Abstract
The androgen role in the maintenance of prostate epithelium is subject to conflicting opinions. While androgen ablation drives the regression of normal and cancerous prostate, testosterone may cause both proliferation and apoptosis. Several investigators note decreased proliferation and stronger response to chemotherapy of the prostate cancer cells stably expressing androgen receptor (AR), however no mechanistic explanation was offered. In this paper we demonstrate in vivo anti-tumor effect of the AR on prostate cancer growth and identify its molecular mediators. We analyzed the effect of AR on the tumorigenicity of prostate cancer cells. Unexpectedly, the AR-expressing cells formed tumors in male mice at a much lower rate than the AR-negative controls. Moreover, the AR-expressing tumors showed decreased vascularity and massive apoptosis. AR expression lowered the angiogenic potential of cancer cells, by increasing secretion of an anti-angiogenic protein, thrombospondin-1. AR activation caused a decrease in RelA, a subunit of the pro-survival transcription factor NFκB, reduced its nuclear localization and transcriptional activity. This, in turn, diminished the expression of its anti-apoptotic targets, Bcl-2 and IL-6. Increased apoptosis within AR-expressing tumors was likely due to the NFκB suppression, since it was restricted to the cells lacking nuclear (active) NFκB. Thus we for the first time identified combined decrease of NFκB and increased TSP1 as molecular events underlying the AR anti-tumor activity in vivo. Our data indicate that intermittent androgen ablation is preferable to continuous withdrawal, a standard treatment for early-stage prostate cancer.
Keywords: prostate cancer, androgen receptor, NFκB, angiogenesis, apoptosis
Androgen withdrawal, common treatment for prostate cancer (PrCa), frequently leads to androgen independence.1,2 Androgen binding to AR facilitates AR dimerization and binding to the androgen response element (ARE) CGTACAnnnTGTTCT and transcription. In addition, AR mediates nongenomic androgen effects, intracellular calcium flux and kinase activation.3 In androgen-independent cell lines, AR may cause cell growth in the absence of ligand.4 Unlawful AR activation can occur without steroids via surface receptors, like HER-2,5 or by growth factors, like interleukin-6, oncostatin-M or bombesin.6,7 AR gene amplification can also lead to increased transcriptional activity.8 PTEN, a tumor suppressor, along with membrane protein caveolin dampen AR activity.9,10 Thus AR can be active even in low androgen environment. AR mutations cluster in an area that defines AR protein interactions,11–13 they are rare in local disease14–16 but frequent in metastases where they enable binding with estradiols, glucocorticoids and anti-androgens11,17 (reviewed in Ref. 17).
The role of androgens in cell survival and proliferation remains controversial. In androgen-sensitive LNCaP cells, physiologic levels of dihydroxytestosterone (DHT) fail to induce prostate-specific genes but enhance growth, possibly via Rb phosphorylation,18 or via CDK2, CDK4 and p16 genes19; moreover AR blocking agents inhibit proliferation.20,21 Blocking AR with antisense oligonucleotides, ribozymes, or Hsp90 hampers PrCa expansion.11 At the same time, androgen may halt cell cycle via p27,18 and facilitates differentiation,22 AR expression in null PC-3 cells causes growth arrest, apoptosis and decreased invasion,23–30 and in DU145 cells, growth arrest and differentiation.31 Moreover, AR activation by mitogentic androgen doses sensitizes prostate cancer cells to the cytotoxic insult by taxanes.32
High microvascular density (MVD) in PrCa marks poor prognoses and metastases.33 Testosterone stimulates endothelial proliferation and vascular regrowth (angiogenesis) after castration, however these may be secondary, due to hypoxia.34,35 In culture, androgens stimulate angiogenic factors via HIF-1.36 The loss of angiogenesis inhibitors in PrCa has been demonstrated,33,37 however direct androgen suppression was only shown for pigment epithelial-derived factor (PEDF).37 Conversely, thrombospondin-1 (TSP1) is decreased or lost in hormone refractory disease.38
NFκB transcription factor is highly active in PrCa due to hyper-active regulatory IκB kinase complex.39 NFκB promotes proliferation and inhibits apoptosis via c-myc, cyclin D, IL-6 and Bcl-2, or by suppressing Bax.40 Noteworthy, in PrCa AR status inversely correlates with NFκB activity.25,41,42
We analyzed how inducible AR affects the tumorigenicity of AR-null PC-3 cells. Unexpectedly, the AR(+) PC-3 cells became less tumorigenic on ambient testosterone background. Moreover, AR(+) tumors displayed low MVD and massive apoptosis. The diminished angiogenesis was due to elevated TSP1, while increased apoptosis may be due to dramatically decreased NFκB activity. AR expression lowered NFκB RelA, mRNA and protein, and reduced RelA activity and nuclear localization. This, in turn, dramatically decreased pro-survival Bcl-2 and IL-6. Thus we have shown the anti-tumor activity of AR in vivo and identified some of its mediators.
Material and methods
Cells
Bovine adrenal capillary endothelial cells (BAMVEC) were grown in MCDB131 (Sigma) with supplements (BioWhittacker). PC-3 were maintained in RPMI1640 (Invitrogen), 10% FBS and 1% Penicillin/Streptomycin. PC-3 cells expressing tetracycline (tet) repressor (PC3-TR) were grown in tet-free serum (HyClone), and Blasticidin (1μg/ml, Invitrogen).
To collect conditioned media (CM), 80% confluent cells were rinsed, incubated 48 hr in serum-free RPMI, media collected, cleared of debris, and concentrated in Millipore Ultrafree filters (5 kDa).
Cell growth was measured using WST-1 kit (Roche). The cells were plated in 96-well plates (5 × 102 cells/well), and induced with Doxycycline (Dox) (1 μg/ml, Fluka).
AR-inducible cells
We used T-REX inducible system (Invitrogen). The wild-type AR cDNA (Dr. X. Liao, University of Chicago, IL) and AR-877 mutant (Dr. Z. Culig, Innsbruck Medical University, Austria) were amplified, cloned into BamHI-Age I sites of pcDNA4/TO/myc-His vector and verified by sequencing. PC-3 cells were transfected with pcDNA6/TR (tet repressor) conferring Blasticidin resistance (FuGENE6, Roche). Transfectants were screened with β-gal reporter (pcDNA4/TO/lacZ, Invitrogen). PC3-TR cells were transfected with pcDNA4/TO/myc-His-AR. Cells resistant to Blasticidin/Zeocin were expanded and screened for AR expression. Clones with the lowest background expression were chosen (PC3-V, PC3-ARWT, PC3-AR877).
Western blotting
The cells were lyzed in PBS, 1% NP40, 0.5% Na deoxycholate, 0.1% SDS, and protease inhibitor cocktail (Sigma). Cleared lysates were resolved by SDS-PAGE and transferred to PVDF membranes. After blocking (5% Blotto in TBS-T, 20 mM TBS, pH7.4, 0.1% Tween-20) the membranes were probed and developed with ECL kit (Amersham). For IκB, total lysates were collected, resolved by SDS-PAGE, transferred to PVDF, blocked and probed in 0.5% BSA/TBS-T. For TSP1, CM (10 μg/lane) were resolved by 8% SDS-PAGE, membranes blocked in 7% Blotto and probed in 1% Blotto/PBS. For Bcl-2, membranes were blocked in 10% Blotto/TBS-T. The antibodies were: AR rabbit PAb (Ab-2, Santa Cruz), IκB-α rabbit PAb (Cell Signaling), TSP1 MAb (A4.1, Novus), Cytokeratin 8 pAb (Santa Cruz) and Bcl-2 antibodies (Santa Cruz). U19 antibodies were raised against GST-fusion protein and purified as described.43
IL-6 measurement
IL-6 was detected in conditioned media (CM) collected as above, using human IL-6 ELISA kit (BD Biosciences, San Diego, CA), as recommended by the manufacturer.
RT-PCR
RNA were extracted with GenElute kit (Sigma), converted to cDNA and amplified 30 cycles in 0.2 mM dNTPs, 1.5 mM MgCl2, 0.1 μM primers and 1 U Taq polymerase (Fermentas); 2′ denaturation (94°C), 45″ annealing (55°C for actin, IL-6 and NFκB, 60°C for TSP1), 45″ elongation (72°C) with the following primers (5′-3′):
Actin, TGTTGGCGTACAGGTCTTTGC/GCTACGAGCTGCCTGACGG (182 bp);
TSP1, ACCGCATTCCAGAGTCTG/GACGTCCAACTCAGCATT (488 bp);
RelA, TATCAGTCAGCGCATCCAGACCAA/AGAGTTTCGGTTCACTCGGCAGAT (222 bp);
IL-6, AAGCCAGAGCTGTGCAGATGAGTA/AACAACAATCTGAGGTGCCCATGC (246 bp).
Luciferase assay
Cells were plated (3 × 105/well) in 6-well plates, induced 24 hr with 1.0 μg/ml Dox and transfected with 1 μg Firefly luciferase (FL) reporter, and 25 ng pRL-TK (Renilla luciferase, RL, Promega). R1881, (DHT), progesterone (Prg), flutamide (Fl) (Sigma) or vehicle (EtOH), were added for 24 hr. Luciferase activity was measured using Dual Luciferase Reporter Assay (Promega). Luminiscence was assessed with Monolight 2010 Luminometer and the FL activity normalized against RL. Background (pGL3-TATA-Luc vector) was subtracted and fold induction calculated. The experiments were repeated in triplicate.
pGL3-TATA-Luc and AR reporter pGL3-GRE-Luc were form Dr. C. Kao, University of Indiana, Indianapolis. For TSP1 we used –2033/+150 promoter fragment44 driving a RL reporter. The following AR/NFκB constructs were used: κB-FL reporter (5x κB promoter, Dr. WC.Greene, Gladstone Institute, UCSF); MMTV-FL reporter for steroid receptors, (Clontech, Palo Alto; CA); pcDNA3.1-CMV-p50 and pcDNA3.1-CMV-p65 (Dr. S. Okret, Karolinska Institutet, Sweden); pcDNA3.1-CMV-AR (Drs. O.A. Janne and J.J. Palvimo, University of Helsinki, Helsinki, Finland); and pcDNA-CMV-dnIκB-α (Dr. I.Verma, Salk Institute, La Jolla, CA).
For NFκB assays, 50% confluent PC-3 or LNCaP were transfected with indicated plasmids. After 36 hr the cells were harvested and Luciferase activity measured. Where shown, the cells were pre-treated 24 hr with DHT (Sigma).
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed using E-ZChIP kit (Upstate). Formaldehyde was added (final 1%, 10 min, 37°C), the cells washed in PBS, lyzed in 1% SDS, 10 mM EDTA, 50 mM Tris pH 8.1 and sonicated to produce ~1 Kb DNA fragments. The samples diluted 1:10 in 0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-HCl, pH 8.1, 150 mM NaCl, were incubated with AR antibody (1:500, BD Biosciences). DNA/protein complexes were isolated on salmon sperm DNA agarose and extracted with 1% SDS, 0.1M NaHCO3. Crosslinking was reversed, proteins digested with proteinase K and removed. DNA was precipitated, re-dissolved and amplified with TSP1 primers localized to the 1st intron (5′-3′): TGAGGCTTCAGTCCCTCTGGT and AGTACAGACTCTTCCCTGAGTGCT (225 bp).
Migration assay
Migration assay was performed as in Ref. 45. BAMVECs starved in MCDB131, 0.1% BSA (Sigma), were plated at 1.5 × 106 ml–1 in Boyden chambers on the lower surface of gelatinized membranes (8 μm, Nucleopore). After attachment, serial dilutions of CM from PC3-V, PC3-ARWT or PC3-AR877 were placed in top wells for 4 hr. Background migration (BSA) was subtracted and the data presented as percent maximal migration (10 ng/ml bFGF). All samples were tested in quadruplicate.
Tumorigenicity assay
PC3-V, PC3-ARWT or PC3-AR877 cells were injected s.c. in hindquarters of athymic male mice (nu/nu, National Cancer Institute, 4–6 weeks), 106 cells/site, 5 animals/group, 2 sites/animal. To induce AR expression, Dox (1mg/ml) was given in drinking water. The tumors were measured every 3 days and the volumes calculated as length × width2 × 0.52. The experiment was repeated using the same numbers of female athymic mice ± DHT pellets. The pellets were generated in the lab as described in Ref. 43. Flutamide (Fl) (40 mg/kg, Sigma) was given daily p.o. At the endpoint tumors were removed, snap-frozen or fixed in 4% formaldehyde. The animals were handled following the National Institute of Health guidelines, protocols approved by Northwestern University Animal Care and Use Committee.
Immunostaining
Five micrometer cryosections were fixed in cold acetone, 1:1 acetone/chloroform and acetone (10 min ea), rinsed in PBS, blocked with Avidin-Biotin Blocking kit, mouse Ig (Vector) and incubated 30 min with rat CD31 (1:125, PharMingen) and mouse TSP1 antibodies (1:100, Neomarkers). The slides were washed in PBS and incubated 15 min with donkey anti-rat RhodamineX antibodies (1:200, Jackson Immunoresearch) and biotinylated anti-mouse antibodies (1:200, Vector). Slides were developed with FITC-conjugated Avidin D (20 μg/ml, Vector). Biotinylated anti-rabbit antibodies were applied in blocking solution (1:200, 30 min) and followed by 1 μg/ml Streptavidin-Cy5 (Jackson Immunoresearch). To visualize apoptosis, the sections were evaluated by TUNEL (ApopTag kit, Serologicals).
For AR, 5 μm sections were deparaffinized, rehydrated, washed, antigen retrieved 15 min at 20–25 psi, 100°C in citric buffer pH 6.0 and 20 min at room temperature. Endogenous peroxidase was inhibited with blocking solution (Dako) and AR antibody added (30 min, N-20, Santa Cruz, 1:200) followed by HRP-conjugated anti-rabbit antibodies (30 min). Slides were developed with diaminobenzidine and counterstained with hematoxylin. Nonimmune rabbit serum served as negative control.
For NFκB, the sections after antigen retrieval were blocked with 20% goat serum in PBS, and incubated with mouse mAb for human p65/RelA (Cell Signaling), followed by fluorescent goat anti-mouse Ab (Jackson Immunoresearch). Representative experiments of 4 are shown.
Image quantification
Fluorescent images were obtained using Nikon fluorescent microscope (Diaphot 200) and converted to digital files using MetaMorph software. The same software was used to measure fluorescence intensity and compare the values to DAPI counterstain used as background. CD31-positive structures (MVD) were counted in 10 40× fields using MetaMorph software. Apoptotic cells were quantified in 10 random fields using MetaMorph software.
Statistical analysis
Mean and standard error values were calculated and compared using paired Student's t test and ANOVA. p values < 0.05 were considered significant.
Results
AR induction reduced tumorigenicity
We generated PC-3 cells inducibly expressing wild-type AR (PC3-ARWT) and promiscuous AR T877A46 (PC3-AR877) (Fig. 1a). Induced AR levels were comparable to LNCaP cells (not shown). The AR axis was restored and two AR-dependent genes, U1943 and cytokeratin 847 robustly induced upon AR activation (Fig. 1a). Both wild-type and mutant AR became nuclear in the presence of DHT (Fig. 1a) and induced transcription of the ARE-luciferase reporter (Fig. 1c). AR877 was also activated by Flutamide and progesterone (Fig. 1c). The expression and nuclear localization of AR were induced in vivo in the PC-3 cells implanted in male mice upon Dox treatment (Fig. 2a, insets). However AR re-expression failed to enhance PC-3 growth in response to DHT (Fig. 1d). Moreover, AR(+) cells were less tumorigenic in male mice in the presence of Dox (Fig. 2a). When we used oral Flutamide to block endogenous testosterone, PC3-ARWT regained tumorigenicity while PC3-AR877 did not (Fig. 2b), suggesting that weak activation by Fl was sufficient to suppress tumor growth. Finally, PC3-ARWT cell formed tumors in Dox-treated female mice, obviously lacking endogenous testosterone, but not when they received DHT implants, underscoring the repression by androgen (Fig. 2c).
Figure 1.
Characterization of AR-expressing cells. (a) Inducible expression of AR and regulated genes. PC-3 clones expressing ARWT and AR877 were treated with Dox and DHT, where shown, lysates resolved by SDS-PAGE and Western blots probed for AR (top), AR-dependent U19 (middle) and cytokeratin 8 (K8, bottom). (b) AR expression and localization was examined by IHC in similarly treated cells. (c) AR activity was tested with ARE reporter. Note AR877 activation by DHT (R1887), Flutamide (Fl) and Progesterone (Prg). (d) Growth curves of PC3-ARWT and PC3-AR877 generated in media with DHT, ± Dox.
Figure 2.
The effect of inducible AR expression on the prostate carcinoma in vivo. (a) Male nude mice with flank injections of PC3-V, PC3-ARWT and PC3-AR877 received Dox (▲) to induce AR expression, or plain drinking water (△). The expression and nuclear localization of AR has been confirmed by IHC (insets). Note delayed tumorigenesis by AR(+) cells. (b) Male nude mice were all treated with Dox and received flank injections of tumor cells as in (a). Half of the animals was treated with Fl (•), another half with vehicle (○). Note restored growth of PC3-ARWT by Fl compared to the control and the lack of response to Fl by PC3-AR877. (c) Female nude mice received flank injections of PC3-V and PC3-ARWT, as in (a). The animals received Dox (•) or plain water (○). One half of the animals implanted with PC3-ARWT were given DHT implants. Note the lack of growth inhibition by AR (Dox) in the absence of DHT and the delayed growth in Dox-treated animals bearing DHT implants.
AR(+) PC-3 tumors had lower MVD and higher apoptosis rate
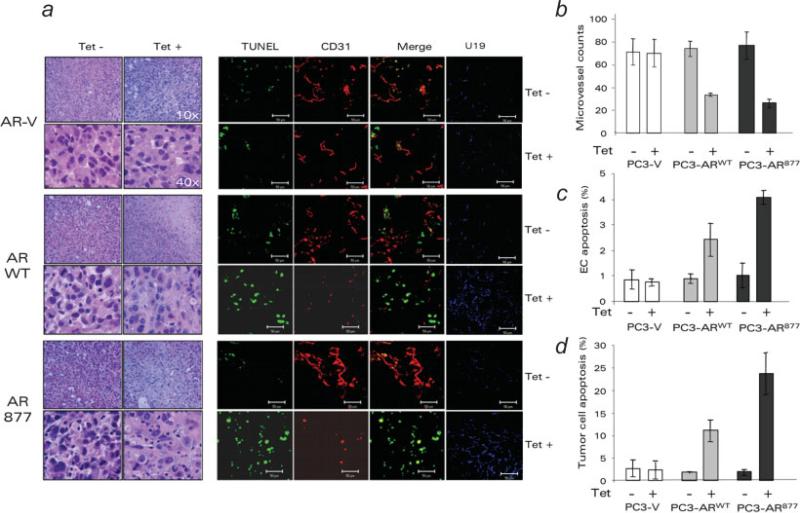
We measured MVD in the AR(+) and AR(–) PC-3 tumors. PC3-ARWT and PC3-AR877 tumors in Dox-treated animals had 2.2–2.6 times lower MVD (p < 0.01) than untreated controls, or the AR(–) controls (Figs. 3a and 3b). TUNEL showed more endothelial and nonendothelial apoptotic cells in the AR(+) PC-3 tumors (Figs. 3a, 3c and 3d). AR remained functional in these tumors: its localization was predominantly nuclear in Dox treated males (Fig. 2a) and, AR responsive protein, U19 was strongly upregulated (Fig. 3a). Thus restoring AR axis in the androgen-insensitive cells delayed tumor progression, lowered MVD and increased apoptosis.
Figure 3.
AR-expressing tumors showed increased apoptosis and decreased MVD. (a) Paraffinized tumor sections were stained with H&E (left) and snap-frozen sections (right) for endothelial marker CD31 (red) and apoptosis (TUNEL, green). To confirm AR functional activity, the same sections were stained for U19 (blue). (b) Quantitative analysis of MVD. (c) Endothelial cell apoptosis calculated as percent of TUNEL-positive of total CD31-positive structures (merge, yellow). (d) Tumor cell apoptosis (total TUNEL-positive cells minus TUNEL positive endothelial cells). All measurements were performed with MetaMorph software.
AR activation upregulated angioinhibitory TSP1
Seeking AR-dependent changes affecting MVD, we investigated angiogenic mediators in AR(+) and AR(–) cells. Three pro-angiogenic cytokines, VEGF, bFGF and IL-8, previously identified in PrCa,36,48–53 remained unaltered. We were unable to detect changes in VEGF mRNA or protein using quantitative RT-PCR, ELISA, or immunostaining (data not shown). TSP1 is a critical angiogenesis inhibitor, whose expression is significantly lower in cancerous compared to the normal prostate51,54; an index integrating TSP1 with angiogenesis independently predicts survival.52 In our model, TSP1 was low in parental PC-3 and PC3-V cells. In PC3-ARWT and PC3-AR877, TSP1 mRNA and secreted protein became high upon Dox/DHT stimulation (Figs. 4a and 4b). In PC3-ARWT Dox/DHT increased activity of the luciferase reporter containing –2033/+150 TSP1 promoter fragment44 (Fig. 4c). Moreover, ChIP demonstrated AR binding to the TSP1 promoter (Fig. 4d).
Figure 4.
AR decreased angiogenesis via angioinhibitory TSP1. (a) RT-PCR detection of TSP1 mRNA in PC3-V, PC3-ARWT and PC3-AR.877 Note decreased TSP1 mRNA by Fl in PC3-ARWT but not AR.877 (b) Western blot of Conditioned Media from PC3-V, PC3-ARWT and PC3-AR877 ± Dox. All cells were DHT-stimulated. (c) The induction of TSP1-Luc reporter in AR(+) and control cells. (d) ChIP of the putative ARE in the 1st intron of TSP1 gene. IC: isotype control. PCR control: cloned TSP1 promoter amplified with the same primers. DNA input is shown. (e) Endothelial cell chemotaxis with the CM from PC3-V, PC3-ARWT and PC3-AR877. The cells were treated with Dox and stimulated with DHT, where indicated and the CM collected and tested at increasing concentrations with the TSP1 neutralizing antibody (•) or with isotype control antibody (IgA) (○). Note the that CM from AR(+) cells are less potent at inducing endothelial cell chemotaxis, and that TSP1 neutralizing antibody, but not isotype control improves migration (arrowheads). (f) Immunostaining of the PC3-V, PC3-ARWT and PC3-AR877 tumors in control and Dox-treated male mice. Cryosections were stained for CD31 (red) and TSP1 (green).
TSP1 suppressed angiogenesis in AR(+) cells
The migration of endothelial cells up the gradient of angiogenic factors is an important component of angiogenesis and an indicator of angiogenic activity of a given cell line.55 The majority of natural inhibitors block endothelial cell chemotaxis induced by VEGF or by bFGF. To determine if TSP1 was responsible for the decrease of angiogenesis in AR(+) tumors, we examined endothelial cell chemotaxis to CM from the PC3-V and PC3-AR. PC3-V CM induced migration, with or without Dox and/or DHT, with EC50 = 2.4 μg/ml. CM from nonstimulated PC3-ARWT and PC3-AR877 were also angiogenic, with similar EC50 (1.9–2.2 μg/ml), and not significantly altered by TSP1 antibodies (Fig. 4e). However, CM from PC3-ARWT and PC3-AR877 stimulated to express AR and activated with DHT became less angiogenic (EC50 > 10 μg/ml). This lower angiogenic activity was due to TSP1, since TSP1 neutralizing antibody restored angiogenic activity (Fig. 4e). IHC showed MVD reduction in AR(+) tumors, paralleled by a dramatic increase in TSP1 (Fig. 4f), pointing to a similar course of events in vivo.
AR activation lowered NFκB levels and activity
Seeking reasons for the decreased viability/increased apoptosis in AR(+) tumors we investigated NFκB status of AR(+) and (–) PC-3 populations. Constitutive NFκB activation and subsequent Bcl-2 increase mark hormone refractory PrCa.42,56,57 Conversely, AR and NFκB counteract in transcription assays.58 Indeed, reporter assays showed high basal NFκB activity in PC-3 cells, which was decreased upon transient transfection with ARWT and diminished further by DHT (Fig. 5a, left). Moreover, ARE-Luc reporter activity, moderate in PC3 transfected with ARWT, doubled in DHT-treated cells when NFκB was blocked with dnIκB-α (Fig. 5a, center). Conversely in AR-sensitive LNCaP p50/p65 dramatically reduced ARE-Luc transactivation, with or without DHT (Fig. 5a, right). EMSA showed that DHT significantly reduced NFκB DNA binding in PC3-AR cells (Fig. 5b). NFκB is chiefly regulated via cytoplasmic retention by IκB-α. However, the IκB-α levels in the AR(+) cells showed only modest increase, after Dox treatment (Fig. 5c) Unexpectedly, DHT significantly decreased the RelA mRNA in PC3-AR but not in PC3-V cells (Fig. 5d).
Figure 5.
The interference between AR and NFκB. (a) Reporter assays in cells transfected with the combination of NFκB-Luc, ARE-Luc, AR or p65/p50. pcDNA-neo was used to equalize the total DNA input. DHT (black bars) or control vehicle (ethanol: gray bars), where added, where indicated. Note decreased NFκB activity in the PC3-AR and increased AR activity in the presence of dnIκBα (NFκB superrepressor). (b) PC3-ARWT cells were treated with Dox or Dox/DHT, nuclear extracts collected and analyzed by EMSA. Cold probe (Comp) or IKK inhibitor, BMS345543 (10 μM, BMS) added where indicated. (c) Total IκB-α in Dox/DHT stimulated cells. PC3-V -ARWT and -AR877 cells were treated for 24 hours with vehicle ethanol or DHT, as indicated, and IκB-α detected in total cell lysates by Western blot. The blot was re-probed for β-tubulin to assess loading (lower panel). (d) RT-PCR of p65 (RelA) mRNA in similarly treated cells. Note a decrease upon AR activation. (e) PC3-ARWT cells were grown on coverslips, treated as indicated and stained for AR (top) or NFκB (bottom). Note the lack of nuclear NFκB upon DHT stimulation. (f, g) IL-6 levels in Dox/DHT stimulated cells. The cells were treated as in (c), total RNA collected and RT-PCR performed with primers for IL-6 (F). C1, no cDNA; C2, no primers. (g) IL-6 detected by ELISA, in the media conditioned by similarly treated cells. (h) Western blot for Bcl-2.
In addition, in PC3-ARWT and AR877, DHT lowered nuclear p65/RelA (Fig. 5e and data not shown). Nuclear localization of AR and p65 were mutually exclusive: in PC3-ARWT, AR was predominantly cytoplasmic in the absence of DHT, while p65 was mostly nuclear. Conversely, in DHT-treated cells AR was predominantly nuclear, while p65 became cytoplasmic (Fig. 5e).
AR blocked pro-survival NFκB targets
DHT severely decreased the two NFκB targets, IL-6, as was measured at mRNA level and secreted protein (Figs. 5f and 5g), and Bcl2 (Fig. 5h). Both proteins are capable of increasing cell survival.
AR diminished nuclear NFκB and increased apoptosis in vivo
The decrease in active NFκB remained true in vivo. While AR(–) tumors showed NFκB staining in the cytoplasm and nuclei, in AR(+) tumors RelA resided mainly in the cytoplasm (Fig. 6a). Similar to the in vitro results, RelA immunoreactivity was much weaker in AR(+) tumors (Figs. 6a and 6c). Higher incidence of nuclear NFκB was accompanied by low apoptosis rates, while in Dox-treated male mice AR(+) tumors showed less nuclear NFκB and higher apoptosis (Figs. 6b and 6d).
Figure 6.
AR effect on NFκB nuclear localization and apoptosis in vivo. (a, c) Immunostaining of AR(+) (PC3-AR) and control (PC3-V) tumors for p65 (RelA). The mice were given drinking water ± Dox, the resultant tumors stained for RelA (NFκB, red). (a) Representative images of the stained sections. Note nuclear RelA (solid arrowheads) in the absence of Dox and the lack of nuclear staining in Dox-treated tumors (empty arrowheads). (c) Immunofluorescence intensity was measured using MetaMorph software in a minimum of 12 random fields on 3 independent sections. (b, d) Inverse correlation between nuclear NFκB localization and apoptosis. Sections of Dox-treated or untreated (control) AR(+) tumors were stained for RelA (NFκB, red). Apoptosis was visualized by TUNEL (green). (b) Representative images of the stained sections. Upper panels show merged images. Empty arrowheads indicate cells lacking nuclear RelA. (d) TUNEL-positive cells were quantified in 6 random fields, 3 independent sections.
Discussion
Current in vivo models include tumor grafting in syngeneic or immune compromised animals, or autochthonous tumors in genetically manipulated mice. The differences in structure, physiology and cancer progression in mouse and human prostate59 make it essential to complement the findings from genetically altered mice with those from xenografted tumors. Indeed, stroma and the smooth muscle are major structural and functional components in human, but not in mouse prostate. Lobular structure is seen in the mouse but not in human prostate, while mice have no transitional zone, prostatic urethra and capsule.59 Most importantly, prostate cancer does not occur spontaneously in wild-type mice; the majority of mouse models are driven by SV-40 large and small T viral oncogenes. Other suspect oncogenes and tumor suppressors yield intraepithelial neoplasia (PIN) but not PrCa.60 Only three genes have been found critical for prostate carcinogenesis in mice: an oncogenic IGF-1 and cMyc, and a tumor suppressor PTEN.61,62
Surprisingly, AR failed as prostate-specific oncogene in transgenic models, its overexpression yields PIN but no invasive carcinoma.63 In another study, wild-type and promiscuous AR mutant T857A (T877A analogue) fail to induce PIN in young animals suggesting that ligand driven AR activation does not induce epithelial hyperproliferation in the whole prostate.64 Thus androgen role in PrCa is not unequivocal. In normal prostate it likey maintains homeostasis of proliferation vs. apoptosis, while androgen ablation changes AR targets from apoptotic to survival/proliferation. Interestingly, studies form Liao and coworkers demonstrate that AR-positive LNCaP cells, conditioned by long-term androgen withdrawal become hypersensitive to androgen and could be suppressed by androgen in vivo65 and identify decreased cMyc and increased Bax as responsible genes.66 Moreover. LNCaP sublines rendered androgen-independent, could be suppressed by androgen and then reversed to androgen-dependent phenotype.67
According to Greenberg and coworkers, transgenes encoding either AR-WT or AR-T857A, a mouse analog of human T877A mutant, did not cause prostate cancer in mice.64 Consistent with their data, we showed that inducible wild-type and T877A AR failed to expedite tumor progression in a subcutaneous xenograft model, but instead caused dramatic delay in tumor progression, decreased MVD and increased apoptosis. Interestingly, these changes occurred predominantly in vivo. Other investigators observed decreased proliferation upon re-expression of AR,26 however in our hands, AR(+) and (–) cells in vitro grew at the same rate. This difference may be due to the use of inducible AR expression, while stable transfectans may have acquired additional changes due to the constitutive AR overexpression. The molecular effects of AR expression/activation in PC-3 cells were twofold: decreased activation of NFκB, a pro-survival transcription factor in prostate epithelium,40,56,57 and decreased overall angiogenic activity due to increased angioinhibitory TSP1, which translated into AR-dependent decrease of tumor MVD. The inverse correlation between TSP1 levels and prostate cancer progression and vascularization has been previously shown,33,51,52,54 however TSP1 induction by AR has not been demonstrated.
The crosstalk between AR and NFκB has been previously shown in vitro, where NFκB inactivation resulted in higher apoptosis rates.25,68 However, others indicate that AR also may increase NFκB activity.69,70 Despite NFκB blockade, AR expression failed to increase apoptosis in vitro. Increased tumor apoptosis in vivo suggests that NFκB deactivation lowered the survival of AR(+) cells under stress. This is consistent with potentiated response to genotoxic stress by AR.24 In our system AR(+) cells low in NFκB activity, become apoptotic in response to hypoxia due to insufficient angiogenesis. Conversely, AR(–) cells remain resistant. In addition, NFκB may contribute to the angiogenic properties of prostate epithelium by increasing NOS and cyclooxygenase-271: its inactivation would further reduce tumor MVD.
It is widely accepted that functional AR is expressed in a large portion of advanced prostate cancers. However the majority of AR pathway genes (HERPUD1, STK39, DHCR24, and SOCS2) are suppressed in metastatic prostate cancer,72 underscoring the fact that many of the AR targets counter cancer progression.
Our study indicates that both wild-type AR and AR with altered ligand specificity, lack the ability to transform prostate epithelium. Conversely, Greenberg and coworkers identified carcinogenic AR mutations in the transactivation domain.73 Interestingly, somatic mutations associated with male infertility are in the DNA and lingand binding domains and the hinge, while ~40% cancer-associated mutations are in the transactivation domain, where they affect cofactor interactions. Although >80% AR point mutations have been identified in cancer specimens, their functional consequences are not verified, except for a few isolated cases. Combined data by Greenberg's group64 and our's suggest that while AR maintains interactions with proper coactivators and corepressors, it continues to control homeostatic proliferation, apoptosis, and angiogenesis. One possible explanation is the release of AR control over NFκB activity: once disrupted, NFκB activation, in turn, favors increased survival, dampens stress responses and favors tumor progression. The mechanism of AR interference with NFκB remains unclear: although weak AR/NFκB interaction was observed in vitro,74 the result has never been reproduced. Other investigators suggest competitive binding to adjacent cis-regulatory elements on the DNA.75 We observed modest IκB-α increase in the AR(+) cells, however higher RelA mRNA and protein levels are more likely to play a role. Indeed, DHT stimulation on the AR(+) tumors produced the decrease in general NFκB immunoreactivity.
Our results suggest that persistent androgen ablation promotes the progression to androgen independent phenotype and indicate possible benefits of the treatment where androgen application and ablation are used in succession or intermittently.
Acknowledgements
We thank Dr. Wang and Dr. Levenson for helpful discussion.
Grant sponsor: NIH; Grant number: 1R01 HL077471-01; Grant sponsor: CDA, Northwestern University Prostate SPORE; Grant number: 5P50 CA90386; Grant sponsor: DOD PCRP; Grant number: DAMD17-03-1-0522; Grant sponsor: PPA, Northwestern University Prostate SPORE; Grant number: 5P50 CA90386.
References
- 1.Scott WW, Menon M, Walsh PC. Hormonal therapy of prostatic cancer. Cancer. 1980;45(7 Suppl):1929–36. [PubMed] [Google Scholar]
- 2.Denis L, Murphy GP. Overview of phase III trials on combined androgen treatment in patients with metastatic prostate cancer. Cancer. 1993;72(12 Suppl):3888–95. doi: 10.1002/1097-0142(19931215)72:12+<3888::aid-cncr2820721726>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Heinlein CA, Chang C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol Endocrinol. 2002;16:2181–7. doi: 10.1210/me.2002-0070. [DOI] [PubMed] [Google Scholar]
- 4.Zegarra-Moro OL, Schmidt LJ, Huang H, Tindall DJ. Disruption of androgen receptor function inhibits proliferation of androgen-refractory prostate cancer cells. Cancer Res. 2002;62:1008–13. [PubMed] [Google Scholar]
- 5.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5:280–5. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 6.Smith PC, Hobisch A, Lin DL, Culig Z, Keller ET. Interleukin-6 and prostate cancer progression. Cytokine Growth Factor Rev. 2001;12:33–40. doi: 10.1016/s1359-6101(00)00021-6. [DOI] [PubMed] [Google Scholar]
- 7.Godoy-Tundidor S, Hobisch A, Pfeil K, Bartsch G, Culig Z. Acquisition of agonistic properties of nonsteroidal antiandrogens after treatment with oncostatin M in prostate cancer cells. Clin Cancer Res. 2002;8:2356–61. [PubMed] [Google Scholar]
- 8.Koivisto P, Visakorpi T, Kallioniemi OP. Androgen receptor gene amplification: a novel molecular mechanism for endocrine therapy resistance in human prostate cancer. Scand J Clin Lab Invest. 1996;226:57–63. [PubMed] [Google Scholar]
- 9.Nan B, Snabboon T, Unni E, Yuan XJ, Whang YE, Marcelli M. The PTEN tumor suppressor is a negative modulator of androgen receptor transcriptional activity. J Mol Endocrinol. 2003;31:169–83. doi: 10.1677/jme.0.0310169. [DOI] [PubMed] [Google Scholar]
- 10.Lu ML, Schneider MC, Zheng Y, Zhang X, Richie JP. Caveolin-1 interacts with androgen receptor. A positive modulator of androgen receptor mediated transactivation. J Biol Chem. 2001;276:13442–51. doi: 10.1074/jbc.M006598200. [DOI] [PubMed] [Google Scholar]
- 11.Culig Z, Klocker H, Bartsch G, Steiner H, Hobisch A. Androgen receptors in prostate cancer. J Urol. 2003;170(4 Part 1):1363–9. doi: 10.1097/01.ju.0000075099.20662.7f. [DOI] [PubMed] [Google Scholar]
- 12.Hirawat S, Budman DR, Kreis W. The androgen receptor: structure, mutations, and antiandrogens. Cancer Invest. 2003;21:400–17. doi: 10.1081/cnv-120018232. [DOI] [PubMed] [Google Scholar]
- 13.Huang H, Tindall DJ. The role of the androgen receptor in prostate cancer. Crit Rev Eukaryot Gene Expr. 2002;12:193–207. doi: 10.1615/critreveukaryotgeneexpr.v12.i3.30. [DOI] [PubMed] [Google Scholar]
- 14.Culig Z, Klocker H, Eberle J, Kaspar F, Hobisch A, Cronauer MV, Bartsch G. DNA sequence of the androgen receptor in prostatic tumor cell lines and tissue specimens assessed by means of the polymerase chain reaction. Prostate. 1993;22:11–22. doi: 10.1002/pros.2990220103. [DOI] [PubMed] [Google Scholar]
- 15.Tilley WD, Buchanan G, Hickey TE, Bentel JM. Mutations in the androgen receptor gene are associated with progression of human prostate cancer to androgen independence. Clin Cancer Res. 1996;2:277–85. [PubMed] [Google Scholar]
- 16.Hyytinen ER, Haapala K, Thompson J, Lappalainen I, Roiha M, Rantala I, Helin HJ, Janne OA, Vihinen M, Palvimo JJ, Koivisto PA. Pattern of somatic androgen receptor gene mutations in patients with hormone-refractory prostate cancer. Lab Invest. 2002;82:1591–8. doi: 10.1097/01.lab.0000038924.67707.75. [DOI] [PubMed] [Google Scholar]
- 17.Marcelli M, Ittmann M, Mariani S, Sutherland R, Nigam R, Murthy L, Zhao Y, DiConcini D, Puxeddu E, Esen A, Eastham J, Weigel NL, et al. Androgen receptor mutations in prostate cancer. Cancer Res. 2000;60:944–9. [PubMed] [Google Scholar]
- 18.Kokontis JM, Hay N, Liao S. Progression of LNCaP prostate tumor cells during androgen deprivation: hormone-independent growth, repression of proliferation by androgen, and role for p27Kip1 in androgen-induced cell cycle arrest. Mol Endocrinol. 1998;12:941–53. doi: 10.1210/mend.12.7.0136. [DOI] [PubMed] [Google Scholar]
- 19.Lu S, Tsai SY, Tsai MJ. Regulation of androgen-dependent prostatic cancer cell growth: androgen regulation of CDK2, CDK4, and CKI p16 genes. Cancer Res. 1997;57:4511–6. [PubMed] [Google Scholar]
- 20.Culig Z, Hobisch A, Herold M, Hittmair A, Thurnher M, Eder IE, Cronauer MV, Rieser C, Ramoner R, Bartsch G, Klocker H, Konwalinka G. Interleukin 1b mediates the modulatory effects of monocytes on LNCaP human prostate cancer cells. Br J Cancer. 1998;78:1004–11. doi: 10.1038/bjc.1998.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell SH, Zhu W, Young CY. Resveratrol inhibits the expression and function of the androgen receptor in LNCaP prostate cancer cells. Cancer Res. 1999;59:5892–5. [PubMed] [Google Scholar]
- 22.Berger R, Febbo PG, Majumder PK, Zhao JJ, Mukherjee S, Signoretti S, Campbell KT, Sellers WR, Roberts TM, Loda M, Golub TR, Hahn WC. Androgen-induced differentiation and tumorigenicity of human prostate epithelial cells. Cancer Res. 2004;64:8867–75. doi: 10.1158/0008-5472.CAN-04-2938. [DOI] [PubMed] [Google Scholar]
- 23.Whitacre DC, Chauhan S, Davis T, Gordon D, Cress AE, Miesfeld RL. Androgen induction of in vitro prostate cell differentiation. Cell Growth Differ. 2002;13:1–11. [PubMed] [Google Scholar]
- 24.Heisler LE, Evangelou A, Lew AM, Trachtenberg J, Elsholtz HP, Brown TJ. Androgen-dependent cell cycle arrest and apoptotic death in PC-3 prostatic cell cultures expressing a full-length human androgen receptor. Mol Cell Endocrinol. 1997;126:59–73. doi: 10.1016/s0303-7207(96)03970-6. [DOI] [PubMed] [Google Scholar]
- 25.Altuwaijri S, Lin HK, Chuang KH, Lin WJ, Yeh S, Hanchett LA, Rahman MM, Kang HY, Tsai MY, Zhang Y, Yang L, Chang C. Interruption of nuclear factor κB signaling by the androgen receptor facilitates 12-O-tetradecanoylphorbolacetate-induced apoptosis in androgen-sensitive prostate cancer LNCaP cells. Cancer Res. 2003;63:7106–12. [PubMed] [Google Scholar]
- 26.Yuan S, Trachtenberg J, Mills GB, Brown TJ, Xu F, Keating A. Androgen-induced inhibition of cell proliferation in an androgen-insensitive prostate cancer cell line (PC-3) transfected with a human androgen receptor complementary DNA. Cancer Res. 1993;53:1304–11. [PubMed] [Google Scholar]
- 27.Lu S, Liu M, Epner DE, Tsai SY, Tsai MJ. Androgen regulation of the cyclin-dependent kinase inhibitor p21 gene through an androgen response element in the proximal promoter. Mol Endocrinol. 1999;13:376–84. doi: 10.1210/mend.13.3.0254. [DOI] [PubMed] [Google Scholar]
- 28.Grierson AJ, Shaw CE, Miller CC. Androgen induced cell death in SHSY5Y neuroblastoma cells expressing wild-type and spinal bulbar muscular atrophy mutant androgen receptors. Biochim Biophys Acta. 2001;1536:13–20. doi: 10.1016/s0925-4439(01)00029-1. [DOI] [PubMed] [Google Scholar]
- 29.Lin HK, Yeh S, Kang HY, Chang C. Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor. Proc Natl Acad Sci USA. 2001;98:7200–5. doi: 10.1073/pnas.121173298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fu M, Wang C, Wang J, Zhang X, Sakamaki T, Yeung YG, Chang C, Hopp T, Fuqua SA, Jaffray E, Hay RT, Palvimo JJ, et al. Androgen receptor acetylation governs trans activation and MEKK1-induced apoptosis without affecting in vitro sumoylation and trans-repression function. Mol Cell Biol. 2002;22:3373–88. doi: 10.1128/MCB.22.10.3373-3388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sigala S, Tognazzi N, Rizzetti MC, Faraoni I, Missale C, Bonmassar E, Spano P. Nerve growth factor induces the re-expression of functional androgen receptors and p75(NGFR) in the androgen-insensitive prostate cancer cell line DU145. Eur J Endocrinol. 2002;147:407–15. doi: 10.1530/eje.0.1470407. [DOI] [PubMed] [Google Scholar]
- 32.Hess-Wilson JK, Daly HK, Zagorski WA, Montville CP, Knudsen KE. Mitogenic action of the androgen receptor sensitizes prostate cancer cells to taxane-based cytotoxic insult. Cancer Res. 2006;66:11998–2008. doi: 10.1158/0008-5472.CAN-06-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Moorselaar RJ, Voest EE. Angiogenesis in prostate cancer: its role in disease progression and possible therapeutic approaches. Mol Cell Endocrinol. 2002;197:239–50. doi: 10.1016/s0303-7207(02)00262-9. [DOI] [PubMed] [Google Scholar]
- 34.Franck-Lissbrant I, Haggstrom S, Damber JE, Bergh A. Testosterone stimulates angiogenesis and vascular regrowth in the ventral prostate in castrated adult rats. Endocrinology. 1998;139:451–6. doi: 10.1210/endo.139.2.5683. [DOI] [PubMed] [Google Scholar]
- 35.Lissbrant IF, Lissbrant E, Persson A, Damber JE, Bergh A. Endothelial cell proliferation in male reproductive organs of adult rat is high and regulated by testicular factors. Biol Reprod. 2003;68:1107–11. doi: 10.1095/biolreprod.102.008284. [DOI] [PubMed] [Google Scholar]
- 36.Mabjeesh NJ, Willard MT, Frederickson CE, Zhong H, Simons JW. Androgens stimulate hypoxia-inducible factor 1 activation via auto-crine loop of tyrosine kinase receptor/phosphatidylinositol 3′-kinase/protein kinase B in prostate cancer cells. Clin Cancer Res. 2003;9:2416–25. [PubMed] [Google Scholar]
- 37.Doll JA, Stellmach VM, Bouck NP, Bergh AR, Lee C, Abramson LP, Cornwell ML, Pins MR, Borensztajn J, Crawford SE. Pigment epithelium-derived factor regulates the vasculature and mass of the prostate and pancreas. Nat Med. 2003;9:774–80. doi: 10.1038/nm870. [DOI] [PubMed] [Google Scholar]
- 38.Colombel M, Filleur S, Fournier P, Merle C, Guglielmi J, Courtin A, Degeorges A, Serre CM, Bouvier R, Clezardin P, Cabon F. Androgens repress the expression of the angiogenesis inhibitor thrombospondin-1 in normal and neoplastic prostate. Cancer Res. 2005;65:300–8. [PubMed] [Google Scholar]
- 39.Gasparian AV, Yao YJ, Kowalczyk D, Lyakh LA, Karseladze A, Slaga TJ, Budunova IV. The role of IKK in constitutive activation of NF-κB transcription factor in prostate carcinoma cells. J Cell Sci. 2002;115(Part 1):141–51. doi: 10.1242/jcs.115.1.141. [DOI] [PubMed] [Google Scholar]
- 40.Ghosh S, Karin M. Missing pieces in the NF-κB puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 41.Supakar PC, Jung MH, Song CS, Chatterjee B, Roy AK. Nuclear factor κ B functions as a negative regulator for the rat androgen receptor gene and NF-κ B activity increases during the age-dependent desensitization of the liver. J Biol Chem. 1995;270:837–42. doi: 10.1074/jbc.270.2.837. [DOI] [PubMed] [Google Scholar]
- 42.Shukla S, Maclennan GT, Marengo SR, Resnick MI, Gupta S. Constitutive activation of P I3 K-Akt and NF-κB during prostate cancer progression in autochthonous transgenic mouse model. Prostate. 2005;64:224–39. doi: 10.1002/pros.20217. [DOI] [PubMed] [Google Scholar]
- 43.Xiao W, Zhang Q, Jiang F, Pins M, Kozlowski JM, Wang Z. Suppression of prostate tumor growth by U19, a novel testosterone-regulated apoptosis inducer. Cancer Res. 2003;63:4698–704. [PubMed] [Google Scholar]
- 44.Framson P, Bornstein P. A serum response element and a binding site for NF-Y mediate the serum response of the human thrombospondin 1 gene. J Biol Chem. 1993;268:4989–96. [PubMed] [Google Scholar]
- 45.Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, Bouck NP. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA. 1990;87:6624–8. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Culig Z, Hobisch A, Bartsch G, Klocker H. Androgen receptor—an update of mechanisms of action in prostate cancer. Urol Res. 2000;28:211–9. doi: 10.1007/s002400000111. [DOI] [PubMed] [Google Scholar]
- 47.Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57:3325–30. [PubMed] [Google Scholar]
- 48.Ferrer FA, Miller LJ, Andrawis RI, Kurtzman SH, Albertsen PC, Laudone VP, Kreutzer DL. Angiogenesis and prostate cancer: in vivo and in vitro expression of angiogenesis factors by prostate cancer cells. Urology. 1998;51:161–7. doi: 10.1016/s0090-4295(97)00491-3. [DOI] [PubMed] [Google Scholar]
- 49.Strohmeyer D, Rossing C, Bauerfeind A, Kaufmann O, Schlechte H, Bartsch G, Loening S. Vascular endothelial growth factor and its correlation with angiogenesis and p53 expression in prostate cancer. Prostate. 2000;45:216–24. doi: 10.1002/1097-0045(20001101)45:3<216::aid-pros3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 50.Meyer GE, Yu E, Siegal JA, Petteway JC, Blumenstein BA, Brawer MK. Serum basic fibroblast growth factor in men with and without prostate carcinoma. Cancer. 1995;76:2304–11. doi: 10.1002/1097-0142(19951201)76:11<2304::aid-cncr2820761119>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 51.Doll JA, Reiher FK, Crawford SE, Pins MR, Campbell SC, Bouck NP. Thrombospondin-1, vascular endothelial growth factor and fibro-blast growth factor-2 are key functional regulators of angiogenesis in the prostate. Prostate. 2001;49:293–305. doi: 10.1002/pros.10025. [DOI] [PubMed] [Google Scholar]
- 52.Kwak C, Jin RJ, Lee C, Park MS, Lee SE. Thrombospondin-1, vascular endothelial growth factor expression and their relationship with p53 status in prostate cancer and benign prostatic hyperplasia. BJU Int. 2002;89:303–9. doi: 10.1046/j.1464-4096.2001.01417.x. [DOI] [PubMed] [Google Scholar]
- 53.Burchardt M, Burchardt T, Chen MW, Hayek OR, Knight C, Shabsigh A, de L. Taille A, Buttyan R. Vascular endothelial growth factor-A expression in the rat ventral prostate gland and the early effects of castration. Prostate. 2000;43:184–94. doi: 10.1002/(sici)1097-0045(20000515)43:3<184::aid-pros4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 54.Mehta R, Kyshtoobayeva A, Kurosaki T, Small EJ, Kim H, Stroup R, McLaren CE, Li KT, Fruehauf JP. Independent association of angiogenesis index with outcome in prostate cancer. Clin Cancer Res. 2001;7:81–8. [PubMed] [Google Scholar]
- 55.Polverini PJ, Bouck NP, Rastinejad F. Assay and purification of naturally occurring inhibitor of angiogenesis. Methods Enzymol. 1991;198:440–50. doi: 10.1016/0076-6879(91)98044-7. [DOI] [PubMed] [Google Scholar]
- 56.Sweeney C, Li L, Shanmugam R, Bhat-Nakshatri P, Jayaprakasan V, Baldridge LA, Gardner T, Smith M, Nakshatri H, Cheng L. Nuclear factor-κB is constitutively activated in prostate cancer in vitro and is overexpressed in prostatic intraepithelial neoplasia and adenocarcinoma of the prostate. Clin Cancer Res. 2004;10:5501–7. doi: 10.1158/1078-0432.CCR-0571-03. [DOI] [PubMed] [Google Scholar]
- 57.Fradet V, Lessard L, Begin LR, Karakiewicz P, Masson AM, Saad F. Nuclear factor-κB nuclear localization is predictive of biochemical recurrence in patients with positive margin prostate cancer. Clin Cancer Res. 2004;10:8460–4. doi: 10.1158/1078-0432.CCR-04-0764. [DOI] [PubMed] [Google Scholar]
- 58.Keller ET, Chang C, Ershler WB. Inhibition of NFκB activity through maintenance of IκBα levels contributes to dihydrotestosterone-mediated repression of the interleukin-6 promoter. J Biol Chem. 1996;271:26267–75. doi: 10.1074/jbc.271.42.26267. [DOI] [PubMed] [Google Scholar]
- 59.Roy-Burman P, Wu H, Powell WC, Hagenkord J, Cohen MB. Genetically defined mouse models that mimic natural aspects of human prostate cancer development. Endocr Relat Cancer. 2004;11:225–54. doi: 10.1677/erc.0.0110225. [DOI] [PubMed] [Google Scholar]
- 60.Kasper S. Survey of genetically engineered mouse models for prostate cancer: analyzing the molecular basis of prostate cancer development, progression, and metastasis. J Cell Biochem. 2005;94:279–97. doi: 10.1002/jcb.20339. [DOI] [PubMed] [Google Scholar]
- 61.DiGiovanni J, Kiguchi K, Frijhoff A, Wilker E, Bol DK, Beltran L, Moats S, Ramirez A, Jorcano J, Conti C. Deregulated expression of insulin-like growth factor 1 in prostate epithelium leads to neoplasia in transgenic mice. Proc Natl Acad Sci USA. 2000;97:3455–60. doi: 10.1073/pnas.97.7.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kwabi-Addo B, Giri D, Schmidt K, Podsypanina K, Parsons R, Greenberg N, Ittmann M. Haploinsufficiency of the Pten tumor suppressor gene promotes prostate cancer progression. Proc Natl Acad Sci USA. 2001;98:11563–8. doi: 10.1073/pnas.201167798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stanbrough M, Leav I, Kwan PW, Bubley GJ, Balk SP. Prostatic intraepithelial neoplasia in mice expressing an androgen receptor transgene in prostate epithelium. Proc Natl Acad Sci USA. 2001;98:10823–8. doi: 10.1073/pnas.191235898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Han G, Buchanan G, Ittmann M, Harris JM, Yu X, Demayo FJ, Tilley W, Greenberg NM. Mutation of the androgen receptor causes oncogenic transformation of the prostate. Proc Natl Acad Sci USA. 2005;102:1151–6. doi: 10.1073/pnas.0408925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Umekita Y, Hiipakka RA, Kokontis JM, Liao S. Human prostate tumor growth in athymic mice: inhibition by androgens and stimulation by finasteride. Proc Natl Acad Sci USA. 1996;93:11802–7. doi: 10.1073/pnas.93.21.11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin Y, Kokontis J, Tang F, Godfrey B, Liao S, Lin A, Chen Y, Xiang J. Androgen and its receptor promote Bax-mediated apoptosis. Mol Cell Biol. 2006;26:1908–16. doi: 10.1128/MCB.26.5.1908-1916.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chuu CP, Hiipakka RA, Fukuchi J, Kokontis JM, Liao S. Androgen causes growth suppression and reversion of androgen-independent prostate cancer xenografts to an androgen-stimulated phenotype in athymic mice. Cancer Res. 2005;65:2082–4. doi: 10.1158/0008-5472.CAN-04-3992. [DOI] [PubMed] [Google Scholar]
- 68.Norata GD, Tibolla G, Seccomandi PM, Poletti A, Catapano AL. Dihydrotestosterone decreases tumor necrosis factor-alpha and lipopolysaccharide-induced inflammatory response in human endothelial cells. J Clin Endocrinol Metab. 2006;91:546–54. doi: 10.1210/jc.2005-1664. [DOI] [PubMed] [Google Scholar]
- 69.Death AK, McGrath KC, Sader MA, Nakhla S, Jessup W, Handelsman DJ, Celermajer DS. Dihydrotestosterone promotes vascular cell adhesion molecule-1 expression in male human endothelial cells via a nuclear factor-κB-dependent pathway. Endocrinology. 2004;145:1889–97. doi: 10.1210/en.2003-0789. [DOI] [PubMed] [Google Scholar]
- 70.Lee SO, Lou W, Nadiminty N, Lin X, Gao AC. Requirement for NF-(κ)B in interleukin-4-induced androgen receptor activation in prostate cancer cells. Prostate. 2005;64:160–7. doi: 10.1002/pros.20218. [DOI] [PubMed] [Google Scholar]
- 71.Chiarugi V, Magnelli L, Chiarugi A, Gallo O. Hypoxia induces pivotal tumor angiogenesis control factors including p53, vascular endothelial growth factor and the NFκB-dependent inducible nitric oxide synthase and cyclooxygenase-2. J Cancer Res Clin Oncol. 1999;125:525–8. doi: 10.1007/s004320050312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hendriksen PJ, Dits NF, Kokame K, Veldhoven A, van Weerden WM, Bangma CH, Trapman J, Jenster G. Evolution of the androgen receptor pathway during progression of prostate cancer. Cancer Res. 2006;66:5012–20. doi: 10.1158/0008-5472.CAN-05-3082. [DOI] [PubMed] [Google Scholar]
- 73.Han G, Foster BA, Mistry S, Buchanan G, Harris JM, Tilley WD, Greenberg NM. Hormone status selects for spontaneous somatic androgen receptor variants that demonstrate specific ligand and cofactor dependent activities in autochthonous prostate cancer. J Biol Chem. 2001;276:11204–13. doi: 10.1074/jbc.M008207200. [DOI] [PubMed] [Google Scholar]
- 74.Palvimo JJ, Reinikainen P, Ikonen T, Kallio PJ, Moilanen A, Janne OA. Mutual transcriptional interference between RelA and androgen receptor. J Biol Chem. 1996;271:24151–6. doi: 10.1074/jbc.271.39.24151. [DOI] [PubMed] [Google Scholar]
- 75.Cinar B, Yeung F, Konaka H, Mayo MW, Freeman MR, Zhau HE, Chung LW. Identification of a negative regulatory cis-element in the enhancer core region of the prostate-specific antigen promoter: implications for intersection of androgen receptor and nuclear factor-κB signalling in prostate cancer cells. Biochem J. 2004;379(Part 2):421–31. doi: 10.1042/BJ20031661. [DOI] [PMC free article] [PubMed] [Google Scholar]