Abstract
AIMS
To investigate whether dose adjustment of the once-daily human glucagon-like peptide-1 analogue liraglutide is required in patients with varying stages of renal impairment.
METHODS
A cohort of 30 subjects, of whom 24 had varying degrees of renal impairment and six had normal renal function, were given a single dose of liraglutide, 0.75 mg subcutaneously, and completed serial blood sampling for plasma liraglutide measurements for pharmacokinetic estimation.
RESULTS
No clear trend for change in pharmacokinetics was evident across groups with increasing renal dysfunction. While the between-group comparisons of the area under the liraglutide concentration–curve (AUC) did not demonstrate equivalence [estimated ratio AUCsevere/AUChealthy 0.73, 90% confidence interval (CI) 0.57, 0.94; and AUC (continuous ambulatory peritoneal dialysis)CAPD/AUChealthy 0.74, 90% CI 0.56, 0.97], the regression analysis of log(AUC) for subjects with normal renal function and mild-to-severe renal impairment showed no significant effect of decreasing creatinine clearance on the pharmacokinetics of liraglutide. The expected AUC ratio between the two subjects with the lowest and highest creatinine clearance in the study was estimated to be 0.88 (95% CI 0.58, 1.34) (NS). Degree of renal impairment did not appear to be associated with an increased risk of adverse events.
CONCLUSIONS
This study indicated no safety concerns regarding use of liraglutide in patients with renal impairment. Renal dysfunction was not found to increase exposure of liraglutide, and patients with Type 2 diabetes and renal impairment should use standard treatment regimens of liraglutide. There is, however, currently limited experience with liraglutide in patients beyond mild-stage renal disease.
Keywords: incretin, liraglutide, pharmacokinetics, renal function, Type 2 diabetes mellitus
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Patients with Type 2 diabetes are likely to have or to develop renal impairment, which affects the pharmacokinetics of some antidiabetic treatments.
Whether dosing of the once-daily human glucagon-like peptide-1 analogue liraglutide should be modified in patients with renal impairment has not previously been studied.
WHAT THIS STUDY ADDS
Renal dysfunction was not found to increase the exposure of liraglutide.
Hence, no dose adjustment is expected to be required in patients with Type 2 diabetes and renal impairment treated with liraglutide.
Introduction
Type 2 diabetes mellitus is a progressive, multifactorial, debilitating disease marked by a gradual decrease in pancreatic β-cell function, and a concomitant deterioration in insulin secretion, against a background of elevated insulin resistance. Many patients with Type 2 diabetes have renal impairment as a late complication of inadequate glycaemic control [1]. Microalbuminuria, the earliest indicator of nephropathy attributable to diabetes, affects 25% of patients with Type 2 diabetes within 10 years of diagnosis [2]. Diabetic nephropathy develops in 5–10% of patients with both Type 2 diabetes and microalbuminuria each year [3–7]. Therefore, therapies that seek to normalize glycaemic control in Type 2 diabetes and that at the same time are safe and effective for use in the presence of renal dysfunction are needed. A number of current therapies for Type 2 diabetes are known to have pharmacokinetics influenced by renal dysfunction: reduced clearance, increased half-life and higher peak levels reported for metformin, glimepiride and acarbose, respectively [8–11]. As a result, metformin is contraindicated in patients with renal dysfunction [8], acarbose is not recommended for use in such patients [11], and conservative dosing of sulphonylureas is recommended [9, 10]. Exenatide (exendin-4), an incretin mimetic, shows significant changes in pharmacokinetics in renal dysfunction and is not recommended for use by patients with severe renal impairment or end-stage renal disease (ESRD) [12, 13].
Glucagon-like peptide-1 (GLP-1) belongs to the incretin class of hormones, which exert an influence over multiple physiological functions, including a rapid blood glucose-lowering effect in response to enteral nutrient absorption. These effects make GLP-1 a potent blood glucose-lowering agent with multiple potential beneficial effects [14], potentially able to modulate the progression of Type 2 diabetes [15–17]. It is therefore of interest from the point of view of Type 2 diabetes therapy [16]. Native GLP-1 is rapidly metabolized by dipeptidyl peptidase-4, which is found in multiple tissues and cell types, as well as in the circulation [18]. Clearance of native GLP-1 and its metabolites is largely mediated by the kidneys [18].
Liraglutide is a once-daily human GLP-1 analogue under development for the treatment of hyperglycaemia in patients with Type 2 diabetes. Liraglutide has a high degree of sequence identity to human GLP-1, but differs in having a Arg34Lys substitution, and a glutamic acid and 16-C free fatty acid addition to Lys26 [19]. Its half-life in humans is approximately 13 h after subcutaneous (s.c.) injection [20], allowing once-daily administration. The metabolism of liraglutide is similar to that of large peptide, i.e. fully degraded in the body [21], and there is no indication that the kidney is a main organ for elimination.
In vitro receptor studies have shown liraglutide to be a potent and selective agonist on the cloned human GLP-1 receptor, and animal studies have shown it to be capable of lowering blood glucose, stimulating insulin secretion, reducing plasma glucagon levels, inhibiting appetite and gastric emptying, reducing body weight, and increasing β-cell volume or mass [22–27].
Liraglutide used as monotherapy has been shown to significantly improve glycaemic control and reduce body weight, with a low risk of hypoglycaemia, in patients with Type 2 diabetes [28, 29]. Liraglutide has also shown favourable effects on several parameters of β-cell function [30–34] and to improve early markers of cardiovascular disease [35].
The aim of this study was to examine the pharmacokinetics of liraglutide in people with varying degrees of renal impairment, to investigate whether dose adjustment of liraglutide is required.
Methods
Design
This was a single-centre, single-dose, parallel-group, open-label trial, investigating the pharmacokinetic and safety profile of liraglutide in healthy vs. renally impaired subjects. The study was conducted according to published guidance for such studies [36, 37].
Thirty subjects, of whom 24 had varying degrees of renal impairment and six had normal renal function, were included in the trial. Subjects were male and female adults, aged between 18 and 85 years, with body mass index (BMI) ≤40 kg m−2 and who met pre-defined criteria for sub-categorization according to renal function [i.e. creatinine clearance (CrCL), which was estimated using the Cockcroft–Gault formula [38]]. The categories were: normal renal function (CrCL > 80 ml min−1); mild (CrCL > 50 to ≤80 ml min−1), moderate (CrCL > 30 to ≤50 ml min−1) or severe renal impairment (CrCL ≤30 ml min−1) and one group with ESRD requiring dialysis. The ESRD group included subjects on continuous ambulatory peritoneal dialysis (CAPD) only and CAPD was continued during the sampling period. Subjects receiving haemodialysis were excluded, as were renally transplanted subjects, those with serious cardiac disease [heart failure New York Heart Association (NYHA) functional class III or IV; myocardial infarction within 3 months; unstable angina pectoris], uncontrolled hypertension (diastolic blood pressure ≥100 mmHg or systolic blood pressure ≥180 mmHg) and those with known hepatic disease or elevated liver enzymes (≥2.5 times upper normal range).
Subjects in the group of normal renal function were aimed to be gender and body weight matched with the renally impaired groups. Medications that could change the tubulary secretion of creatinine were not allowed during the trial. Renal patients were allowed to continue their current treatment for the renal disease.
All subjects gave their written consent prior to any trial-related procedures. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. The study protocol and informed-consent information were approved by the Ethics Committee, Ministry of Health, Christchurch, New Zealand.
Liraglutide administration, sample collection and other assessments
Subjects attended a screening visit to assess their eligibility. Within 21 days of that visit, subjects attended a second visit where inclusion and exclusion criteria were confirmed. A dose visit immediately followed during which all subjects received a single 0.75-mg s.c. dose of liraglutide into the abdomen using a pen-injector. To determine the plasma concentrations of liraglutide, blood samples were drawn in the period from 30 min before to 72 h after administration of trial product (to cater for a potentially prolonged elimination half-life in subjects with renal impairment). The samples (3 ml each) were taken 30 and 15 min prior to administration of liraglutide and 2, 4, 6, 8, 9, 9.5, 10, 10.5, 11, 11.5, 12, 12.5, 13, 13.5, 14, 15, 16, 21, 24, 36, 48, 60 and 72 h after administration. There was a follow-up visit up to 2 weeks later to evaluate safety parameters.
Adverse events were recorded throughout the trial. Other safety assessments included physical examination prior to dosing and following the last blood sample. In addition, supine blood pressure, pulse and ECG were recorded and laboratory safety parameters were measured prior to liraglutide administration, 10–15 h following dose administration and following completion of the blood sampling. During the dose visit subjects remained at the study site from dose administration between 21.00 and 22.00 h and the following 48 h; thereafter subjects were allowed to leave the clinic and return for the last samples. When subjects attended the clinic they were served standard meals for breakfast, lunch, dinner and snacks at predefined time points, and plasma glucose levels were measured prior to each meal and at bedtime.
Laboratory assessments
The bioanalysis of liraglutide in plasma was performed by Capio Diagnostik (Copenhagen, Denmark) using a validated enzyme-linked immunosorbent assay as described by Agersøet al.[20]. The assay measures total liraglutide plasma concentration and is specific for intact liraglutide, the repeatability is 2.4–6.5%, the day-to-day variation is 3.7–10%, the lower limit of quantification is 18 pM, and dilution is documented up to 16-fold while maintaining linearity.
The fraction of liraglutide bound to plasma proteins was measured on spiked samples by reiterated stepwise equilibrium dialysis by Novo Nordisk A/S (Bagsvaerd, Denmark). A blood sample was drawn from each subject 30 min prior to dose administration for estimation of plasma protein binding. Unbound fraction of liraglutide was calculated as (100-bound fraction) %.
Plasma glucose levels were measured for safety reasons when subjects were at the site during the blood sampling period, using a glucometer. Analyses for clinical chemistry and haematology were performed by Canterbury Health Laboratories (Christchurch, New Zealand).
Pharmacokinetic and statistical analyses
The primary pharmacokinetic end-point used was the area under the liraglutide concentration curve from time zero to infinity (AUC) estimated by standard noncompartment methods and was derived as the sum of two areas: the area under the curve from zero to last valid measurement t (AUC0–t) and the area from the last valid measurement to infinity. AUC0–t was calculated by the trapezoidal method. The last area was approximated by the area from t to infinity under an exponential curve with terminal rate λz. In addition, the maximum concentration, Cmax, and the time for maximum concentration, tmax, were reported. Secondarily, the trial also included the estimation of other pharmacokinetic endpoints such as t1/2 and CL/F using standard methods for estimation.
An anova of the log-transformed pharmacokinetic end-points, adjusted for renal group, age and log(weight), was performed. Equivalence between the group of healthy subjects and the group of subjects with severe renal impairment was considered to be demonstrated if the 90% confidence interval (CI) for an end-point ratio was wholly contained in the interval 0.70–1.43. The analysis of tmax was performed by nonparametric methods for independent samples (Hodges–Lehmann estimator). An exploratory statistical analysis including albumin levels as covariate in the analysis of plasma liraglutide concentrations was made.
Regression analyses of log AUC and secondary pharmacokinetic end-points were performed in which log(creatinine clearance), log(weight) and age were accounted for. Statistical analyses were performed using SAS version 8.0, PROC MIXED (SAS Institute Inc., Cary, NC, USA).
Results
Subjects: baseline characteristics
All enrolled and exposed subjects were White, except for two men of Maori and Asian/Pacific Island origin, and all were aged between 31 and 82 years. A total of 30 subjects were exposed to liraglutide and completed the trial. Two subjects with moderate renal impairment and one of the ESRD group had Type 2 diabetes.
Of those subjects entering the trial, six had normal renal function, six had mild renal impairment, seven moderate impairment, five severe impairment, and six had ESRD requiring CAPD dialysis (Table 1). All groups included both female and male subjects. Creatinine clearance at entry varied between groups, ranging from 132 ml min−1 in the group with normal renal function to 14 ml min−1 in the severely impaired group. The groups had generally similar average BMI and body weight, although mean body weight was lower in the severe renally impaired group. There was a difference in mean age between groups, with highest mean age in the ESRD group (70.8 years) and lowest in the healthy subjects (41.8 years). The mean baseline serum albumin levels were 43.3, 42.0, 39.3, 40.8 and 38.2 g l−1 across the five groups; and slightly below the reference range for only a few subjects with moderate impairment and ESRD, respectively.
Table 1.
Baseline demographics and characteristics by severity of renal impairment ('renal group’)
| Healthy | Mild | Moderate | Severe | End stage | |
|---|---|---|---|---|---|
| Sex (n) | |||||
| Male | 4 | 4 | 5 | 4 | 5 |
| Female | 2 | 2 | 2 | 1 | 1 |
| Age (years) | |||||
| Mean | 41.8 | 58.5 | 56.6 | 57.2 | 70.8 |
| Min–max | 36.0–52.0 | 46.0–70.0 | 31.0–82.0 | 36.0–75.0 | 61.0–81.0 |
| Body weight (kg) | |||||
| Mean | 84.7 | 82.2 | 85.2 | 71.7 | 82.8 |
| Min–max | 62.0– 99.0 | 64.0–99.0 | 72.0–104.0 | 62.5–81.0 | 63.5–107.0 |
| BMI (kg m−2) | |||||
| Mean | 27.4 | 30.1 | 27.3 | 25.7 | 28.8 |
| Min–max | 22.8–33.1 | 22.9–34.3 | 23.1–30.4 | 23.5–31.8 | 22.9–37.4 |
| Creatinine clearance (ml min−1) | |||||
| Mean | 110.0 | 68.5 | 35.6 | 22.2 | – |
| Min–max | 84.0–132.0 | 60.0–77.0 | 31.0–46.0 | 14.0–30.0 | – |
BMI, body mass index; min–max, minimum to maximum.
Pharmacokinetic characteristics of liraglutide
Concentration profiles were similar in all groups (Figure 1). Values for AUC showed no consistent trend with decreasing renal function, ranging from a lowest mean exposure of 219.8 nmol h l−1 in the mild renal impairment group to the largest mean exposure of 274.3 nmol h l−1 in healthy subjects (Table 2). Comparisons between renal impairment groups and the healthy group did not, however, demonstrate equivalence according to the predefined criteria for 90% CI (Table 3). All comparisons showed lower exposure in the renally impaired groups relative to the group with normal renal function (Table 3).
Figure 1.

Plasma concentration–time curve of liraglutide in healthy and renally impaired subjects. Healthy ( ); Mild (- -•- -); Moderate (- -□- -); Severe (- -▵- -); End stage (—○—)
); Mild (- -•- -); Moderate (- -□- -); Severe (- -▵- -); End stage (—○—)
Table 2.
Pharmacokinetics of liraglutide by renal impairment group
| Healthy | Mild | Moderate | Severe | End stage | |
|---|---|---|---|---|---|
| n | 6 | 6 | 7 | 5 | 6 |
| AUC (nmol h l−1) | |||||
| Mean (SD) | 274.3 (71.4) | 219.8 (76.6) | 256.7 (63.2) | 273.6 (61.4) | 265.4 (104.2) |
| Cmax (nmol l−1) | |||||
| Mean (SD) | 9.25 (2.47) | 7.87 (2.79) | 9.17 (2.45) | 9.17 (1.96) | 10.48 (4.87) |
| tmax (h) | |||||
| Median (min–max) | 12.50 (11.50–21.00) | 12.00 (9.50–16.00) | 12.50 (11.00– 16.00) | 11.00 (10.00–14.00) | 10.25 (6.00–12.50) |
| t1/2 (h) | |||||
| Mean (SD) | 14.25 (3.21) | 11.90 (1.40) | 11.90 (1.01) | 11.88 (1.80) | 11.13 (0.91) |
| CL/F (l h−1) | |||||
| Mean (SD) | 0.79 (0.29) | 1.00 (0.32) | 0.82 (0.22) | 0.76 (0.18) | 0.86 (0.33) |
t1/2, harmonic mean (SD).
Table 3.
Estimated ratios of pharmacokinetics of liraglutide in subjects with renal impairment vs. healthy subjects
| Mild: healthy | Moderate: healthy | Severe: healthy | End stage: healthy | |
|---|---|---|---|---|
| n | 6 | 7 | 5 | 6 |
| AUC | ||||
| Estimate (90% CI) | 0.67 (0.54, 0.85) | 0.86 (0.70, 1.07) | 0.73 (0.57, 0.94) | 0.74 (0.56, 0.97) |
| Cmax | ||||
| Estimate (90% CI) | 0.75 (0.57, 0.98) | 0.96 (0.74, 1.23) | 0.77 (0.57, 1.03) | 0.92 (0.67, 1.27) |
| tmax (h) | ||||
| Estimate (90% CI) | −2.00 (−6.00, 2.00) | 0.00 (−4.50, 2.50) | −1.50 (−7.00, 0.50) | –2.50 (−8.50, −1.00) |
| t1/2 (h) | ||||
| Estimate (90% CI) | 0.79 (0.68, 0.91) | 0.79 (0.69, 0.91) | 0.79 (0.68, 0.93) | 0.71 (0.60, 0.84) |
| CL/F | ||||
| Estimate (90% CI) | 1.48 (1.18, 1.87) | 1.16 (0.93, 1.44) | 1.37 (1.07, 1.76) | 1.36 (1.04, 1.78) |
Estimates are ratio except for tmax, where differences are shown.
There were no consistent trends in liraglutide Cmax with decreasing renal function (Table 2); mean maximum concentration, Cmax, ranged from 10.48 nmol l−1 in the ESRD group to 7.87 nmol l−1 in the mild impairment group. Mean t1/2 was reported between 11 h in the ESRD group and 14 h in the healthy group. The median time to maximum liraglutide concentration appeared around 10–12.5 h. The mean total apparent clearance (CL/F) was also similar across the trial groups (Table 2), ranging from 0.76 l h−1 in the severely impaired group to slowest (1.00 l h−1) in the mildly impaired group. Equivalence was not demonstrated between any of the renal impairment groups and the healthy group for Cmax (except between moderate and healthy, for which equivalence was demonstrated), t1/2 or CL/F; and estimates showed lower Cmax, shorter t1/2 and faster CL/F for the renal impairment groups compared with the healthy group (Table 3). Likewise, no clear trend was seen for extent of renal impairment and binding of liraglutide to plasma proteins; protein binding values were high in all groups (ranging from 98.3% in a subject with mild renal impairment to 99.8% measured in a subject with normal renal function) but overall with highly variable results. Consistent with total liraglutide exposure, the fraction of unbound liraglutide did not increase across renal groups (data not shown).
In contrast to the analysis of equivalence, continuous regression analysis showed renal function (creatinine clearance for subjects with normal and mild-to-severe renal impairment) to have no statistically significant effect on the primary end-point, AUC (covariate estimate for renal function = 0.056; 95% CI [−0.132 to 0.245]; P = 0.539). Based on the regression model, the expected ratio of AUC between a subject with the lowest creatinine clearance (14 ml min−1 in a subject from the severe group of renal impairment) and the highest clearance (132 ml min−1, subject from the healthy group), with the same age and weight, had an estimate of 0.88 (95% CI 0.58, 1.34), which was not statistically significantly different from 1. Body weight, but not age, was found to be a significant covariate in the regression analysis (covariate estimate for age = 0.003; 95% CI [−0.005 to 0.012]; P = 0.439; covariate estimate for weight =−1.096; 95% CI [−1.774 to −0.417]; P = 0.003). Scatterplot of AUC versus creatinine clearance is shown in Figure 2.
Figure 2.
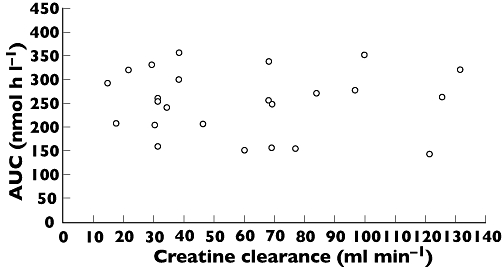
Scatter plot of liraglutide AUC vs. creatinine clearance
Regression analyses also showed no relationship between creatinine clearance and Cmax or other pharmacokinetic end-points, while the exploratory analysis showed no significant association between liraglutide exposure and albumin levels (data not shown). It should be noted, however, that most values for albumin levels were within the reference range and albumin levels were below the reference range only for a few subjects.
Adverse events and other safety assessments
Treatment-emergent adverse events reported as probably or possibly related to trial drug exposure are shown in Table 4. The most frequently reported adverse events were headache and symptoms related to the gastrointestinal system organ class. All events were mild or moderate in intensity. In total, nine subjects (three healthy, one in each of the mild and moderate renal impairment groups and two subjects in each of the severe and ESRD groups) reported 11 adverse events related to the gastrointestinal system and assessed as probably or possibly related to trial drug (Table 4). The three events in the ESRD group were reported by two subjects, one reporting nausea and vomiting with onset a few hours apart.
Table 4.
Treatment-emergent adverse events
| Healthy | Mild | Moderate | Severe | End stage | |
|---|---|---|---|---|---|
| n | 6 | 6 | 7 | 5 | 6 |
| Gastrointestinal | |||||
| Nausea | 1 [1] | 0 | 1 [1] | 1 [1] | 1 [1] |
| Vomiting | 0 | 0 | 0 | 1 [1] | 2 [2] |
| Diarrhoea | 1 [1] | 1 [1] | 0 | 0 | 0 |
| Abdominal discomfort | 1 [1] | 0 | 0 | 0 | 0 |
| Abdominal distension | 1 [1] | 0 | 0 | 0 | 0 |
| Nervous system | |||||
| Headache | 1 [1] | 0 | 1 [1] | 2 [2] | 0 |
| Dizziness | 0 | 0 | 1 [1] | 0 | 0 |
| Lethargy | 0 | 0 | 0 | 0 | 1 [1] |
| Musculoskeletal and connective tissue | |||||
| Pain in extremity | 1 [1] | 0 | 0 | 0 | 0 |
| Respiratory, thoracic, mediastinal | |||||
| Productive cough | 0 | 1 [1] | 0 | 0 | 0 |
| Vascular | |||||
| Hot flush | 0 | 1 [1] | 0 | 0 | 0 |
| All events | 3 [6] | 2 [3] | 1 [3] | 2 [4] | 3 [4] |
Data show the number of subjects with events [number of events] in each group, for events considered possibly or probably related to trial product.
In addition to the adverse events summarized in Table 4, one subject from the ESRD group reported three events of vomiting, all considered unlikely to be related to trial drug, one of the events with onset prior to dose administration and two events approximately 1 week following dose administration. Additionally, one subject reported one episode of application site inflammation. This event occurred 3 days after dosing and was thought unlikely to be related to the trial product. The subject recovered within 3 days. No serious adverse event was reported.
One healthy subject had one hypoglycaemic event 15 h after administration of liraglutide (plasma glucose 2.5 mmol l−1). One renally impaired subject (severe group) experienced four separate hypoglycaemic events between 14 and 29 h after liraglutide administration (plasma glucose values between 2.8 and 3.6 mmol l−1). All events were minor or symptomatic only.
No clinically significant values or changes in vital signs (blood pressure and pulse), ECG, physical examinations or clinical laboratory assessments were reported.
Discussion
We examined the once-daily human GLP-1 analogue liraglutide in subjects with varying degrees of renal impairment, to investigate if pharmacokinetics are changed across groups resulting in the need for dose adjustments in this group of patients, and, in particular, if the liraglutide dose should be reduced. The trial included renally impaired subjects of all severities: mild to severe and one group of subjects undergoing peritoneal dialysis. The trial population therefore represents a broad range of subjects with renal impairment. In addition to their renal disorder, three subjects were diagnosed with Type 2 diabetes.
Although renal dysfunction is common in people with diabetes, a number of established oral antidiabetic drugs are unsuitable for use in these subjects or require special precautions [8–11, 39]. Poor tolerability and significant changes in the pharmacokinetics of a newer antidiabetic product, the incretin mimetic exenatide (exendin-4), would argue against use of the available therapeutic doses of that drug in subjects with significant renal impairment, and exenatide is not recommended for use by patients with severe renal impairment or ESRD [12, 13].
The results of our trial showed that, overall, none of the renally impaired groups presented with higher mean liraglutide exposure than the healthy reference group. Although the comparison between the group with normal renal function and the renally impaired groups did not demonstrate equivalence, the estimated levels of exposure in the groups of renally impaired subjects were lower than that in the healthy subjects. The continuous regression analyses of log(AUC) and log(Cmax) of liraglutide with creatinine clearance as a continuous covariate showed that the estimated ratio of AUC between a subject with the lowest creatinine clearance and a subject with the highest creatinine clearance was not statistically significantly different from 1, suggesting no effect of renal impairment on exposure of liraglutide.
From these analyses a minor lowering of liraglutide exposure with decreasing creatinine clearance cannot be ruled out. Liraglutide half-life was not found to be increased and clearance was not found to be decreased in subjects with renal dysfunction, and the trial results therefore support the observation that the kidneys are not a major site for elimination and degradation of liraglutide. In contrast, the kidney is the primary route for elimination and degradation of exenatide [40].
Albumin levels in this population of renally impaired subjects were not lowered significantly, which may explain why no association was found between liraglutide exposure and albumin levels. It should be noted that a statistically positive association between liraglutide exposure and albumin level was observed in another trial including subjects with hepatic impairment with albumin levels significantly lower than the reference range. However, it was not possible to distinguish if albumin levels or the hepatic impairment was associated with liraglutide exposure [41].
As expected, most reported adverse events were gastrointestinal. Although the group of subjects with ESRD experienced, in total, two events of vomiting compared with one event in the severe renal impairment group and none in the other groups, this was in contrast to pharmacokinetic analyses, which demonstrated that an increase in liraglutide exposure among renally impaired subjects would not be expected (potentially the opposite), and subsequently an increase in reported gastrointestinal adverse events is not foreseen.
In conclusion, the results of this study indicate that the pharmacokinetics of liraglutide is essentially independent of renal function. However, a lower exposure with renal impairment cannot be excluded based on these data. No safety concerns were raised during the trial, and the degree of renal impairment of subjects in this trial did not appear to be associated with an increased risk of adverse events. Thus, we can expect that subjects with Type 2 diabetes who also suffer from renal impairment, including subjects with ESRD, will be able to use standard treatment regimens for liraglutide without dose adjustments. There is, however, currently limited experience with liraglutide in patients beyond mild-stage renal disease.
Acknowledgments
L.V.J., C.H. and M.Z. are employed by Novo Nordisk and hold stocks in the company.
The authors accept direct responsibility for this paper but are grateful for the contribution made by Watermeadow Medical (supported by Novo Nordisk A/S, Bagsvaerd, Denmark) in developing the draft manuscript from an agreed outline and in collating comments.
REFERENCES
- 1.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR. Risk factors for renal dysfunction in type 2 diabetes: UK Prospective Diabetes Study 74. Diabetes. 2006;55:1832–9. doi: 10.2337/db05-1620. [DOI] [PubMed] [Google Scholar]
- 2.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63:225–32. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 3.Parving H-H, Østerby R, Ritz E. Diabetic nephropathy. In: Brenner BM, editor. The Kidney. 6th. Philadelphia, PA: W.B. Saunders; 2000. pp. 1731–73. [Google Scholar]
- 4.Ravid M, Savin H, Jutrin I, Bental T, Katz B, Lishner M. Long-term stabilizing effect of angiotensin-converting enzyme inhibition on plasma creatinine and on proteinuria in normotensive type II diabetic patients. Ann Intern Med. 1993;118:577–81. doi: 10.7326/0003-4819-118-8-199304150-00001. [DOI] [PubMed] [Google Scholar]
- 5.Nelson RG, Bennett PH, Beck GJ, Tan M, Knowler WC, Mitch WE, Hirschman GH, Myers BD. Development and progression of renal disease in Pima Indians with non-insulin-dependent diabetes mellitus. N Engl J Med. 1996;335:1636–42. doi: 10.1056/NEJM199611283352203. [DOI] [PubMed] [Google Scholar]
- 6.Gæde P, Vedel P, Parving H-H, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet. 1999;353:617–22. doi: 10.1016/S0140-6736(98)07368-1. [DOI] [PubMed] [Google Scholar]
- 7.Heart Outcomes Prevention. Evaluation (HOPE) Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253–9. [PubMed] [Google Scholar]
- 8.Glucophage Prescribing Information. Available at http://www.fda.gov/CDER/foi/label/2000/21202lbl.pdf (last accessed 10 November 2008.
- 9.Micronase (glyburide) Tablets Prescribing Information. Available at http://media.pfizer.com/files/products/uspi_micronase.pdf (last accessed 2 November 2008.
- 10.Glipizide Extended Release Tablets Prescribing Information. Available at http://media.pfizer.com/files/products/uspi_glipizide.pdf (last accessed 2 November 2008.
- 11.Precose (acarbose tablets) Prescribing Information. Available at http://www.univgraph.com/bayer/inserts/precose.pdf (last accessed 10 November 2008.
- 12.Linnebjerg H, Kothare PA, Park S, Mace K, Reddy S, Mitchell M, Lins R. Effect of renal impairment on the pharmacokinetics of exenatide. Br J Clin Pharmacol. 2007;64:317–27. doi: 10.1111/j.1365-2125.2007.02890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byetta (exenatide injection) Prescribing Information. Available at http://pi.lilly.com/us/byetta-pi.pdf (last accessed 2 November 2008.
- 14.Holst JJ. Enteroglucagon. Annu Rev Physiol. 1997;59:257–71. doi: 10.1146/annurev.physiol.59.1.257. [DOI] [PubMed] [Google Scholar]
- 15.Meier JJ, Gallwitz B, Nauck MA. Glucagon-like peptide and gastric inhibitory polypeptide: potential applications in type 2 diabetes mellitus. BioDrugs. 2003;17:93–102. doi: 10.2165/00063030-200317020-00002. [DOI] [PubMed] [Google Scholar]
- 16.Nauck MA, Meier JJ, Creutzfeldt W. Incretins and their analogues as new antidiabetic agents. Drug News Perspect. 2003;16:413–22. doi: 10.1358/dnp.2003.16.7.829353. [DOI] [PubMed] [Google Scholar]
- 17.Nauck MA, Hompesch M, Filipczak R, Le TDT, Zdravkovic M, Gumprecht J. Five weeks of treatment with the GLP-1 analogue liraglutide improves glycaemic control and lowers body weight in subjects with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2006;114:417–23. doi: 10.1055/s-2006-924230. [DOI] [PubMed] [Google Scholar]
- 18.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–57. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 19.Knudsen LB, Nielsen PF, Huusfeldt PO, Johansen NL, Madsen K, Pedersen FZ, Thøgersen H, Wilken M, Agersø H. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem. 2000;43:1664–9. doi: 10.1021/jm9909645. [DOI] [PubMed] [Google Scholar]
- 20.Agersø H, Jensen LB, Elbrønd B, Rolan P, Zdravkovic M. The pharmacokinetics, pharmacodynamics, safety and tolerability of NN2211, a new long-acting GLP-1 derivative, in healthy men. Diabetologia. 2002;45:195–202. doi: 10.1007/s00125-001-0719-z. [DOI] [PubMed] [Google Scholar]
- 21.Helleberg H, Malm-Erjefält M, Bjørnsdottir I, Larsen US, Oosterhuis B, van Lier JJ, Zdravkovic M. Metabolism and excretion of [Pal-3H]-liraglutide in human healthy subjects. Diabetes. 2008;57(Suppl. 1):A581. Abstract 2107-PO. [Google Scholar]
- 22.Rolin B, Larsen MO, Gotfredsen CF, Deacon CF, Carr RD, Wilken M, Knudsen LB. The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases beta-cell mass in diabetic mice. Am J Physiol Endocrinol Metab. 2002;283:E745–52. doi: 10.1152/ajpendo.00030.2002. [DOI] [PubMed] [Google Scholar]
- 23.Raun K, von Voss P, Gotfredsen CF, Golozoubova V, Rolin B, Knudsen LB. Liraglutide, a long-acting GLP-1 analogue, reduces body weight and food intake in obese candy fed rats while a DPP-IV inhibitor, LAF237, does not. Diabetes. 2007;56:8–15. doi: 10.2337/db06-0565. [DOI] [PubMed] [Google Scholar]
- 24.Ribel U, Larsen MO, Rolin B, Carr RD, Wilken M, Sturis J, Westergaard L, Deacon CF, Knudsen LB. NN2211: a long-acting glucagon-like peptide-1 derivative with anti-diabetic effects in glucose-intolerant pigs. Eur J Pharmacol. 2002;451:217–25. doi: 10.1016/s0014-2999(02)02189-1. [DOI] [PubMed] [Google Scholar]
- 25.Sturis J, Gotfredsen CF, Romer J, Rolin B, Ribel U, Brand CL, Wilken M, Wassermann K, Deacon CF, Carr RD, Knudsen LB. GLP-1 derivative liraglutide in rats with b-cell deficiencies: influence of metabolic state on b-cell mass dynamics. Br J Pharmacol. 2003;140:123–32. doi: 10.1038/sj.bjp.0705397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bregenholt S, Moldrup A, Blume N, Karlsen AE, Nissen Friedrichsen B, Tornhave D, Knudsen LB, Petersen JS. The long-acting glucagon-like peptide-1 analogue, liraglutide, inhibits beta-cell apoptosis in vitro. Biochem Biophys Res Commun. 2005;330:577–84. doi: 10.1016/j.bbrc.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Friedrichsen BN, Neubauer N, Lee YC, Gram VK, Blume N, Petersen JS, Nielsen JH, Møldrup A. Stimulation of pancreatic β-cell replication by incretins involves transcriptional induction of cyclin D1 via multiple signalling pathways. J Endocrinol. 2006;188:481–92. doi: 10.1677/joe.1.06160. [DOI] [PubMed] [Google Scholar]
- 28.Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM, Zdravkovic M, Bode B, for the LEAD-3 (Mono) Study Group Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomized, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–81. doi: 10.1016/S0140-6736(08)61246-5. [DOI] [PubMed] [Google Scholar]
- 29.Vilsbøll T, Zdravkovic M, Le Thi T, Krarup T, Schmitz O, Courreges J-P, Verhoeven R, Buganova I, Madsbad S. Liraglutide, a once-daily GLP-1 analog, monotherapy significantly improves glycaemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes mellitus. Diabetes Care. 2007;30:1608–10. doi: 10.2337/dc06-2593. [DOI] [PubMed] [Google Scholar]
- 30.Juhl CB, Hollingdal M, Sturis J, Jakobsen G, Agersø H, Veldhuis J, Pørksen N, Schmitz O. Bedtime administration of NN2211, a long-acting GLP-1 derivative, substantially reduces fasting and postprandial glycemia in type 2 diabetes. Diabetes. 2002;51:424–9. doi: 10.2337/diabetes.51.2.424. [DOI] [PubMed] [Google Scholar]
- 31.Chang AM, Jakobsen G, Sturis J, Smith MJ, Bloem CJ, Galecki A, Halter JB. The GLP-1 derivative NN2211 restores beta-cell sensitivity to glucose in type 2 diabetic patients after a single dose. Diabetes. 2003;52:1786–91. doi: 10.2337/diabetes.52.7.1786. [DOI] [PubMed] [Google Scholar]
- 32.Degn KB, Juhl CB, Sturis J, Jakobsen G, Brock B, Chandramouli V, Rungby J, Landau BR, Schmitz O. One week's treatment with the long-acting GLP-1 derivative, liraglutide (NN2211), markedly improves 24-h glycaemia, alpha- and beta-cell function and reduces endogenous glucose release in patients with type 2 diabetes. Diabetes. 2004;53:1187–94. doi: 10.2337/diabetes.53.5.1187. [DOI] [PubMed] [Google Scholar]
- 33.Madsbad S, Brock B, Perrild H, Lervang HH, Kolendorf K, Krarup T, Schmitz O, Le-Thi T, Zdravkovic M, Vilsboll T. Liraglutide significantly improves first-phase insulin secretion and maximal beta-cell secretory capacity. Diabetologia. 2006;49(Suppl. 1):A0004. [Google Scholar]
- 34.Vilsbøll T, Brock P, Perrild H, Levin K, Lervang HH, Kølendorf K, Krarup T, Schmitz O, Zdravkovic M, Le-Thi T, Madsbad S. Liraglutide, a once-daily human GLP-1 analogue, improves pancreatic B-cell function and arginine-stimulated insulin secretion during hyperglycaemia in patients with Type 2 diabetes mellitus. Diabet Med. 2008;25:152–6. doi: 10.1111/j.1464-5491.2007.02333.x. [DOI] [PubMed] [Google Scholar]
- 35.Courreges JP, Zdravkovic M, Vilsboll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, Verhoeven R, Buganova I, Madsbad S. Liraglutide, a once-daily human glucagon-like peptide-1 analogue, shows beneficial effects on cardiovascular risk biomarkers in patients with type 2 diabetes. Diabet Med. 2008;25:1129–31. doi: 10.1111/j.1464-5491.2008.02484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Committee for Medicinal Products for Human Use. London: European Medicines Agency; Note for Guidance on the Evaluation of the Pharmacokinetics of Medicinal Products in Patients with Impaired Renal Function. Available at http://www.tga.gov.au/DOCS/pdf/euguide/ewp/022502en.pdf (last accessed 4 June 2009. [Google Scholar]
- 37.Guidance for Industry: Pharmacokinetics in Patients with Impaired Renal Function—Study design, Data Analysis, and Impact on Dosing and Labeling. Rockville, MD: US Department of Health and Human Services Food and Drug Administration; 1998. Available at http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072127.pdf (last accessed 4 June 2009. [Google Scholar]
- 38.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 39.Runge S, Mayerle J, Warnke C, Robinson D, Roser M, Felix SB, Friesecke S. Metformin-associated lactic acidosis in patients with renal impairment solely due to drug accumulation? Diabetes Obes Metab. 2008;10:91–3. doi: 10.1111/j.1463-1326.2006.00657.x. [DOI] [PubMed] [Google Scholar]
- 40.Copley K, McCowen K, Hiles R, Nielsen LL, Young A, Parkes DG. Investigation of exenatide elimination and its in vivo and in vitro degradation. Curr Drug Metab. 2006;7:367–74. doi: 10.2174/138920006776873490. [DOI] [PubMed] [Google Scholar]
- 41.Flint A, Nazzal K, Jagielski P, Segel S, Zdravkovic M. Influence of hepatic impairment on pharmacokinetics of the long-acting human GLP-1 analogue liraglutide. Diabetes. 2007;56(Suppl. 1):A145. doi: 10.1111/j.1365-2125.2010.03762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


