Abstract
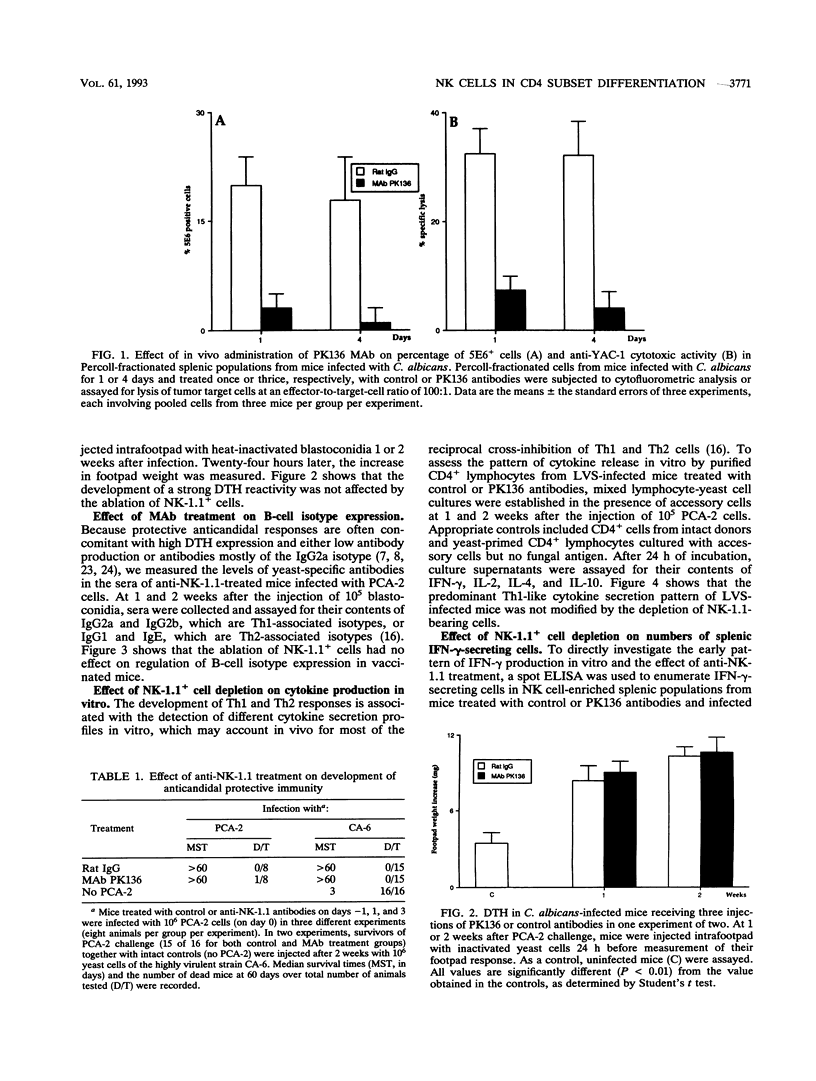
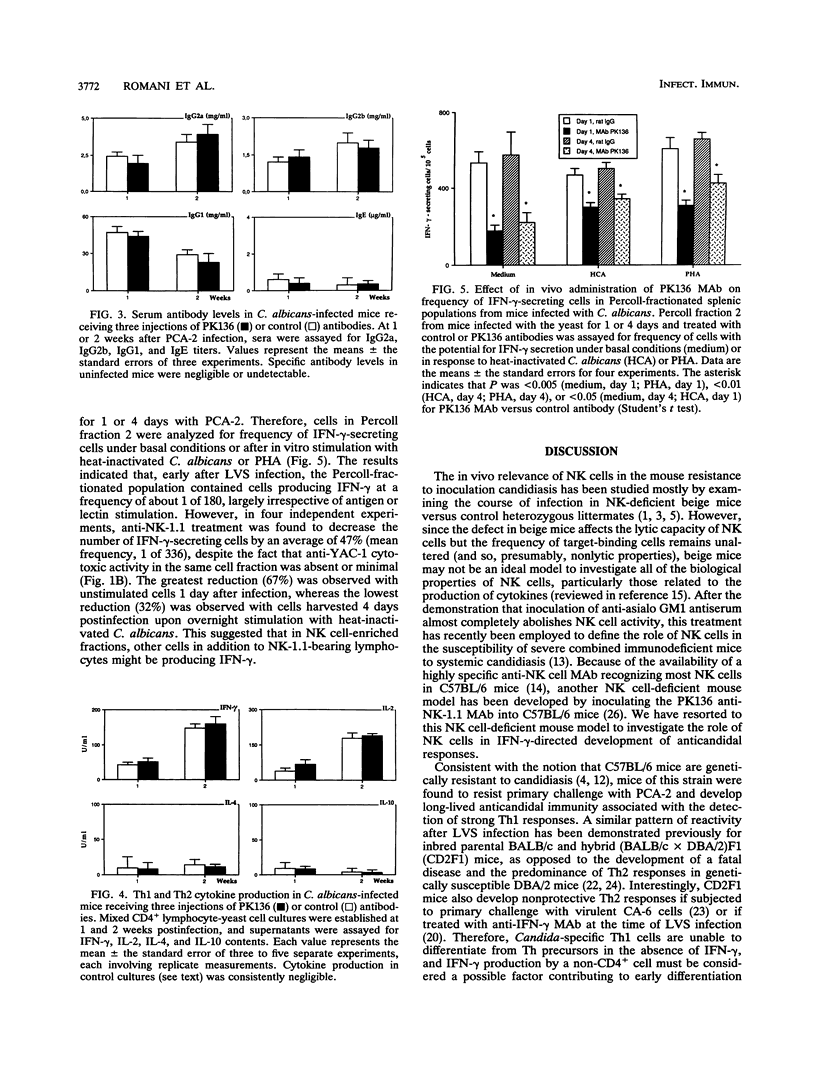
The effects of in vivo administration of monoclonal antibodies against NK-1.1-bearing cells on the early production of gamma interferon (IFN-gamma) in vitro and development of Th1-associated immunity were studied in mice infected with a live vaccine strain of Candida albicans. At 1 and 4 days postinfection, natural killer (NK) cell-enriched fractions from the spleens of antibody-treated mice displayed a dramatic reduction in 5E6+ lymphocytes and negligible anti-YAC-1 cytotoxic activity in vitro. Nevertheless, the frequency of IFN-gamma-producing cells in those fractions was reduced by less than half, on average, by anti-NK-1.1 treatment in vivo. In addition, the antibody-treated and infected mice demonstrated unchanged T helper cell responses, as measured by yeast-specific footpad reactions, resistance to reinfection, occurrence of antibodies of different isotypes, and production in vitro of interleukin-2 (IL-2), IFN-gamma, IL-4, and IL-10 by CD4+ cells. Therefore, although NK cells may contribute to early IFN-gamma production in Candida-vaccinated mice, these cells apparently do not play a dominant role in the qualitative development of yeast-specific T helper responses.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashman R. B., Papadimitriou J. M. Susceptibility of beige mutant mice to candidiasis may be linked to a defect in granulocyte production by bone marrow stem cells. Infect Immun. 1991 Jun;59(6):2140–2146. doi: 10.1128/iai.59.6.2140-2146.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarini M., Bistoni F., Puccetti P., Garaci E. Natural cell-mediated cytotoxicity against Candida albicans induced by cyclophosphamide: nature of the in vitro cytotoxic effector. Infect Immun. 1983 Oct;42(1):1–9. doi: 10.1128/iai.42.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghian A., Lee K. W. Systemic candidosis in beige mice. J Med Vet Mycol. 1989;27(1):51–55. doi: 10.1080/02681218980000071. [DOI] [PubMed] [Google Scholar]
- Beno D. W., Mathews H. L. Growth inhibition of Candida albicans by interleukin-2-activated splenocytes. Infect Immun. 1992 Mar;60(3):853–863. doi: 10.1128/iai.60.3.853-863.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna M. T., Balish E. Acquired immunity to systemic candidiasis in immunodeficient mice. J Infect Dis. 1991 Nov;164(5):936–943. doi: 10.1093/infdis/164.5.936. [DOI] [PubMed] [Google Scholar]
- Cenci E., Romani L., Mencacci A., Spaccapelo R., Schiaffella E., Puccetti P., Bistoni F. Interleukin-4 and interleukin-10 inhibit nitric oxide-dependent macrophage killing of Candida albicans. Eur J Immunol. 1993 May;23(5):1034–1038. doi: 10.1002/eji.1830230508. [DOI] [PubMed] [Google Scholar]
- Cenci E., Romani L., Vecchiarelli A., Puccetti P., Bistoni F. Role of L3T4+ lymphocytes in protective immunity to systemic Candida albicans infection in mice. Infect Immun. 1989 Nov;57(11):3581–3587. doi: 10.1128/iai.57.11.3581-3587.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci E., Romani L., Vecchiarelli A., Puccetti P., Bistoni F. T cell subsets and IFN-gamma production in resistance to systemic candidosis in immunized mice. J Immunol. 1990 Jun 1;144(11):4333–4339. [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K., Halkias D., Friedman H. Growth inhibition of Candida albicans by human polymorphonuclear neutrophils: activation by interferon-gamma and tumor necrosis factor. J Immunol. 1986 Nov 1;137(9):2980–2984. [PubMed] [Google Scholar]
- Djeu J. Y., Blanchard D. K., Richards A. L., Friedman H. Tumor necrosis factor induction by Candida albicans from human natural killer cells and monocytes. J Immunol. 1988 Dec 1;141(11):4047–4052. [PubMed] [Google Scholar]
- Hector R. F., Domer J. E., Carrow E. W. Immune responses to Candida albicans in genetically distinct mice. Infect Immun. 1982 Dec;38(3):1020–1028. doi: 10.1128/iai.38.3.1020-1028.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J., Vazquez-Torres A., Balish E. Poly(I.C)-induced interferons enhance susceptibility of SCID mice to systemic candidiasis. Infect Immun. 1992 Nov;60(11):4549–4557. doi: 10.1128/iai.60.11.4549-4557.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo G. C., Peppard J. R. Establishment of monoclonal anti-Nk-1.1 antibody. Hybridoma. 1984 Fall;3(3):301–303. doi: 10.1089/hyb.1984.3.301. [DOI] [PubMed] [Google Scholar]
- Morelli L., Lusignan Y., Lemieux S. Heterogeneity of natural killer cell subsets in NK-1.1+ and NK-1.1- inbred mouse strains and their progeny. Cell Immunol. 1992 Apr 15;141(1):148–160. doi: 10.1016/0008-8749(92)90134-b. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–147. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- Nabavi N., Murphy J. W. In vitro binding of natural killer cells to Cryptococcus neoformans targets. Infect Immun. 1985 Oct;50(1):50–57. doi: 10.1128/iai.50.1.50-57.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. Induction of TH1 and TH2 responses: a key role for the 'natural' immune response? Immunol Today. 1992 Oct;13(10):379–381. doi: 10.1016/0167-5699(92)90083-J. [DOI] [PubMed] [Google Scholar]
- Romani L., Cenci E., Mencacci A., Spaccapelo R., Grohmann U., Puccetti P., Bistoni F. Gamma interferon modifies CD4+ subset expression in murine candidiasis. Infect Immun. 1992 Nov;60(11):4950–4952. doi: 10.1128/iai.60.11.4950-4952.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L., Mencacci A., Cenci E., Mosci P., Vitellozzi G., Grohmann U., Puccetti P., Bistoni F. Course of primary candidiasis in T cell-depleted mice infected with attenuated variant cells. J Infect Dis. 1992 Dec;166(6):1384–1392. doi: 10.1093/infdis/166.6.1384. [DOI] [PubMed] [Google Scholar]
- Romani L., Mencacci A., Cenci E., Spaccapelo R., Mosci P., Puccetti P., Bistoni F. CD4+ subset expression in murine candidiasis. Th responses correlate directly with genetically determined susceptibility or vaccine-induced resistance. J Immunol. 1993 Feb 1;150(3):925–931. [PubMed] [Google Scholar]
- Romani L., Mencacci A., Grohmann U., Mocci S., Mosci P., Puccetti P., Bistoni F. Neutralizing antibody to interleukin 4 induces systemic protection and T helper type 1-associated immunity in murine candidiasis. J Exp Med. 1992 Jul 1;176(1):19–25. doi: 10.1084/jem.176.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L., Mocci S., Bietta C., Lanfaloni L., Puccetti P., Bistoni F. Th1 and Th2 cytokine secretion patterns in murine candidiasis: association of Th1 responses with acquired resistance. Infect Immun. 1991 Dec;59(12):4647–4654. doi: 10.1128/iai.59.12.4647-4654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L., Mocci S., Cenci E., Rossi R., Puccetti P., Bistoni F. Candida albicans-specific Ly-2+ lymphocytes with cytolytic activity. Eur J Immunol. 1991 Jun;21(6):1567–1570. doi: 10.1002/eji.1830210636. [DOI] [PubMed] [Google Scholar]
- Seaman W. E., Sleisenger M., Eriksson E., Koo G. C. Depletion of natural killer cells in mice by monoclonal antibody to NK-1.1. Reduction in host defense against malignancy without loss of cellular or humoral immunity. J Immunol. 1987 Jun 15;138(12):4539–4544. [PubMed] [Google Scholar]
- Sentman C. L., Hackett J., Jr, Kumar V., Bennett M. Identification of a subset of murine natural killer cells that mediates rejection of Hh-1d but not Hh-1b bone marrow grafts. J Exp Med. 1989 Jul 1;170(1):191–202. doi: 10.1084/jem.170.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varkila K., Chatelain R., Leal L. M., Coffman R. L. Reconstitution of C.B-17 scid mice with BALB/c T cells initiates a T helper type-1 response and renders them capable of healing Leishmania major infection. Eur J Immunol. 1993 Jan;23(1):262–268. doi: 10.1002/eji.1830230141. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli A., Cenci E., Puliti M., Blasi E., Puccetti P., Cassone A., Bistoni F. Protective immunity induced by low-virulence Candida albicans: cytokine production in the development of the anti-infectious state. Cell Immunol. 1989 Dec;124(2):334–344. doi: 10.1016/0008-8749(89)90135-4. [DOI] [PubMed] [Google Scholar]
- Zunino S. J., Hudig D. Interactions between human natural killer (NK) lymphocytes and yeast cells: human NK cells do not kill Candida albicans, although C. albicans blocks NK lysis of K562 cells. Infect Immun. 1988 Mar;56(3):564–569. doi: 10.1128/iai.56.3.564-569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



