 1% dry milk solution for 15 minutes, the sections were incubated with the primary (Table 1) and appropriate Alexa Fluor 488–or Alexa Fluor 555–labeled immunoglobulin G secondary antibody (MobiTec, Göttingen, Germany). For several sections, double-staining was also performed combining calretinin with pan-neuronal antibodies or those against neural cell adhesion molecule (NCAM), calbindin, tyrosine hydroxylase, substance P, neuronal nitric oxide synthase (nNOS), vesicular acetylcholine transporter (VAChT), or α-smooth muscle (SM) actin.
Table 1.
Source and Concentration of the Primary Antibodies
| Antibody | Host | Dilution | Source |
|---|---|---|---|
| Calretinin | Rabbit | 1:1000 | SWant, Bellinzona, Switzerland |
| Calretinin | Goat | 1:1000 | SWant |
| Calbindin | Rabbit | 1:1000 | SWant |
| Protein gene product (PGP) 9.5 | Rabbit | 1:100 | Biotrend, Cologne, Germany |
| Neurofilament 200 | Rabbit | 1:400 | Biotrend |
| Pan neurofilament | Mouse | 1:200 | Zymed, San Francisco, CA |
| NCAM (140 kDa, 180 kDa, 120 kDa) | Mouse | 1:50 | Serotec, Oxford, UK |
| NCAM (180 kDa) | Mouse | 1:50 | Melitta Schachner, Hamburg |
| Synaptophysin | Mouse | 1:20 | Dako, Glostrup, Denmark |
| Tyrosine hydroxylase | Rabbit | 1:40 | Chemicon, Hofheim, Germany |
| Neuropeptide Y | Rat | 1:1000 | Biotrend |
| Substance P | Rabbit | 1:200 | Peninsula Laboratories, San Carlos, CA |
| VAChT | Goat | 1:1000 | Bioscience, Heidelberg, Germany |
| nNOS | Rabbit | 1:1000 | Bernd Mayer, Graz, Austria |
| α-Smooth muscle (SM) actin | Mouse | 1:300 | Sigma, St. Louis, MO |
NCAM, neural cell adhesion molecule; VACht, vesicular acetylcholine transporter; nNOS, neuronal nitric oxide synthase.
Duration of incubation was in accordance with the manufacturer's instructions. Negative control experiments were performed using either PBS or mouse or rabbit preimmune serum substituted for the primary antibody. After washing in PBS three times for 10 to 30 minutes each time, the sections were mounted in Kaiser's glycerin gelatin (Merck, Darmstadt, Germany). The immunostained slides were viewed with a Leica microscope (DMR; Leica Microsystems GmbH, Wetzlar, Germany). Some of the sections were viewed with a confocal laser scanning system (MRC600; Bio-Rad Microscience Ltd., Hemel Hempstead, UK) attached to an inverted fluorescence microscope (Diaphor 800; Nikon, Düsseldorf, Germany) and equipped with a krypton-argon-laser.
Staining of Wholemounts
Ten eyes were used for wholemount preparations. The anterior half of each eye was freed from the lens and cut into four quadrants. Using scissors or blades, the pigment epithelium of the iris and of the pars plana region was carefully scraped off. The tips of the ciliary processes were also cut off with utmost caution to expose undisturbed the underlying connective tissue of the ciliary body.
NADPH Diaphorase
NADPH diaphorase staining was performed using frozen sections and wholemount specimens fixed for 4 to 8 hours in paraformaldehyde. After washing three times in PBS (pH 7.4), the sections or wholemounts were incubated in NADPH diaphorase staining solution containing 2 mg nitroblue tetrazolium chloride (Serva, Heidelberg, Germany), 30 μL Triton X-100, and 10 mg NADPH (Biomol, Hamburg, Germany) in 10 mL of 0.1 M PBS for 2 to 6 hours. After washing again in PBS, the sections or wholemounts, or both were either processed for immunohistochemical staining using calretinin antibodies as described or mounted on slides (wholemounts) with Kaiser's glycerin gelatin.
Electron Microscopy
Specimens were cut from segments from each quadrant of five eyes fixed in Ito's solution and were processed for electron microscopy. After postfixation with 1% osmium tetroxide, the specimens were dehydrated with graded alcohols and embedded in Epon (Roth, Karlsruhe, Germany). Serial meridional, frontal, and tangential semithin sections were cut on a microtome and stained with Richardson's stain. Ultrathin sections were treated with lead citrate and uranyl acetate and were evaluated under an electron microscope (EM 902; Zeiss, Oberkochen, Germany).
Results
Immunohistochemical staining with antibodies against VGLUT 2 and calbindin as markers for afferent innervation in other muscular systems showed only single fine nerve fibers within larger nerve bundles. There were no terminals stained within the ciliary muscle at the muscular tendons or in the connective tissue of the ciliary body. CGRP-, tyrosine hydroxylase-, neuropeptide Y-, and SP-immunoreactive (IR) nerve terminals were present at vessels within the muscle and ciliary processes, whereas CGRP- and SP-IR terminals were also seen at the anterior insertion of the muscle in the trabecular meshwork. The remaining ciliary muscle and the transition zone between muscle tips and tendons were free of these markers. Staining for VAChT was restricted to the nerve terminals around the ciliary muscle cells. There was no staining at the anterior and posterior tendons. Immunoreactivity for nNOS and NADPH-diaphorase staining were seen in fibers and cells that were confined to ganglion cells within the circular and reticular portions of the ciliary muscle, as described previously.10
However, using antibodies against calretinin, there was a consistent, intensive, and specific staining pattern at the posterior muscle tips and within the connective tissue adjacent to the inner portion of the muscle.
Posterior Portion of the Ciliary Muscle
Wholemounts and tangential sections revealed that here the posterior ends of the outer longitudinal ciliary muscle taper and form irregular, slender, starlike configurations connected to the posterior elastic tendons that radiate into the elastic network of the choroid. In this region, calretinin-labeled fibers formed an irregular net surrounding the tips of the muscle fibers and their transition into the elastic tendons with terminals. This could be seen even more clearly in wholemounts and sections double-stained for calretinin and α-SM actin (Fig. 1A). Single calretinin-IR cells were embedded within the calretinin-IR fiber net showing numerous processes connected to the nerve fiber plexus. Double-staining with the neuronal marker PGP 9.5 confirmed the neuronal nature of these cells.
Figure 1.
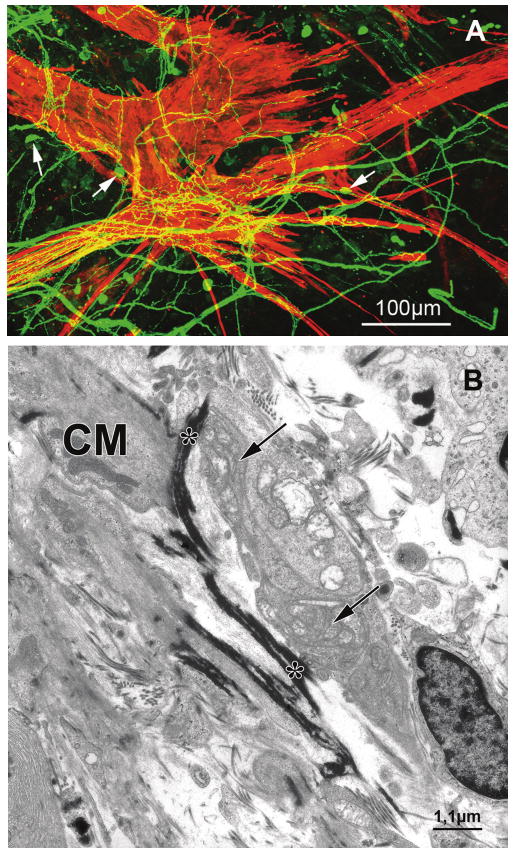
(A) Confocal all-in-focus image of a stack of optical sections showing the wholemount of the posterior ciliary muscle tips of a 58-year-old donor eye double-stained for calretinin (green) and α-SM actin (red). Calretinin-immunolabeled fibers form an irregular web surrounding the muscle fibers. Note the muscle star configuration and its dense innervation by calretinin-IR fibers. Yellow mixed color results from overlay of green calretinin fiber plexus over red smooth ciliary muscle fibers. Single calretinin-IR neurons with processes connected to the fiber net are seen (arrows). (B) Electron micrograph of the posterior ciliary muscle tips inserting into the anterior extension of Bruch's membrane. A nerve terminal filled with numerous mitochondria (arrows) is seen in contact with the electron-dense elastic tendon (asterisks) of a ciliary muscle (CM) cell.
At the inner longitudinal and reticular portion, the muscle developed short tendons anchoring in the continuation of Bruch's membrane and surrounding the parallel-arranged pars plana vessels.35 These inner tips of the muscle and the origin of their tendons were surrounded by calretinin-IR nerves and terminals that appeared less dense than seen further posteriorly. At the ultrastructural level, nerve terminals with numerous mitochondria contacted the origin of the tendons (Fig. 1B).
Anterior Portion of the Ciliary Muscle
At the tips of the anterior longitudinal portion, single calretinin-IR axons were seen running circularly around the circumference of the eye. In some eyes they formed a very loose and delicate net with bouton-like swellings within the uveal and corneoscleral network (Fig. 2A). There were, however, numerous neurofilament- and synaptophysin-IR nerve fibers and endings within the muscle tip itself and at the transition toward the trabecular meshwork and scleral spur. Electron microscopy showed small terminal endings with mitochondria and close connection to the elastic-like tendons, similar to those seen at the posterior inner muscle tips (Fig. 1B). In addition, in this anterior region there were large terminal endings with vesicles, neurofilaments, and lamellar bodies. These terminals were located between muscle fibers and were in close contact with the elastic-like tendinous insertion into the trabecular meshwork (Figs. 2B, C).
Figure 2.
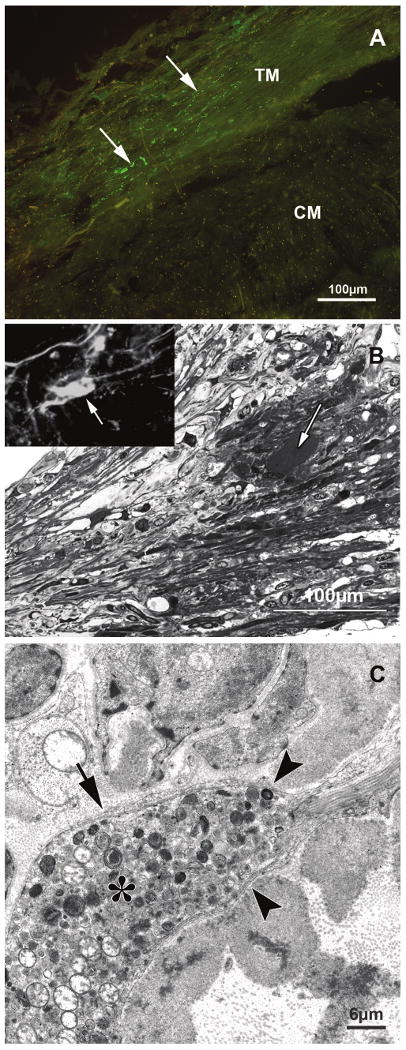
(A) Tangential section through the anterior portion of the ciliary muscle showing the ciliary muscle (CM) tips and the trabecular meshwork (TM), immunolabeled for calretinin (green). Only single calretinin fibers (arrows) are seen within the TM, whereas at the muscle tips no stained fibers are seen. (B) Sagittal semithin section (1 μm) through the anterior ciliary muscle tips (Richardson's stain). The large ovoid structure (arrow) within the ciliary muscle tips next to their tendinous insertion into the connective tissue of the trabecular meshwork stains immunohistochemically for neurofilaments (inset) and synaptophysin (not shown). (C) Electron micrograph of the large structure (asterisk) shown in (B). The structure resembles a mechanoreceptor because it is filled with numerous vesicles, mitochondria, and neurofilaments and is surrounded by a basement membrane (arrow) that is in contact with the sheath of the elastic-like anterior muscle tendon (arrowheads).
Inner Portion of the Ciliary Muscle
In addition, there were calretinin-IR nerves and their terminals that were most prominent in the anterior inner portion of the ciliary body in the connective tissue ground plate located between the circular portion of the ciliary muscle and the ciliary processes. Anteriorly these fibers joined a nerve fiber ring surrounding the major iridal circle at the iris root, whereas posteriorly they reached toward the end of the circular portion and the beginning of the brush-like elastic tendons of the reticular portion of the muscle inserting into the anterior extensions of Bruch's membrane (Fig. 3A). Depending on the state of contraction of the muscle and individual variations, this posterior extension of the calretinin-IR nerve fiber plexus ended either in the transition zone between pars plicata and pars plana (9 of 48 eyes) or more anteriorly in the region of the pars plicata.
Figure 3.
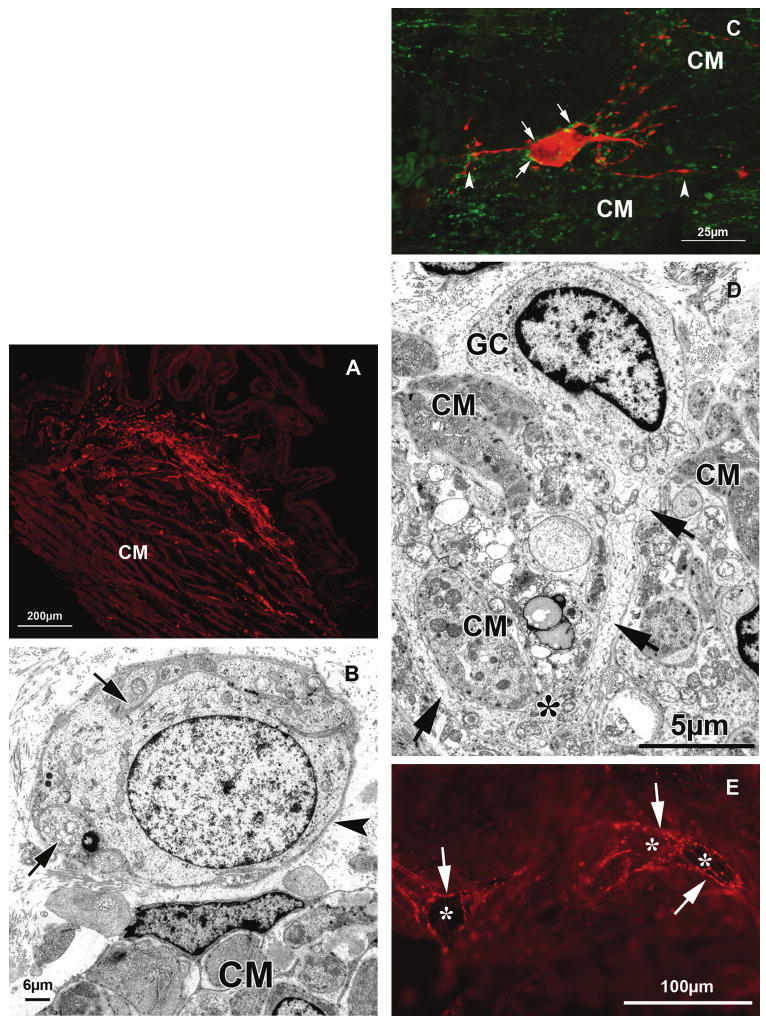
(A) Sagittal frozen section through the anterior inner portion of the ciliary muscle (CM), immunolabeled for calretinin (red). Intense labeling is seen within the ground plate of the ciliary body bordering the circular portion of the muscle. (B) Electron micrograph of a neuron located within the ground plate in the vicinity of the circular portion of the CM. Although parts of the circumference of the ganglion cell are covered with axon varicosities (arrows), others are seen in direct contact with the surrounding connective tissue (arrowhead). (C) Confocal all-in-focus image of a stack of optical sections showing an intrinsic neuron within the circular portion of the CM double-stained for calretinin (red) and synaptophysin (green). Note the long processes contacting CM cells (arrowheads). Varicosities also contact the calretinin-IR ganglion cell and its processes (arrows). (D) Electron micrograph of a ganglion cell (GC) between CM cells. A long cytoplasmic process (arrows) is seen between and partly surrounding the CM cells. Asterisk: varicosity with numerous mitochondria contacting a muscle cell. (E) Frozen tangential section through the CM double-labeled for NADPH-diaphorase and calretinin (red). The dark diaphorase-positive multipolar ganglion cells (asterisks) are surrounded by calretinin fibers and their terminals (arrows).
Using serial sections performed in sagittal, transversal, and frontal planes through the ciliary body, it could be seen that the nerve fibers formed a net-like structure with a circumferential orientation. These findings were confirmed by wholemount preparations that had been carefully freed from the ciliary processes to expose the inner aspect of the connective tissue layer. In these preparations, at places, smaller and larger basket-like networks were seen within this circumferentially arranged calretinin-IR net. Especially dense and fine nerve fibers with bouton-like formations and swellings were seen in the connective tissue of the ground plate connected to the muscle cells of the circular portion and extending into the connective tissue between the innermost muscle cells (Fig. 3A).
Apart from nerve fibers there were also calretinin-IR cells that stained for other neuronal markers (e.g., PGP 9.5). These nerve cells were bipolar or more often star-shaped, showing several processes. At the ultrastructural level, there were ganglion cells in that region that were surrounded by axonal varicosities. At places, the basement membrane of these cells was in direct contact with the surrounding connective tissue (Fig. 3B).
Not only were such calretinin-IR cells present in the region of the calretinin-IR net adjacent to the ciliary muscle, they were even more numerous within the circular and reticular portions of the ciliary muscle. Most of the cells were 15 to 30 μm in diameter and showed several long processes, thus resembling neurons. These cells had their perikarya always within the perimysial connective tissue, whereas their processes ran even over longer distances to approach the ciliary muscle cells and to form varicosities along the surfaces of the muscle cells (Fig. 3C). Other processes were seen to join the calretinin fiber net in the ground plate or to contact other neuronal cells and processes within the ciliary muscle. These cells also stained for neurofilaments and PGP 9.5, confirming their neuronal nature. A few cells did not stain for calretinin but were immunoreactive only for PGP 9.5 and neurofilaments. Using the antibodies for the neuronal marker NCAM, immunoreactive neurons and their dendritic processes were clearly visible.
All neurons were surrounded by synaptophysin IR-boutons (Fig. 3C). At the ultrastructural level, neuronal cells within the ground plate (Fig. 3B) were seen that were surrounded in places by varicosities and showed close connection to the connective tissue. Within the ciliary muscle, neurons were found that had long cytoplasmic processes surrounding the muscle cells and varicosities with numerous mitochondria (Fig. 3D).
Staining for NADPH-diaphorase confirmed our previous results, namely that there were cells positive for this enzyme in the circular and reticular portions of the ciliary muscle.10 Among them only single cells were double stained with calretinin. There were, however, calretinin-IR endings surrounding the NADPH-diaphorase-positive cells (Fig. 3E).
Ground Plate Adjacent to the Zonular Attachment at the Ciliary Epithelium
In addition to the calretinin-IR nerves and terminals within the ciliary muscle and the directly surrounding connective tissue of the ground plate, numerous nerve terminals were seen adjacent to the pigmented epithelium of the anterior-most pars plicata. In tangential sections, the terminals appeared as baskets around the basal portions of the pigmented epithelium. Some of the fibers formed small, others complex, terminal arborizations (Fig. 4). The latter derived from larger parent axons displaying various ramifications with expanded terminal boutons. Although some axons gave rise to a single complex ending, others ramified into two branches developing laminar and tree-like endings. These structures resembled Ruffini endings (Fig. 4). Double immunolabeling with synaptophysin antibody showed a clear staining of the calretinin-IR Ruffini-like endings. There were, however, also laminar endings, often adjacent to calretinin-stained endings, that stained for synaptophysin but not for calretinin. Within the basal portion of the processes, the terminals were located mainly adjacent to the lateral region between process and valley of the pars plicata. In these locations, zonular fibers were seen to be attached to the overlying inner basal membrane of the nonpigmented epithelium. Within the ciliary processes themselves, no fibers or terminals were present. Ultrastructural investigation of the ground plate and the basal stroma of the ciliary processes revealed complexes of terminal endings that showed mitochondria and numerous lysosome-like lamellated structures and vesicles. At places, intimate contact with the extracellular connective tissue was seen. Examination of the entire circumference showed no major differences within or between eyes.
Figure 4.
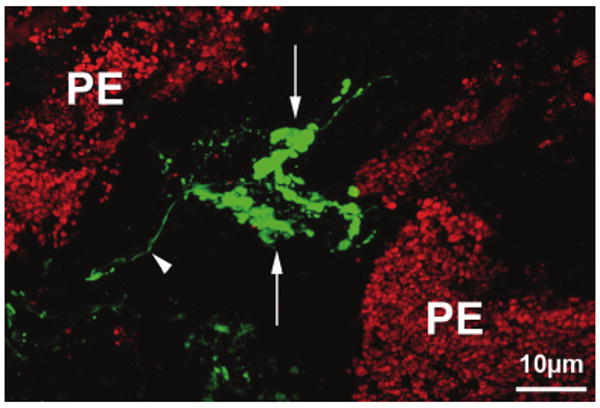
Confocal all-in-focus image of a frozen tangential section through the valley of a ciliary process within the anterior pars plicata, immuno-labeled for calretinin (green). Complex Ruffini ending-like arborizations (arrows) of a calretinin-IR fiber (arrowhead) can be seen next to cells of the pigmented epithelium (PE).
Discussion
The ciliary muscle is unique among all parasympathetically dominated smooth muscles in the body because it has many of the ultrastructural, histochemical, and efferent innervational characteristics of a fast striated muscle. Indeed, accommodation requires excellent central visual resolution, rapid distance/near adjustment of focus, and strong tracking and image stabilization. Even maintaining focus on an object at a fixed distance requires constant fine adjustment of crystalline lens shape and position, mediated by the tension of the zonular apparatus, which is, in turn, dependent on the position of the zonular plexus within the valleys of the ciliary body. Therefore, the structures involved in the accommodative mechanism, and perhaps the muscle itself, would be expected to have some sort of afferent mechanism to monitor the state of the muscle's contraction/relaxation and thus the position of the zonular plexus.
Our results showed that there are, in fact, several structures within the ciliary body that could serve such functions. Most of these structures stained for calretinin.
The calcium-binding protein calretinin is a member of the EF-hand homolog family of calcium-binding proteins.36–38 Calretinin immunoreactivity has been documented in peripheral sensory neurons and in axons innervating muscle spindles, Pacini corpuscles, and Ruffini-like endings. It has, therefore, been suggested that calretinin-expressing neurons innervate particular mechanoreceptors.16,30–33,39 Such mechanoreceptor-like structures staining for calretinin are present in the posterior part of the ciliary muscle, especially in the transition zone between longitudinal and reticular muscle tips and their elastic tendons. These posterior tendons of the ciliary muscle insert into the elastic fiber network of the choroid and the anterior extension of Bruch's membrane. During accommodation, the posterior end of the ciliary muscle moves anteriorly, stretching the elastic tendons. During disaccommodation, the relaxed muscle is pulled backward again by the elastic force of the tendons.40,41
The posterior muscle tips and their elastic tendons exhibit calretinin-IR terminals that, based on their location and morphology, suggest a robust mechanism for monitoring stretch, whereby the afferent arm is mediated via axons providing input presumably mainly to the trigeminal ganglion and eventually modulating the efferent response via the parasympathetic oculomotor pathway to the ciliary ganglion. This would be extremely important in regulating the accommodation/disaccommodation process and even during maintenance of sharp focus when fixing on a stable object. In addition, it is known that strong miotics can cause occasional retinal breaks and detachments (for reviews see Refs. 42–45). Controlled contraction could achieve and maintain the appropriate lens configuration without abruptly stretching the retina. Based on our morphologic studies, however, it cannot be ruled out that the terminals also serve other functions.
In contrast to the posterior elastic tendons, nearly no stained fibers were seen at the anterior elastic-like tendons of the ciliary muscle tips inserting into the trabecular meshwork and scleral spur. However, within the muscle tip itself, large afferent endings were seen, similar to those previously described in the area of the scleral spur.12 These endings contained no calretinin-IR but stained for neurofilament markers and synaptophysin and ultrastructurally contained numerous and densely packed mitochondria, neurofilaments, and membrane-bound vesicles typical for mechanoreceptors (e.g., also in baroreceptors).46–48 The anterior longitudinal muscle serves different functions than the posterior portion. This part has to provide stiffening of the tips before the inner portion of the ciliary muscle can move forward-inward. This stiffening is possible because the anterior elastic tendons contain little elastin, in contrast to the posterior ones; rather, they are surrounded by a cross-linked fibrillar sheath increasing in thickness with age (for review see Ref. 49). It is assumed that afferent endings in the anterior muscle portion, with its mainly isometric contraction, face shear stress within the connective tissue surrounding the muscle fibers. It is well documented that the connective tissue spaces at the muscle tips decrease in size during ciliary muscle contraction.50
The inner portion of the ciliary muscle morphologically differs significantly from the longitudinal portion. Earlier histochemical studies of the primate ciliary muscle3 showed similarities of the longitudinal part to fast type II skeletal muscle, whereas the inner portions had staining characteristics of slow tonic type I fibers. Ultrastructurally, the circular muscle cells contain significantly more mitochondria than the longitudinal ones. Moreover, they do not form tendinous insertion into the overlying connective tissue of the ground plate. During contraction, the circular portion increases in size50 and forms an anterior inner edge important for configurational changes of the lens. Visualization of the accommodating ciliary body in monkeys after surgical iridectomy and midbrain implantation of a stimulating electrode51–53 has confirmed the large amplitude of this forward and inward movement, especially in young animals. It is here in the connective tissue of the ground plate, covering the inner circular muscle portion and separating it from the anterior-most ciliary processes and within this circular muscle portion, that the most numerous and most complex afferent structures are present. Location of the terminals indicates that here, movement of connective tissue fibers by muscle movement and thickening of the muscle fibers themselves can be registered.
Another striking finding, especially in this region, was the presence of a complex intrinsic nerve cell system. These calretinin-IR nerve cells labeled with other neuronal markers and, therefore, representing ganglion cells contacted the muscle cells via their dendrites. It is tempting to speculate that these neurons monitor the state of contraction of the muscle cells directly. Some of the neurons within the calretinin network of the ground plate and within the ciliary muscle show ultrastructural features (e.g., direct connections to the surrounding connective tissue fibers) that suggest direct mechanoreceptive functions as assumed for intrinsic calretinin-positive neurons of the choroid.34 Thus, the calretinin-IR nerve cells and terminals could measure shape changes of the inner circular muscle portion and, therefore, the state of contraction indirectly. Within the inner portions of the ciliary muscle, Tamm et al.11 have also described the presence of nitrergic neurons. Our results show that these neurons are surrounded by calretinin-IR synaptic contacts that at least partly derive from the calretinin-IR ganglia in this region.
Afferent stimuli do, however, derive not only from the ciliary muscle tendons and its inner portion but also from the ciliary epithelium because Ruffini-like terminals were found directly adjacent to or in the vicinity of the epithelium at the base of the processes of the anterior pars plicata. Here, in the valleys between the ciliary processes, the overlying epithelium is the anchorage of the zonular plexus.54
It is tempting to speculate that receptor end organs in these locations are necessary for the registration of pull or traction forces that might occur during the process of fine adjustment of the lens during accommodation and disaccommodation.
This entire nervous system at the inner/apical region of the ciliary muscle suggests the existence of an internal loop regulating fine and rapid microcontraction/relaxation of the region closest and most immediately consequential to the zonular plexus and the lens. We hypothesize that the calretinin-IR nerves and boutons monitor stretch in the ground plate and muscle and transmit information to the NO neurons, whose activation relaxes the muscle and thus dampens and smooths the accommodative and disaccommodative fine control of focus, both when changing the focal distance and when maintaining a fixed focus on a static object (Fig. 5).
Figure 5.
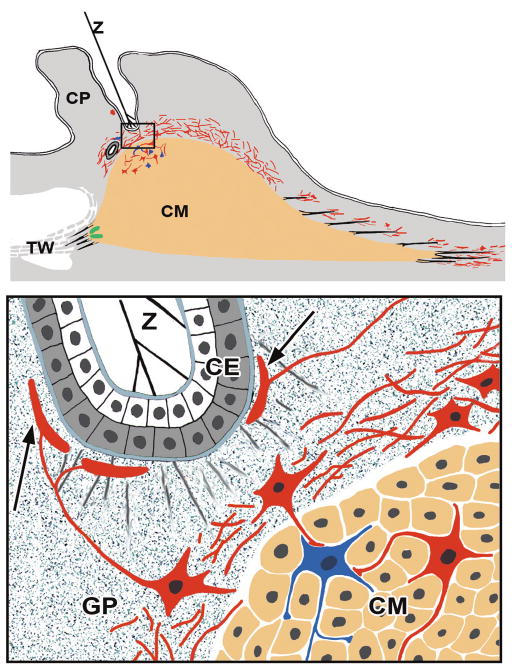
Schematic drawing summarizing our results on calretinin-IR. Top: the posterior tendons (black lines) of the ciliary muscle (CM) are surrounded by calretinin-IR terminals (red). At the anterior muscle tips (black lines), these calretinin-IR endings are absent, but large mechanoreceptor-like endings (green) are present between the muscle tips and in the scleral spur region. The circular muscle portion is covered by a cap-like net containing numerous ganglion cells. Calretinin-IR ganglion cells are also present within the inner portions of the muscle itself (bottom; magnified inset from top). These cells (red) form contact with NADPH diaphorase-positive cells (blue) within the circular and reticular muscle portions. Some ganglion cells within the ground plate (GP) are in contact with Ruffini-like structures (arrows) next to the pigmented ciliary epithelium (CE) in areas where zonular fibers (Z) are attached to the inner limiting membrane of the nonpigmented CE. CP, ciliary process; TW trabecular meshwork
Acknowledgments
The authors thank the cornea bank of Amsterdam and Hans Bloemendal (Department of Biochemistry, University of Nijmegen, The Netherlands) for the intense endeavors in providing, fixation, and sending of human eyes, and they thank Hong Nguyen, Gerti Link, Elke Kretzschmar, Jörg Pekarsky, and Marco Gösswein for expert technical assistance.
Supported by Deutsche Forschungsgemeinschaft Grants DFG Dr 124/71 and SFB 539 Glaukome, and National Institutes of Health Grant EYO 2698.
Footnotes
Disclosure: C. Flügel-Koch, None; W.L. Neuhuber, None; P.L. Kaufman, None; E. Lütjen-Drecoll, None
References
- 1.Van der Zypen E. Licht- und elektronenmikroskopische Untersuchungen über den Bau und die Innervation des Ciliarmuskels bei Mensch und Affe (Cercopithecus aethiops) Graefes Arch Clin Ophthalmol. 1967;174:143–168. doi: 10.1007/BF00413512. [DOI] [PubMed] [Google Scholar]
- 2.Lütjen-Drecoll E, Tamm E, Kaufman PL. Age changes in rhesus monkey ciliary muscle: light and electron microscopy. Exp Eye Res. 1988;47:885–899. doi: 10.1016/0014-4835(88)90070-x. [DOI] [PubMed] [Google Scholar]
- 3.Flügel C, Bárány EH, Lütjen-Drecoll E. Histochemical differences within the ciliary muscle and its function in accommodation. Exp Eye Res. 1990;50:219–226. doi: 10.1016/0014-4835(90)90234-l. [DOI] [PubMed] [Google Scholar]
- 4.Ishikawa T. Fine structure of the ciliary muscle. Invest Ophthalmol. 1962;1:587–608. [PubMed] [Google Scholar]
- 5.Townes-Anderson E, Raviola G. Giant nerve fibers in the ciliary muscle and iris sphincter of Macaca mulatta. Cell Tissue Res. 1976;169:33–40. doi: 10.1007/BF00219305. [DOI] [PubMed] [Google Scholar]
- 6.Townes-Anderson E, Raviola G. Degeneration and regeneration of autonomic nerve endings in the anterior part of the rhesus monkey ciliary muscle. J Neurocytol. 1978;7:583–600. doi: 10.1007/BF01260891. [DOI] [PubMed] [Google Scholar]
- 7.Raviola G, Sagaties MJ, Miller C. Intercellular junctions between fibroblasts in connective tissue of the eyes of macaque monkeys: a thin section and freeze fracture analysis. Invest Ophthalmol Vis Sci. 1987;28:834–841. [PubMed] [Google Scholar]
- 8.Samuel U, Lütjen-Drecoll E, Tamm ER. Gap junctions are found between iris sphincter smooth muscle cells but not in the ciliary muscle of human and monkey eyes. Exp Eye Res. 1996;63:187–192. doi: 10.1006/exer.1996.0107. [DOI] [PubMed] [Google Scholar]
- 9.Tamm E, Lütjen-Drecoll E, Rohen JW. Age-related changes of the ciliary muscle in comparison with changes induced by treatment with prostaglandin F2α: an ultrastructural study in rhesus and cynomolgus monkeys. Mech Ageing Develop. 1990;51:101–120. doi: 10.1016/0047-6374(90)90093-u. [DOI] [PubMed] [Google Scholar]
- 10.Tamm ER, Flügel-Koch C, Mayer B, Lütjen-Drecoll E. Nerve cells in the human ciliary muscle: ultrastructural and immunocytochemical characterization. Invest Ophthalmol Vis Sci. 1995;36:414–426. [PubMed] [Google Scholar]
- 11.Tamm ER, Lütjen-Drecoll E. Nitrergic nerve cells in the primate ciliary muscle are only present in species with a fovea centralis. Opthalmologica. 1997;211:201–204. doi: 10.1159/000310789. [DOI] [PubMed] [Google Scholar]
- 12.Tamm ER, Flügel C, Stefani FH, Lütjen-Drecoll E. Nerve endings with structural characteristics of mechanoreceptors in the human scleral spur. Invest Ophthalmol Vis Sci. 1994;35:1157–1166. [PubMed] [Google Scholar]
- 13.Selbach JM, Gottanka J, Wittmann M, Lütjen-Drecoll E. Efferent and afferent innervation of primate trabecular meshwork and scleral spur. Invest Ophthalmol Vis Sci. 2000;41:2184–2191. [PubMed] [Google Scholar]
- 14.Ruskell GL. Trigeminal innervation of the scleral spur in cynomolgus monkeys. J Anat. 1994;184:511–518. [PMC free article] [PubMed] [Google Scholar]
- 15.Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle : re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev. 2000;34:1–26. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- 16.Neuhuber WL, Raab M, Berthoud HR, Wörl J. Innervation of the mammalian esophagus. Adv Anat Embryol Cell Biol. 2006;185:1–73. [PubMed] [Google Scholar]
- 17.Costa M, Brookes S, Zagorodnyuk V. How many kinds of visceral afferents. Gut. 2004;53(suppl 2):1–4. doi: 10.1136/gut.2003.033407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng Z, Powley TL, Schwaber JS, Doyle FJ. Vagal afferent innervation of the atria of the rat heart reconstructed with confocal microscopy. J Comp Neurol. 1997;38:1–17. doi: 10.1002/(sici)1096-9861(19970428)381:1<1::aid-cne1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 19.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 20.Ichikawa H. Innervation of the carotid body: immunohistochemical, denervation, and retrograde tracing studies. Microsc Res Tech. 2002;59:188–195. doi: 10.1002/jemt.10193. [DOI] [PubMed] [Google Scholar]
- 21.Maeda T, Ochi K, Nakakura-Oshima K, Youn SH, Wakisaka S. The Ruffini ending as the primary mechanoreceptor in the periodontal ligament: its morphology, cytochemical features, regeneration, and development. Crit Rev Oral Biol Med. 1999;10:303–327. doi: 10.1177/10454411990100030401. [DOI] [PubMed] [Google Scholar]
- 22.Wakisaka S, Atsumi Y, Youn SH, Maeda T. Morphological and cytochemical characteristics of periodontal Ruffini ending under normal and regeneration processes. Arch Histol Cytol. 2000;63:91–113. doi: 10.1679/aohc.63.91. [DOI] [PubMed] [Google Scholar]
- 23.De Groat WC. Neuropeptides in pelvic afferent pathways. Experientia. 1987;43:801–813. doi: 10.1007/BF01945358. [DOI] [PubMed] [Google Scholar]
- 24.Wyndaele JJ, de Wachter S. The basics behind bladder pain: a review of data on lower urinary tract sensations. Int J Urol. 2003;10(suppl):S49–S55. doi: 10.1046/j.1442-2042.10.s1.11.x. [DOI] [PubMed] [Google Scholar]
- 25.Widdicombe J. Airway receptors. Resp Physiol. 2001;125:3–15. doi: 10.1016/s0034-5687(00)00201-2. [DOI] [PubMed] [Google Scholar]
- 26.Yu J. Airway mechanosensors. Respir Physiol Neurobiol. 2005;148:217–243. doi: 10.1016/j.resp.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618–627. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brouns I, De Proost I, Pintelon I, Timmermans JP, Adriaensen D. Sensory receptors in the airways: neurochemical coding of smooth muscle-associated airway receptors and pulmonary neuroepithelial body innervation. Auton Neurosci. 2006;126-127:307–319. doi: 10.1016/j.autneu.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Philippe E, Droz B. Calbindin-immunoreactive sensory neurons of dorsal root ganglion project to skeletal muscle in the chick. J Comp Neurol. 1989;283:153–160. doi: 10.1002/cne.902830113. [DOI] [PubMed] [Google Scholar]
- 30.Duc C, Barakat-Walter I, Droz B. Innervation of putative rapidly adapting mechanoreceptors by calbindin- and calretinin-immunoreactive primary sensory neurons in the rat. Eur J Neurosci. 1994;6:264–271. doi: 10.1111/j.1460-9568.1994.tb00269.x. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto Y, Atoji Y, Kuramoto H, Suzuki Y. Calretinin-immunoreactive laminar nerve endings in the laryngeal mucosa of the rat. Cell Tissue Res. 1998;292:613–617. doi: 10.1007/s004410051091. [DOI] [PubMed] [Google Scholar]
- 32.Pintelon I, Brouns I, de Proost I, van Meir F, Timmermans JP, Adriaensen D. Sensory receptors in the visceral pleura: neurochemical coding and live staining in wholemounts. Am J Respir Cell Mol Biol. 2007;36:541–551. doi: 10.1165/rcmb.2006-0256OC. [DOI] [PubMed] [Google Scholar]
- 33.Dütsch M, Eichhorn U, Wörl J, Wank M, Berthoud HR, Neuhuber WL. Vagal and spinal afferent innervation of the rat esophagus: a combined retrograde tracing and immunocytochemical study with special emphasis on calcium-binding proteins. J Comp Neurol. 1998;398:289–307. [PubMed] [Google Scholar]
- 34.May CA, Neuhuber W, Lütjen-Drecoll E. Immunohistochemical classification and functional morphology of human choroidal ganglion cells. Invest Ophthalmol Vis Sci. 2004;45:361–367. doi: 10.1167/iovs.03-0624. [DOI] [PubMed] [Google Scholar]
- 35.Lütjen-Drecoll E. Functional morphology of the ciliary epithelium. In: Lütjen-Drecoll E, editor. Basic Aspects of Glaucoma Research. Stuttgart: FK Schattauer; 1982. pp. 69–87. [Google Scholar]
- 36.Kawasaki H, Kretsinger RH. Calcium-binding proteins–1: EF hands. Protein Profile. 1994;1:343–517. [PubMed] [Google Scholar]
- 37.Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992;15:303–308. doi: 10.1016/0166-2236(92)90081-i. [DOI] [PubMed] [Google Scholar]
- 38.Andressen C, Blümcke L, Celio MR. Calcium-binding proteins: selective markers of nerve cells. Cell Tissue Res. 1993;271:181–208. doi: 10.1007/BF00318606. [DOI] [PubMed] [Google Scholar]
- 39.Ichikawa H, Deguchi T, Nakago T, Jacobowitz DM, Sugimoto T. Parvalbumin, calretinin and carbonic anhydrase in the trigeminal and spinal primary neurons of the rat. Brain Res. 1994;655:241–245. doi: 10.1016/0006-8993(94)91620-9. [DOI] [PubMed] [Google Scholar]
- 40.Rohen J. Abhandlungen der mathematisch-naturwissenschaftlichen Klasse Mainz, Wiesbaden Verlag der Akademie der Wissenschaften und der Literatur. Franz-Steiner Verlag; 1953. Die funktionelle Gestalt des Auges und seiner Hilfsorgane. Untersuchungen zur funktionellen Anatomie des Auges; pp. 69–87. [Google Scholar]
- 41.Lütjen-Drecoll E, Tamm E, Kaufmann PL. Age-related loss of morphologic response to pilocarpine in rhesus monkey ciliary muscle. Arch Ophthalmol. 1988;106:1591–1998. doi: 10.1001/archopht.1988.01060140759051. [DOI] [PubMed] [Google Scholar]
- 42.Heimann K, Kyrieleis E. Retinal detachment from miotic therapy. Klin Monatsbl Augenheilkd. 1970;156:98–104. [PubMed] [Google Scholar]
- 43.Pape LG, Forbes M. Retinal detachment and miotic therapy. Am J Ophthalmol. 1978;85:558–566. doi: 10.1016/s0002-9394(14)75255-9. [DOI] [PubMed] [Google Scholar]
- 44.Alpar JJ. Miotics and retinal detachment: a survey and case report. Ann Ophthalmol. 1979;1:395–401. [PubMed] [Google Scholar]
- 45.Kraushar MF, Steinberg JA. Miotics and retinal detachment: upgrading the community standard. Surv Ophthalmol. 1991;35:311–316. doi: 10.1016/0039-6257(91)90053-i. [DOI] [PubMed] [Google Scholar]
- 46.Krauhs JM. Structure of rat aortic baroreceptors and their relationship to connective tissue. J Neurocytol. 1979;8:401–414. doi: 10.1007/BF01214800. [DOI] [PubMed] [Google Scholar]
- 47.Knoche H, Wiesner-Menzel L, Addicks K. Ultrastructure of baroreceptors in the carotid sinus of the rabbit. Acta Anat (Basel) 1980;106:63–83. doi: 10.1159/000145170. [DOI] [PubMed] [Google Scholar]
- 48.Shin HS, Hulbert WC, Biggs DF. Observations on the fine structure of the baroreceptors and adrenergic innervation of the guinea-pig carotid sinus. J Morphol. 1987;194:65–74. doi: 10.1002/jmor.1051940106. [DOI] [PubMed] [Google Scholar]
- 49.Tektas OY, Lütjen-Drecoll E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res. 2009;88:769–775. doi: 10.1016/j.exer.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 50.Lütjen E. Histometrische Untersuchungen über den Ciliarmuskel der Primaten. Graefes Arch Clin Ophthalmol. 1966;171:121–133. doi: 10.1007/BF00418250. [DOI] [PubMed] [Google Scholar]
- 51.Croft MA, Kaufman PL, Crawford KS, Neider MW, Glasser A, Bito LZ. Accommodation dynamics in aging rhesus monkeys. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1885–R1897. doi: 10.1152/ajpregu.1998.275.6.R1885. [DOI] [PubMed] [Google Scholar]
- 52.Neider MW, Crawford K, Kaufman PL, Bito LZ. In vivo videography of the rhesus monkey accommodative apparatus: age related loss of ciliary muscle response to central stimulation. Arch Ophthalmol. 1990;108:69–74. doi: 10.1001/archopht.1990.01070030075032. [DOI] [PubMed] [Google Scholar]
- 53.Glasser A, Kaufman PL. The mechanisms of accommodation in primates. Ophthalmology. 1999;106:863–873. doi: 10.1016/S0161-6420(99)00502-3. [DOI] [PubMed] [Google Scholar]
- 54.Rohen JW. Scanning electron microscopic studies of the zonular apparatus in human and monkey eyes. Invest Ophthalmol Vis Sci. 1979;18:133–144. [PubMed] [Google Scholar]


