Abstract
AIMS
A long-term and concentration-dependent beneficial effect of efavirenz (EFV) on cholesterol associated with high-density lipoprotein (HDL-c) in human immunodeficiency virus (HIV)-infected patients has been documented. Furthermore, it has been suggested that, instead of the current practice of only measuring HDL-c values, the evaluation of HDL quality might be an improved tool for identifying subjects at increased risk of cardiovascular events. Paraoxonase-1 (PON-1) is an enzyme associated with HDL that is involved in the onset of cardiovascular disease and responsible for HDL antioxidant function. The aim of the present study was to investigate the effect of EFV on the circulating activity of PON-1 in HIV-infected patients.
METHODS
The patients included were adults with a documented HIV-1 infection, nontreated or treated with antiretroviral regimens including EFV 600 mg once daily as first therapeutic regimen for at least 3 months. The influence of treatment with EFV, HDL-c and CD4 cell count on PON-1 activity was analysed.
RESULTS
HIV-infected White patients treated with EFV had higher PON-1 activity [77.35 U l−1 (65.66, 89.04)] (P < 0.05) and higher PON-1 activity : HDL-c ratio [1.88 (1.49, 2.28)] (P < 0.01) than untreated patients. PON-1 activity was higher in Black patients (P < 0.001) and in patients with a CD4 cell count >500 cells ml−1 (P= 0.0120).
CONCLUSIONS
EFV-based antiretroviral regimens are associated with HDL particles with a better antioxidant function, i.e. with a higher PON-1 activity. The PON-1 activity of Black patients is higher than that found in Whites regardless of treatment. Ethnicity should be taken into consideration when studying drug effects on PON-1 activity.
Keywords: antiretrovirals, efavirenz, HDL antioxidant function, high-density lipoprotein function, HIV, paraoxonase activity
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
In previous work, we showed a long-term and concentration-dependent beneficial effect of the non-nucleoside reverse transcriptase inhibitor efavirenz (EFV) on high-density lipoproteins (HDL) in human immunodeficiency virus (HIV)-infected patients.
Furthermore, it has been suggested that instead of the current practice of only measuring HDL-chelesterol values, the evaluation of HDL function, namely its antioxidant properties, might be an improved tool for identifying subjects at increased risk for cardiovascular events.
Paraoxonase-1 (PON-1) is an enzyme associated with HDL that is responsible for HDL antioxidant function.
WHAT THIS STUDY ADDS
In the present work, we studied the effect of EFV on the activity of PON-1 and showed, for the first time, that EFV-based antiretroviral therapy is associated with a better antioxidant function, i.e. with a higher PON-1 activity.
Introduction
The combination of three drugs [two nucleoside analogues (NRTI) and a protease inhibitor (PI) or a non-nucleoside reverse transcriptase inhibitor (NNRTI)] that can inhibit the replication of the human immunodeficiency virus (HIV) leads to a dramatically improved outcome for this chronic infection [1]. However, while the benefits of this highly active antiretroviral therapy (HAART) have revolutionized the life expectancy of HIV-infected patients, treatment-associated dyslipidaemia has frequently been observed [2]. This includes an increase in the atherogenic nonhigh-density-lipoprotein cholesterol (non-HDL-c) [3]. Whereas many of the PI-based regimens are often associated with increased concentrations of triglycerides (TG), total cholesterol and low-density lipoprotein cholesterol (LDL-c), NNRTI-based regimens differ importantly, being associated with increases in HDL [4–6].
It has also been shown that infection, in itself, causes a significant drop in HDL [7]. For instance, HDL-c decreases in HIV-infected individuals [7, 8] and the viral replication is related to cholesterol membrane metabolism [9]. Also, Nef, the accessory protein of HIV-1, impairs cholesterol efflux from the macrophages [10].
The role of HDL-c as a protective factor against cardiovascular disease is well documented [11]. On the other hand, levels of HDL-c have been significantly associated with a better HIV infection course [12]. Furthermore, it has been suggested that, instead of the current practice of only measuring HDL-c values, the evaluation of HDL quality might be an improved tool for identifying subjects at increased risk of cardiovascular events [13].
Regarding quality, HDL are heterogeneous particles containing variable levels of antioxidants that are, in part, related to enzymes associated with HDL [13, 14]. This results in a different functionality/quality and protective effect of HDL.
Paraoxonase-1 (PON-1) is an antioxidant enzyme associated with HDL, mechanistically linked to systemic oxidative stress and prospectively to cardiovascular risk, thus indicating a potential mechanism for its atheroprotective function [15]. Navab et al.[14] showed that patients with coronary atherosclerosis had lower PON-1 activity than healthy controls, despite having the same HDL-c level. This suggests that HDL antioxidant properties are more useful in distinguishing the cardiovascular risk of patients and healthy controls than the amount of HDL-c [13].
PON-1 is highly effective in preventing lipid peroxidation of LDL [16]. However, PON-1 may be partially inactivated in the presence of oxidative stress. Several results corroborate that substantial oxidative stress occurs [17–19] and PON-1 activity decreases [20] during HIV infection. In addition, Masiá and co-authors [19] compared total peroxide concentration in a cohort of patients and reported an association between a NNRTI-based HAART and low peroxide concentrations.
Efavirenz (EFV) is a NNRTI that has been associated with a long-term and concentration-dependent beneficial effect on HDL-c [21]. However its effects on HDL quality are unknown. The present work was aimed to study the effect of EFV-based antiretroviral regimens on HDL quality, particularly on PON-1 activity.
Methods
Patients with documented HIV infection who had received continuous treatment with EFV 600 mg once daily as first therapeutic regimen for at least 3 months were invited to participate. A second group of patients with documented HIV infection but without indication to start antiretroviral treatment was considered as control. Exclusion criteria were aspartate aminotransferase or alanine aminotransferase more then five times the upper limit of normal, drugs for dyslipidaemia and other viral co-infections besides HIV. The protocol received prior approval from the Ethics Committee of Centro Hospitalar de Lisboa, Central (Desterro). All patients gave their written informed consent and agreed on adherence to treatment. Before sample collection patients were asked about compliance issues and only those patients who confirmed adherence were included.
PON-1 activity was obtained from 46 patients assessed by spectrophotometry, through the quantification of p-nitrophenol formation as previously described by Batuca et al.[22]. The reaction medium was a glycine buffer (0.05 mol l−1, pH 10.5) with 1 mmol l−1 CaCl2. The temperature of the reaction was 37 °C. PON-1 activity was calculated with a molar extinction coefficient of 18.290 M cm−1 and expressed as U l−1, which is defined as 1 µmol of p-nitrophenol generated per minute per litre, under assay conditions. As paraoxon is very unstable, the reagent was prepared immediately before use. Regarding paraoxon toxicity, stock solutions were handled in an air-extraction fumehood and the operator took appropriate safety precautions, such as wearing a mask and gloves to protect against accidental contact or inhalation of the toxic fumes.
Drug plasma concentrations were obtained from 25 patients by high-performance liquid chromatography with UV detection method described elsewhere [23].
EFV is generally taken once daily at bedtime to improve its tolerability and minimize its psychiatric and central nervous system adverse effects, which makes it more difficult to determine through concentrations in an outpatient setting. Blood samples were thus taken during the morning, 8–16 h post dosing. It has been previously demonstrated that, given the long half-life of EFV, mid-interval sampling at 8–16 h post dose influences the total variance in EFV concentrations only slightly [23].
The samples for PON-1 activity and EFV concentration determination were time matched.
Plasma samples for lipid profile determination were analysed in the local clinical pathology laboratory by standard enzymatic assays.
Results were shown as mean (95% confidence interval). Normally distributed variables were analysed using the unpaired Student's t-test while non-normally distributed variables were compared using the Mann–Whitney U-test. Correlations between Gaussian variables were performed using the Pearson correlation test, while nonparametric correlation was performed with Spearman's test. Statistical significance was assumed at P < 0.05. GraphPad Prism version 4 (GraphPad Software Inc., San Diego, CA, USA) was used to perform data and statistical analysis. Determination of independent predictors was done using SPSS (SPSS Inc., Chicago, IL, USA).
Results
The study was conducted in 46 HIV-infected patients (Table 1): (i) 21 untreated patients (control group), and (ii) 25 patients who had received continuous treatment with EFV 600 mg once daily as first therapeutic regimen for at least 3 months (study group).
Table 1.
Diagram of the groups and subgroups of the study population
| Total | HIV-infected individuals | |||
| (n= 46) | ||||
| Treatment | Naive | on efavirenz | ||
| (n= 21) | (n= 25) | |||
| Ethnicity | Black | White | Black | White |
| (n= 3) | (n= 18) | (n= 7) | (n= 18) | |
| Antiretroviral backbone | Lamivudine+zidovudine(n= 7) | |||
| Tenofovir+emtricitabine(n= 8) | ||||
| other drug association(n= 3) | ||||
n, number of patients.
PON-1 activity was higher in treated patients [n= 25; 85.46 U l−1 (75.49, 95.44)] than in the nontreated group [n= 21; 65.58 U l−1 (55.77, 75.39)] (P= 0.0067; unpaired Student's t-test).
As the demographic characteristics of the patients may influence PON-1 activity, a multivariate analysis was conducted to ensure that this was an independent association. The possible independent effect of age, sex, ethnicity and treatment with EFV on PON-1 activity was assessed in a regression model that revealed ethnicity and treatment with EFV as being independently associated with PON-1 activity (t= 2.440, P= 0.019 and t= 4.879, P < 0.001, respectively).
Race-related differences were found in PON-1 activity (P < 0.01; one-way anova plus Bonferroni's multiple comparison test; Figure 1). Within the Black patients, treated and nontreated groups were different in sample size and demographic characteristics. No differences in sample size, demographic characteristics (age, sex and body mass index), CD4 cell count, lipid and liver function profile were found within Whites (n= 36) (Table 2).
Figure 1.
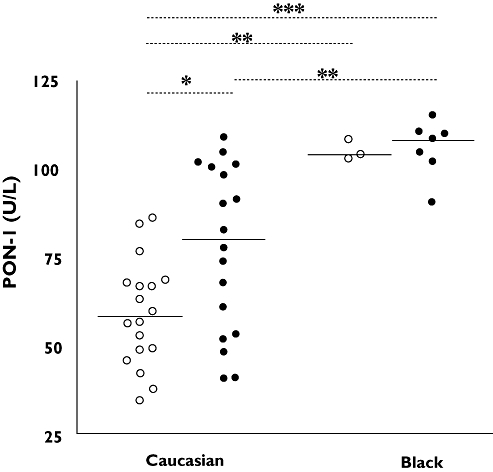
Paraoxonase-1 (PON-1) activity in human immunodeficiency virus (HIV)-infected individuals. PON-1 activity was lower in untreated [58.80 U l−1 (51.36, 66.23); n= 18] than in treated [77.35 U l−1 (65.66, 89.04); n= 18] White patients and both were lower than Black patients despite treatment (*P < 0.05, **P < 0.01 and ***P < 0.001, one-way anova plus Bonferroni's multiple comparison test). No treatment (○); EFV (•)
Table 2.
Comparison between demographic and biochemical variables between White HIV-infected patients without treatment and HIV-infected patients treated with efavirenz (EFV)
| HIV–infected patients | |||
|---|---|---|---|
| Parameter | Untreated | EFV | P-value |
| n | 18 | 18 | |
| Male | 11 | 15 | |
| Age (years) | 38 (32, 43) | 44 (37, 51) | NS |
| Body mass index (kg m−2) | 25 (22, 28) | 23 (22, 25) | NS |
| GGT (U l−1) | 32 (13, 50) | 49 (20, 77) | NS |
| AP (U l−1) | 65 (52, 78) | 87 (69, 104) | NS |
| AST (U l−1) | 24 (15, 33) | 20 (17, 23) | NS |
| ALT (U l−1) | 38 (20, 55) | 29 (20, 39) | NS |
| CD4 cell count (cells mm−3) | 492 (397, 588) | 594 (445, 742) | NS |
| TC (mg dl−1) | 179 (159, 200) | 187 (173, 200) | NS |
| LDL-c (mg dl−1) | 118 (98, 138) | 115 (99, 131) | NS |
| HDL-c (mg dl−1) | 48 (42, 54) | 46 (39, 52) | NS |
| TG (mg dl−1) | 89 (68, 109) | 126 (90, 162) | NS |
| TC : HDL-c ratio | 3.86 (3.30, 4.14) | 4.35 (3.71, 4.98) | NS |
| PON-1 activity (U l−1) | 58.80 (51.36, 66.23) | 77.35 (65.66, 89.04) | <0.05 |
| PON-1 activity : HDL-c ratio | 1.30 (1.11, 1.49) | 1.88 (1.49, 2.28) | <0.01 |
Data are expressed has mean (95% CI). NS, nonsignificant (P > 0.05); n, number of patients; GGT, γ-glutamyltranspeptidase; reference range (RR) < 36 U l−1; AP, alkaline phosphatase; RR 35–104 U l−1; AST, aspartate aminotransferase; RR < 31 U l−1; ALT, alanine aminotransferase; RR < 31 U l−1. To convert total cholesterol (TC) high-density (HDL-c) and low-density (LDL-c) lipoprotein cholesterol to mmol l−1 multiply by 0.0259. For triglycerides (TG), the conversion value is 0.0113. RR, TC < 200 mg dl−1; TG < 150 mg dl−1, HDL-c > 40 mg dl−1, LDL-c < 150 mg dl−1.
The PON-1 activity : HDL-c ratio and PON-1 activity and were higher in White individuals on EFV (P= 0.0082; unpaired Student's t-test) (Table 2).
In patients with CD4 >500 cells ml−1, PON-1 activity was positively related with CD4 cell count (Pearson r= 0.6105; P= 0.0120, n= 16). The CD4 cell count was higher with increasing HDL values (Pearson r= 0.3786; P= 0.0249, n= 35) and TG values (Pearson r= 0.3728; P= 0.0299, n= 34). These relations were observed from an analysis conducted on the entire cohort. However, as the differences in CD4 count between these patients could create a high likelihood of bias, multivariate analysis was further performed in the group of patients on EFV. The possible independent effects of age, sex, CD4 count and treatment duration on PON-1 activity were assessed in a regression model that revealed that none of the variables was independently associated with PON-1 activity.
Mean EFV plasma concentration was 2.47 mg l−1 (1.80, 3.15) and no association was found between drug plasma level and PON-1 activity.
The NNRTI backbone of HAART did not influence PON-1 activity [seven patients lamivudine + zidovudine 78.94 U l−1 (54.01, 103.9) and eight patients on tenofovir + emtricitabine 78.75 U l−1 (58.92, 98.58)].
Discussion and conclusions
In the present work, a positive effect of EFV on PON-1 activity in HIV-infected patients has been described for the first time. Also, we have shown that ethnicity can not be excluded in studies of PON-1 activity.
PON-1 activity in HIV-infected patients on EFV was higher than in HIV-infected patients without treatment. Recently, a relation between this enzyme and its capacity to promote systemic antioxidant effects in humans with systemic oxidative stress and cardiovascular risk has been suggested [15, 24]. It has also been shown that concentrations and activity of PON-1 are altered in chronic diseases [20, 25], probably as a response to the enhanced oxidative stress observed in the earlier stages of these diseases. Furthermore, changes in oxidative stress markers, antioxidant capacity, lipid profiles in HIV-infected patients and cardiovascular risk have been recently described [8, 19].
The mechanism behind the effect of EFV on PON-1 activity remains speculative. In untreated patients, the lower PON-1 activity can be the result of increased inactivation of the enzyme due to increased generation of reactive oxygen species [19]. EFV could also be acting as a scavenger of reactive oxygen species, enabling higher PON-1 activity. In addition, it has been shown that alterations in HDL structure and composition can affect PON-1 activity and function [26]. The effect of HIV infection on HDL metabolism is well documented [8], and we have previously shown that EFV is associated with higher values of HDL-c [21]. Thus the alterations of HDL-c caused by EFV could be stabilizing the link of the enzyme to HDL, hence allowing higher enzymatic activity. In short, we can speculate that EFV can balance the inactivation of the enzyme by reducing oxidative stress status and/or inducing new HDL structural alterations, counteracting the effects of the infection. Another hypothesis is that the drug is acting as an inducer of the enzyme. Further studies are warranted to establish the underlying mechanism.
As the study design was not prospective, the percentage increase in both HDL-c concentrations and PON-1 activity, in relation to basal values (before start of treatment), can not be evaluated. However, in the treated group, in addition to the higher PON-1 activity, a higher PON-1 activity : HDL-c ratio was also found. This observation raises an interesting consideration. As PON-1 has been described as preferentially associated to HDL and EFV increases HDL, changes in the PON-1 activity : HDL-c ratio were not expected. This higher ratio observed suggests that, for patients on EFV, at least part of the higher PON-1 activity found appears to be independent of HDL-c variation.
It has been suggested that within the patients with the same HDL-c value, lower PON-1 activity is predictive of coronary heart disease [14]. Moreover, other authors have also suggested that the ability of HDL to inhibit oxidation of lipoproteins is more important than the HDL-c concentration [13]. Thus, the present study suggests that EFV elicits HDL with more antioxidant capacity.
Another conclusion of our work is that ethnicity should be taken into consideration when studying PON-1 activity. It has been known that the distribution of PON-1 polymorphisms differs with ethnicity [27–29]. The focal approach of the association of different end-points of cardiovascular problems and PON-1 arrived from genotype studies [30]. However, some authors have highlighted the role of PON-1 activity determinations [30, 31], safeguarding the necessity to perform functional studies. Among the different ethnic populations, there are also marked differences in PON-1 activity [32], although in most studies no references have been made to ethnicity.
In the present work we have shown that PON-1 activity in the entire cohort was lower in untreated patients and in those with lower CD4 cell count, indicating that lower PON-1 activity is associated with more severe infection. A relation between CD4 cell counts and PON-1 activity was previously described [20]. However, this work was performed in HIV-infected patients under different antiretroviral regimens and with >60% being co-infected with hepatitis C virus (HCV). The authors described a negative influence of HCV co-infection on PON-1 activity, but the hepatic function of these patients was not categorized. In the present work, viral hepatitis co-infection was an exclusion factor, given that PON-1 is mainly synthesized by the liver and PON-1 measurement has been proposed as a marker of hepatic function [25].
It has been suggested that a strong association between viral replication and both HDL [9, 10, 12] and host antioxidant defence [33–35] exists. The positive relationship between CD4 cell count and PON-1 activity suggests that higher PON-1 activity is associated with a better course of HIV infection and antiviral properties.
No correlations were found between plasma concentrations of EFV and PON-1 activity. However, study of this relation requires the percentage of variation of PON-1 activity instead of absolute values. The association of PON-1 activity with EFV concentrations is important to distinguish if the effect relies on the response to treatment or on EFV per se. Nevertheless, excluding NNRTI [21, 36], the majority of antiretroviral drugs have been described as not affecting HDL-c [2,7].
Regarding methodological aspects, there have been some issues regarding the measurement of PON-1 activity [37]. There are no standardized methods for measuring PON1 esterase activity. The differences in procedure imply that values obtained from different laboratories vary considerably, which complicates interlaboratory comparisons. However, the method used here is the most widely used [37].
In conclusion, EFV is associated with increased PON-1 activity, which is higher in Black patients and is associated with a better disease course. Further studies are required to clarify the mechanism by which EFV-based HAART is associated with HDL particles with a better antioxidant function, namely, with higher PON-1 activity.
Competing interests
None declared.
Financial support was provided by the Portuguese Foundation for Science and Technology (FCT)/Centro de Estudos de Doenças Crónicas (CEDOC). Samples of pure efavirenz were kindly provided by Merck Sharp and Dohme. The authors are grateful to Mr Michael Bright for reviewing the English. A preliminary account of this work has been presented at the 9th International Workshop on Clinical Pharmacology of HIV Therapy, New Orleans, USA, 2008.
REFERENCES
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study investigator. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet. 2000;356:1423–30. doi: 10.1016/S0140-6736(00)02854-3. [DOI] [PubMed] [Google Scholar]
- 3.Behrens G, Dejam A, Schmidt H, Balks HJ, Brabant G, Körner T, Stoll M, Schmidt RE. Impaired glucose tolerance, beta cell function and lipid metabolism in HIV patients under treatment with protease inhibitors. AIDS. 1999;13:F63–70. doi: 10.1097/00002030-199907090-00001. [DOI] [PubMed] [Google Scholar]
- 4.Negredo E, Ribalta J, Ferre R, Salazar J, Rey-Joly C, Sirera G, Masana L, Clotet B. Efavirenz induces a striking and generalized increase of HDL-cholesterol in HIV-infected patients. AIDS. 2004;18:819–21. doi: 10.1097/00002030-200403260-00017. [DOI] [PubMed] [Google Scholar]
- 5.van Leth F, Phanuphak P, Stroes E, Gazzard B, Cahn P, Raffi F, Wood R, Bloch M, Katlama C, Kastelein JJ, Schechter M, Murphy RL, Horban A, Hall DB, Lange JM, Reiss P. Nevirapine and efavirenz elicit different changes in lipid profiles in antiretroviral-therapy-naive patients infected with HIV-1. PLoS Med. 2004;1:64–73. doi: 10.1371/journal.pmed.0010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisac C, Fumero E, Crespo M, Roson B, Ferrer E, Virgili N, Ribera E, Gatell JM, Podzamczer D. Metabolic benefits 24 months after replacing a protease inhibitor with abacavir, efavirenz or nevirapine. AIDS. 2005;19:917–25. doi: 10.1097/01.aids.0000171405.46113.bf. [DOI] [PubMed] [Google Scholar]
- 7.Riddler SA, Smit E, Cole SR, Li R, Chmiel JS, Dobs A, Palella F, Visscher B, Evans R, Kingsley LA. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289:2978–82. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 8.Rose H, Hoy J, Woolley I, Tchoua U, Bukrinsky M, Dart A, Sviridov D. HIV infection and high density lipoprotein metabolism. Atherosclerosis. 2008;199:79–86. doi: 10.1016/j.atherosclerosis.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ono A, Freed EO. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc Natl Acad Sci USA. 2001;98:13925–30. doi: 10.1073/pnas.241320298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mujawar Z, Rose H, Morrow MP, Pushkarsky T, Dubrovsky L, Mukhamedova N, Fu Y, Dart A, Orenstein JM, Bobryshev YV, Bukrinsky M, Sviridov D. Human immunodeficiency virus impairs reverse cholesterol transport from macrophages. PLoS Biol. 2006;1:e365. doi: 10.1371/journal.pbio.0040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR, Jr, Bangdiwala S, Tyroler HA. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 12.Alonso-Villaverde C, Segues T, Coll-Crespo B, Pérez-Bernalte R, Rabassa A, Gomila M, Parra S, Gozález-Esteban MA, Jiménez-Expósito MJ, Masana L. High-density lipoprotein concentrations relate to the clinical course of HIV viral load in patients undergoing antiretroviral therapy. AIDS. 2003;17:1173–8. doi: 10.1097/00002030-200305230-00009. [DOI] [PubMed] [Google Scholar]
- 13.Ansell BJ, Navab M, Hama S, Kamranpour N, Fonarow G, Hough G, Rahmani S, Mottahedeh R, Dave R, Reddy ST, Fogelman AM. Inflammatory/anti-inflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favourably affected by simvastatin treatment. Circulation. 2003;108:2751–6. doi: 10.1161/01.CIR.0000103624.14436.4B. [DOI] [PubMed] [Google Scholar]
- 14.Navab M, Hama-Levy S, Van Lenten BJ, Fonarow GC, Cardinez CJ, Castellani LW, Brennan ML, Lusis AJ, Fogelman AM, La Du BN. Mildly oxidized LDL induces an increased apolipoprotein J/paraoxonase ratio. J Clin Invest. 1997;99:2005–19. doi: 10.1172/JCI119369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, Fu X, Shao M, Brennan DM, Ellis SG, Brennan ML, Allayee H, Lusis AJ, Hazen SL. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299:1265. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackness MI, Arrol S, Abbott C, Durrington PN. Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase. Arteriosclerosis. 1993;104:129–35. doi: 10.1016/0021-9150(93)90183-u. [DOI] [PubMed] [Google Scholar]
- 17.Gil L, Martínez G, González I, Tarinas A, Alvarez A, Giuliani A, Molina R, Tápanes R, Pérez J, León OS. Contribution to characterization of oxidative stress in HIV/AIDS patients. Pharmacol Res. 2003;47:217–24. doi: 10.1016/s1043-6618(02)00320-1. [DOI] [PubMed] [Google Scholar]
- 18.Ngondi JL, Oben J, Forkah DM, Etame LH, Mbanya D. The effect of different combination therapies on oxidative stress markers in HIV infected patients in Cameroon. AIDS Res Ther. 2006;22:3–19. doi: 10.1186/1742-6405-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masiá M, Padilla S, Bernal E, Almenar MV, Molina J, Hernández I, Graells ML, Gutiérrez F. Influence of antiretroviral therapy on oxidative stress and cardiovascular risk: a prospective cross-sectional study in HIV-infected patients. Clin Ther. 2007;29:1448–55. doi: 10.1016/j.clinthera.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Parra S, Alonso-Villaverde C, Coll B, Ferré N, Marsillach J, Aragonès G, Mackness M, Mackness B, Masana L, Joven J, Camps J. Serum paraoxonase-1 activity and concentration are influenced by human immunodeficiency virus infection. Atherosclerosis. 2007;194:175–81. doi: 10.1016/j.atherosclerosis.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Pereira SA, Branco T, Côrte-Real RM, Germano I, Lampreia F, Caixas U, Monteiro EC. Long-term and concentration-dependent beneficial effect of efavirenz on HDL-cholesterol in HIV-infected patients. Br J Clin Pharmacol. 2006;61:601–4. doi: 10.1111/j.1365-2125.2006.02619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batuca JR, Ames PR, Isenberg DA, Alves JD. Antibodies toward high-density lipoprotein components inhibit paraoxonase activity in patients with systemic lupus erythematosus. Ann NY Acad Sci. 2007;1108:137–46. doi: 10.1196/annals.1422.016. [DOI] [PubMed] [Google Scholar]
- 23.Pereira SA, Branco T, Caixas U, Côrte-Real RM, Germano I, Lampreia F, Monteiro EC. Intra-individual variability in efavirenz plasma concentrations supports therapeutic drug monitoring based on quarterly sampling in the first year of therapy. Ther Drug Monit. 2008;30:60–6. doi: 10.1097/FTD.0b013e318160ce76. [DOI] [PubMed] [Google Scholar]
- 24.Mackness B, Durrington P, McElduff P, Yarnell J, Azam N, Watt M, Mackness M. Low paraoxonase activity predicts coronary events in the Caerphilly Prospective Study. Circulation. 2003;107:2775–9. doi: 10.1161/01.CIR.0000070954.00271.13. [DOI] [PubMed] [Google Scholar]
- 25.Ferré N, Marsillach J, Camps J, Mackness B, Mackness M, Riu F, Coll B, Tous M, Joven J. Paraoxonase-1 is associated with oxidative stress, fibrosis and FAS expression in chronic liver diseases. J Hepatol. 2006;45:51–9. doi: 10.1016/j.jhep.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 26.James RW, Deakin SP. The importance of high-density lipoproteins for paraoxonase-1 secretion, stability, and activity. Free Radic Biol Med. 2004;37:1986–94. doi: 10.1016/j.freeradbiomed.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 27.Chen Q, Reis SE, Kammerer CM, McNamara DM, Holubkov R, Sharaf BL, Sopko G, Pauly DF, Merz CN, Kamboh MI, WISE Study Group Association between the severity of angiographic coronary artery disease and paraoxonase gene polymorphisms in the National Heart, Lung, and Blood Institute-sponsored Women's Ischemia Syndrome Evaluation (WISE) study. Am J Hum Genet. 2003;72:13–22. doi: 10.1086/345312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tripi LM, Manzi S, Chen Q, Kenney M, Shaw P, Kao A, Bontempo F, Kammerer C, Kamboh MI. Relationship of serum paraoxonase 1 activity and paraoxonase 1 genotype to risk of systemic lupus erythematosus. Arthritis Rheum. 2006;54:1928–39. doi: 10.1002/art.21889. [DOI] [PubMed] [Google Scholar]
- 29.Thyagarajan B, Jacobs DR, Jr, Carr JJ, Alozie O, Steffes MW, Kailash P, Hayes JH, Gross MD. Factors associated with paraoxonase genotypes and activity in a diverse, young, healthy population: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Clin Chem. 2008;54:738–46. doi: 10.1373/clinchem.2007.099044. [DOI] [PubMed] [Google Scholar]
- 30.Mackness M, Durrington P, Mackness B. Paraoxonase 1 activity, concentration and genotype in cardiovascular disease. Curr Opin Lipidol. 2004;15:399–404. doi: 10.1097/01.mol.0000137227.54278.29. [DOI] [PubMed] [Google Scholar]
- 31.Richter RJ, Furlong CE. Determination of paraoxonase (PON1) status requires more than genotyping. Pharmacogenetics. 1999;9:745–53. [PubMed] [Google Scholar]
- 32.Playfer JR, Eze LC, Bullen MF, Evans DA. Genetic polymorphism and interethnic variability of plasma paraoxonase activity. J Med Genet. 1976;13:337–42. doi: 10.1136/jmg.13.5.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allard JP, Aghdassi E, Chau J, Tam C, Kovacs CM, Salit IE, Walmsley SL. Effects of vitamin E and C supplementation on oxidative stress and viral load in HIV-infected subjects. Eur J Clin Invest. 1998;12:1653–9. doi: 10.1097/00002030-199813000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Sandstrom PA, Murray J, Folks TM, Diamond AM. Antioxidant defenses influence HIV-1 replication and associated cytopathic effects. Free Radic Biol Med. 1998;24:1485–91. doi: 10.1016/s0891-5849(98)00023-9. [DOI] [PubMed] [Google Scholar]
- 35.Treitinger A, Spada C, Verdi JC, Miranda AF, Oliveira OV, Silveira MV, Moriel P, Abdalla DS. Decreased antioxidant defence in individuals infected by the human immunodeficiency virus. Eur J Clin Invest. 2000;5:454–9. doi: 10.1046/j.1365-2362.2000.00642.x. [DOI] [PubMed] [Google Scholar]
- 36.van der Valk M, Kastelein JJ, Murphy RL, van Leth F, Katlama C, Horban A, Glesby M, Behrens G, Clotet B, Stellato RK, Molhuizen HO, Reiss P, Atlantic Study Team Nevirapine-containing antiretroviral therapy in HIV-1 infected patients results in an anti-atherogenic lipid profile. AIDS. 2001;15:2407–14. doi: 10.1097/00002030-200112070-00008. [DOI] [PubMed] [Google Scholar]
- 37.Camps J, Marsillach J, Joven J. The paraoxonases: role in human diseases and methodological difficulties in measurement. Crit Rev Clin Lab Sci. 2009;46:83–106. doi: 10.1080/10408360802610878. [DOI] [PubMed] [Google Scholar]


