Abstract
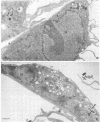
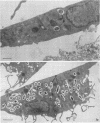
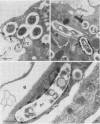
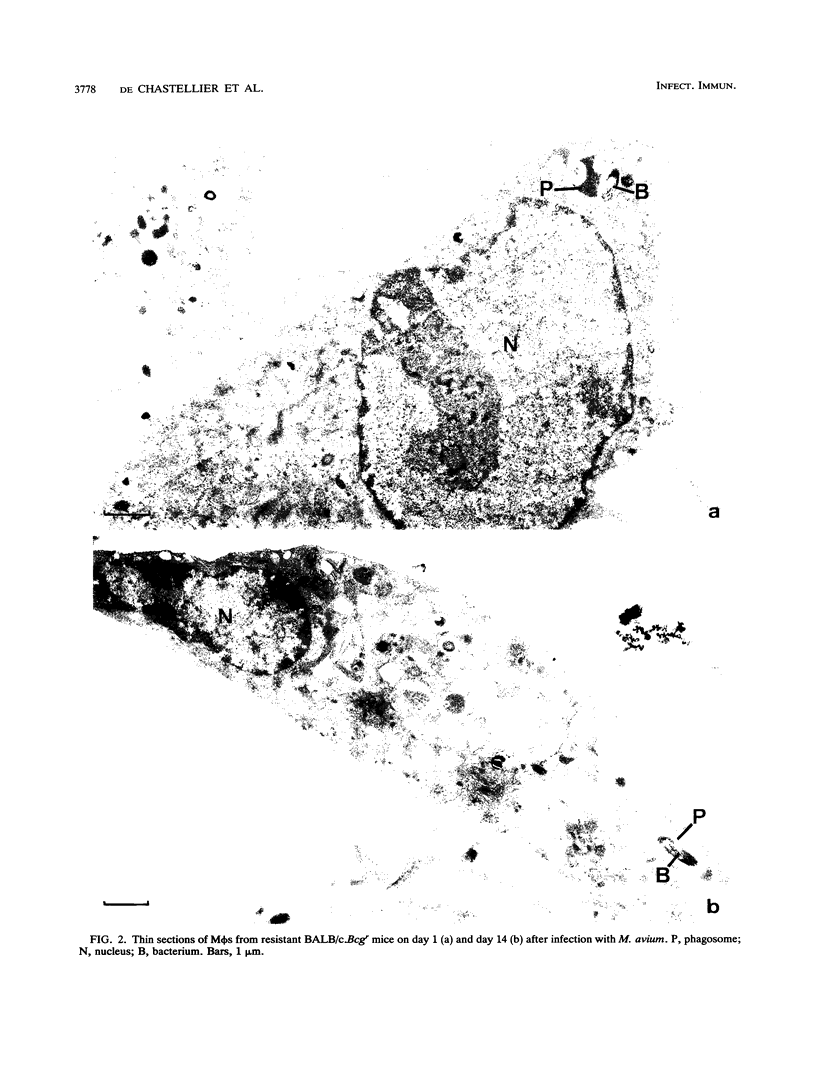
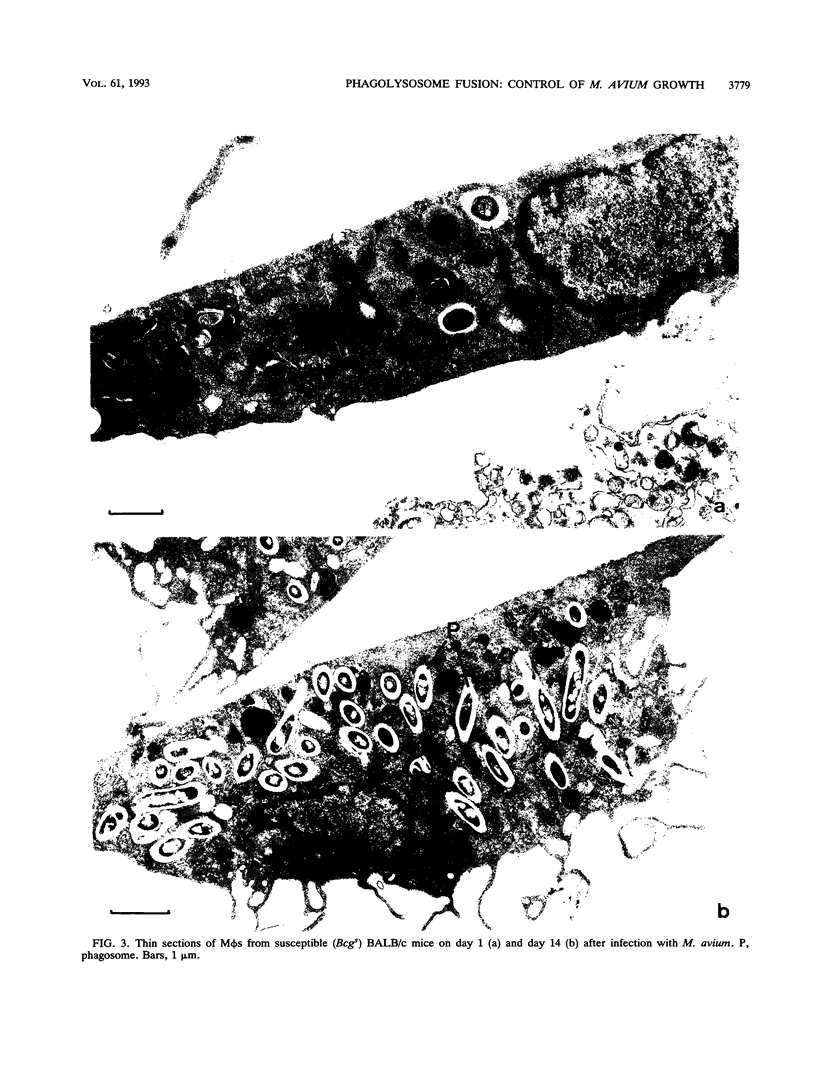
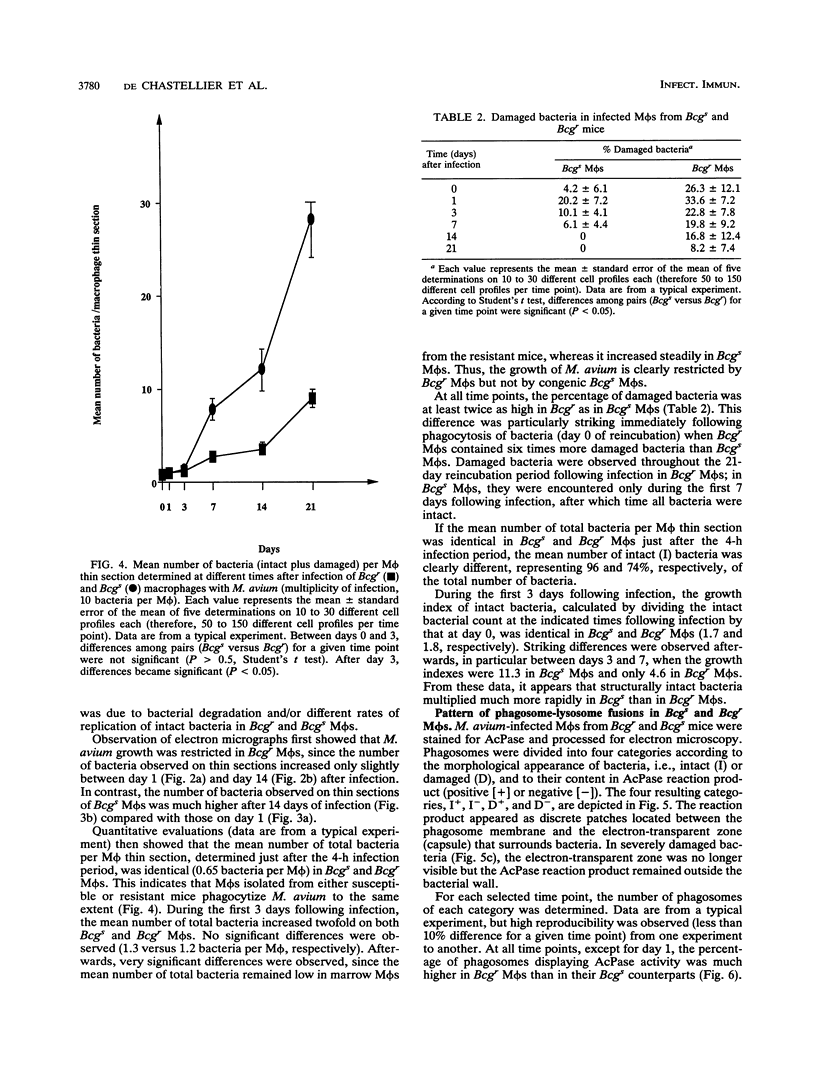
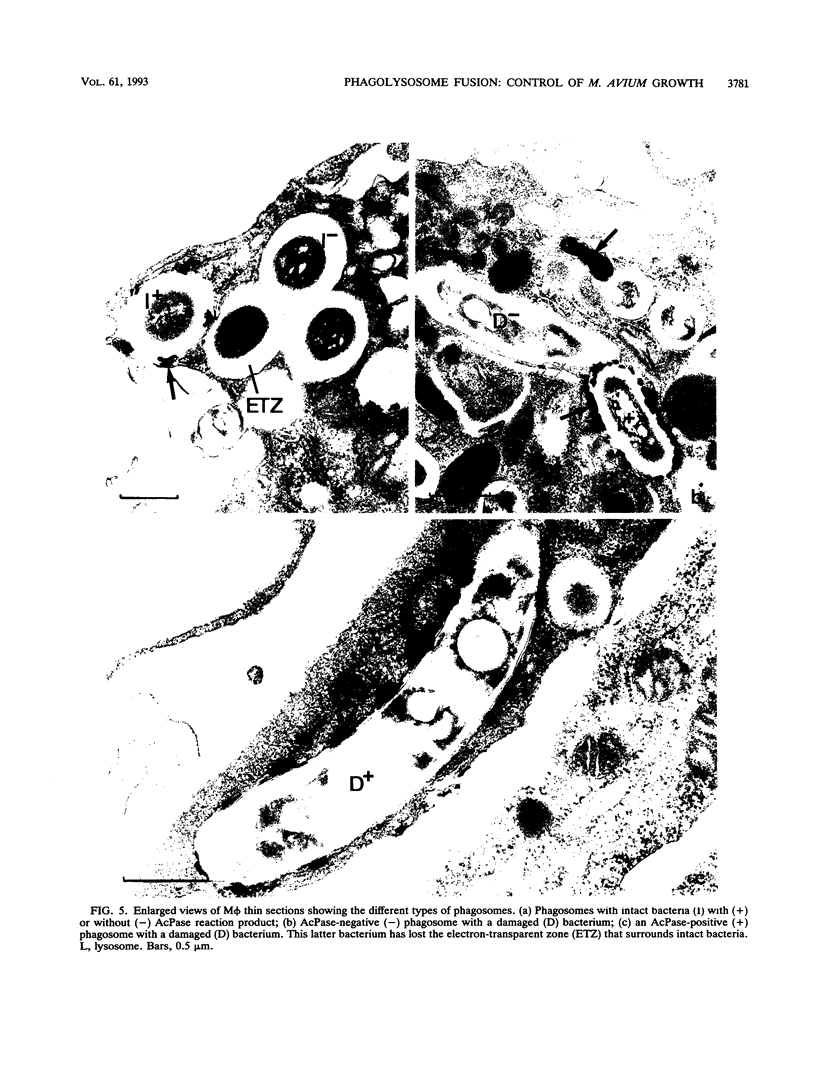
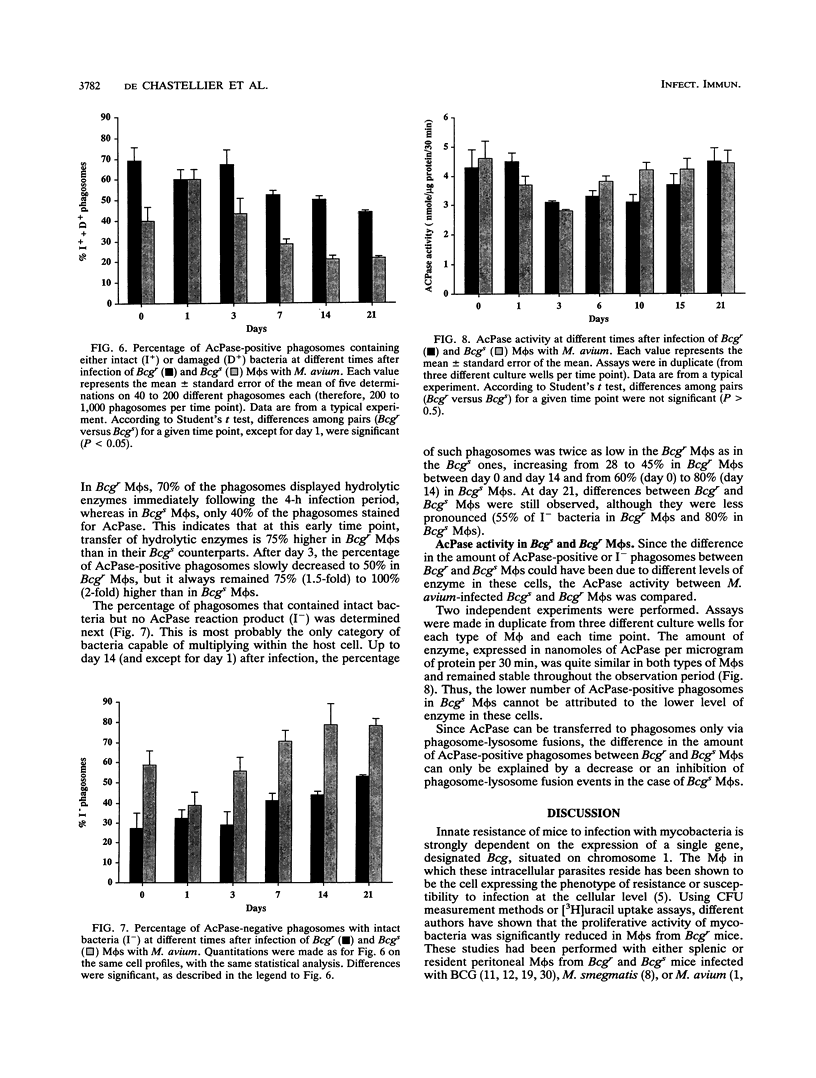
The ability of the host to resist infection to a variety of intracellular pathogens, including mycobacteria, is strongly dependent upon the expression of the Bcg gene. Mouse strains which express the resistance phenotype (Bcgr) restrict bacterial growth, whereas susceptible strains (Bcgs) allow bacterial growth. Expression of the Bcg allele is known to influence the priming of host macrophages (M phi s) for bactericidal function. In the present work, bone marrow-derived M phi s from congenic BALB/c (Bcgs) and C.D2 (BALB/c.Bcgr) mice were infected with the virulent strain Mycobacterium avium TMC 724 to define the mechanism involved in growth restriction of M. avium. By combining CFU measurements and ultrastructural analyses, we show that growth of this bacterium is restricted in marrow M phi s from resistant mice. Using acid phosphatase as a lysosomal marker, we provide evidence that the hydrolytic activity of M phi s, as measured by the capacity of lysosomes to fuse with and transfer active hydrolytic enzymes to phagosomes in which M. avium resides, is an expression of the Bcg gene and that this phenomenon is a key antibacterial activity responsible for growth restriction of M. avium: (i) the percentage of phagosome-lysosome fusions was twice as high in Bcgr M phi s as in Bcgs M phi s, and (ii) the percentage of intact viable bacteria residing in acid phosphatase-negative phagosomes was twice as low in Bcgr M phi s as in the Bcgs counterparts. These differences are not due to a lower activity of the enzyme in Bcgr M phi s. The mechanism by which the Bcg gene exerts control over the phagolysosomal fusion is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelberg R., Sarmento A. M. The role of macrophage activation and of Bcg-encoded macrophage function(s) in the control of Mycobacterium avium infection in mice. Clin Exp Immunol. 1990 Jun;80(3):324–331. doi: 10.1111/j.1365-2249.1990.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S., Enkel H. Interaction of Mycobacterium avium complex with human macrophages: roles of membrane receptors and serum proteins. Infect Immun. 1991 May;59(5):1697–1702. doi: 10.1128/iai.59.5.1697-1702.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Inhibition of macrophage phagosome-lysosome fusion by Salmonella typhimurium. Infect Immun. 1991 Jul;59(7):2232–2238. doi: 10.1128/iai.59.7.2232-2238.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman E., Taniyama T., Nakamura R., Skamene E. Functional expression of the Bcg gene in macrophages. Res Immunol. 1989 Oct;140(8):793–797. doi: 10.1016/0923-2494(89)90035-7. [DOI] [PubMed] [Google Scholar]
- Catanzaro A., Wright S. D. Binding of Mycobacterium avium-Mycobacterium intracellulare to human leukocytes. Infect Immun. 1990 Sep;58(9):2951–2956. doi: 10.1128/iai.58.9.2951-2956.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. Mycobacterial disease, immunosuppression, and acquired immunodeficiency syndrome. Clin Microbiol Rev. 1989 Oct;2(4):360–377. doi: 10.1128/cmr.2.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M., Forget A., Pelletier M., Gervais F., Skamene E. Killing of Mycobacterium smegmatis by macrophages from genetically susceptible and resistant mice. J Leukoc Biol. 1990 Jan;47(1):25–30. doi: 10.1002/jlb.47.1.25. [DOI] [PubMed] [Google Scholar]
- Denis M., Forget A., Pelletier M., Skamene E. Pleiotropic effects of the Bcg gene. I. Antigen presentation in genetically susceptible and resistant congenic mouse strains. J Immunol. 1988 Apr 1;140(7):2395–2400. [PubMed] [Google Scholar]
- Denis M., Forget A., Pelletier M., Skamene E. Pleiotropic effects of the Bcg gene: III. Respiratory burst in Bcg-congenic macrophages. Clin Exp Immunol. 1988 Sep;73(3):370–375. [PMC free article] [PubMed] [Google Scholar]
- Denis M., Forget A., Pelletier M., Turcotte R., Skamene E. Control of the Bcg gene of early resistance in mice to infections with BCG substrains and atypical mycobacteria. Clin Exp Immunol. 1986 Mar;63(3):517–525. [PMC free article] [PubMed] [Google Scholar]
- Forget A., Skamene E., Gros P., Miailhe A. C., Turcotte R. Differences in response among inbred mouse strains to infection with small doses of Mycobacterium bovis BCG. Infect Immun. 1981 Apr;32(1):42–47. doi: 10.1128/iai.32.1.42-47.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frehel C., Rastogi N. Mycobacterium leprae surface components intervene in the early phagosome-lysosome fusion inhibition event. Infect Immun. 1987 Dec;55(12):2916–2921. doi: 10.1128/iai.55.12.2916-2921.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frehel C., Ryter A., Rastogi N., David H. The electron-transparent zone in phagocytized Mycobacterium avium and other mycobacteria: formation, persistence and role in bacterial survival. Ann Inst Pasteur Microbiol. 1986 Nov-Dec;137B(3):239–257. doi: 10.1016/s0769-2609(86)80115-6. [DOI] [PubMed] [Google Scholar]
- Frehel C., de Chastellier C., Lang T., Rastogi N. Evidence for inhibition of fusion of lysosomal and prelysosomal compartments with phagosomes in macrophages infected with pathogenic Mycobacterium avium. Infect Immun. 1986 Apr;52(1):252–262. doi: 10.1128/iai.52.1.252-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frehel C., de Chastellier C., Offredo C., Berche P. Intramacrophage growth of Mycobacterium avium during infection of mice. Infect Immun. 1991 Jun;59(6):2207–2214. doi: 10.1128/iai.59.6.2207-2214.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y., Buschman E., Skamene E. Regulation of host resistance to Mycobacterium intracellulare in vivo and in vitro by the Bcg gene. Immunogenetics. 1989;30(3):218–221. doi: 10.1007/BF02421210. [DOI] [PubMed] [Google Scholar]
- Gros P., Skamene E., Forget A. Genetic control of natural resistance to Mycobacterium bovis (BCG) in mice. J Immunol. 1981 Dec;127(6):2417–2421. [PubMed] [Google Scholar]
- Horwitz M. A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983 Dec 1;158(6):2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi Y., Arai T. Effect of gamma-interferon on phagosome-lysosome fusion in Salmonella typhimurium-infected murine macrophages. FEMS Microbiol Immunol. 1990 Sep;2(2):75–82. doi: 10.1111/j.1574-6968.1990.tb03503.x. [DOI] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagaya K., Watanabe K., Fukazawa Y. Capacity of recombinant gamma interferon to activate macrophages for Salmonella-killing activity. Infect Immun. 1989 Feb;57(2):609–615. doi: 10.1128/iai.57.2.609-615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F. A simple method for counting adherent cells: application to cultured human monocytes, macrophages and multinucleated giant cells. J Immunol Methods. 1983 Jan 28;56(2):261–268. doi: 10.1016/0022-1759(83)90418-0. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Hibbs J. B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Potter M., O'Brien A. D., Skamene E., Gros P., Forget A., Kongshavn P. A., Wax J. S. A BALB/c congenic strain of mice that carries a genetic locus (Ityr) controlling resistance to intracellular parasites. Infect Immun. 1983 Jun;40(3):1234–1235. doi: 10.1128/iai.40.3.1234-1235.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzioch D., Hudson T., Boulé M., Barrera L., Urbance J. W., Varesio L., Skamene E. Genetic resistance/susceptibility to mycobacteria: phenotypic expression in bone marrow derived macrophage lines. J Leukoc Biol. 1991 Sep;50(3):263–272. doi: 10.1002/jlb.50.3.263. [DOI] [PubMed] [Google Scholar]
- Sibley L. D., Franzblau S. G., Krahenbuhl J. L. Intracellular fate of Mycobacterium leprae in normal and activated mouse macrophages. Infect Immun. 1987 Mar;55(3):680–685. doi: 10.1128/iai.55.3.680-685.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stach J. L., Gros P., Forget A., Skamene E. Phenotypic expression of genetically-controlled natural resistance to Mycobacterium bovis (BCG). J Immunol. 1984 Feb;132(2):888–892. [PubMed] [Google Scholar]
- Stokes R. W., Collins F. M. Growth of Mycobacterium avium in activated macrophages harvested from inbred mice with differing innate susceptibilities to mycobacterial infection. Infect Immun. 1988 Sep;56(9):2250–2254. doi: 10.1128/iai.56.9.2250-2254.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes R. W., Orme I. M., Collins F. M. Role of mononuclear phagocytes in expression of resistance and susceptibility to Mycobacterium avium infections in mice. Infect Immun. 1986 Dec;54(3):811–819. doi: 10.1128/iai.54.3.811-819.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S. M., Malo D., Vogan K., Skamene E., Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993 May 7;73(3):469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- Young L. S., Inderlied C. B., Berlin O. G., Gottlieb M. S. Mycobacterial infections in AIDS patients, with an emphasis on the Mycobacterium avium complex. Rev Infect Dis. 1986 Nov-Dec;8(6):1024–1033. doi: 10.1093/clinids/8.6.1024. [DOI] [PubMed] [Google Scholar]