Abstract
Microvascular dysfunction, loss of vascular support, ischaemia and sub-acute vascular instability in surviving blood vessels contribute to secondary injury following SCI (spinal cord injury). Neither the precise temporal profile of the cellular dynamics of spinal microvasculature nor the potential molecular effectors regulating this plasticity are well understood. TGFβ (transforming growth factor β) isoforms have been shown to be rapidly increased in response to SCI and CNS (central nervous system) ischaemia, but no data exist regarding their contribution to microvascular dysfunction following SCI. To examine these issues, in the present study we used a model of focal spinal cord ischaemia/reperfusion SCI to examine the cellular response(s) of affected microvessels from 30 min to 14 days post-ischaemia. Spinal endothelial cells were isolated from affected tissue and subjected to focused microarray analysis of TGFβ-responsive/related mRNAs 6 and 24 h post-SCI. Immunohistochemical analyses of histopathology show neuronal disruption/loss and astroglial regression from spinal microvessels by 3 h post-ischaemia, with complete dissolution of functional endfeet (loss of aquaporin-4) by 12 h post-ischaemia. Coincident with this microvascular plasticity, results from microarray analyses show 9 out of 22 TGFβ-responsive mRNAs significantly up-regulated by 6 h post-ischaemia. Of these, serpine 1/PAI-1 (plasminogen-activator inhibitor 1) demonstrated the greatest increase (>40-fold). Furthermore, uPA (urokinase-type plasminogen activator), another member of the PAS (plasminogen activator system), was also significantly increased (>7.5-fold). These results, along with other select up-regulated mRNAs, were confirmed biochemically or immunohistochemically. Taken together, these results implicate TGFβ as a potential molecular effector of the anatomical and functional plasticity of microvessels following SCI.
Keywords: endothelin, insulin-like growth factor binding protein 3 (IGFBP-3), interleukin-6 (IL-6), matrix metalloproteinase 9 (MMP-9), plasminogen-activator inhibitor 1 (PAI-1), urokinase-type plasminogen activator (uPA)
Abbreviations: Aqp-4, aquaporin-4; BMP, bone morphogenetic protein; BSCB, blood-spinal cord-barrier; CNS, central nervous system; EC, endothelial cell; ET, endothelin; GFAP, glial fibrillary acidic protein; HUVEC, human umbilical vein endothelial cell; IGF, insulin-like growth factor; IGFBP-3, IGF-binding protein 3; IL, interleukin; LEA, Lycopersicon esculentum agglutinin; LLC, large latent complex; Map2, microtubule-associated protein 2; MCAO, middle cerebral artery occlusion; MMP, matrix metalloproteinase; NVU, neurovascular unit; PA, plasminogen activator; PAI, PA inhibitor; PAS, PA system; SCI, spinal cord injury; smvEC, spinal microvascular EC; TBS, Tris-buffered saline; TGFβ, transforming growth factor β; tPA, tissue-type PA; TSP-1, thrombospondin-1; uPA, urokinase-type PA; uPAR, uPA receptor; VEGF, vascular endothelial growth factor
INTRODUCTION
Following traumatic SCI (spinal cord injury), significant vascular disruption occurs at the site(s) of injury. This interruption of vascular support is thought to be a key mediator of multiple secondary injury cascades, all of which contribute to loss of functional tissue (Nelson et al., 1977). In the intact CNS (central nervous system), the microvasculature is composed of an integrated unit consisting of ECs (endothelial cells), pericytes, astrocytes and neurons. Any perturbation of the normal functional and/or anatomical integration of the microvasculature results in neural pathology (Hawkins and Davis, 2005). Ultrastructural studies have documented vascular pathology minutes after SCI (Goodman et al., 1979; Koyanagi et al., 1993) and this persists throughout the acute injury phase (Whetstone et al., 2003; Benton et al., 2008a). In fact, ECs appear to be the first cells to die following contusive SCI (Griffiths et al., 1978; Casella et al., 2006). These immediate vascular events, including increased permeability of the BSCB (blood-spinal cord-barrier), induce oedema and contribute to detrimental inflammation (Amar and Levy, 1999; Mautes et al., 2000). In the subacute phase of injury, the penumbral microvasculature is also pathologically transformed by loss of astrocytic investment (Whetstone et al., 2003), regression of pericytes (Benton et al., 2008a) and the perivascular localization of infiltrating inflammatory cells (Popovich and Jones, 2003). This second and more prolonged phase of microvascular instability has been hypothesized to be a primary event leading to chronic histopathology after SCI (Casella et al., 2002; Loy et al., 2002). Cellular protection/stabilization of microvascular elements within penumbral microvasculature remains a largely unexplored therapeutic avenue due to a relative lack of understanding of key molecular pathways pathologically induced in smvECs (spinal microvascular ECs). This is a critical issue as preservation of metabolic support of spinal tissue spared by the primary injury event should result in enhanced substrate for chronic recovery.
A number of effectors influence BSCB function following traumatic SCI, including the critical vasoactive molecules ephs/ephrins, VEGF (vascular endothelial growth factor), and functionally related co-factor(s), the angiopoeitins (Sharma, 2005). The neurotrophins BDNF (brain-derived neurotrophic factor), NGF (nerve growth factor) and NT3 (neurotrophin 3) also modulate EC survival and proliferation in vivo (Ward and LaManna, 2004), but their role in SCI-induced microvascular plasticity is unknown. Several secreted cytokines, including TNFα (tumour necrosis factor α) and TGFβ (transforming growth factor β) isoforms are increased following SCI and are thought to be potent regulators of EC survival, proliferation and function, as well as BSCB integrity (O'Brien et al., 1994; McTigue et al., 2000; Han and Suk, 2005), acting, in part, via the induction of VEGF expression (ten Dijke and Arthur, 2007). Previous in vitro evidence suggests that TGFβ1 can act in concert with VEGF to induce EC apoptosis (Ferrari et al., 2006), a surprising finding with potentially important implications for microvascular stability in the injured spinal cord. Furthermore, MMPs (matrix metalloproteinases) are established regulators of vascular destabilization and EC dysfunction following SCI (Noble et al., 2002) and cortical ischaemia (Cunningham et al., 2005). A pathological connection may exist between TGFβ1 and MMPs, as TGFβ1 signalling increases MMP expression (ten Dijke and Arthur, 2007). Furthermore, MMPs are activators of latent TGFβ1 in various in vivo contexts (ten Dijke and Arthur, 2007), suggesting the potential for a potent, reciprocal, feed-forward pathological loop in the microvasculature following SCI.
Thus the principal goal of the present study was to determine whether ECs are induced by TGFβ in the early phases of SCI utilizing multiple novel approaches. Using a previously described model of focal spinal ischaemia (Benton et al., 2005), we sought to replicate the non-traumatic stress/ischaemia present in penumbral spinal tissue (i.e. immediately surrounding the traumatic injury site). Furthermore, we utilized new methodology to isolate spinal cord microvascular ECs (Benton et al., 2008b) from this ischaemic spinal cord. These approaches lead to the identification of multiple TGFβ/BMP (bone morphogenetic protein)-responsive/related mRNAs expressed in the EC compartment concomitant to the onset of significant histopathology following spinal ischaemia. Taken together, the present results indeed implicate TGFβ, known to be pathologically altered after SCI, in the acute pathological transformation of the spinal microvasculature.
MATERIALS AND METHODS
Focal ischaemic SCIs
All surgical intervention and subsequent care and treatment of all animals used in the present study were in strict accordance with the PHS Policy on Humane Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, 1996) and approved by the University of Louisville IACUC guidelines. Focal ischaemic grey matter SCI was accomplished by slight modification of methods described previously (Benton et al., 2005). A total of 70 adult (approx. 6–8 week old) female Sprague–Dawley rats (180–200 g; Harlan) were anaesthetized using sodium pentobarbital [Nembutal; 50 mg/kg, i.p. (intraperitoneally)]. Once anaesthesia was achieved, the surgical site was prepared by shaving, and a betadine scrub and laminectomies were performed at the T13 vertebral level, exposing the L1 spinal cord segment. Animals were randomized into groups receiving a 0.75 μl microinjection of vehicle (saline+1 mg/ml rat serum albumin) or rET-1 (rat endothelin-1; 15 pmol; Sigma, E7764). A single midline incision was made in the exposed dura and rET-1 was delivered in one microinjection into the anterior sulcus using bevelled glass micropipettes custom-pulled to an external diameter of 50–60 μm. The incision sites were then sutured in layers and the topical antibiotic Bacitracin was applied to the incision site. Animals received prophylactic injections of gentamycin to prevent infection [1 mg/kg, i.m. (intramuscularly)], and a 10 cc bolus subcutaneous injection of saline was given to prevent peri-operative dehydration. For longer survival times, rats were housed two per cage and cages were placed on a 37°C heating pad overnight. Immediately following surgery and for 48 h post-operatively, animals also received twice-daily injections of buprenorphine [Bupronex; 0.075 mg/kg, s.c. (subcutaneously), b.i.d. (twice daily)] and their bladders were manually expressed twice daily.
Isolation of smvECs
Spinal microvessels in control and injured rat spinal cords were intravascularly labelled using 200 μg of FITC-conjugated LEA (Lycopersicon esculentum agglutinin) lectin (FL-1171, 2 mg/ml; Vector Laboratories) as described previously (Benton et al., 2008b). Following anaesthesia, 100 μl of FITC–LEA was delivered systemically by intravenous injection into the surgically exposed right external jugular vein at a rate of 1.2 ml/h using a syringe pump (model # 780100, KD Scientific). FITC–LEA was allowed to circulate for 15 min prior to perfusion with saline. Spinal cord tissue containing intravascularly labelled microvessels was then processed for microvascular EC isolation. Briefly, 6 and 24 h post-SCI, approx. 1 cm of spinal cord tissue spanning the injection site was isolated, and the ventral intact/lesioned grey matter was dissected and homogenized in ice-cold HBSS (Hanks balanced salt solution). The crude homogenate was triturated using multiple passes through 26 and 30 gauge needles and filtered through a 70 μm mesh. smvECs were purified by FACS using a MoFlo system (DAKO) using identical parameters with those previously described (Benton et al., 2008b); approx. 90% enrichment of microvascular fragments is achieved using this technique. This microvascular fraction contains mainly small-diameter vessels (i.e. <10 μm in diameter) with little evidence of preserved mural cell ensheathment (Supplementary Figure S1 at http://www.asnneuro.org/an/001/an001e015.add.htm). Following FACS isolation, microvascular fragments were collected by centrifugation (16000 g) at 4°C and stored at −80°C.
TGFβ/BMP-related gene microarray analysis
Focused microarray analysis of smvEC gene expression was accomplished as previously described (Benton et al., 2008b). Total RNA was extracted from microvascular ECs using the PicoPure™ RNA Isolation Kit (Arcturus Bioscience). Isolated RNA was reverse-transcribed using the Reaction Ready™ First Strand cDNA synthesis Kit (SuperArray Bioscience). Differential gene expression was conducted using the RT2Profiler™ PCR Array (rat, TGFβ signalling PCR array; catalogue number PARN-035, SuperArray Bioscience). qRT-PCR (quantitative real-time-PCR) was performed using an ABI 7900 real-time PCR instrument. Results were analysed using the Microsoft Excel™ analysis template provided by SuperArray Bioscience using the ΔΔCt method. For each gene, the fold-changes were calculated as the difference in gene expression between smvECs from control spinal tissue and that in smvECs from ischaemic spinal tissue.
Tissue processing and immunohistochemical analysis
At multiple experimental timepoints post-ischaemia, animals were perfused using 200 ml of 4% (w/v) PFA (paraformaldehyde). Spinal cords were dissected and transversely sectioned at 20 μm on a cryostat. Sections were thaw-mounted on microscope slides and stored at −80°C. On the day of staining, slides were warmed at 37°C for 20 min and the mounting matrix was removed with forceps. Tissue was blocked in 0.1 M TBS (Tris-buffered saline; 100 mM Tris and 150 mM NaCl, pH 7.4), 0.1% Triton X-100, 0.5% BSA and 10% normal donkey serum for 1 h at room temperature (22°C). Primary antibodies were applied in 0.1 M TBS, 0.1% Triton X-100, 0.5% BSA and 5% normal donkey serum overnight in a humidified chamber at 4°C. Table 1 contains all information regarding the primary antibodies used in the present study, including specificity data. Sections were then incubated with TRITC- (tetramethylrhodamine β-isothiocyanate; 1:200), FITC- (1:100) or AMCA- (1:100) conjugated secondary antibodies (Jackson ImmunoResearch Laboratories). Negative controls for each epitope consisted of experimental tissue exposed to species-specific pre-immune IgG and did not result in significant staining. All photomicrographs were captured using a Nikon Ti-E automated inverted microscope equipped with a DS-Ri1 digital camera or an LS-500 laser confocal microscope (Olympus) and Nikon Elements BR analysis software or an Eclipse C1 laser confocal microscope (Nikon Instruments). To eliminate the possibility of ‘cross-over' signal, sequential excitation and capture was performed with tissue containing multiple fluorophores analysed by laser confocal microscopy.
Table 1. List of antibodies used in the present study.
| Primary antibody | Cells identified | Immunogen | Species | Catalogue number/company | Reference |
| GFAP | Astrocytes(1:500) | GFAP from bovine spinal cord | Rabbit poly-IgG | ZO334/Dako | Single 50 kDa band on immunoblot (Jakovcevski et al., 2007) |
| GFAP | Astrocytes(1:250) | GFAP from porcine spinal cord | Mouse mono-IgG | MAB3402/Chemicon | Single 51 kDa band on immunoblot (Debus et al., 1983) |
| Desmin | Pericytes(1:50) | Desmin protein isolated from chicken gizzard | Rabbit poly-IgG | AB907/Chemicon | Single 53 kDa band on immunoblot (Corti et al., 2005) |
| Iba1 | Microglia/ macrophages(1:250) | Synthetic peptide fragment corresponding to amino acids 118–131 within the C-terminus of Iba1 (LRMILMYEEKNKEH-C) | Rabbit poly-IgG | # 019-19741; lot HNM3505/Wako | Single 17 kDa band on immunoblot (Jakovcevski et al., 2007) |
| Map2 | Neurons(1:200) | Map2 from bovine brain | Mouse mono-IgG | M1406/Sigma | Single 280 kDa band on immunoblot (Binder et al., 1986) |
| Aqp4 | Perivascular astrocytic endfeet(1:100) | Synthetic peptide fragment corresponding to amino acids 244–323 within the C-terminus of human Aqp4 (AGGLYEYVFCPDVEFKRRFKEAFSKAAQQTKGSYMEVEDNRSQVETDDLILKPGVVHVIDVDRGEEKKGKDQSGEVLSSV-C) | Rabbit poly-IgG | Aqp4 (H-80); sc-20812/Santa Cruz Biotechnology | Single 32 kDa band on immunoblot (Quick and Cipolla, 2005) |
| PAI-1 | Perivascular astrocytic endfeet(1:100) | Secretion product of a dexamethasone-stimulated HTC rat hepatoma cell line | Rabbit poly-IgG | #1062/American Diagnostica | Single 50 kDa band on immunoblot (Allaire et al., 1998) |
| IGFBP-3 | Perivascular astrocytic endfeet(1:50) | Synthetic peptide corresponding to amino acids 241–291 within the C-terminus of mouse IGFBP-3 (DKKGFYKKKRCRPSKGRKQSFCWCVDKYGQRLPGYDTKGKDDVHCLSV QSQ-C) | Goat poly-IgG | M-19; sc-6004/Santa Cruz Biotechnology | Single 42 kDa band on immunoblot (Ye et al., 2003) |
| IL-6 | Perivascular astrocytic endfeet(1:50) | Recombinant rat IL-6 (rrIL-6) derived from Escherichia coli | Goat poly-IgG | AF506; lot # BCZ04/R&D Systems | Single 27 kDa band on immunoblot (Wang et al., 2004) |
| RECA-1 | Rat ECs (1:25) | Stromal cells from rat lymph node | Mouse mono-IgG | MCA970GR/Serotec | Not appropriate for immunoblot (Duijvestijn et al., 1992) |
| SMI-71 | Blood–brain barrier (1:100) | Rat brain homogenate | Mouse mono-IgM | #SMI71/Sternberger Monoclonals | Not appropriate for immunoblot (Sternberger and Sternberger, 1987) |
| MMP-9 | Blood–brain barrier(1:100) | NS0-derived, recombinant mouse MMP-9 (rmMMP-9) | Goat poly-IgG | AF909; lot # EFP02/R&D Systems | Single 110 kDa band on immunoblot (Yin et al., 2006) |
uPA [urokinase-type PA (plasminogen activator)] zymography
uPA enzymatic activity was determined as described previously (Benton et al., 2008b), which has been adapted with slight modification of an established protocol (Heussen and Dowdle, 1980). Briefly, 5 mm of spinal tissue, centered on the ET-1 injection site, was rapidly removed from the spinal column. Tissue was then bisected into ventral and dorsal portions. Protein was extracted from the ventral tissue sample containing ischaemic grey matter. Total protein (10 μg) from spinal cord tissues was resolved under non-reducing conditions on SDS/PAGE (10% gels) containing 0.01 unit/ml plasminogen and 1.5 mg/ml gelatin (Sigma). For standardization, 10 pg of rat uPA (American Diagnostica) was loaded on to each gel. After electrophoresis, the gels were washed three times in 2.5% Triton X-100 solution and then incubated in 100 mmol/l Tris/HCl (pH 8.2) buffer for 18 h at 37°C. The gels were stained with 0.1% Amido Black solution and zymograms were quantified by densitometric analysis using ImageQuant software (Molecular Devices). Specifically, gels were scanned and the images coverted into greyscale and inverted so areas of enzymatic activity appeared as dark staining. A rectangular box was then placed over the largest band and the total area of dark pixels was counted. The same box was used to quantify each band for individual experiments. Background densities for each lane were then subtracted from enzymatic values obtained from density measurements of each band. This method has been used routinely for the detection of uPA activity in diseased brain (Burk et al., 2008) and spinal cord (Glas et al., 2007).
Statistical analyses
All quantitative data are expressed as means±S.D. Differences between two groups (SCI microarray results) were compared using a Student's t test. One-way ANOVA followed by Tukey's HSD (Honestly Significant Difference) post-hoc analysis was used to compare results for uPA enzymatic activity. Quantitative results from fold-changes in mRNA levels after SCI in smvECs were compared using a two-tailed unpaired Student's t test, assuming unequal variances. For all analyses, statistical significance was defined at P≤0.05.
RESULTS
Neuronal integrity/survival is compromised as early as 3 h following onset of ET-1-mediated focal ischaemia
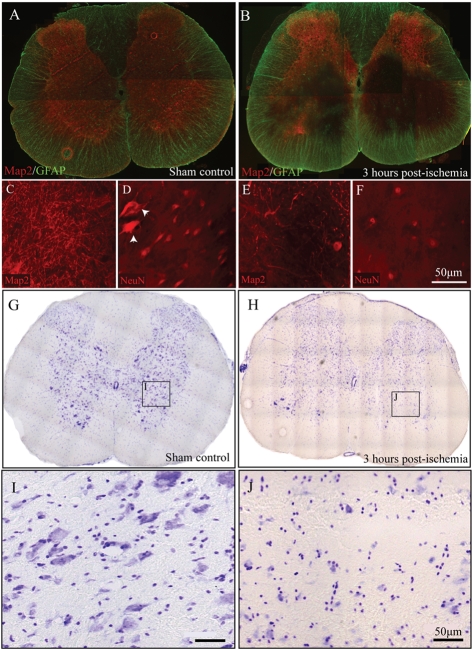
Although anatomically isolated from the microvasculature, neurons are linked metabolically with ECs and mural cells (i.e. astrocytes, pericytes and smooth muscle cells) in the CNS. Thus neuronal death/dysfunction may precipitate pathological transformation of the CNS vasculature (Zlokovic, 2008). The results of the present study show that neuronal pathology occurs very early following focal spinal grey matter ischaemia. Significant grey matter pathology is observed by 3 h post-ET-1 injection, as demonstrated by the overt loss of Map2 (microtubule-associated protein 2)- and NeuN-immunoreactivity (Figures 1B, 1E and 1F). Large spinal motor neurons (Figure 1D; arrowheads) were notably absent in the acute phase of insult in affected grey matter by 3 h following onset of ET-1-mediated ischaemia (Figure 1F). At early timepoints following focal ischaemia, only grey matter perfused by target vessels emanating from the anterior spinal artery demonstrate significant pathology (Figure 1B). This decreased Map2- and NeuN-immunoreactivity in affected spinal grey matter is the result of neuronal death and not down-regulation of epitopes as evidenced by loss of Nissl-stained neurons in adjacent sections (Figures 1G–1J).
Figure 1. Temporal course of neuronal loss following focal ischaemic SCI.
Intact spinal grey matter staining in the rostral lumbar spinal cord of a sham-control rat (A). As early as 3 h following ET-1 microinjection, significant loss of Map2-immunoreactive tissue in focal grey matter supplied by targeted supply microvessels is observed (B). At higher magnification, a dense network of neuronal processes in ventral grey matter can be seen (C), a pattern that is diminished by 3 h post-ischaemia (E). Furthermore, NeuN-immunoreactive motor neurons are apparent in control tissue (D; arrowheads), but are lost by 3 h following ET-1 microinjection (F). Nissl staining of adjacent spinal cord sections (G and H) corroborates this neuronal loss. Many Nissl-positive neurons are apparent in control tissue (I) with obvious loss of these profiles in ventral grey matter 3 h following ET-1-induced focal ischaemia (J). Scale bar = 50 μm.
Temporal course of astrocyte loss from microvessels affected by ischaemic SCI
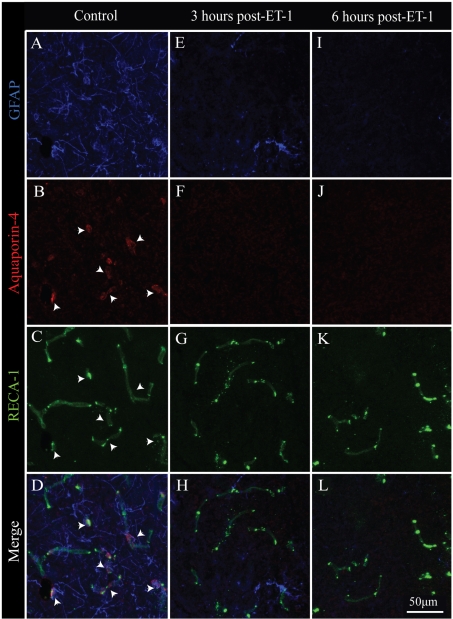
Following experimental stroke, loss of perivascular astrocytic endfeet has been documented by 6 h post-ischaemia, resulting in substantial permeability and oedema in and around affected microvessels (Polavarapu et al., 2007). In the present study, we examined the temporal loss of functional astrocytic contribution to microvessels following spinal cord ischemia by Aqp-4 (aquaporin-4) immunostaining (Figures 2A–2D; arrowheads). Aqp-4 is a water channel associated with perivascular astrocytic endfeet (Abbott et al., 2006) and is characteristic of an intact BSCB. At 3 h post-ischaemia, when overt neuronal pathology is initially observed, affected microvessels begin to exhibit partial loss of astroglial investment, with most microvessels devoid of Aqp-4 immunoreactivity (Figures 2E–2H). By 6 h post-ischaemia, no GFAP (glial fibrillary acidic protein)- or Aqp-4-immunoreactive elements are observed in affected grey matter (Figures 2I–2L). This astroglial loss becomes more pronounced by 3 days post-ischaemia, with astrocytes becoming limited to the perimeter of spinal white matter and completely absent from the ischaemic core as determined by GFAP-immunoreactivity (results not shown). Importantly, this temporal course of astrocyte and anatomical/functional decoupling from the EC compartment correlates with the appearance of abnormal EC tight-junctional phenotypes (results not shown), consistent with what is observed after contusive SCI in the adult mouse (Benton et al., 2008a).
Figure 2. Acute astroglial loss from ischaemic microvessels.
In intact spinal grey matter, Aqp-4- and GFAP-immunoreactive astrocytic endfeet are associated with perfused microvessels (A–D; arrowheads). By 3 h post-ischaemia, significant loss of GFAP-immunoreactivity is observed in affected tissue (E), with no detectable Aqp-4 staining associated (F) with microvascular elements (G). By 6 h post-ischaemia, all GFAP- (I) and Aqp-4- (J) immunoreactivity is lost in affected grey matter, despite a preservation of intact microvessels (K and L). Scale bar = 50 μm (C–F).
Spinal endothelial expression of TGFβ-related/responsive mRNAs is induced concomitant with microvascular plasticity
Table 2 summarizes the greatest magnitude changes in TGFβ/BMP signalling-related mRNA expression acutely after SCI (see Supplementary Figure S2 at http://www.asnneuro.org/an/001/an001e015.add.htm for the complete mRNA data set). Of the 83 represented genes, approx. 10% were induced at 6 and/or 24 h post-ischaemia. Of these, nearly all were transcripts known to be TGFβ1-responsive. More specifically, several within this class were significantly up-regulated at both 6 and 24 h post-ischaemia including serpine 1/PAI-1 (PA inhibitor-1) (41- and 24-fold), c-fos (34- and 8-fold), plau/uPA (8- and 3-fold) and Tgif (7- and 3-fold). A smaller subset of mRNAs, including IL (interleukin)-6 (27-fold), JunB (5-fold), p27Cip1/Waf1 (4-fold) and Jun (2-fold), had significantly higher expression at 6 h, but not 24 h, post-ischaemia. Interestingly, a number of the genes regulating TGFβ superfamily signalling in the EC compartment responded to ET-1-induced focal ischaemia by significantly down-regulating transcript levels. For example, cystatin C expression was significantly decreased at both 6 and 24 h after SCI (2- and 5-fold respectively), whereas decreased expression of Fkbp1b and Nbl1 (3-fold) and noggin (4-fold) mRNAs were seen only at 24 h post-ischaemia. A mixed response of TGFβ superfamily receptor genes was observed, with IGFBP-3 [IGF (insulin-like growth factor)-binding protein 3]/TGFβRV mRNA levels exhibiting a 12-fold increase at 24 h post-ischaemia, whereas significant reductions were observed in Itgb5 (6-fold), Bmpr1b (3-fold) and Acvr1 (2-fold) mRNA levels only at 24 h.
Table 2. Selected microarray results.
| GenBank® assession number | Symbol and gene name | 6 h post-ET-1 fold-change (P value) | 24 h post-ET-1 fold-change (P value) |
| TGFβ-responsive genes | |||
| NM_012620 | Serpine 1, serine peptidase inhibitor, clade E, member 1 (PAI-1A/Pai1) | +40.67 (0.001) | +24.28 (0.001) |
| NM_022197 | c-fos, FBJ murine osteosarcoma viral oncogene homologue | +34.46 (<0.001) | +7.66 (<0.001) |
| NM_012589 | Il6, IL-6 (IL-6/Ifnb2) | +27.07 (<0.001) | +2.27 (0.085) |
| NM_013085 | Plau, plasminogen activator, urokinase (UPAM) | +7.93 (0.042) | +3.05 (0.032) |
| NM_001015020 | Tgif, transforming growth factor interacting factor | +6.71 (0.01) | +3.30 (0.004) |
| NM_021836 | Junb, jun-B oncogene | +5.49 (0.008) | +2.04 (0.142) |
| NM_012603 | Myc, myelocytomatosis viral oncogene homologue (RNCMYC/c-myc) | +3.93 (0.85) | +3.52 (0.018) |
| NM_080782 | Cdkna, cyclin-dependent kinase inhibitor 1A (p27Cip1/Waf1) | +3.92 (0.014) | +1.19 (0.520) |
| NM_021835 | Jun, Jun oncogene | +2.07 (0.029) | +1.86 (0.072) |
| Genes regulating TGFβ superfamily signalling | |||
| NM_012837 | Cst3, cystatin C (CYSC) | −2.10 (0.017) | −5.19 (0.001) |
| NM_022675 | Fkbp1b, FK506 binding protein 1b | +1.12 (0.84) | −2.69 (0.02) |
| NM_031609 | Nbl1, neuroblastoma, suppression of tumorigenicity 1 | −1.64 (0.38) | −3.10 (0.005) |
| XM_343954 | Nog, noggin | +1.70 (0.56) | −3.98 (<0.001) |
| TGFβ superfamily receptor genes | |||
| NM_012588 | Igfbp3, IGFBP-3 (TβR-V) | +1.32 (0.76) | +11.72 (0.003) |
| NM_147139 | Itgb5, integrin, β5 (RGD1563276) | +1.22 (0.83) | −5.82 (0.003) |
| NM_001024259 | Bmpr1b, BMP receptor, type 1B (CFK-43a) | +2.11 (0.43) | −2.89 (<0.001) |
| NM_024486 | Acvr1, activin A receptor, type 1 | +1.17 (0.83) | −2.48 (0.004) |
Tissue levels of uPA are increased acutely following ischaemic SCI
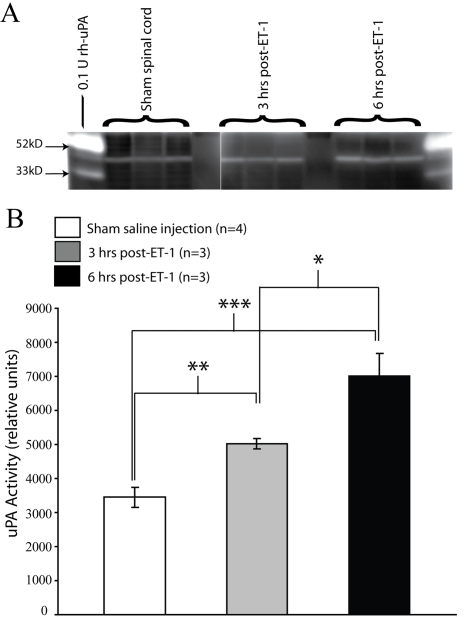
Previous results have shown uPA mRNA expression and bioactivity to be acutely induced in smvECs by traumatic SCI (Benton et al., 2008b). Consistent with these findings, we show that uPA mRNA levels are significantly increased as early as 6 h following ischaemic SCI (Table 2). To determine whether this induction may have biological consequences in the ischaemic spinal cord, we examined uPA enzymatic activity in affected spinal tissue. As early as 3 h post-ET-1 microinjection, uPA proteolytic activity was significantly increased, with levels further increasing by 6 h post-ischaemia (Figures 3A and 3B). The temporal course of this increased enzymatic activity parallels both the loss of astrocytic endfeet in affected microvessels (Figure 2), as well the induction of TGFβ activation of smvECs as demonstrated by microarray results. This result is important in that it implicates uPA in the activation of TGFβ within the ischaemic grey matter and in ECs within affected tissue soon after the onset of ischaemic/reperfusive insult.
Figure 3. Tissue levels of uPA enzymatic activity are significantly increased in response to spinal cord ischaemia.
At 3 and 6 h post-ET-1 microinjection, 5 mm of experimental spinal cords (centered on the microinjection sites) were rapidly isolated, ventral spinal tissue was dissected, and 10 μg of total protein was analysed by uPA zymography (A). Densitometric analysis of zymographic results revealed that by 3 h, levels of uPA enzymatic activity were significantly increased compared with sham-injected tissue (B). Levels of uPA activity are further increased by 6 h post-ischaemia, and were significantly higher than both sham and 3 h levels (B). Quantitative data are the mean uPA activity±S.D. (*P≤0.05, **P≤0.01 and ***P≤0.001; F = 48.79, df = 9).
MMP-9, a potent modulator of TGFβ bioactivity, is expressed in affected microvessels
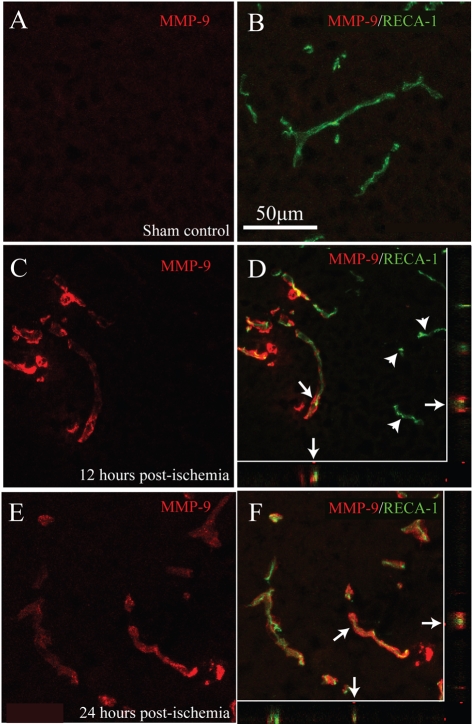
In most tissues, including the CNS, pools of TGFβ exist in inactive forms within the pericellular space and may be rendered bioactive by one or more activators, which include proteases, integrins and thrombospondins (ten Dijke and Arthur, 2007). Of these potential activators, expression and/or activity of MMP-9 is induced in traumatic SCI (Noble et al., 2002). In control ventral grey matter, no MMP-9 expression was observed in smvECs (Figures 4A and 4B). The earliest timepoint post-ischaemia where MMP-9 immunoreactivity was observed was 12 h, where a marked increase was seen in affected microvessels (Figures 4C and 4D), although some affected microvascular elements did not exhibit detectable immunoreactivity (Figure 4D; arrowheads). By 24 h post-ischaemia, levels of MMP-9 expression remained elevated in all affected microvessels (Figures 4E and 4F). This result suggests a role for MMP-9 in regulation of TGFβ activity in/at affected microvessels in the subacute phases, but not in the immediate phase, of smvECs activation following ischaemic SCI.
Figure 4. Microvascular MMP-9 expression is induced by ischaemic SCI.
In control ventral grey matter, no detectable MMP-9 immunoreactivity is present in smvECs (A and B). By 12 h post-ischaemia, significant MMP-9-immunoreactvity is observed in affected spinal microvessels (C and D), although a subset of microvessels does not express MMP-9 (D; arrowheads). This increase in MMP-9 immunoreactivity is maintained 24 h post-ischaemia (E and F), with all microvessels in affected tissue expressing detectable levels of MMP-9. Confocal analysis confirms co-localization of RECA-1 and MMP-9 in the xz and yz planes (D and F; arrows). Scale bar = 50 μm (A–F).
Detection of multiple TGFβ-responsive proteins in spinal microvessels in ischaemic SCI
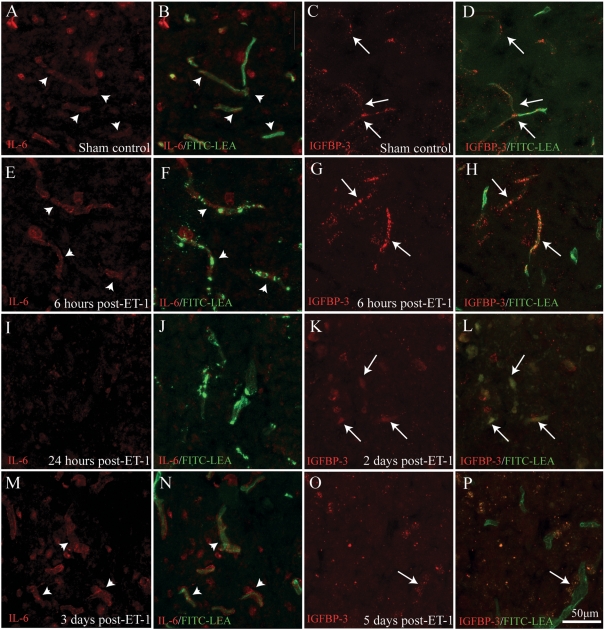
The rationale for the selection of mRNAs to validate at the protein level was based upon (i) the magnitude of the transcriptional changes revealed by microarray analysis and (ii) the novelty of their role in acute microvascular dysfunction in the context of SCI. Based on these criteria, we chose to define the temporal dynamic of protein expression levels of PAI-1, IL-6 and IGFBP-3, which represent the three most highly up-regulated mRNAs examined in smvECs acutely following ischaemic SCI. Surprisingly for IL-6, we observed a low level of immunoreactivity in grey matter microvessels in control tissue (Figures 5A and 5B; arrowheads). By 6 h post-ischaemia, levels of IL-6 were qualitatively increased in activated microvessels (Figures 5E and 5F; arrowheads). Unexpectedly, little IL-6 expression was detectable in spinal microvessels 24 h post-ischaemia (Figures 5I and 5J), but was again observed in vessels 3 days following ischaemia (Figures 5M and 5N; arrowheads). This phasic response is consistent with that quantitatively determined for mRNA levels (Table 2). Microvascular expression of IGFBP-3, another TGFβ-responsive molecule involved in cytokine function, was also complex. Low levels of immunoreactivity where observed in control ventral grey matter (Figures 5C and 5D; arrows), with an apparent increase of smvEC expression by 6 h post-ischaemia (Figures 5G and 5H; arrows). Expression levels appear to decrease in affected microvessels by 2 days post-ischaemia (Figures 5K and 5L), with little detectable IGFBP-3 immunoreactivity apparent in perfused microvessels 5 days post-ischaemia (Figures 5O and 5P). This result suggests a transient role for IGFBP-3 in the activation of the smvECs following ischaemic SCI.
Figure 5. smvEC expression of IL-6 and IGFBP-3 after SCI.
Basal levels of expression for both IL-6 (A and B; arrowheads) and IGFBP-3 (C and D; arrows) were detected in the vasculature in control spinal grey matter. Increased IL-6 immunoreactivity was observed in smvECs at 6 h post-ischaemia (E and F; arrowheads). Interestingly, this expression appeared to exhibit a biphasic pattern, with qualitative levels diminished at 1 day (I and J), but reappearing in activated microvascular profiles by 3 days post-ischaemia (M and N; arrowheads). Similar to IL-6 staining, levels of IGFBP-3 immunoreactivity were intensified by 6 h post-ET-1 microinjection (G and H; arrows). However, levels were then found to diminish progressively beginning at 2 days post-ischaemia (K and L; arrows), with little IGFBP-3 immunoreactivity associated with perfused microvessels 5 days post-ischaemia (O and P; arrow). Scale bar = 50 μm (A–P).
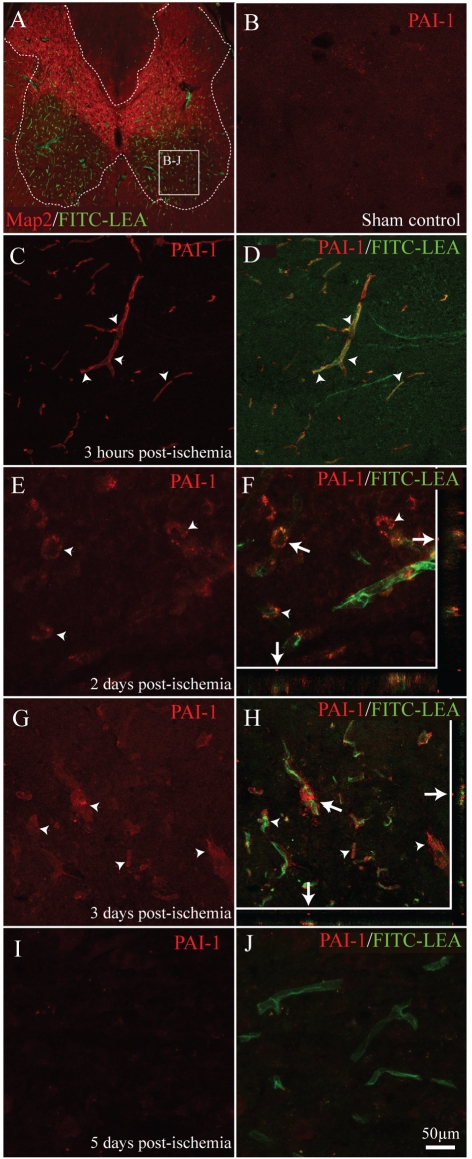
The most highly up-regulated mRNA observed in the present study (40- and 24-fold at 6 and 24 h post-ischaemia; Table 1) was that encoding the endogenous uPA inhibitor PAI-1. In sham-control spinal grey matter, no detectable PAI-1 expression was observed in spinal microvessels (Figure 6B). As early as 3 h post-ET-1 microinjection, PAI-1 immunoreactivity was associated with perfused microvessels in affected tissue (Figures 6C and 6D; arrowheads). This increase in PAI-1 expression was maintained throughout the subacute period post-ischaemia, with detectable levels of PAI-1 expression associated with activated smvECs at 2 and 3 days (Figures 6E–6H; arrowheads). Interestingly, 5 days post-ischaemia, little PAI-1 immunoreactivity was observed in perfused microvessels in affected ventral grey matter (Figures 6I and 6J). These results implicate PAI-1 in contributing to the early pathological activation of the microvasculature following focal spinal ischaemia. Moreover, these immunohistochemical data showing correlative protein expression and mRNA levels in ECs support the conclusion that results obtained from FACS-isolated ECs reflect their in situ transcriptional state. This is an important issue considering the stress induced by the isolation protocol.
Figure 6. smvEC expression of PAI-1 is rapidly induced by ischaemic SCI.
No detectable PAI-1 immunoreactivity was observed in control spinal grey matter (B). As early as 3 h post-ischaemia, robust PAI-1 immunoreactivity is observed in affected smvECs (C and D; arrowheads). This expression level is maintained in smvECs for up to 3 days post-ischaemia (E–H; arrowheads). By 5 days post-ischaemia, PAI-1 immunoreactivity returned to basal levels in perfused spinal microvessels (I and J). Confocal imaging definitively co-localizes PAI-1 to FITC–LEA activated/perfused spinal microvessels in the xz and yz planes (F and H; arrows). Scale bar = 50 μm (A–J).
DISCUSSION
In the present study, we show that induction of focal spinal cord ischaemia results in rapid dissolution of NVU (neurovascular unit) integrity. The use of ET-1 to induce vasospastic spinal cord ischaemia is based upon previous experimental models of ET-1-mediated stoke (O'Neill and Clemens, 2001). In mammals, ET exerts its bioactivity via three structurally related peptides (ET-1, ET-2 and ET-3), which bind two subtypes of G-protein-coupled receptors (ETA and ETB), which are highly conserved across multiple species (Schinelli, 2006). In the healthy CNS, receptor expression is observed in ependymal/subependymal cells (Tuschick et al., 1997), Bergmann glia of the cerebellum (Tuschick et al., 1997), epithelial cells of the choroid plexus (Tsaur et al., 1997), neurons (Yamada and Kurokawa, 1998) and astrocytes (Schinelli et al., 2001), with the highest levels of expression observed in the vasculature (Yu et al., 1995; Chen et al., 2000; Hansen-Schwartz et al., 2002). The ET system is up-regulated in response to brain ischaemia (Edvinsson, 2009), suggesting that it may play a role in the aetiology of clinical stroke. While less is known about the precise role of ET-1 in CNS trauma, blockade of ET-1 receptors has been shown to ameliorate tissue loss and facilitate functional sparing following experimental SCI in rats (Uesugi et al., 1998; Peters et al., 2003; Ogawa et al., 2008). Furthermore, blood levels of ET-1 are elevated in patients suffering from traumatic SCI (Wang et al., 2007). These data, combined with the results of the present study, support the idea that ET-1 does play a role in secondary injury cascades following SCI.
Although the primary action in nervous tissue is in regulation of intrinsic vascular tone (Schinelli, 2006), it has more recently been implicated in the modulation of nociception and sensory function (Khodorova et al., 2009). Although this implicates direct activation of neurons, little data exist demonstrating receptor-mediated neurotoxic actions of ET-1. Indeed, previous results show that direct application of 1 μM ET-1 to mixed cultures of neonatal neurons and astrocytes results in neither cell death nor potentiation of excitotoxicity, whereas as little as 50 nM is sufficient for cellular activation, primarily via ETB (Benton et al., 2005). In the present study, acute grey matter pathology following ET-1 microinjection is limited to areas corresponding to patterns of intrinsic blood supply of targeted penetrating microvasculature (Mautes et al., 2000). This supports the idea that the mechanism(s) of insult observed is likely to be due to interruption of blood flow to affected grey matter and not a direct cytotoxic action.
Results from the present study demonstrate early and robust transcriptional activation of TGFβ-responsive genes in the EC compartment in response to spinal ischaemia/reperfusion injury. Previous studies have determined that it is primarily the TGFβ1 isoform that is acutely increased following traumatic SCI (Semple-Rowland et al., 1995; Streit et al., 1998; Nakamura et al., 2003; Wang et al., 2009). In clinical cases of SCI, TGFβ1 appears to be primarily associated with acute injury responses, with other isoforms being expressed at more chronic phases of injury (Buss et al., 2008). Among the postulated effects of this early TGFβ1 response in traumatic SCI are included altered microglial chemotaxis (Yao et al., 1990) and astrocyte proliferation (Lindholm et al., 1992). Present results suggest that TGFβ1 activation of the EC compartment in the early phase of spinal insult may play a role in acute microvascular dysfunction/plasticity following SCI. Also, these results suggest conservation in the TGFβ1 response in traumatic and atraumatic SCI.
The results of the present study suggest de novo synthesis of TGFβ isoforms is unchanged in ECs following ischaemic SCI (see Supplementary Figure S2), a result confirmed by ELISA analyses (results not shown). These observations support the conclusion that EC stimulation by TGFβ results from EC exposure to extracellular sources of bioactive cytokine. Platelets are a rich source of TGFβ1, containing 40–100 times as much as other cells (Assoian et al., 1983). Early studies of traumatic SCI established platelet aggregation as a precipitating event in secondary vascular responses to injury (Griffiths et al., 1978; Goodman et al., 1979). However, observations of acute platelet aggregation in acute ET-1 lesions using CD61 immunohistochemistry shows little/no detectable staining (results not shown). Other potential cellular sources for TGFβ isoform(s) in this context include inflammatory cells (McTigue et al., 2000) and astrocytes (O'Brien et al., 1994). Additional experiments will be necessary to definitively identify stimulatory cellular sources of TGFβ in the acute smvEC response to SCI.
Another possibility supported by the present results, is that local inactive pools of TGFβ are rendered bioactive by one of several potential pathways induced by focal spinal ischaemia. TGFβ1 exists in a biologically inactive form in complex with the remaining portion of its precursor molecule, LAP (latency-associated peptide), which is bound to a LTBP (latent TGFβ1 binding protein) (LTBP-1, 3 or 4) forming the LLC (large latent complex). Virtually all of the TGFβ1 released from platelets and other cells is in the LLC (Annes et al., 2003). In the CNS, TSP-1 (thrombospondin-1) is a very potent activator of TGFβ1, as are the proteases plasmin, mast cell chymase, metalloproteases [including MMP-9 and MT1-MMP (membrane-type 1-MMP)] and thrombin (ten Dijke and Arthur, 2007). Interestingly, 24 h after contusive SCI in the mouse, up-regulated expression of both TSP-1 (58-fold) and uPA (21-fold) was observed in purified microvascular ECs (Benton et al., 2008b). uPA cleaves plasminogen to plasmin which directly activates TGFβ (Tkachuk et al., 1996) as well as indirectly by cleaving pro-MMP-9 to active MMP-9 enhancing bioactivation of TGFβ from extracellular stores (Zhang et al., 2005). Thus not only is TGFβ activated acutely after SCI, it is also done so in a positive-feedback loop further enhancing its actions. The results of the present study support the local activation of TGFβ by uPA in the immediate phase of ischaemia/reperfusion, with other possible effectors including, but not limited to, MMP-9, resulting in TGFβ bioactivity/bioavailability in the subacute phase of microvascular plasticity in response to ischaemic SCI. Furthermore, these results suggest that the molecular mechanism of NVU dysfunction (i.e. increased expression of uPA, MMP-9 and TSP-1) may be similar in both traumatic and atraumatic (i.e. ischaemic and excitotoxic) forms of SCI.
Of the TGFβ-responsive genes examined, the largest change was the 11-fold increase in IGFBP-3 24 h post-ischaemia, a result also demonstrated at the protein level by immunohistochemistry. The role of IGFBP-3 on EC function in the injured spinal cord is unknown, but its overexpression may have implications for smvEC plasticity following SCI. IGFBP-3 is normally expressed at high levels by hepatic ECs (Zimmermann et al., 2000) and is the most abundant circulating binding protein (Guler et al., 1989). The main function of IGFBP-3 is to bind IGF, which results in enhanced bioavailability and activity (Guler et al., 1989). In addition, there are a number of critical IGF-independent actions of IGFBP-3, including wound healing, cell adhesion/migration, gene transcription, cytosolic trafficking and neuronal protection (for a review see Yamada and Lee, 2009). Data on EC-specific responses suggest a controversial and context-dependent role in vascular plasticity. IGFBP-3 accelerates EC senescence in vitro (Kim et al., 2007) and inhibits angiogenesis in HUVECs (human umbilical vein endothelial cells) and chick chorioallantoic in vitro models (Oh et al., 2006). By contrast, IGFBP-3 enhances differentiation of EC precursors in vitro and enhances angiogenesis in an oxygen-induced retinopathy mouse model (Chang et al., 2007). IGFBP-3 remains a poorly understood biomarker of CNS pathology. Increased IGFBP-3 expression is associated with amyloid-β plaque formation in affected microvasculature in brains of Alzheimer's disease patients (Rensink et al., 2002). Furthermore, the IGF system has been implicated in both experimental stroke recovery/susceptibility in patients. Specifically, IGFBP-3 mRNA is acutely up-regulated in the mouse brain in response to transient ischaemia (O'Donnell et al., 2002), a response that was associated with the post-ischaemic inflammatory response. Interestingly, plasma levels of IGFBP-3 are inversely correlated with both increased risk of (Johnsen et al., 2005) and recovery from (Schwab et al., 1997) clinical stroke. Taken together, the latter findings suggest a protective role for IGFBP-3 in CNS pathology. To date, no results exist regarding the putative role of the IGF/IGFBP-3 system in microvascular function and/or neuroprotection following SCI.
Also of note is the present result demonstrating mRNA levels of IL-6 to be significantly up-regulated in activated ECs following SCI, with protein levels increased acutely, but not sustained beyond, 24 h post-SCI. With respect to IL-6, in addition to its role as a multifunctional pro-inflammatory cytokine involved in the regulation of the acute inflammatory response (Papassotiropoulos et al., 2001; Rose-John et al., 2007), IL-6 protects HUVECs from H2O2-induced cell death (Waxman et al., 2003). On the other hand, IL-6 induces tight junction dysfunction and hyperpermeability in rat heart microvascular ECs in vitro (Tinsley et al., 2008). In the context of traumatic SCI, IL-6 mRNA levels are increased 3–24 h post-injury in astrocytes, neurons and resident microglia (Pineau and LaCroix, 2007). Similarly, IL-6 mRNA is elevated 3–24 h in response to permanent MCAO (middle cerebral artery occlusion) in rats, with expression peaking at 12 h post-ischaemia (Wang et al., 1995). More recently, serum levels of IL-6 were shown to significantly increase 24 h following transient MCAO (Martinez-Revelles et al., 2008). Similar results have been observed in patients within hours following stroke onset, with a positive correlation seen between plasma levels of IL-6 and both infarct size and clinical outcome (Tarkowski et al., 1995; Huang et al., 2006). While no data currently exist regarding whether the action of IL-6 is deleterious in SCI, recent findings have linked IL-6 signalling in the EC compartment with blood–brain barrier failure in EAE (experimental autoimmune encephalomyelitis) (Linker et al., 2008). Additional investigations will be needed to determine the contribution of increased EC expression of IL-6 on inflammation and/or angiogenesis following SCI.
The temporal response of uPA mRNA and activity observed in the present study is consistent with observations made in experimental stroke (Hosomi et al., 2001). Specific inhibition of uPA in experimental stroke decreases infarct size by protecting blood–CNS barrier integrity (Hamann et al., 2004). The acute increase in uPA mRNA in the EC compartment observed in the present study is likely to be of functional consequence as uPA activity is increased in smvECs isolated 72 h post-ischaemia (R. L. Benton, unpublished data). Furthermore, significant inductions of PAI-1 (mRNA and protein) and uPAR (uPA receptor) (R. L. Benton, unpublished data) in ECs after ischaemic SCI suggest a role for the PAS (PA system) in vascular dysfunction and secondary injury progression following SCI, as has been established in stroke. The implications of the response observed here, as well as in other types of neuropathology, are likely to be a fundamental event underlying secondary degeneration. The PAS is best known for its function in thrombolysis, but it also plays substantial roles in many cellular processes outside of the bloodstream, especially in the CNS (Zhang et al., 2005). The central active protease of the PAS is plasmin, which is generated by cleavage of plasminogen by the PAs, tPA (tissue-type PA) and uPA. While both PAs are secreted proteases, uPA is unique in that following secretion it binds its cell-surface receptor (uPAR) where it is then activated, directing proteolysis to the pericellular space (Cubellis et al., 1986). tPA appears to be the primary PA expressed in the intact CNS and is involved in neuronal migration and synaptic plasticity (Seeds et al., 1997, 1999, 2003). By contrast, little uPA expression is observed in the adult CNS in the absence of pathology (Tsirka et al., 1997), with a single report demonstrating uPA up-regulation in the injured spinal motor neurons (Minor and Seeds, 2008). The clinical relevance of this observation is substantiated by the observation of increased uPAR expression in ECs in human cases of both TBI (traumatic brain injury) and stroke (Beschorner et al., 2000). Furthermore, several members of the PAS (uPA, uPAR and PAI-1) are expressed in ECs and astrocytes within human stroke foci (Dietzmann et al., 2000).
In conclusion, the increased TGFβ signalling demonstrated in the present study might be a double-edged sword. On one hand, TGFβ1 is known to confer neuroprotection against various insults, including stroke (Dhandapani and Brann, 2003). However, increased TGFβ signalling probably has destabilizing actions in pathologically activated vessels, in part via induction of both VEGF and MMP expression (ten Dijke and Arthur, 2007). While uPA and plasmin are likely regulators of the action of TGFβ1 on spinal microvasculature (Sato and Rifkin, 1989; Sato et al., 1990; Rainger and Nash, 2001), the present study suggests a comparable role for regulating spinal microvascular plasticity and/or dysfunction following SCI. Furthermore, it may be possible that the activation of the EC compartment by TGFβ may be downstream of a yet-to-be identified cytokine cascade, involving IL-6 and/or other growth factors. Acutely attenuating the action of TGFβ1 in microvessels following SCI may have therapeutic potential.
Online data
ACKNOWLEDGEMENTS
We thank Christine Nunn for surgical support, Kim Fentress for post-operative animal care and Christopher Worth for his technical expertise with FACS.
Footnotes
This work was supported by the University of Louisville School of Medicine Foundation [grant numbers RR15576, NS045734 (to R.L.B.)]; Norton Healthcare; and the Commonwealth of Kentucky Challenge for Excellence (to S.R.W. and T.H.).
REFERENCES
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Allaire E, Hasenstab D, Kenagy RD, Starcher B, Clowes MM, Clowes AW. Prevention of aneurysm development and rupture by local overexpression of plasminogen activator inhibitor-1. Circulation. 1998;98:249–255. doi: 10.1161/01.cir.98.3.249. [DOI] [PubMed] [Google Scholar]
- Amar AP, Levy ML. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 1999;44:1027–1039. doi: 10.1097/00006123-199905000-00052. [DOI] [PubMed] [Google Scholar]
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFβ activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB. Transforming growth factor-β in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983;258:7155–7160. [PubMed] [Google Scholar]
- Benton RL, Woock JP, Gozal E, Hetman M, Whittemore SR. Intraspinal application of endothelin results in focal ischemic injury of spinal gray matter and restricts the differentiation of engrafted neural stem cells. Neurochem Res. 2005;30:809–823. doi: 10.1007/s11064-005-6875-7. [DOI] [PubMed] [Google Scholar]
- Benton RL, Maddie MA, Minnillo DR, Hagg T, Whittemore SR. Griffonia simplicifolia isolectin B4 identifies a specific subpopulation of angiogenic blood vessels following contusive spinal cord injury in the adult mouse. J Comp Neurol. 2008a;507:1031–1052. doi: 10.1002/cne.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton RL, Maddie MA, Worth CA, Mahoney ET, Hagg T, Whittemore SR. Transcriptomic screening of microvascular endothelial cells implicates novel molecular regulators of vascular dysfunction after spinal cord injury. J Cereb Blood Flow Metab. 2008b;28:1771–1185. doi: 10.1038/jcbfm.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beschorner R, Schluesener HJ, Nguyen TD, Magdolen V, Luther T, Pedal I, Mattern R, Meyermann R, Schwab JM. Lesion-associated accumulation of uPAR. Neuropathol Appl Neurobiol. 2000;26:522–527. doi: 10.1046/j.0305-1846.2000.287.x. [DOI] [PubMed] [Google Scholar]
- Binder LI, Frankfurter A, Rebhun LI. Differential localization of MAP-2 and tau in mammalian neurons in situ. Ann N Y Acad Sci. 1986;466:145–166. doi: 10.1111/j.1749-6632.1986.tb38392.x. [DOI] [PubMed] [Google Scholar]
- Burk J, Burggraf D, Vosko M, Dichgans M, Hamann GF. Protection of cerebral microvasculature after moderate hypothermia following experimental focal cerebral ischemia in mice. Brain Res. 2008;1226:248–255. doi: 10.1016/j.brainres.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Buss A, Pech K, Kakulas BA, Martin D, Schoenen J, Noth J, Brook GA. TGF-β1 and TGF-β2 expression after traumatic human spinal cord injury. Spinal Cord. 2008;46:364–371. doi: 10.1038/sj.sc.3102148. [DOI] [PubMed] [Google Scholar]
- Casella GT, Marcillo A, Bunge MB, Wood PM. New vascular tissue rapidly replaces neural parenchyma and vessels destroyed by a contusion injury to the rat spinal cord. Exp Neurol. 2002;173:63–76. doi: 10.1006/exnr.2001.7827. [DOI] [PubMed] [Google Scholar]
- Casella GT, Bunge MB, Wood PM. Endothelial cell loss is not a major cause of neuronal and glial cell death following contusion injury of the spinal cord. Exp Neurol. 2006;202:8–20. doi: 10.1016/j.expneurol.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Chang KH, Chan-Ling T, McFarland EL, Afzal A, Pan H, Baxter LC, Shaw LC, Caballero S, Sengupta N, Li CS, Sullivan SM, Grant MB. IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development. Proc Natl Acad Sci USA. 2007;104:10595–10600. doi: 10.1073/pnas.0702072104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, McCarron RM, Ohara Y, Bembry J, Azzam N, Lenz FA, Shohami E, Mechoulam R, Spatz M. Human brain capillary endothelium: 2-arachidonoglycerol (endocannabinoid) interacts with endothelin-1. Circ Res. 2000;87:323–327. doi: 10.1161/01.res.87.4.323. [DOI] [PubMed] [Google Scholar]
- Corti S, Locatelli F, Papadimitriou D, Donadoni C, Del Bo R, Fortunato F, Strazzer S, Salani S, Bresolin N, Comi GP. Multipotentiality, homing properties, and pyramidal neurogenesis of CNS-derived LeX(ssea-1)+/CXCR4+ stem cells. FASEB J. 2005;13:1860–1862. doi: 10.1096/fj.05-4170fje. [DOI] [PubMed] [Google Scholar]
- Cubellis MV, Nolli ML, Cassani G, Blasi F. Binding of single-chain prourokinase to the urokinase receptor of human U937 cells. J Biol Chem. 1986;261:15819–15822. [PubMed] [Google Scholar]
- Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50:329–339. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- Debus E, Weber K, Osborn M. Monoclonal antibodies specific for glial fibrillary acidic (GFA) protein and for each of the neurofilament triplet polypeptides. Differentiation. 1983;25:193–203. doi: 10.1111/j.1432-0436.1984.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Brann DW. Transforming growth factor-β: a neuroprotective factor in cerebral ischemia. Cell Biochem Biophys. 2003;39:13–22. doi: 10.1385/CBB:39:1:13. [DOI] [PubMed] [Google Scholar]
- Dietzmann K, von Bossanyi P, Krause D, Wittig H, Mawrin C, Kirches E. Expression of the plasminogen activator system and the inhibitors PAI-1 and PAI-2 in posttraumatic lesions of the CNS and brain injuries following dramatic circulatory arrests: an immunohistochemical study. Pathol Res Pract. 2000;196:15–21. doi: 10.1016/s0344-0338(00)80017-5. [DOI] [PubMed] [Google Scholar]
- Duijvestijn AM, van Goor H, Klatter F, Majoor GD, van Bussel E, van Breda Vriesman PJ. Antibodies defining rat endothelial cells: RECA-1, a pan-endothelial cell-specific monoclonal antibody. Lab Invest. 1992;66:459–466. [PubMed] [Google Scholar]
- Edvinsson L. Cerebrovascular endothelin receptor upregulation in cerebral ischemia. Curr Vasc Pharmacol. 2009;7:26–33. doi: 10.2174/157016109787354178. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Pintucci G, Seghezzi G, Hyman K, Galloway AC, Mignatti P. VEGF, a prosurvival factor, acts in concert with TGF-β1 to induce endothelial cell apoptosis. Proc Natl Acad Sci USA. 2006;103:17260–17265. doi: 10.1073/pnas.0605556103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glas M, Popp B, Angele B, Koedel U, Chahli C, Schmalix WA, Anneser JM, Pfister HW, Lorenzl S. A role for the urokinase-type plasminogen activator system in amyotrophic lateral sclerosis. Exp Neurol. 2007;207:350–356. doi: 10.1016/j.expneurol.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Goodman JH, Bingham WG, Jr, Hunt WE. Platelet aggregation in experimental spinal cord injury. Ultrastructural observations. Arch Neurol. 1979;36:197–201. doi: 10.1001/archneur.1979.00500400051006. [DOI] [PubMed] [Google Scholar]
- Griffiths IR, Burns N, Crawford AR. Early vascular changes in the spinal grey matter following impact injury. Acta Neuropathol. 1978;41:33–39. doi: 10.1007/BF00689554. [DOI] [PubMed] [Google Scholar]
- Guler HP, Zapf J, Schmid C, Froesch ER. Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates. Acta Endocrinol (Copenh) 1989;121:753–758. doi: 10.1530/acta.0.1210753. [DOI] [PubMed] [Google Scholar]
- Hamann GF, Burggraf D, Martens HK, Liebetrau M, Jager G, Wunderlich N, DeGeorgia M, Krieger DW. Mild to moderate hypothermia prevents microvascular basal lamina antigen loss in experimental focal cerebral ischemia. Stroke. 2004;35:764–769. doi: 10.1161/01.STR.0000116866.60794.21. [DOI] [PubMed] [Google Scholar]
- Han HS, Suk K. The function and integrity of the neurovascular unit rests upon the integration of the vascular and inflammatory cell systems. Curr Neurovasc Res. 2005;2:409–423. doi: 10.2174/156720205774962647. [DOI] [PubMed] [Google Scholar]
- Hansen-Schwartz J, Szok D, Edvinsson L. Expression of ET(A) and ET(B) receptor mRNA in human cerebral arteries. Br J Neurosurg. 2002;16:149–153. doi: 10.1080/02688690220131750. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Heussen C, Dowdle EB. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Hosomi N, Lucero J, Heo JH, Koziol JA, Copeland BR, del Zoppo GJ. Rapid differential endogenous plasminogen activator expression after acute middle cerebral artery occlusion. Stroke. 2001;32:1341–1348. doi: 10.1161/01.str.32.6.1341. [DOI] [PubMed] [Google Scholar]
- Huang J, Urvashi M, Upadhyay BS, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol. 2006;66:232–245. doi: 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Jakovcevski I, Wu J, Karl N, Leshchyns'ka I, Sytnyk V, Chen J, Irintchev A, Schachner M. Glial scar expression of CHL1, the close homolog of the adhesion molecule L1, limits recovery after spinal cord injury. J Neurosci. 2007;27:7222–7233. doi: 10.1523/JNEUROSCI.0739-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen SP, Hundborg HH, Sørensen HT, Ørskov H, Tjønneland A, Overvad K, Jørgensen JOL. Insulin-like growth factor (IGF) I, -II, and IGF binding protein-3 and risk of ischemic stroke. J Clin Endocrinol Metab. 2005;90:5937–5941. doi: 10.1210/jc.2004-2088. [DOI] [PubMed] [Google Scholar]
- Khodorova A, Montmayeur JP, Strichartz G. Endothelin receptors and pain. J Pain. 2009;10:4–28. doi: 10.1016/j.jpain.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Kim MS, Seu YB, Chung HY, Kim JH, Kim JR. Regulation of replicative senescence by insulin-like growth factor-binding protein 3 in human umbilical vein endothelial cells. Aging Cell. 2007;6:535–545. doi: 10.1111/j.1474-9726.2007.00315.x. [DOI] [PubMed] [Google Scholar]
- Koyanagi I, Tator CH, Lea PJ. Three-dimensional analysis of the vascular system in the rat spinal cord with scanning electron microscopy of vascular corrosion casts. Part 2: Acute spinal cord injury. Neurosurgery. 1993;33:285–291. [PubMed] [Google Scholar]
- Lindholm D, Castren E, Kiefer R, Zafra F, Thoenen H. Transforming growth factor-β1 in the rat brain: increase after injury and inhibition of astrocyte proliferation. J Cell Biol. 1992;117:395–400. doi: 10.1083/jcb.117.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker RA, Lühder F, Kallen K, Lee D, Engelhardt B, Rose-John S, Gold R. IL-6 transsignalling modulates the early effector phase of EAE and targets the blood-brain barrier. J Neuroimmunol. 2008;205:64–72. doi: 10.1016/j.jneuroim.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Loy DN, Crawford CH, Darnall JB, Burke DA, Onifer SM, Whittemore SR. Temporal progression of angiogenesis and basal lamina deposition after contusive spinal cord injury in the adult rat. J Comp Neurol. 2002;445:308–324. doi: 10.1002/cne.10168. [DOI] [PubMed] [Google Scholar]
- Martinez-Revelles S, Jiménez-Altayó F, Caracuel L, Pérez-Asensio FJ, Planas AM, Vila E. Endothelial dysfunction in rat mesenteric resistance artery after transient middle cerebral artery occlusion. J Pharmacol Exp Therap. 2008;325:363–369. doi: 10.1124/jpet.107.134619. [DOI] [PubMed] [Google Scholar]
- Mautes AE, Weinzierl MR, Donovan F, Noble LJ. Vascular events after spinal cord injury: contribution to secondary pathogenesis. Phys Ther. 2000;80:673–687. [PubMed] [Google Scholar]
- McTigue DM, Popovich PG, Morgan TE, Stokes BT. Localization of transforming growth factor-β1 and receptor mRNA after experimental spinal cord injury. Exp Neurol. 2000;163:220–230. doi: 10.1006/exnr.2000.7372. [DOI] [PubMed] [Google Scholar]
- Minor KH, Seeds NW. Plasminogen activator induction facilitates recovery of respiratory function following spinal cord injury. Mol Cell Neurosci. 2008;37:143–152. doi: 10.1016/j.mcn.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Houghtling RA, MacArthur L, Bayer BM, Bregman BS. Differences in cytokine gene expression profile between acute and secondary injury in adult rat spinal cord. Exp Neurol. 2003;184:313–325. doi: 10.1016/s0014-4886(03)00361-3. [DOI] [PubMed] [Google Scholar]
- Nelson E, Gertz SD, Rennels ML, Ducker TB, Blaumanis OR. Spinal cord injury. The role of vascular damage in the pathogenesis of central hemorrhagic necrosis. Arch Neurol. 1977;34:332–333. doi: 10.1001/archneur.1977.00500180026005. [DOI] [PubMed] [Google Scholar]
- Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 2002;22:7526–7535. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien MF, Lenke LG, Lou J, Bridwell KH, Joyce ME. Astrocyte response and transforming growth factor-β localization in acute spinal cord injury. Spine. 1994;19:2321–2329. doi: 10.1097/00007632-199410150-00012. [DOI] [PubMed] [Google Scholar]
- O'Donnell SL, Frederick TJ, Krady JK, Vannucci SJ, Wood TL. IGF-1 and microglia/macrophage proliferation in the ischemic mouse brain. Glia. 2002;38:85–97. doi: 10.1002/glia.10081. [DOI] [PubMed] [Google Scholar]
- O'Neill MJ, Clemens JA. Rodent models of focal cerebral ischemia. Curr Protoc Neurosci. 2001;9:9. doi: 10.1002/0471142301.ns0906s12. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Sasatomi K, Hiragata S, Seki S, Nishizawa O, Chermansky CJ, Pflug BR, Nelson JB, Chancellor MB, Yoshimura N. Therapeutic effects of endothelin-A receptor antagonist on bladder overactivity in rats with chronic spinal cord injury. Urology. 2008;71:341–345. doi: 10.1016/j.urology.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SH, Kim WY, Kim JH, Younes MN, El-Naggar AK, Myers JN, Kies M, Cohen P, Khuri F, Hong WK, Lee HY. Identification of insulin-like growth factor binding protein-3 as a farnesyl transferase inhibitor SCH66336-induced negative regulator of angiogenesis in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12:653–661. doi: 10.1158/1078-0432.CCR-05-1725. [DOI] [PubMed] [Google Scholar]
- Papassotiropoulos A, Hock C, Nitsch RM. Genetics of interleukin 6: implications for Alzheimer's disease. Neurobiol Aging. 2001;22:863–871. doi: 10.1016/s0197-4580(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Peters CM, Rogers SD, Pomonis JD, Egnaczyk GF, Keyser CP, Schmidt JA, Ghilardi JR, Maggio JE, Mantyh PW. Endothelin receptor expression in the normal and injured spinal cord: potential involvement in injury-induced ischemia and gliosis. Exp Neurol. 2003;180:1–13. doi: 10.1016/s0014-4886(02)00023-7. [DOI] [PubMed] [Google Scholar]
- Pineau I, LaCroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. 2007;500:267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- Polavarapu R, Gongora MC, Yi H, Ranganthan S, Lawrence DA, Strickland D, Yepes M. Tissue-type plasminogen activator-mediated shedding of astrocytic low-density lipoprotein receptor-related protein increases the permeability of the neurovascular unit. Blood. 2007;109:3270–3278. doi: 10.1182/blood-2006-08-043125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovich PG, Jones TB. Manipulating neuroinflammatory reactions in the injured spinal cord: back to basics. Trends Pharmacol Sci. 2003;24:13–17. doi: 10.1016/s0165-6147(02)00006-8. [DOI] [PubMed] [Google Scholar]
- Quick AM, Cipolla MJ. Pregnancy-induced up-regulation of aquaporin-4 protein in brain and its role in eclampsia. FASEB J. 2005;19:170–175. doi: 10.1096/fj.04-1901hyp. [DOI] [PubMed] [Google Scholar]
- Rainger GE, Nash GB. Cellular pathology of atherosclerosis: smooth muscle cells prime cocultured endothelial cells for enhanced leukocyte adhesion. Circ Res. 2001;88:615–622. doi: 10.1161/01.res.88.6.615. [DOI] [PubMed] [Google Scholar]
- Rensink AM, Gellekink H, Otte-Höller I, ten Konkelaar HJ, de Waal RM, Verbeek MM, Kremer B. Expression of the cytokine leukemia inhibitory factor and pro-apoptotic insulin-like growth factor binding protein-3 in Alzheimer's disease. Acta Neuropathol. 2002;104:525–533. doi: 10.1007/s00401-002-0585-x. [DOI] [PubMed] [Google Scholar]
- Rose-John S, Waetzig GH, Scheller J, Grotzinger J, Seegert D. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin Ther Targets. 2007;11:613–624. doi: 10.1517/14728222.11.5.613. [DOI] [PubMed] [Google Scholar]
- Sato Y, Rifkin DB. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-β1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309–315. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Tsuboi R, Lyons R, Moses H, Rifkin DB. Characterization of the activation of latent TGF-β by co-cultures of endothelial cells and pericytes or smooth muscle cells: a self-regulating system. J Cell Biol. 1990;111:757–763. doi: 10.1083/jcb.111.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinelli S, Zanassi P, Paolillo M, Wang H, Feliciello A, Gallo V. Stimulation of endothelin B receptors in astrocytes induces cAMP response element-binding protein phosphorylation and c-fos expression via multiple mitogen-activated protein kinase signaling pathways. J Neurosci. 2001;21:8842–8853. doi: 10.1523/JNEUROSCI.21-22-08842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinelli S. Pharmacology and physiopathology of the brain endothelin system: an overview. Curr Med Chem. 2006;13:627–638. doi: 10.2174/092986706776055652. [DOI] [PubMed] [Google Scholar]
- Schwab S, Spranger M, Krempien S, Hacke W, Bettendorf M. Plasma insulin-like growth factor 1 and IGF binding protein 3 levels in patients with acute cerebral ischemic injury. Stroke. 1997;28:1744–1748. doi: 10.1161/01.str.28.9.1744. [DOI] [PubMed] [Google Scholar]
- Seeds NW, Siconolfi LB, Haffke SP. Neuronal extracellular proteases facilitate cell migration, axonal growth, and pathfinding. Cell Tissue Res. 1997;290:367–370. doi: 10.1007/s004410050942. [DOI] [PubMed] [Google Scholar]
- Seeds NW, Basham ME, Haffke SP. Neuronal migration is retarded in mice lacking the tissue plasminogen activator gene. Proc Natl Acad Sci USA. 1999;96:14118–14123. doi: 10.1073/pnas.96.24.14118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds NW, Basham ME, Ferguson JE. Absence of tissue plasminogen activator gene or activity impairs mouse cerebellar motor learning. J Neurosci. 2003;23:7368–7375. doi: 10.1523/JNEUROSCI.23-19-07368.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple-Rowland SL, Mahatme A, Popovich PG, Green DA, Hassler G, Jr, Stokes BT, Streit WJ. Analysis of TGF-β1 gene expression in contused rat spinal cord using quantitative RT-PCR. J Neurotrauma. 1995;12:1003–1014. doi: 10.1089/neu.1995.12.1003. [DOI] [PubMed] [Google Scholar]
- Sharma HS. Pathophysiology of blood-spinal cord barrier in traumatic injury and repair. Curr Pharm Des. 2005;11:1353–1389. doi: 10.2174/1381612053507837. [DOI] [PubMed] [Google Scholar]
- Sternberger NH, Sternberger LA. Blood-brain barrier protein recognized by monoclonal antibody. Proc Natl Acad Sci USA. 1987;84:8169–8173. doi: 10.1073/pnas.84.22.8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Semple-Rowland SL, Hurley SD, Miller RC, Popovich PG, Stokes BT. Cytokine mRNA profiles in contused spinal cord and axotomized facial nucleus suggest a beneficial role for inflammation and gliosis. Exp Neurol. 1998;152:74–87. doi: 10.1006/exnr.1998.6835. [DOI] [PubMed] [Google Scholar]
- Tarkowski E, Rosengren L, Blomstrand C, Wikkelsö C, Jensen C, Ekholm S, Tarkowski A. Early intrathecal production of interleukin-6 predicts the size of brain lesion in stroke. Stroke. 1995;26:1393–1398. doi: 10.1161/01.str.26.8.1393. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Arthur HM. Extracellular control of TGFβ signalling in vascular development and disease. Nat Rev Mol Cell Biol. 2007;8:857–869. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- Tinsley JH, Hunter FA, Childs EW. PKC and MLCK-dependent, cytokine-induced rat coronary endothelial dysfunction. J Surg Res. 2009;152:76–83. doi: 10.1016/j.jss.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Tkachuk V, Stepanova V, Little PJ, Bobik A. Regulation and role of urokinase plasminogen activator in vascular remodelling. Clin Exp Pharmacol Physiol. 1996;23:759–765. doi: 10.1111/j.1440-1681.1996.tb01177.x. [DOI] [PubMed] [Google Scholar]
- Tsaur ML, Wan YC, Lai FP, Cheng HF. Expression of B-type endothelin receptor gene during neural development. FEBS Lett. 1997;417:208–212. doi: 10.1016/s0014-5793(97)01295-7. [DOI] [PubMed] [Google Scholar]
- Tsirka SE, Rogove AD, Bugge TH, Degen JL, Strickland S. An extracellular proteolytic cascade promotes neuronal degeneration in the mouse hippocampus. J Neurosci. 1997;17:543–552. doi: 10.1523/JNEUROSCI.17-02-00543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuschick S, Kirischuk S, Kirchhoff F, Liefeldt L, Paul M, Verkhratsky A, Kettenmann H. Bergmann glial cells in situ express endothelinB receptors linked to cytoplasmic calcium signals. Cell Calcium. 1997;21:409–419. doi: 10.1016/s0143-4160(97)90052-x. [DOI] [PubMed] [Google Scholar]
- Uesugi M, Kasuya Y, Hayashi K, Goto K. SB209670, a potent endothelin receptor antagonist, prevents or delays axonal degeneration after spinal cord injury. Brain Res. 1998;786:235–239. doi: 10.1016/s0006-8993(97)01431-5. [DOI] [PubMed] [Google Scholar]
- Wang S, Lim G, Zeng Q, Sung B, Ai Y, Guo G, Yang L, Mao J. Expression of central glucocorticoid receptors after peripheral nerve injury contributes to neuropathic pain behaviors in rats. J Neurosci. 2004;24:8595–8605. doi: 10.1523/JNEUROSCI.3058-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TD, Wang YH, Huang TS, Su TC, Pan SL, Chen SY. Circulating levels of markers of inflammation and endothelial activation are increased in men with chronic spinal cord injury. J Formos Med Assoc. 2007;106:919–928. doi: 10.1016/S0929-6646(08)60062-5. [DOI] [PubMed] [Google Scholar]
- Wang X, Yue TL, Young PR, Barone FC, Feuerstein GZ. Expression of interleukin-6, c-fos, and zif268 mRNAs in rat ischemic cortex. J Cereb Blood Flow Metab. 1995;15:166–171. doi: 10.1038/jcbfm.1995.18. [DOI] [PubMed] [Google Scholar]
- Wang X, Chen W, Liu W, Wu J, Shao Y, Zhang X. The role of thrombospondin-1 and transforming growth factor-β after spinal cord injury in the rat. J Clin Neurosci. 2009;16:818–821. doi: 10.1016/j.jocn.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Ward NL, LaManna JC. The neurovascular unit and its growth factors: coordinated response in the vascular and nervous systems. Neurol Res. 2004;26:870–883. doi: 10.1179/016164104X3798. [DOI] [PubMed] [Google Scholar]
- Waxman AB, Mahboubi K, Knickelbein RG, Mantell LL, Manzo N, Pober JS, Elias JA. Interleukin-11 and interleukin-6 protect cultured human endothelial cells from H2O2-induced cell death. Am J Respir Cell Mol Biol. 2003;29:513–522. doi: 10.1165/rcmb.2002-0044OC. [DOI] [PubMed] [Google Scholar]
- Whetstone WD, Hsu JY, Eisenberg M, Werb Z, Noble-Haeusslein LJ. Blood-spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J Neurosci Res. 2003;74:227–239. doi: 10.1002/jnr.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Kurokawa K. Histochemical studies on endothelin and the endothelin-A receptor in the hypothalamus. J Cardiovasc Pharmacol. 1998;31(Suppl 1):S215–S218. doi: 10.1097/00005344-199800001-00060. [DOI] [PubMed] [Google Scholar]
- Yamada PM, Lee KW. Perspectives in mammalian IGFBP-3 biology: local vs. systemic action. Am J Physiol Cell Physiol. 2009;296:C954–C976. doi: 10.1152/ajpcell.00598.2008. [DOI] [PubMed] [Google Scholar]
- Yao J, Harvath L, Gilbert DL, Colton CA. Chemotaxis by a CNS macrophage, the microglia. J Neurosci Res. 1990;27:36–42. doi: 10.1002/jnr.490270106. [DOI] [PubMed] [Google Scholar]
- Ye P, Price W, Kassiotis G, Kollias G, D'Ercole AJ. Tumor necrosis factor-α regulation of insulin-like growth factor-I, type 1 IGF receptor, and IGF binding protein expression in cerebellum of transgenic mice. J Neurosci Res. 2003;71:721–731. doi: 10.1002/jnr.10512. [DOI] [PubMed] [Google Scholar]
- Yin KJ, Cirrito JR, Yan P, Hu X, Xiao Q, Pan X, Bateman R, Song H, Hsu FF, Turk J, Xu J, Hsu CY, Mills JC, Holtzman DM, Lee JM. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-β peptide catabolism. J Neurosci. 2006;26:10939–10948. doi: 10.1523/JNEUROSCI.2085-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JC, Pickard JD, Davenport AP. Endothelin ETA receptor expression in human cerebrovascular smooth muscle cells. Br J Pharmacol. 1995;116:2441–2446. doi: 10.1111/j.1476-5381.1995.tb15093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Pothakos K, Tsirka SA. Extracellular proteases: biological and behavioral roles in the mammalian central nervous system. Curr Top Dev Biol. 2005;66:161–188. doi: 10.1016/s0070-2153(05)66005-x. [DOI] [PubMed] [Google Scholar]
- Zimmermann EM, Li L, Hoyt EC, Pucilowska JB, Lichtman S, Lund PK. Cell-specific localization of insulin-like growth factor binding protein mRNAs in rat liver. Am J Physiol Gastrointest Liver Physiol. 2000;278:G447–G457. doi: 10.1152/ajpgi.2000.278.3.G447. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.