Abstract
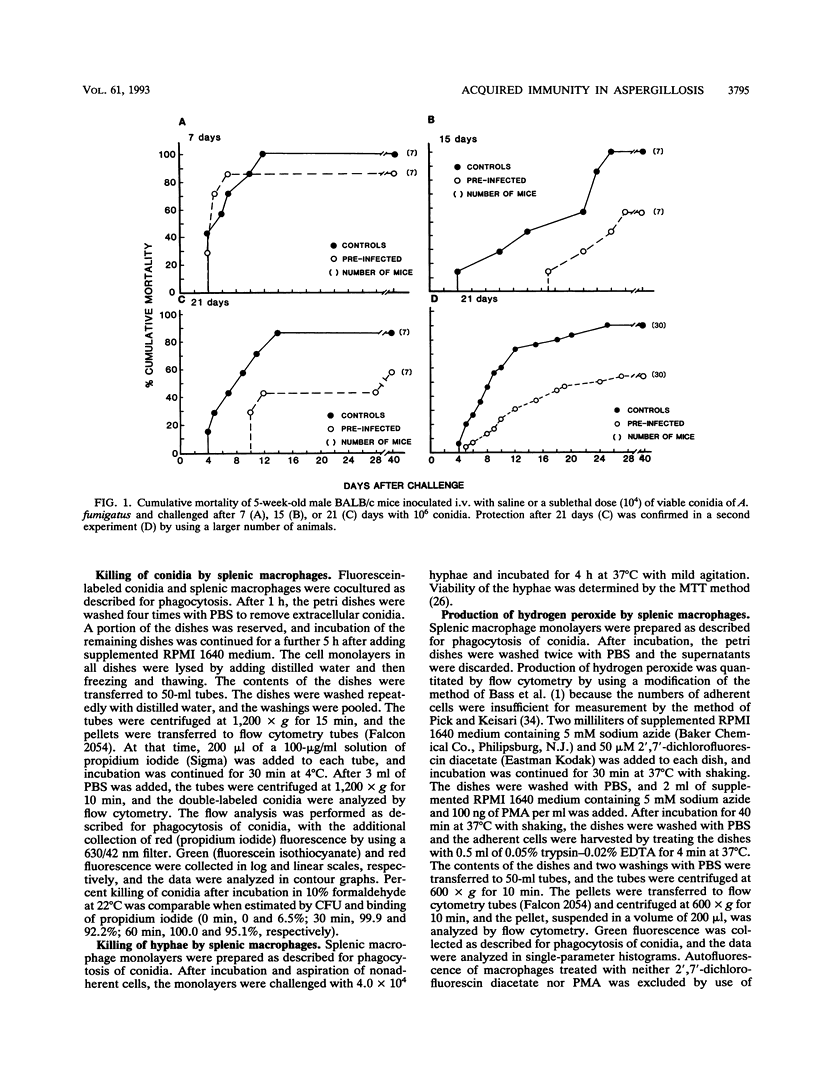
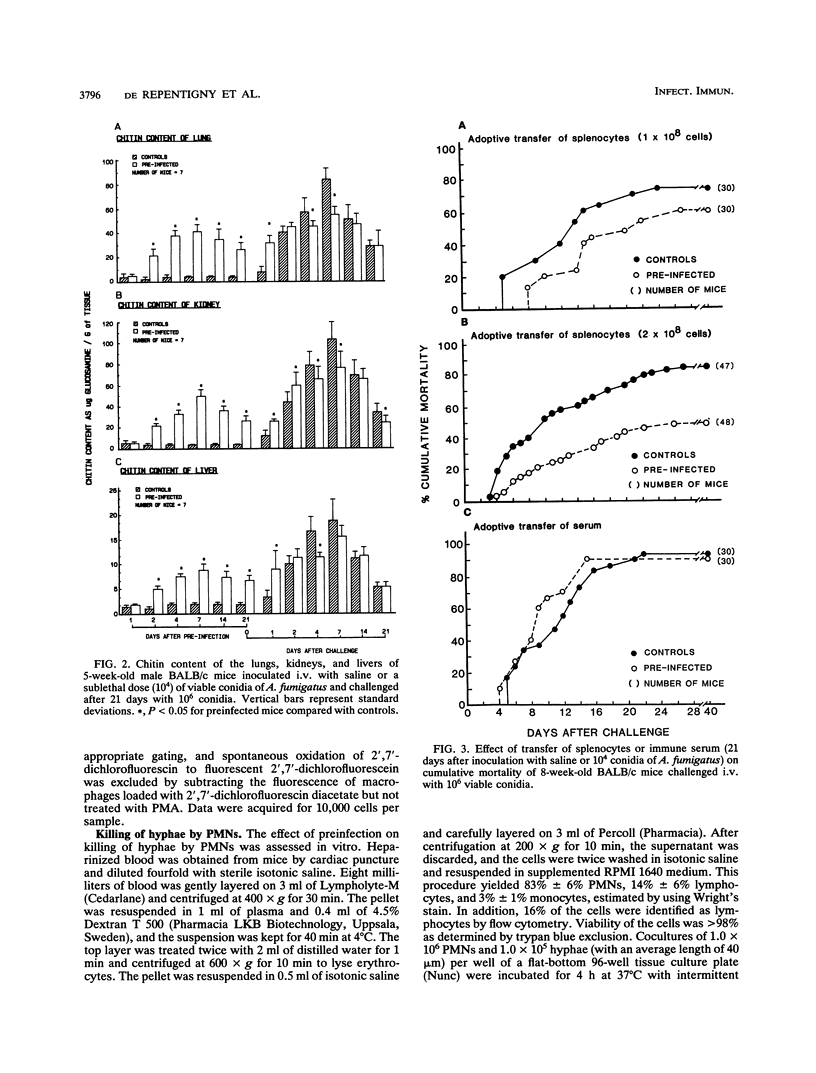
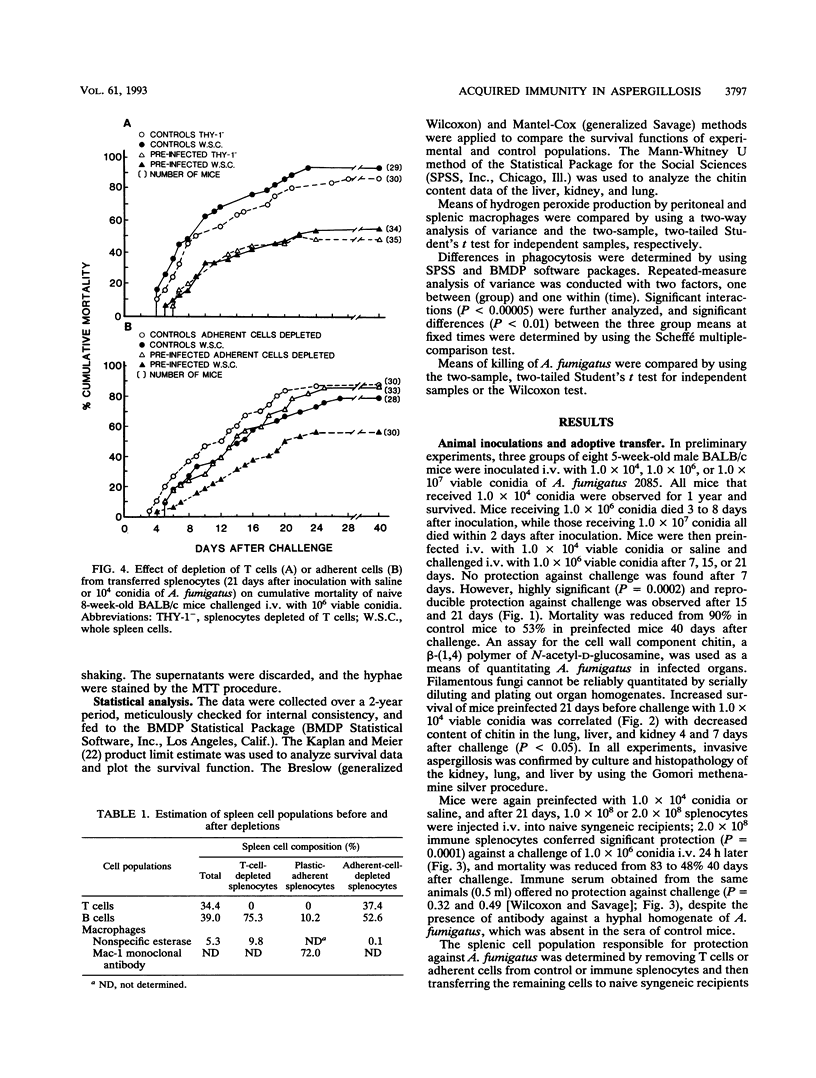
A number of studies have substantiated the pivotal role of innate defense mechanisms in protection against invasive aspergillosis. However, experiments demonstrating increased resistance to lethal intravenous (i.v.) infection with Aspergillus fumigatus conidia in cortisone-treated or untreated mice preinfected with a sublethal dose of conidia and protection of turkeys inoculated subcutaneously with a killed A. fumigatus germling vaccine against subsequent aerosol challenge led us to speculate that acquired immunity may also contribute to host defense against Aspergillus infection. Five-week-old male BALB/c mice were inoculated i.v. with 1.0 x 10(4) viable conidia or saline and challenged i.v. with 1.0 x 10(6) conidia after 7, 15, or 21 days. No protection against challenge was found after 7 days. However, significant and reproducible protection was observed after 15 and 21 days. Mortality was reduced from 90% in control mice to 53% in preinfected mice 40 days after challenge (P = 0.0002). Increased survival was correlated with decreased content of chitin in lungs, liver, and kidneys 4 and 7 days after challenge (P < 0.05). Mice were again inoculated with 1.0 x 10(4) conidia or saline, and after 21 days, 1.0 x 10(8) or 2.0 x 10(8) splenocytes were transferred to naive syngeneic recipients; 2.0 x 10(8) immune splenocytes conferred significant protection (P = 0.0001) against i.v. challenge with 1.0 x 10(6) conidia, and mortality decreased from 83 to 48% 40 days after challenge. Transfer of immune serum offered no protection despite the presence of antibody against a hyphal homogenate of A. fumigatus, which was absent in the sera of control mice. Protection by immune splenocytes was maintained after selective depletion of T cells but was abolished after removal of plastic-adherent splenocytes. Adherent cells were characterized as macrophages by using morphological criteria, nonspecific esterase, and MAC-1 monoclonal antibody. Production of hydrogen peroxide by peritoneal and splenic macrophages from preinfected mice was the same as and lower than, respectively, that from uninfected controls. However, phagocytosis of conidia by peritoneal or splenic macrophages from mice preinfected i.v. or intratracheally was significantly increased after 2 and 3 h of coculture compared with that from uninfected animals, whereas in vitro killing of conidia by splenic macrophages was unaltered. Peritoneal or splenic macrophages from control or preinfected mice failed to kill hyphae in vitro. Killing of hyphae by polymorphonuclear leukocytes was not significantly different between mice preinfected i.v. and uninfected controls. Taken together, the results indicate that acquired immunity mediated by activated macrophages can be demonstrated in experimental murine aspergillosis.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass D. A., Parce J. W., Dechatelet L. R., Szejda P., Seeds M. C., Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983 Apr;130(4):1910–1917. [PubMed] [Google Scholar]
- Beaman L., Benjamini E., Pappagianis D. Activation of macrophages by lymphokines: enhancement of phagosome-lysosome fusion and killing of Coccidioides immitis. Infect Immun. 1983 Mar;39(3):1201–1207. doi: 10.1128/iai.39.3.1201-1207.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Benjamini E., Pappagianis D. Role of lymphocytes in macrophage-induced killing of Coccidioides immitis in vitro. Infect Immun. 1981 Nov;34(2):347–353. doi: 10.1128/iai.34.2.347-353.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L. Fungicidal activation of murine macrophages by recombinant gamma interferon. Infect Immun. 1987 Dec;55(12):2951–2955. doi: 10.1128/iai.55.12.2951-2955.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistoni F., Vecchiarelli A., Cenci E., Puccetti P., Marconi P., Cassone A. Evidence for macrophage-mediated protection against lethal Candida albicans infection. Infect Immun. 1986 Feb;51(2):668–674. doi: 10.1128/iai.51.2.668-674.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistoni F., Verducci G., Perito S., Vecchiarelli A., Puccetti P., Marconi P., Cassone A. Immunomodulation by a low-virulence, agerminative variant of Candida albicans. Further evidence for macrophage activation as one of the effector mechanisms of nonspecific anti-infectious protection. J Med Vet Mycol. 1988;26(5):285–299. doi: 10.1080/02681218880000401. [DOI] [PubMed] [Google Scholar]
- Brummer E., Hanson L. H., Restrepo A., Stevens D. A. Intracellular multiplication of Paracoccidioides brasiliensis in macrophages: killing and restriction of multiplication by activated macrophages. Infect Immun. 1989 Aug;57(8):2289–2294. doi: 10.1128/iai.57.8.2289-2294.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer E., Morrison C. J., Stevens D. A. Recombinant and natural gamma-interferon activation of macrophages in vitro: different dose requirements for induction of killing activity against phagocytizable and nonphagocytizable fungi. Infect Immun. 1985 Sep;49(3):724–730. doi: 10.1128/iai.49.3.724-730.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer E., Stevens D. A. Fungicidal mechanisms of activated macrophages: evidence for nonoxidative mechanisms for killing of Blastomyces dermatitidis. Infect Immun. 1987 Dec;55(12):3221–3224. doi: 10.1128/iai.55.12.3221-3224.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall J. R., Black C. M., Leventhal J. P., Rizk N. W., Wachtel J. S., Remington J. S. Nonoxidative microbicidal activity in normal human alveolar and peritoneal macrophages. Infect Immun. 1987 Jul;55(7):1635–1640. doi: 10.1128/iai.55.7.1635-1640.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbel M. J., Eades S. M. Examination of the effect of age and acquired immunity on the susceptibility of mice to infection with Aspergillus fumigatus. Mycopathologia. 1977 Feb 18;60(2):79–85. doi: 10.1007/BF00490376. [DOI] [PubMed] [Google Scholar]
- DeMaria T. F., Kapral F. A. Pulmonary infection of mice with Staphylococcus aureus. Infect Immun. 1978 Jul;21(1):114–123. doi: 10.1128/iai.21.1.114-123.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker T., Lohmann-Matthes M. L., Baccarini M. Heterogeneous activity of immature and mature cells of the murine monocyte-macrophage lineage derived from different anatomical districts against yeast-phase Candida albicans. Infect Immun. 1986 Nov;54(2):477–486. doi: 10.1128/iai.54.2.477-486.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Clark R. A. Damage to Aspergillus fumigatus and Rhizopus oryzae hyphae by oxidative and nonoxidative microbicidal products of human neutrophils in vitro. Infect Immun. 1982 Nov;38(2):487–495. doi: 10.1128/iai.38.2.487-495.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Huber E., Haudenschild C. C. Mechanisms of destruction of Aspergillus fumigatus hyphae mediated by human monocytes. J Infect Dis. 1983 Mar;147(3):474–483. doi: 10.1093/infdis/147.3.474. [DOI] [PubMed] [Google Scholar]
- Diamond R. D. Inhibition of monocyte-mediated damage to fungal hyphae by steroid hormones. J Infect Dis. 1983 Jan;147(1):160–160. doi: 10.1093/infdis/147.1.160. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Epstein B., Jao W. Damage to hyphal forms of fungi by human leukocytes in vitro. A possible host defense mechanism in aspergillosis and mucormycosis. Am J Pathol. 1978 May;91(2):313–328. [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Lyman C. A., Wysong D. R. Disparate effects of interferon-gamma and tumor necrosis factor-alpha on early neutrophil respiratory burst and fungicidal responses to Candida albicans hyphae in vitro. J Clin Invest. 1991 Feb;87(2):711–720. doi: 10.1172/JCI115050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford S., Friedman L. Experimental study of the pathogenicity of aspergilli for mice. J Bacteriol. 1967 Oct;94(4):928–933. doi: 10.1128/jb.94.4.928-933.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Lehrer R. I. Defensins. Eur J Haematol. 1990 Jan;44(1):1–8. doi: 10.1111/j.1600-0609.1990.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Greenberg D., Ammann A. J., Wara D. W., Kaltreider H. B. Immunity to Aspergillus in patients with chronic granulomatous disease. J Pediatr. 1977 Apr;90(4):601–603. doi: 10.1016/s0022-3476(77)80377-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehmann P. F., White L. O. Acquired immunity to Aspergillus fumigatus. Infect Immun. 1976 Apr;13(4):1296–1298. doi: 10.1128/iai.13.4.1296-1298.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann P. F., White L. O. Chitin assay used to demonstrate renal localization and cortisone-enhanced growth of Aspergillus fumigatus mycelium in mice. Infect Immun. 1975 Nov;12(5):987–992. doi: 10.1128/iai.12.5.987-992.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., Diamond R. D. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J Infect Dis. 1985 Nov;152(5):938–945. doi: 10.1093/infdis/152.5.938. [DOI] [PubMed] [Google Scholar]
- Levitz S. M., Diamond R. D. Mechanisms of resistance of Aspergillus fumigatus Conidia to killing by neutrophils in vitro. J Infect Dis. 1985 Jul;152(1):33–42. doi: 10.1093/infdis/152.1.33. [DOI] [PubMed] [Google Scholar]
- Morrison C. J., Brummer E., Stevens D. A. In vivo activation of peripheral blood polymorphonuclear neutrophils by gamma interferon results in enhanced fungal killing. Infect Immun. 1989 Oct;57(10):2953–2958. doi: 10.1128/iai.57.10.2953-2958.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Byrne G. I., Rothermel C. D., Cartelli D. M. Lymphokine enhances oxygen-independent activity against intracellular pathogens. J Exp Med. 1983 Jul 1;158(1):234–239. doi: 10.1084/jem.158.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W. Interferon-gamma, the activated macrophage, and host defense against microbial challenge. Ann Intern Med. 1988 Apr;108(4):595–608. doi: 10.7326/0003-4819-108-4-595. [DOI] [PubMed] [Google Scholar]
- Ouellette A. J., Greco R. M., James M., Frederick D., Naftilan J., Fallon J. T. Developmental regulation of cryptdin, a corticostatin/defensin precursor mRNA in mouse small intestinal crypt epithelium. J Cell Biol. 1989 May;108(5):1687–1695. doi: 10.1083/jcb.108.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect J. R., Hobbs M. M., Granger D. L., Durack D. T. Cerebrospinal fluid macrophage response to experimental cryptococcal meningitis: relationship between in vivo and in vitro measurements of cytotoxicity. Infect Immun. 1988 Apr;56(4):849–854. doi: 10.1128/iai.56.4.849-854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Rex J. H., Bennett J. E., Gallin J. I., Malech H. L., DeCarlo E. S., Melnick D. A. In vivo interferon-gamma therapy augments the in vitro ability of chronic granulomatous disease neutrophils to damage Aspergillus hyphae. J Infect Dis. 1991 Apr;163(4):849–852. doi: 10.1093/infdis/163.4.849. [DOI] [PubMed] [Google Scholar]
- Richard J. L., Thurston J. R., Cutlip R. C., Pier A. C. Vaccination studies of aspergillosis in turkeys: subcutaneous inoculation with several vaccine preparations followed by aerosol challenge exposure. Am J Vet Res. 1982 Mar;43(3):488–492. [PubMed] [Google Scholar]
- Schaffner A. Acquired immune deficiency syndrome: is disseminated aspergillosis predictive of underlying cellular immune deficiency? J Infect Dis. 1984 May;149(5):828–829. doi: 10.1093/infdis/149.5.828-a. [DOI] [PubMed] [Google Scholar]
- Schaffner A., Davis C. E., Schaffner T., Markert M., Douglas H., Braude A. I. In vitro susceptibility of fungi to killing by neutrophil granulocytes discriminates between primary pathogenicity and opportunism. J Clin Invest. 1986 Aug;78(2):511–524. doi: 10.1172/JCI112603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Braude A. I., Davis C. E. Killing of Aspergillus spores depends on the anatomical source of the macrophage. Infect Immun. 1983 Dec;42(3):1109–1115. doi: 10.1128/iai.42.3.1109-1115.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest. 1982 Mar;69(3):617–631. doi: 10.1172/JCI110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P., James S., Sher A. The respiratory burst is not required for killing of intracellular and extracellular parasites by a lymphokine-activated macrophage cell line. Eur J Immunol. 1985 Jun;15(6):553–558. doi: 10.1002/eji.1830150605. [DOI] [PubMed] [Google Scholar]
- Smith G. R. Experimental aspergillosis in mice: aspects of resistance. J Hyg (Lond) 1972 Dec;70(4):741–754. doi: 10.1017/s0022172400022580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ste-Marie L., Sénéchal S., Boushira M., Garzon S., Strykowski H., Pedneault L., de Repentigny L. Production and characterization of monoclonal antibodies to cell wall antigens of Aspergillus fumigatus. Infect Immun. 1990 Jul;58(7):2105–2114. doi: 10.1128/iai.58.7.2105-2114.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Hayashi Y. Skin reaction and macrophage migration inhibition tests for polysaccharides from Aspergillus fumigatus and Candida albicans. Jpn J Microbiol. 1975 Oct;19(5):355–362. doi: 10.1111/j.1348-0421.1975.tb00892.x. [DOI] [PubMed] [Google Scholar]
- Waldorf A. R., Levitz S. M., Diamond R. D. In vivo bronchoalveolar macrophage defense against Rhizopus oryzae and Aspergillus fumigatus. J Infect Dis. 1984 Nov;150(5):752–760. doi: 10.1093/infdis/150.5.752. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Weiner M. H., Drutz D. J. Immunologic studies of disseminated infection with Aspergillus fumigatus in the nude mouse. J Infect Dis. 1981 May;143(5):726–733. doi: 10.1093/infdis/143.5.726. [DOI] [PubMed] [Google Scholar]
- Wolf J. E., Abegg A. L., Travis S. J., Kobayashi G. S., Little J. R. Effects of Histoplasma capsulatum on murine macrophage functions: inhibition of macrophage priming, oxidative burst, and antifungal activities. Infect Immun. 1989 Feb;57(2):513–519. doi: 10.1128/iai.57.2.513-519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Hsieh B. A., Howard D. H. Inhibition of the intracellular growth of Histoplasma capsulatum by recombinant murine gamma interferon. Infect Immun. 1987 Apr;55(4):1014–1016. doi: 10.1128/iai.55.4.1014-1016.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Hsieh B., Howard D. H. Inhibition of growth of Histoplasma capsulatum by lymphokine-stimulated macrophages. J Immunol. 1984 May;132(5):2593–2597. [PubMed] [Google Scholar]
- Wu-Hsieh B., Zlotnik A., Howard D. H. T-cell hybridoma-produced lymphokine that activates macrophages to suppress intracellular growth of Histoplasma capsulatum. Infect Immun. 1984 Jan;43(1):380–385. doi: 10.1128/iai.43.1.380-385.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]