Abstract
AIMS
To assess the pharmacokinetics (PK) of selective substrates of CYP1A2 (caffeine), CYP2C9 (S-warfarin), CYP2C19 (omeprazole), CYP2D6 (metoprolol) and CYP3A (midazolam) when administered orally and concurrently as a cocktail relative to the drugs administered alone.
METHODS
This was an open-label, single-dose, randomized, six-treatment six-period six-sequence William's design study with a wash-out of 7 or 14 days. Thirty healthy male subjects received 100 mg caffeine, 100 mg metoprolol, 0.03 mg kg−1 midazolam, 20 mg omeprazole and 10 mg warfarin individually and in combination (cocktail). Poor metabolizers of CYP2C9, 2C19 and 2D6 were excluded. Plasma samples were obtained up to 48 h for caffeine, metoprolol and omeprazole, 12 h for midazolam, 312 h for warfarin and the cocktail. Three different validated liquid chromatography tandem mass spectrometry methods were used. Noncompartmental PK parameters were calculated. Log-transformed Cmax, AUClast and AUC for each analyte were analysed with a linear mixed effects model with fixed term for treatment, sequence and period, and random term for subject within sequence. Point estimates (90% CI) for treatment ratios (individual/cocktail) were computed for each analyte Cmax, AUClast and AUC.
RESULTS
There was no PK interaction between the probe drugs when administered in combination as a cocktail, relative to the probes administered alone, as the 90% CI of the PK parameters was within the prespecified bioequivalence limits of 0.80, 1.25.
CONCLUSION
The lack of interaction between probes indicates that this cocktail could be used to evaluate the potential for multiple drug–drug interactions in vivo.
Keywords: cocktail, CYP, in vivo drug–drug interaction
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Numerous cocktails using concurrent administration of several cytochrome P450 (CYP) isoform-selective probe drugs have been reported to investigate drug–drug interactions in vivo.
This approach has several advantages: characterize the inhibitory or induction potential of compounds in development toward the CYP enzymes identified in vitro in an in vivo situation, assess several enzymes in the same trial, and have complete in vivo information about potential CYP-based drug interactions.
WHAT THIS STUDY ADDS
This study describes a new cocktail containing five probe drugs that has never been published.
This cocktail can be used to test the effects of a new chemical entity on multiple CYP isoforms in a single clinical study: CYP1A2 (caffeine), CYP2C9 (warfarin), CYP2C19 (omeprazole), CYP2D6 (metoprolol), and CYP3A (midazolam) and was designed to overcome potential liabilities of other reported cocktails.
Introduction
Multiple drug therapy is common in clinical practice. The resultant potential for drug–drug interactions (DDIs) is a safety concern. Therefore, there is a need to identify possible DDIs during early drug development. This typically begins with in vitro evaluation of a new chemical entity as an inhibitor or inducer of CYP isoforms. Positive in vitro findings then lead to follow-up studies in vivo. However, conducting multiple clinical drug interaction trials is costly and time-consuming. With a drug cocktail, more than one interaction can be tested in a single crossover study, as it involves the simultaneous administration of two or more probe drugs.
Numerous cocktails using concurrent administration of several cytochrome P450 (CYP) isoform-selective probe drugs have been reported to investigate DDI in vivo, like the ‘Inje’ or ‘Cooperstown 5 + 1’ cocktails [1, 2]. This approach was successfully used to investigate the effect of drugs on various CYP isoforms (e.g. the effect of propiverine on the activity of intestinal 3A and of hepatic CYP3A, CYP2C9, CYP2C19 and CYP1A2 [3]).
The main advantage is to characterize the inhibitory or induction potential of compounds in development toward the several CYP enzymes identified in vitro in an in vivo situation and therefore have complete in vivo information about potential CYP-based drug interactions in the same trial.
Drugs used in such a cocktail should be selective for individual CYP isoforms and should not interact with each other. This study describes the validation of a new cocktail containing five probe drugs for five isoforms: CYP1A2 (caffeine), CYP2C9 (warfarin), CYP2C19 (omeprazole), CYP2D6 (metoprolol) and CYP3A (midazolam).
The present clinical study was conducted to test for pharmacokinetic (PK) interactions between these probe drugs by administering them each alone and simultaneously. In addition, the overall safety of this cocktail was assessed during the study.
Materials and methods
Clinical study design
A single-centre, open-label, single oral dose study was carried out in healthy male subjects in a six-treatment six-period six-sequence William's design with a wash-out of 7 days after caffeine, omeprazole, metoprolol and midazolam treatments and 14 days after cocktail and warfarin treatments. The study was conducted at Optimed (Gieres, France) in accordance with the ethical principles set forth in the Declaration of Helsinki and in compliance with local regulations. The protocol was reviewed and approved by the Comite Consultatif pour la Protection des Personnes dans la Recherche Biomedicale de LYON B. Prior to any study procedures, subjects provided written informed consent after having received verbal and written information on the procedure and possible hazards and risks of the studies.
Thirty-three subjects met the inclusion/exclusion criteria of the study. Exclusion criteria included any medication or nutraceutical (e.g. St John's Wort) received within 20 days prior to the drug administration, or within six times the elimination half-life, whichever was longest, except occasional use of paracetamol; administration of enzyme-inducing or enzyme-inhibiting drugs within 3 months of trial initiation; history or presence of drug or alcohol abuse; excessive consumption of beverages containing caffeine (more than four cups or glasses per day) or unable to stop consumption during the hospitalization; smoking more than five cigarettes or equivalent per day or not able to stop smoking during hospitalization; and grapefruit and grapefruit-containing products or orange juice were prohibited from 48 h prior to each dosing until the last sample was taken for each period. CYP2C9, CYP2C19 and CYP2D6 poor metabolizers were excluded, based on genotyping of genomic DNA from blood samples.
International Normalized Ratio (INR) was monitored during the study and subjects were institutionalized until INR returned to baseline value ±10% or INR < 2 of each period with warfarin administration only. In cases where a major therapeutic effect was observed (e.g. INR > 5 and/or occurrence of haemorrhage) after warfarin or cocktail administration, the investigator had the responsibility to investigate the appropriateness of administering the antidote (vitamin K). If such a case occurred at the first warfarin administration, the subject should have been excluded from the second study period with warfarin administration.
Subjects, after attending the unit for the laboratory value check in the morning, were institutionalized on the afternoon the day before administration, until 24 h post administration (day 2) of each period.
Drug, dose and administration route
On day 1 of each corresponding period, subjects received one of the following treatments with 200 ml of water at approximately 08.00 h while fasting: caffeine 100 mg (four vials of 50 mg of Cafeine Cooper containing 25 mg of caffeine base); warfarin 10 mg (two tablets of 5 mg); omeprazole 20 mg (one capsule of 20 mg); metoprolol 100 mg (one coated tablet of 100 mg); midazolam 0.03 mg kg–1 (5 mg/5 ml vials mixed in 120 ml of 5% dextrose in water) administered; the cocktail: all five drugs administered together in the same doses and conditions. Blood samples were collected for 48 h for caffeine, metoprolol and omeprazole, 12 h for midazolam and 312 h for warfarin and the cocktail.
Except for metoprolol, these drugs are all listed as sensitive substrates in the Food and Drug Administration draft guidance. Yet metoprolol is a well-documented CYP2D6 probe drug. The doses chosen were based on the clinical tolerability of these drugs and the ability to assess reliably their PK parameters. For caffeine, 100 mg was chosen as it is approximately the amount found in one cup of coffee. For warfarin, in patients 10 mg is often used as a maintenance dose. For omeprazole, the dose of 20 mg once a day is used in the treatment of symptomatic gastro-oesophageal reflux disease and duodenal ulcer. For midazolam, as it has an absolute bioavailability of 74.5%, a dose of 0.03 mg kg–1 of the intravenous formulation given orally should lead to significant systemic exposure without major sedative effect [4]. Metoprolol is currently used in clinic at a daily dose of 100 mg. This dose allows metoprolol plasma concentrations to be quantified up to 24 h.
Bioanalytical methods
Caffeine and its metabolite (paraxanthine), metoprolol and omeprazole plasma samples were analysed together, while midazolam and R- and S-warfarin plasma concentrations were analysed separately. All procedures associated with this analysis were conducted following methods previously validated by Covance (Indianapolis, IN, USA).
Regarding caffeine, paraxanthine, metoprolol and omeprazole, plasma samples were spiked with an internal standard {caffeine-(trimethyl)-13C3, theophylline-(2-13C,1-3-15N2) [for paraxanthine], metoprolol-d7(+) tartrate, lansoprazole [for omeprazole]}. The analytes and their internal standards were then extracted from human plasma by Oasis HLB solid-phase extraction using sodium acetate, 5% methanol in water. The solid-phase extraction cartridges were subsequently placed on top of an Axygen 96-well plate and, to finish, 90% methanol in water was added to them. Next, the samples were analysed using a Sciex API 4000 liquid chromatography coupled with tandem mass spectrometry system (LC-MS/MS). Chromatographic separation of the compounds was accomplished using a Genesis C18 column, with 1% acetic acid in methanol : water (90:10, v : v) as the mobile phase at a flow rate of 1.00 ml min–1. The MS/MS system was operated using an electrospray in positive ionization mode. The precursor-to-product ion reactions monitored are presented in Table 1. The lower limit of quantification (LLOQ) was 25 ng ml–1 for caffeine and paraxanthine and 5 ng ml–1 for metoprolol and omeprazole.
Table 1.
Mass transition monitored for each probe and its internal standard
| Drug | Transition monitored | IS name | Transition monitored |
|---|---|---|---|
| Caffeine | 195.1 → 110.1 | Caffeine-(trimethyl)-13C3 | 198.1 → 140.0 |
| Paraxanthine | 181.1 → 68.9 | Theophylline-(2-13C,1-3-15N2 | 183.9 → 125.0 |
| Metoprolol | 268.1 → 116.2 | Metoprolol-d7(+) tartrate | 275.3 → 122.9 |
| Omeprazole | 346.1 → 198.0 | Lansoprazole | 370.0 → 252.0 |
| Midazolam | 326.1 → 291.1 | α-Hydroxytriazolam | 359.1 → 176.1 |
| R- and S-warfarin | 307.1 → 161.0 | R- and S-warfarin-d6 | 313.1 → 161.0 |
Regarding midazolam, plasma samples were spiked with an internal standard (α-hydroxytriazolam), alkalinized with 5% ammonium hydroxide, and extracted with methyl-tert-butyl ether. After evaporation of the organic solvent under nitrogen, reconstituted residues of the organic phase were analysed using a Sciex API 4000 LC-MS/MS. Chromatographic separation of the compounds was accomplished using a Chromolith SpeedROD RP-18e with 0.15% trifluoroacetic acid in ammonium formate and methanol (30:70, v/v) as the mobile phase at a flow rate of 1.00 ml min–1. The MS/MS system was operated using an electrospray in positive ionization mode. For midazolam and α-hydroxytriazolam, the precursor-to-product ion reactions monitored are presented in Table 1. The LLOQ for midazolam was 0.1 ng ml–1.
Finally regarding R- and S-warfarin, plasma samples were spiked with an internal standard (R- and S-warfarin-d6), acidified with 1.0 N sulphuric acid and extracted with 5% ethyl acetate in hexane. After evaporation of the organic solvent under nitrogen, reconstituted residues of the organic phase were analysed using a Sciex API 3000 negative ion atmospheric pressure chemical ionization LC-MS/MS. Chromatographic separation of the compounds was accomplished using an Astec β-cyclodextrin cyclobond I with acetonitrile : acetic acid : triethylamine (1000:3:2.5, v : v : v) as the mobile phase at a flow rate of 1.00 ml min–1. For R- and S-warfarin and R- and S-warfarin-d6, the precursor-to-product ion reactions monitored are presented in Table 1. The LLOQ for R- and S-warfarin was 1.0 ng ml–1.
Calibration standards and quality control (QC) samples were analysed in at least one batch to determine intra-assay precision and mean accuracy, and in a minimum of three batches to determine interassay precision and mean accuracy. Calibration standards and QC samples were interspersed throughout each batch. The accuracy of the method was determined by comparing the means of the measured concentrations of the calibration standards and QC samples with their theoretical concentrations. The ranges, interassay precisions and accuracy values for the calibration standards are presented in Table 2. The interassay results demonstrated a relative standard deviation for calibration standards and QC samples of <13%. The accuracy results demonstrated calculated deviation of mean value from nominal values in the range of –8.0 to 6.9% for calibration standards and QC samples.
Table 2.
Ranges, interassay precisions and accuracy values for each probe calibration standards
| Drug | Calibration range (ng ml–1) | Interassay precision (RSD%) | Accuracy (DMT%) |
|---|---|---|---|
| Caffeine | 25–20 000 | 2.9–6.6 | −4 to 6.9 |
| Paraxanthine | 25–20 000 | 3.7–7.4 | −3.5 to 6.3 |
| Metoprolol | 5–2500 | 2.1–7.3 | −1.2 to 1.6 |
| Omeprazole | 5–2 500 | 3–7.2 | −3.2 to 3.5 |
| Midazolam | 0.1–100 | 2.6–7.6 | −4.8 to 2.5 |
| R-warfarin | 1–100 | 2.8–8.2 | −3 to 3 |
| S-warfarin | 1–100 | 2.5–6.3 | −1.5 to 1 |
For all analytes, no significant interfering peaks were detected in matrix blanks.
Data analysis
PK parameters of caffeine, paraxanthine, metoprolol, midazolam, omeprazole, R-warfarin and S-warfarin were determined on day 1 of each treatment period by noncompartmental analysis of plasma concentrations and real time values using WinNonlin Professional, Version 4.01. The peak plasma concentration (Cmax), the time to reach Cmax (tmax), and time corresponding to the last concentration above the limit of quantification (tlast) were obtained directly from experimental observations. The area under the plasma concentration–time curve extrapolated to infinity (AUC) was calculated according to the following equation: AUC = AUClast+ (Clast/λz), where AUClast was calculated using the trapezoidal method from time 0 to the real time tlast. The terminal half-life (t1/2z) was determined according to the following equation: t1/2z= 0.693/λz, where λz is the slope of the regression line of the terminal phase of the plasma concentration–time curve, on a semi-logarithmic scale.
Statistical analysis
Transformed pharmacokinetic parameters (Cmax, AUClast and AUC values were log transformed, tmax was rank transformed and t1/2z values were replaced with λz values) were analysed with a linear mixed effects model with fixed term for treatment (cocktail and probe alone), sequence and period, and random term for subject within sequence, parameter = sequence + subject(sequence) + treatment + period, fit using SAS PROC MIXED. For Cmax, AUClast and AUC, estimates and 90% confidence intervals (CIs) for the ratios of treatments were obtained by computing estimates and 90% CIs for the differences between treatment means within the mixed model framework, and converting to ratios by the antilog transformation. Equivalence was concluded if the 90% CI for the ratio was entirely within the 0.80, 1.25 equivalence specifications. For tmax and t1/2z, differences between treatments were tested for significance with P-values from the mixed model analysis of rank tmax and λz, respectively.
Results
All subjects were healthy White men. The mean age was 26 years (range 19–35 years). The mean body mass index was 22.6 kg m–2 with a range of 18.2–25.8 kg m–2. Three of the 33 subjects who participated in the study discontinued study treatment, but none for safety-related reasons. Thus, 30 subjects were available for PK analyses.
Plots of the mean caffeine, paraxanthine, metoprolol, midazolam, omeprazole, R-warfarin and S-warfarin plasma concentrations administered alone or concurrently as a cocktail are shown in Figure 1.
Figure 1.
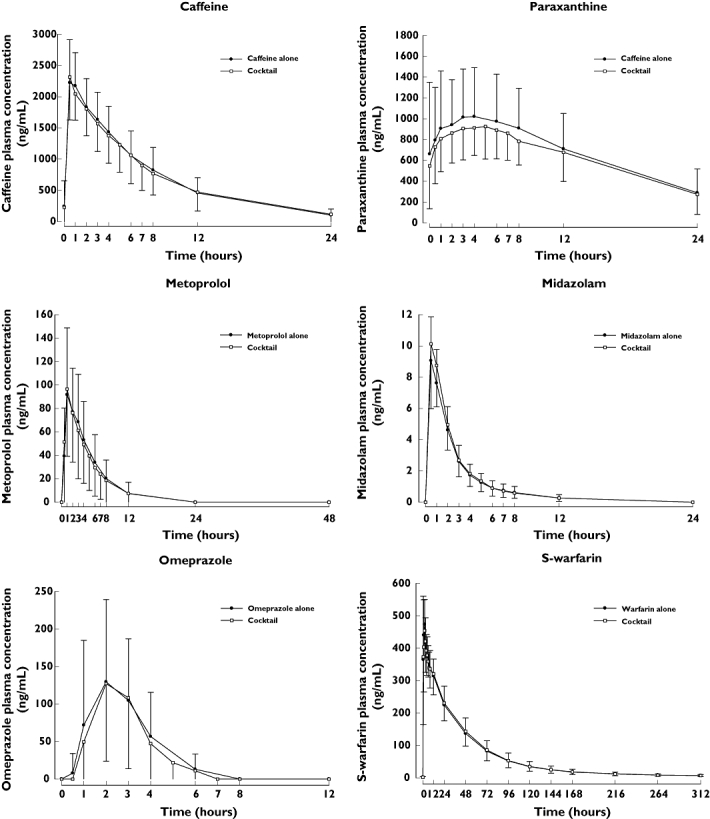
Mean (±SD) plasma concentrations for each probe administered alone or concurrently as a cocktail (n ≤ 33)
PK parameters for caffeine, paraxanthine, metoprolol, midazolam, omeprazole and S-warfarin administered alone or concurrently as a cocktail are summarized in Table 3.
Table 3.
Summary of PK parameters for each probe administered alone or concurrently as a cocktail
| Drug | Period | n | Cmax (ng ml–1) | tmax (h)* | AUClast (ng h–1 ml–1) | AUC (ng h–1 ml–1) | t1/2z (h) | tlast (h)* |
|---|---|---|---|---|---|---|---|---|
| Caffeine | Alone | 30 | 2390 (559) | 0.500 (0.500, 2.00) | 21 300 (9 530) | NC† | NC† | 48.0 (12.0, 48.0) |
| Cocktail | 33 | 2430 (625) | 0.500 (0.500, 48.0) | 22 900 (11 500) | NC† | NC† | 48.0 (12.0, 48.0) | |
| Paraxanthine | Alone | 30 | 1230 (684) | 4.00 (1.00, 48.0) | 28 300 (18 600) | NC† | NC† | 48.0 (48.0, 48.0) |
| Cocktail | 33 | 1100 (457) | 5.00 (0.500, 48.0) | 27 100 (12 500) | NC† | NC† | 48.0 (24.0, 48.0) | |
| Metoprolol | Alone | 31 | 101 (54.2) | 2.00 (0.500, 3.00) | 449 (303) | 494 (321) | 2.76 (0.738) | 12.0 (4.00, 24.0) |
| Cocktail | 33 | 105 (55.7) | 1.00 (0.500, 4.00) | 445 (344) | 510 (383)‡ | 3.04 (1.49) | 12.0 (2.00, 24.0) | |
| Midazolam | Alone | 30 | 9.43 (2.60) | 0.500 (0.500, 1.00) | 23.7 (7.96) | 25.1 (9.24) | 3.33 (0.925) | 12.0 (8.00, 12.0) |
| Cocktail | 33 | 10.7 (3.58) | 0.500 (0.500, 1.00) | 26.1 (10.7) | 27.0 (11.4)‡ | 3.41 (1.19)‡ | 12.0 (8.00, 48.0) | |
| Omeprazole | Alone | 31 | 187 (98.0) | 2.00 (1.00, 4.00) | 368 (250) | 396 (263)§ | 0.858 (0.313)§ | 6.00 (3.00, 8.00) |
| Cocktail | 33 | 184 (118) | 2.00 (1.00, 3.00) | 340 (230) | 350 (236) | 0.802 (0.224) | 6.00 (3.00, 8.00) | |
| S-warfarin | Alone | 31 | 316 (57.0) | 12.0 (12.0, 24.0) | 16 000 (4 340) | 17 200 (4 800) | 120 (41.7) | 312 (312, 312) |
| Cocktail | 33 | 321 (45.5) | 12.0 (12.0, 12.0) | 16 000 (4 780) | 17 400 (4 780)¶ | 113 (30.1)¶ | 312 (24.0, 312) |
Tabulated values are mean (SD) except for tmax and tlast, where values are median (min, max).
No half-life for caffeine and paraxanthine was calculated due to the rebound in plasma concentration at 48 h. This rebound is probably due to ingestion of caffeinated drinks or food, as the subjects were discharged 24 h after the dose.
n= 31.
n= 28.
n= 32. NC, not calculated; AUC values extrapolated by >30% were excluded from the analysis.
Geometric mean (%CV) and point estimates (90% CI) of the pharmacokinetic parameters of caffeine, paraxanthine, metoprolol, midazolam, omeprazole and S-warfarin administered alone or concurrently as a cocktail are presented in Table 4.
Table 4.
PK parameter point estimates (90% CI) for each probe
| Drug | Parameter | n | Ratio estimate (90% CI) |
|---|---|---|---|
| Caffeine | Cmax | 30 | 1.03 (0.97, 1.10) |
| AUClast | 30 | 1.10 (1.01, 1.20) | |
| Paraxanthine | Cmax | 30 | 0.95 (0.84, 1.08) |
| AUClast | 30 | 1.05 (0.89, 1.23) | |
| Metoprolol | Cmax | 31 | 1.06 (0.95, 1.18) |
| AUClast | 31 | 0.95 (0.86, 1.05) | |
| AUC | 29 | 0.97 (0.88, 1.06) | |
| Midazolam | Cmax | 30 | 1.11 (1.02, 1.21) |
| AUClast | 30 | 1.08 (0.99, 1.18) | |
| AUC | 29 | 1.06 (0.98, 1.16) | |
| Omeprazole | Cmax | 31 | 0.99 (0.85, 1.16) |
| AUClast | 31 | 0.93 (0.84, 1.04) | |
| AUC | 28 | 0.91 (0.81, 1.02) | |
| S-warfarin | Cmax | 31 | 1.02 (0.95, 1.09) |
| AUClast | 31 | 1.03 (0.99, 1.08) | |
| AUC | 31 | 1.02 (0.98, 1.07) |
One subject experienced two adverse events (dizziness and headache following caffeine and midazolam administration, respectively) during the study. There were sporadic abnormalities in haematology (decreases in haemoglobin), ECG, and vital sign (minor orthostatic changes in heart rate or blood pressure) parameters, but these were not clinically relevant and none occurred following cocktail administration. In addition, the mean value of INR in this study was <1.5. Only one subject had a value reaching the threshold of therapeutic efficacy (INR of 2) on two occasions.
Discussion
Drugs to be included in a cocktail should meet several criteria [5]. Each drug should be a selective substrate for a single CYP isoform (i.e. clearance of the probe drug should predominantly be due to a single CYP isoform). Drugs within a cocktail should not interfere with the metabolism and clearance of other drugs in the cocktail. The drugs should be safe for trial subjects, readily available and straightforward to administer. Assays for quantifying probe drugs should be sensitive and rigorous. PK of the drugs should be easily interpretable, even with significant inhibition or induction of one or more CYP isoforms.
This new cocktail was designed to overcome potential liabilities of other reported cocktails [5]. The probe drugs (caffeine, warfarin, omeprazole, metoprolol and midazolam) were selected to test the five CYP isoforms predominantly involved in metabolism of most small-molecule drugs [6]. Drugs and doses were carefully selected based on described metabolism and PK characteristics to minimize or eliminate potential interference between probes. The probes to be used in this cocktail approach were chosen based not only on their CYP specificity, but also on their availability on the major global markets and recommendations in regulatory guidance. The doses selected are commonly used in clinical drug interaction trials and thought not to interfere with clearance of the other probe drugs, which was verified in this study. Drugs were administered orally to reflect both liver and intestinal activities, which are particularly relevant for CYP3A (i.e. midazolam probe).
Ideally, evaluation of probe drugs would be non-invasive, allowing quantification of drug or metabolites in urine or breath, but that was not practical with the use of drugs selected as probes in this cocktail. Therefore, quantification of the probe drugs and metabolites in plasma was intentional, to overcome potential problems with assays utilizing urine, including issues of urine collection and interference with quantification due to changes in pH, fluid intake or output, or other documented problems.
For this cocktail, blood samples must be collected in a clinical protocol where blood is already drawn to quantify concentrations of the new chemical entity and clinical chemistry parameters. Bioanalysis of clinical samples to quantify concentrations of probe drugs or metabolites used to be problematic due to the need for large sample sizes, lack of sensitivity and analytical chemical interference between compounds. However, the use of LC-MS/MS to quantify drug concentrations eliminated these issues for the probe drugs used in this cocktail.
The aim of our approach was the determination of a potential metabolic-based DDI as well as its magnitude. That is why we assumed that the determination of the AUC, reflecting the clearance, was the best end-point for this kind of study. In addition, the CV values of the AUC parameter are smaller than those found for a single sampling point, as already reported for Cmax[7]. Moreover the European Medicines Agency has recommended assessing complete AUCs for the probe drugs in order to estimate effects on (oral) clearance. Simpler ratios such as metabolite-to-parent drug ratios in urine are usually not a satisfactory parameter, as results may have more confounding factors, and as the magnitude of an effect is difficult to translate into inhibition or induction potency and into treatment recommendations in the Summary of Product Characteristics [8].
These probe drugs were tested to determine their utility in a cocktail. Each drug exhibited equivalent PK characteristics when administered alone or together in the cocktail. The lack of interaction between probes indicates that this cocktail can be used for the in vivo evaluation of metabolism-based DDIs. This cocktail should be useful for testing DDI due to CYP inhibition or induction, and could be used in various combinations to test five or fewer potential interactions, as appropriate.
Another critical point is the safety of co-administering multiple drugs. The safety profile of this Sanofi-Aventis cocktail was excellent.
As with all previously reported cocktails, it should be noted that the validation protocol reported here demonstrates the lack of interaction of the cocktail in the ‘control’ state that is without the additional effect of an enzyme inducer or inhibitor. The probe drugs and doses in this cocktail were chosen to be selective for individual CYP isoforms, with the expectation of no or minimal interference between probes [5, 9–17]. However, loss of selectivity of a probe drug may be possible if the CYP isoform responsible for the metabolism of the probe drug is inhibited by the test compound and probe concentrations are elevated. Induction of one or more CYP isoforms by the new chemical entity could also potentially interfere with interpretation of a cocktail drug interaction study. Because of this limitation, it may be prudent to consider all positive data obtained from any cocktail study as requiring further in vivo investigations.
There were no PK interactions between the probe drugs, caffeine (CYP1A2), warfarin (CYP2C9), omeprazole (CYP2C19), metoprolol (CYP2D6) or midazolam (CYP3A) when administered in combination as a cocktail, relative to the probes administered alone. The safety profile of this cocktail was excellent. The lack of interaction between probes indicates that this cocktail could be used for in vivo evaluation of the potential DDIs involving multiple CYP isoforms.
Competing interests
None to declare.
REFERENCES
- 1.Chainuvati S, Nafziger AN, Leeder JS, Gaedigk A, Kearns GL, Sellers E, Zhang Y, Kashuba ADM, Rowland E, Bertino JS. Combined phenotypic assessment of CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP3A, NAT2 and XO with the Cooperstown 5+1 Cocktail. Clin Pharmacol Ther. 2003;74:437–47. doi: 10.1016/S0009-9236(03)00229-7. [DOI] [PubMed] [Google Scholar]
- 2.Ryu JY, Song IS, Sunwoo YE, Shon JH, Liu KH, Cha IJ, Shin JG. Development of the ‘Inje Cocktail’ for high-throughput evaluation of five human cytochrome P450 isoforms in vivo. Clin Pharmacol Ther. 2007;82:531–40. doi: 10.1038/sj.clpt.6100187. [DOI] [PubMed] [Google Scholar]
- 3.Tomalik-Scharte D, Jetter A, Kinzig-Schippers M, Skott A, Sörgel F, Klaassen T, Kasel D, Harlfinger S, Doroshyenko O, Frank D, Kirchheiner J, Bräter M, Richter K, Gramatté T, Fuhr U. Effect of propiverine on cytochrome P450 enzymes: a cocktail interaction study in healthy volunteers. Drug Metab Dispos. 2005;33:1859–66. doi: 10.1124/dmd.105.005272. [DOI] [PubMed] [Google Scholar]
- 4.Schwagmeier R, Alincic S, Striebel HW. Midazolam pharmacokinetics following intravenous and buccal administration. Br J Clin Pharmacol. 1998;46:203–6. doi: 10.1046/j.1365-2125.1998.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou H, Tong Z, McLeod JF. ‘Cocktail’ approaches and strategies in drug development: valuable tool or flawed science? J Clin Pharmacol. 2004;44:120–34. doi: 10.1177/0091270003261333. [DOI] [PubMed] [Google Scholar]
- 6.Guengerich FP. Cytochromes P450, drugs, and diseases. Mol Interv. 2003;3:194–204. doi: 10.1124/mi.3.4.194. [DOI] [PubMed] [Google Scholar]
- 7.Yuen KH, Wong JW, Yap SP, Billa N. Estimated coefficient of variation values for sample size planning in bioequivalence studies. Int J Clin Pharmacol Ther. 2001;39:37–40. doi: 10.5414/cpp39037. [DOI] [PubMed] [Google Scholar]
- 8.Efficacy working party: questions and answers on the use of Cocktail studies for investigating in vivo drug interaction potential. EMEA/CHMP/EWP/490784/2007.
- 9.Andersson T, Miners J-O, Tassaneeyakul W, Veronese ME, Meyer UA, Birkett DJ. Identification of human liver cytochrome P450 isoforms mediating omeprazole metabolism. Br J Clin Pharmacol. 1993;36:521–30. doi: 10.1111/j.1365-2125.1993.tb00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersson T, Bredberg E, Lagerstrom PO, Naesdal J, Wilson I. Lack of drug–drug interaction between three different non-steroidal anti-inflammatory drugs and omeprazole. Eur J Clin Pharmacol. 1998;54:399–404. doi: 10.1007/s002280050482. [DOI] [PubMed] [Google Scholar]
- 11.Andersson T, Lundborg P, Regårdh CG. Lack of effect of omeprazole treatment on steady-state plasma levels of metoprolol. Eur J Clin Pharmacol. 1991;40:61–5. doi: 10.1007/BF00315140. [DOI] [PubMed] [Google Scholar]
- 12.Andersson T, Bergstrand R, Cederberg C, Eriksson S, Lagerström PO, Skånberg I. Omeprazole treatment does not affect the metabolism of caffeine. Gastroenterology. 1991;101:943–7. doi: 10.1016/0016-5085(91)90719-2. [DOI] [PubMed] [Google Scholar]
- 13.Li G, Klotz U. Inhibitory effect of omeprazole on the metabolism of midazolam in vitro. Arzneimittelforschung. 1990;40:1105–7. [PubMed] [Google Scholar]
- 14.McGourty JC, Silas JS, Lennard MS, Tucker GT, Woods HF. Metoprolol metabolism and debrisoquine oxidation polymorphism—population and family studies. Br J Clin Pharmacol. 1985;20:555–66. doi: 10.1111/j.1365-2125.1985.tb05112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmider J, Brockmöller J, Arold G, Bauer S, Roots I. Simultaneous assessment of CYP3A4 and CYP1A2 activity in vivo with alprazolam and caffeine. Pharmacogenetics. 1999;9:725–34. doi: 10.1097/01213011-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Prueksaritanont T, Gorham LM, Ma B, Liu L, Yu X, Zhao JJ, Slaughter DE, Arison BH, Vyas KP. In vitro metabolism of simvastatin in humans [SBT] identification of metabolizing enzymes and effect of the drug on hepatic P450s. Drug Metab Dispos. 1997;25:1191–9. [PubMed] [Google Scholar]
- 17.Streetman DS, Bleakley JF, Kim JS, Nafziger AN, Leeder JS, Gaedigk A, Gotschall R, Kearns GL, Bertino JS., Jr Combined phenotypic assessment of CYP1A2, CYP2C19, CYP2D6, CYP3A, N-acetyltransferase-2, and xanthine oxidase with the ‘Cooperstown cocktail’. Clin Pharmacol Ther. 2000;68:375–83. doi: 10.1067/mcp.2000.109519. [DOI] [PubMed] [Google Scholar]


