Abstract
Age-related macular degeneration (AMD) is a complex disease that has potential involvement of inflammatory and oxidative stress-related pathways in its pathogenesis. In search of effective therapeutic agents, we tested curcumin, a naturally-occurring compound with known anti-inflammatory and anti-oxidative properties, in rat model of light induced retinal degeneration (LIRD) and in retina derived cell lines. We hypothesized that any compound effective against LIRD, which involves significant oxidative stress and inflammation, would be a candidate for further characterization for its potential application in AMD.
We observed significant retinal neuroprotection in rats fed diets supplemented with curcumin (0.2% in diet) for 2 weeks. The mechanism of retinal protection from LIRD by curcumin involves inhibition of NF-κB activation and down-regulation of cellular inflammatory genes. When tested on retina-derived cell lines (661W and ARPE-19), pre-treatment of curcumin protected these cells from H2O2-induced cell death by up-regulating cellular protective enzymes, such as HO-1, thioredoxin.
Since, curcumin with its pleiotropic activities can modulate the expression and activation of many cellular regulatory proteins such as NF-κB, AKT, NRF2 and growth factors, which in turn inhibit cellular inflammatory responses and protect cells; we speculate that curcumin would be an effective nutraceutical compound for preventive and augmentative therapy of AMD.
Keywords: Curcumin, AMD, light-induced retinal degeneration, photoreceptors
Introduction
Traditional medicines provide frontline pharmacotherapy for many millions of people worldwide [1]. Curcumin is a naturally occurring yellow pigment, isolated from the rhizomes of the plant Curcuma longa (Linn), that is commonly used in Asian cooking as a coloring and flavoring agent. It has been used in both Oriental and Ayurvedic medicine since ancient times [2]. Studies have shown that curcumin has a wide range of beneficial properties, including anti-inflammatory, antioxidant, chemopreventive and chemotherapeutic activities [3–5]. This pleiotropic effect derives from curcumin’s ability to influence multiple survival and cytoprotective signaling pathways including pathways that inhibit inflammatory responses and those regulated by NF-κB, AKT, growth factors and NRF2 transcription factor [6–14]. In the past three decades, detailed studies and analysis of different molecular pathways modulated by curcumin identified it as a promising therapeutic and nutraceutical compound that could be used for treatment or prevention of many diseases. As summarized by Hatcher et al., [3] and Goel et al., [4] there are at least 16 ongoing and several completed clinical trials examining the effects of curcumin on various types of carcinomas and conditions linked to inflammation such as psoriasis and Alzheimer’s disease.
Owing to its multipotent activities and especially as an agent for anti-oxidative and anti-inflammatory therapies, we hypothesize that curcumin could represent a preventive treatment option for inflammatory retinal diseases such as age-related macular degeneration (AMD) and diabetic retinopathy (DR). This hypothesis is based on studies suggesting the significant contribution of oxidative and inflammatory stresses on the pathogenesis of AMD and DR [15–19]. Dietary supplementation of curcumin has been shown to be effective in modulating redox status in a rat model of streptozotocin (STZ)-induced diabetic retinopathy [20]. However, the protective effect of curcumin on retinal dystrophies has not been tested in vivo.
Here we measured the efficacy of dietary supplementation of curcumin on retinal neuroprotection using an in vivo model of light-induced retinal degeneration (LIRD) in rats. The pathogenesis of LIRD involves the generation of oxidants [21] and the accumulation of oxidatively-modified lipids, nucleic acids, and proteins [22–25]. Furthermore, several reports describe protection against LIRD by a variety of antioxidants, including ascorbate [26], dimethylthiourea [27], thioredoxin [28], NG-nitro-L-arginine-methyl ester (L-NAME) [29], and phenyl-N-tert-butylnitrone (PBN) [30]. We maintained Wistar rats on a curcumin-supplemented diet for two weeks and then exposed them to damaging light and evaluated retinal protection by morphological and functional analyses. We further evaluated the effect of pre-treatment of curcumin on oxidative stress-mediated cell death in retina-derived cell lines (661W and ARPE-19). Finally, we tested the potential mechanism(s) of curcumin-mediated protection of retinal cells by employing various biochemical and molecular assays.
Materials and Methods
Animal Care
All procedures were performed according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the University of Oklahoma Health Sciences Center (OUHSC) Guidelines for Animals in Research. All protocols were reviewed and approved by the Institutional Animal Care and Use Committees of the OUHSC and the Dean A. McGee Eye Institute (DMEI). Wistar (Charles River Laboratories, Wilmington, MA) rats were born and raised in the DMEI vivarium and maintained under dim cyclic light (5 lux, 12 hours on/off, 7 AM–7 PM central time).
Dietary Supplementation of Curcumin and Exposure to Light
Five to six week-old Wistar rats reared in dim cyclic light (5 lux) were divided into two groups (12 animal/ group) for each experiment. One group was fed with powdered control lab diet AIN-76A (Con-) and the other group the AIN-76A diet supplemented with 2000 ppm (0.2%) curcumin (Cur-) for two weeks. Purified and crystallized curcumin (>98% pure by HPLC) was obtained from the National Cancer Institute Chemopreventive Agent Repository. Rats were housed two to a cage and the diets were supplied in the cage in a standard feeding bowl. Water was provided ad libitum. After two weeks, the rats were placed individually in clear plastic cages with wire tops and exposed to 1,000 lux light (white cool light) for 3 hours (9 AM to 12 PM). Drinking water was supplied by a bottle attached to the side of cage so that there was no obstruction between the light and the animal. During the exposure to light, the right eye of each rat was covered with a black-painted polypropylene eye cap attached to the facial skin with an adhesive (no. 454; Loctite Corp., Hartford, CT) to serve as a non–light-damaged control (covered eye, NLD) [31]. The left eye of each rat was left uncovered and was considered the light-damaged eye (uncovered eye, LD). For morphological and functional assessment, rats were returned to the dim cyclic light environment after exposure to light and retinal function was measured by ERG, 5 to 7 days later. The rats were then euthanized and the eyes processed for quantitative morphology. For RNA and protein extraction, rats were euthanized immediately or at 3 h after completion of bright light exposure and the retinas were harvested.
Electroretinography
Flash ERGs were recorded with an ERG recording system (Diagnosys Espion E2 ERG System, Lowell, MA). Rats were maintained in total darkness overnight and prepared for ERG recording under dim red light. They were anesthetized with ketamine [120 mg/kg body weight intramuscularly (IM)] and xylazine (6 mg/kg body weight, IM). One drop of 10% phenylephrine was applied to the cornea to dilate the pupil and one drop of 0.5% proparacaine HCl was applied for local anesthesia. A reference electrode was positioned in the mouth and a ground electrode on the foot, and the rat was placed inside of a Ganzfeld illuminating sphere. Responses were differentially amplified, averaged, and stored. For the assessment of rod photoreceptor function (scotopic ERG), five strobe flash stimuli were presented in a Ganzfeld with flash intensities at 0.005, 0.05, 5, 500 and 5000 cd × sec/mm2. The amplitude of the a-wave was measured from the pre-stimulus baseline to the a-wave trough. The amplitude of the b-wave was measured from the trough of the a-wave to the peak of the b-wave. For the evaluation of cone function (photopic ERG), a strobe flash stimulus was presented to 5 min light-adapted, dilated eyes in a Ganzfeld with a 2000 cd × sec/mm2 flash intensity. The amplitude of the cone b-wave was measured from the trough of the a-wave to the peak of the b-wave.
Measurement of the ONL Thickness
After ERG recording, animals were euthanized by carbon dioxide asphyxiation, and eyes were removed, fixed, and embedded in paraffin. Sections (5 µm thick) were taken through the optic nerve head along the vertical meridian, to allow comparison of all regions of the retina in the superior and inferior hemispheres. In each hemisphere, the outer nuclear layer (ONL) thickness was measured at 480-µm intervals in nine defined areas, starting at the optic nerve head and extending toward the superior and inferior ora serrata. In addition, mean ONL thickness was calculated for the inferior and the superior regions of the retina.
Cell Culture
Mouse photoreceptor-derived 661W cells [32] were kindly provided by Dr. Muayyad Al-Ubaidi (University of Oklahoma Health Sciences Center, Oklahoma City, OK) and human retinal pigment epithelium (RPE)-derived ARPE-19 cells were purchased from American Type Culture Collection (Manassas, VA). 661W cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM), while ARPE-19 cells were maintained in DMEM-F12 (1:1) media. Each media contained 10% fetal bovine serum, 1mM sodium pyruvate, 100 units/ml penicillin, and 100 µg/ml streptomycin. Cells were grown in 5% CO2, 95% humidity at 37 °C.
Cell Viability Assays
The 661W and ARPE-19 cells (4×106) were cultured for 24 h in 10 ml medium in 10 cm plates. To test the cytoprotective effect of curcumin against H2O2-mediated cell death, the cells were pre-treated for 3 h with 10 to 20 µM of curcumin. This dose of curcumin is below the cytotoxic level as 20 µM curcumin caused moderate cell death in human colon carcinoma cell (HCT-116) only when treated for more than 30 h [33]. The cells were then washed and stressed with various doses of H2O2 (Sigma, St. Louis, MO) (100 µM to 2 mM for 661W cells and 1 mM to 10 mM for ARPE-19 cells) for 3 h. Cell viability was determined indirectly by measuring released lactate dehydrogenase (LDH) into the medium using a commercial kit from Promega (CytoTox-One Homogenous Membrane Integrity Assay kit) following the manufacturer’s instructions. For the LDH release assay, fluorescent measure of the release of LDH from cells with a damaged membrane was calculated by subtraction of culture medium background and the cell viability (%) was calculated from 100% viability (mean value of untreated cells) to 0% viability (mean value of 2% Triton X-100 treated cells). To isolate RNA and protein, 661W and ARPE-19 cells are scrapped from 10-cm dishes, washed twice with RNase-free ice-cold phosphate buffered-saline (PBS) and collected by centrifugation. The cell pellets were stored at −80 °C until extraction of RNA and proteins.
RNA Isolation, cDNA Synthesis, and Quantitative Reverse-transcriptase PCR (qRT-PCR)
RNA was isolated and purified from frozen rat retinas and cultured 661W cells using PureLink™ Micro-to Midi Total RNA Purification System from Invitrogen following the manufacturer’s protocol. Equal quantities (2.0 µg) of total RNA from each tissue were converted to first-strand cDNA using SuperScript III First-Strand Synthesis SuperMix (Invitrogen, Carlsbad, CA) for RT-PCR. First-strand cDNA was used for qRT-PCR. Primers for qRT-PCR were designed in such a way that they spanned at least one intron, which eliminated the chance of amplification from residual genomic DNA contamination. Sequences of the primers are provided in the supplement table (Table S1). Quantitative PCR and melt-curve analyses were performed using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) and an iCycler machine. Relative quantities of expression of the genes of interest in different samples were calculated by the comparative Ct (threshold cycle) value method [34, 35].
Western Dot Blot for 4-HNE- and Nitrotyrosine-Modified Proteins
Whole retinal homogenates were prepared from individual retinas by homogenization in T-PER reagent (Pierce, Rockford, IL) containing protease inhibitors. Protein concentrations were measured using Coomassie Reagent (Pierce, Rockford, IL). Equal aliquots (5.0 µg) of retinal proteins were applied to a 96-well dot blot apparatus (Bio-Rad, Hercules, CA) and then transferred to 0.45 µ m nitrocellulose membranes (Bio-Rad, Hercules, CA) by vacuum filtration. Equivalent sample loading was monitored by staining the membrane with Ponceau Red (Sigma, St. Louis, MO). After Ponceau Red staining, membranes were washed and blocked with 5% non-fat dry milk (Bio-Rad, Hercules, CA) for 2 h at room temperature followed by incubation with either goat anti-4-HNE antibodies coupled with horseradish peroxidase enzyme (1:1000) or rabbit polyclonal anti-nitrotyrosine antibodies (1:1000) for 15h. Chemi-luminescence was developed with Supersignal West Femto Chemiluminescent Substrate (Pierce, Rockford, IL) and the signal was detected with a digital imaging system (IS4000R; Kodak, New Haven, CT). Care was taken to ensure that the intensities of detected spots were within the linear range of the camera and that no pixels were saturated. Intensities of dots stained with Ponceau red, anti-4-HNE, and anti-nitrotyrosine were determined using Kodak Molecular Imaging software (Rochester, NY).
Western Blotting
Retinal extracts and 661W whole-cell lysates were prepared for Western blotting by sonicating in T-PER reagent (Pierce, Rockford, IL) containing protease inhibitor cocktail (Roche, Indianapolis, IN) and then centrifuging at 10,000g for 15 min at 4 °C to collect the supernatants. After protein concentrations were determined using BCA reagent (Pierce, Rockford, IL), equal aliquots (20–30 µg) of protein samples were applied to 10 % or 4–20% gradient sodium dodecylsulfate polyacrylamide gel (Invitrogen, Carlsbad, CA) and electrophoretically separated. Resolved proteins were electrophoretically transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA) and blocked with 5 % nonfat dry milk for 1 h at room temperature. The membranes were incubated with anti-HO-1 (1:1000), anti-TRX1 (1:500), anti-TRXR (1:1000), anti-IκBα (1:1000), anti-pIκBα (1:1000), or anti-β-Actin (1:2000) antibodies for 16 h at 4 °C, after which they were incubated with the appropriate peroxidase-linked secondary antibody for 1 h at room temperature. Chemiluminescence signals were developed as described for the Western dot blot experiment. The intensities of protein bands were determined using Image J 1.32j software.
Statistical Analysis
Statistical analyses were performed using Microsoft excel data analysis tools. The quantitative data are expressed as mean ± SD or SEM for each group. The Student’s t-test and paired t-test was performed to assess the differences between the means.
Results
Functional Evaluation by ERG
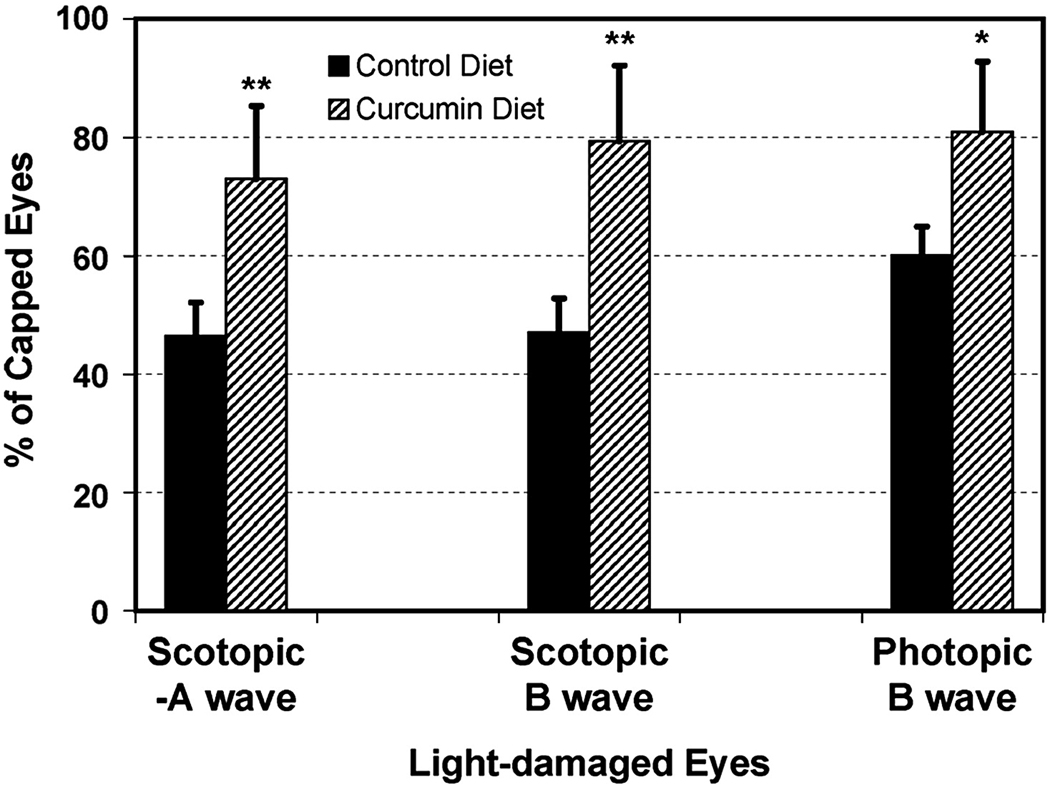
In rats fed the control diet (AIN-76A), we observed significant reductions in scotopic a- and b-wave amplitudes (57% and 56%, respectively) and a 40% reduction in photopic b-wave amplitude in uncovered compared to covered eyes (P < 0.001) (Fig. 1). In rats fed the same diet supplemented with 2000 ppm curcumin, the reduction in ERG amplitudes was 26%, 21 % and 19%, respectively, in uncovered compared to covered eyes (P < 0.001) (Fig. 1). In comparison to control-fed rats, curcumin supplementation resulted in significant protection of photoreceptors from light-induced cell death with preservation of scotopic ERG a-wave (P < 0.05), b-wave (P < 0.05), and photopic b-wave (P < 0.001) amplitudes in uncovered eyes (Fig. 1).
FIGURE 1.
Electroretinography. The mean (±SD) of scotopic –A wave, B-wave and photopic B-wave amplitudes for Light-damaged (uncovered) eyes are shown as % of amplitudes in the capped or non light-damaged eyes for control (AIN-76A) and curcumin supplemented diet fed rats (n = 8 rats). *P < 0.01, and **P < 0.001, by paired t-test.
Morphologic Evaluation by Quantitative Histology
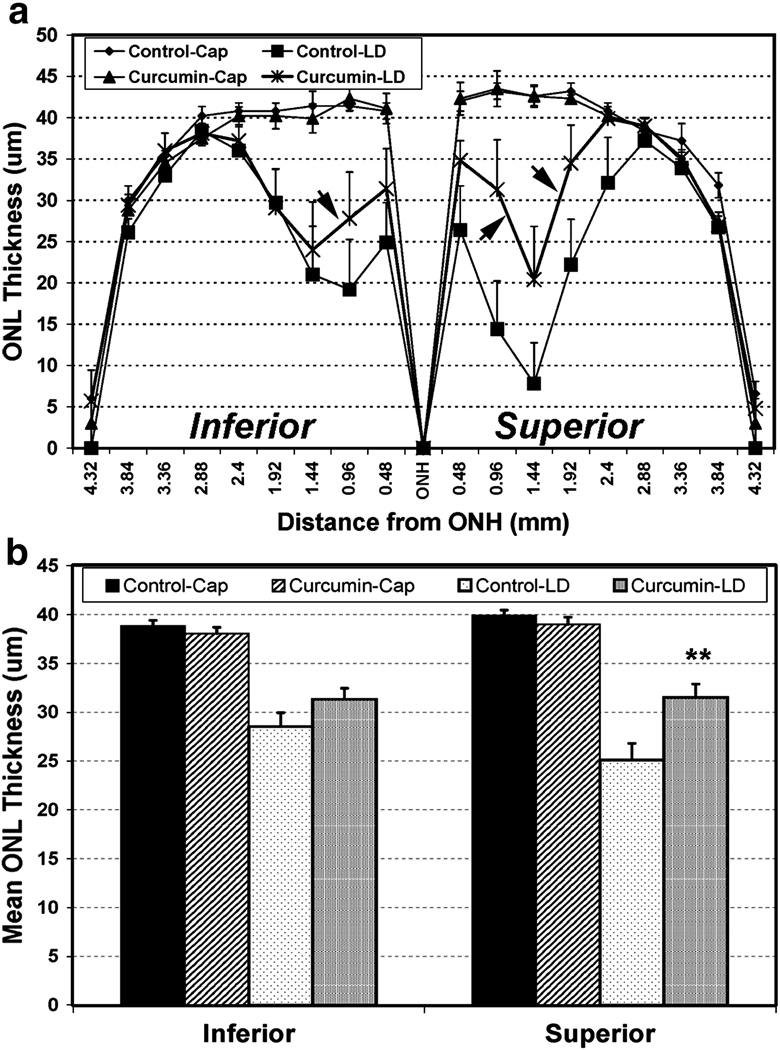
As curcumin was found to protect against light-induced loss of retinal function, we next determined whether retinal structure was also preserved by curcumin supplementation. Five to seven days after light exposure, a decrease in ONL thickness was observed in both the inferior and the superior hemispheres of uncovered eyes from rats fed the control diet, with the superior hemisphere showing more pronounced damage than the inferior hemisphere (Fig. 2A). Superior hemisphere is usually more sensitive to acute light-induced damage, which has been shown earlier [36]. The light-induced thinning of the ONL in uncovered eyes was reduced in curcumin-fed rats (Fig. 2A, arrow). It should be noted that ONL thickness was indistinguishable in covered eyes from rats fed either diet, indicating that curcumin supplementation alone did not cause any significant photoreceptor loss (Fig. 2A) [36].
FIGURE 2.
Retina outer nuclear layer (ONL) thickness. The ONL thickness measured in µM at each defined area from superior to inferior through optic nerve (A). ONL thickness of curcumin fed retina is indicated by arrows. The mean ONL thickness in the inferior and the superior hemispheres (B). The mean (±SD) of the thickness is shown (n = 8 rats for the 2000 ppm curcumin diet and n = 8 rats for control diet group groups). **P < 0.001, by Student’s t-test.
A 27% (P < 0.001) and 37% (P < 0.001) reduction in mean ONL thickness was observed in the inferior and the superior hemispheres, respectively, in uncovered compared to covered eyes from control diet-fed rats (Fig. 2B). On the other hand, there was only 19% (P < 0.001) and 21% (P < 0.001) reduction in mean ONL thickness in inferior and the superior hemisperes, respectively, in rats fed with curcumin (Fig. 2B). Thus, curcumin supplementation caused a decrease in the ONL thickness in the inferior (P < 0.1), and a significant decrease in the superior (P < 0.001) hemispheres of the uncovered eyes exposed to light damage compared to controls. Collectively, the results indicate that dietary curcumin supplementation protects the structure and function of retina from light-induced damage.
Expression of Inflammatory and Oxidant Stress Related Genes
To understand the molecular mechanism of curcumin-mediated protection of photoreceptors against light-induced degeneration, we performed qRT-PCR to measure the expression of genes known to be involved in cellular stress and inflammatory processes. As shown in Table 1, intense light exposure significantly induced the expression of Mmp3, Fosl1, Egr1, Cxcl1, Icam1, Timp1, Lox12, Ho-1, Ccl2, Cxcl10 and Tnf-a in the retinas of control diet fed rats. After supplementation with curcumin, the basal level of expression of some of these genes was altered. Expression of Mmp3, Cccl10, and Nrf2 genes were significantly reduced in retinas from curcumin-fed rats not exposed to light damage (Cur-NLD) and the expression of Egr1, Trx1, Cxcl1, Icam1, and Ccl2 appeared to be increased (Table 1). These data suggest that curcumin possesses immunomodulatory properties by altering expression of inflammatory genes. When curcumin-fed animals were exposed to damaging light (Cur-LD), the expression of the above genes followed a similar change in expression (i.e. induction) as in control diet-fed, light-exposed retinas (Con-LD) with only one exception: the expression of Trx1 was induced (1.69-fold) instead of repressed in Con-LD retina (−1.22). Although the expression of Mmp3, Fosl1, Egr1, Cxcl1, Timp1, Lox12, Ho-1, and Ccl2 genes was up-regulated due to light exposure in curcumin-fed rats, the level of expression was significantly lower than in the control diet-fed animals. The values for Icam1, Cxcl10 and Tnf-a were not significantly different in Cur-LD compared to Con-LD retinas. In summary, intense light induced expression of many pro-inflammatory (Mmp3, Egr-1, Cxcl1, Icam1, Timp1, Lox12, Ccl2), pro-apoptotic (Fosl1), and oxidant stress (Ho-1) related genes in the retina, but supplementation of curcumin significantly reduced their expression.
Table 1. Quantitative expression of some inflammatory and oxidative stress related genes in the retinas of Control and Curcumin fed rats after light-damage (LD).
Expression values are normalized with house keeping gene, Rpl-19 and represents a relative quantity of 10,000 in Rpl19. Four samples analyzed with all the genes and ‘±’ indicates the SE. In column 3–6, the fold change ‘+’ indicates up-regulation and ‘−’ indicates down-regulation. Con-NLD, control-diet no-light-damaged; Con-LD, control-diet light-damaged; Cur-NLD, curcumin-diet no-light-damaged; Cur-LD, curcumin-diet light-damaged. The expression values are statistically analyzed using Student’s t-test where * P < 0.1 and ** P < 0.001.
| Con-NLD | Con-LD | Cur-NLD | Cur-LD | Cur-LD | |
|---|---|---|---|---|---|
| Gene | Expression values | Fold / Con-NLD | Fold / Con-NLD | Fold / Con-NLD | Fold / Con-LD |
| Mmp3 | 2.84 ± 1.8 | + 172.09** | − 6.66** | + 70.66** | − 2.44** |
| Fosl1 | 2.48 ± 0.6 | + 95.00** | − 1.41 | + 38.72** | − 2.45** |
| Egr1 | 41.51 ± 14.5 | + 36.43** | + 1.46 | + 14.57** | − 2.50** |
| Trx1 | 2925.80 ± 830 | − 1.22 | + 1.41 | + 1.69* | + 2.06** |
| CxCl1 | 1.86 ± 0.3 | + 55.82** | + 1.62 | + 35.72** | − 1.56 |
| Icam1 | 6.95 ± 0.5 | + 23.26** | + 1.55 | + 37.01** | + 1.59 |
| Timp1 | 35.97 ± 10.6 | + 10.36** | − 1.03 | + 4.48** | − 2.31** |
| Lox12 | 22.14 ± 5.3 | + 3.27** | + 1.12 | + 1.63* | − 2.00** |
| Ho-1 | 3.96 ± 1.0 | + 16.34** | − 1.54 | + 9.91** | − 1.65* |
| Ccl2 | 14.79 ± 7.2 | + 5.85** | + 1.76* | + 2.52** | − 2.32** |
| Nrf2 | 254.40 ± 66.0 | − 1.08 | − 2.08* | − 1.31 | − 1.21 |
| CxCl10 | 9.90 ± 2.3 | + 12.87** | − 3.85** | + 8.49** | − 1.52 |
| Tnf-a | 1.62 ± 0.1 | + 3.71** | − 1.26 | + 3.89** | 1.05 |
Effects of Curcumin on 4-HNE- and Peroxynitrite-mediated Protein Modifications in the Retina
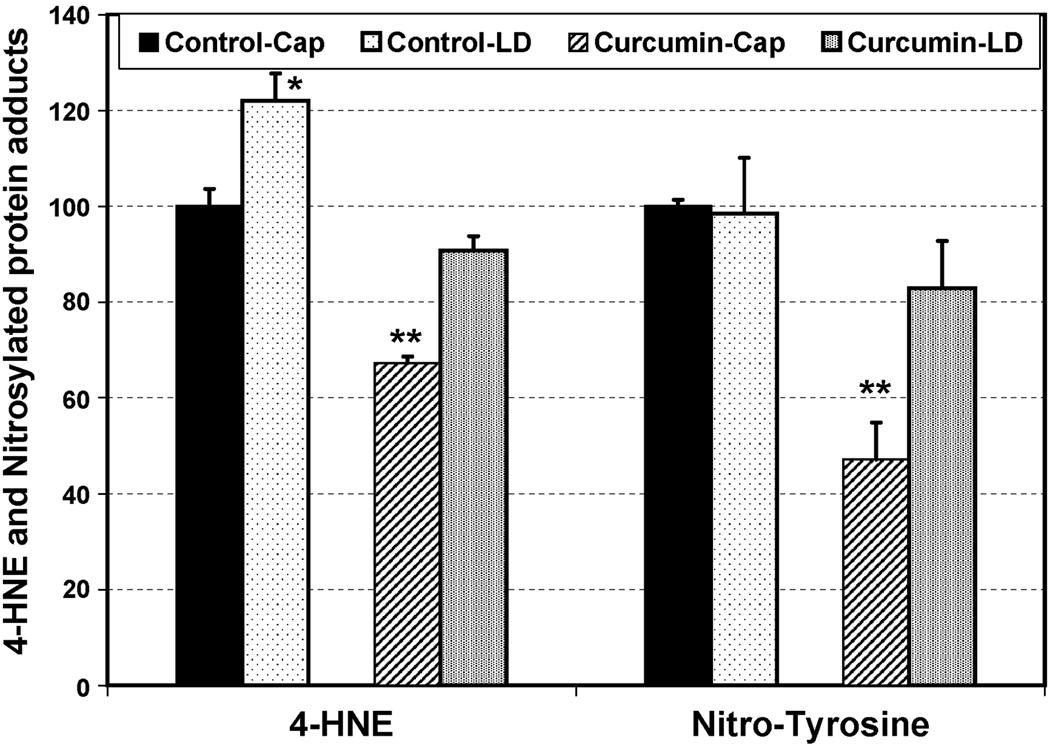
4-HNE is generated by the non-enzymatic oxidation of n-6 polyunsaturated fatty acids and is not only a marker and mediator of cellular oxidative stress, but also a major initiator of lipid-mediated inflammation [37]. 4-HNE forms Michael adducts with lysine, histidine, and cysteine residues of protein, which can be detected with a specific antibody. To explore a possible mechanism of curcumin protection, we used Western dot blot analysis to quantify the effects of supplementation on the level of 4-HNE in the retina. Bright light exposure increased the levels of 4-HNE-modified proteins in both control diet-fed (21%, P < 0.05) and curcumin-fed animals (35%, P <0.001) when compared with the covered eyes (Fig. 3). Interestingly, the basal level in covered eyes was significantly lower in curcumin fed animals (33%, P< 0.001) (Fig. 3). As a result, the light-induced level of 4-HNE-modified proteins in curcumin-fed animals was still below the basal level of control diet fed animals (Fig. 3).
FIGURE 3.
Western dot blot quantification of 4-HNE- and Nitrotyrosine- modified proteins in rat retina. Retinas were harvested from curcumin and control diet-fed-rats after 3h of light exposure followed by 3h in darkness. The data (±SEM) is presented as percentage of the non-light exposed (capped) control diet-fed animals (n = 4 retina × 3 replication). *P < 0.05, and **P < 0.001, by Student’s t-test.
In addition to 4-HNE, we measured nitrotyrosine, a modification resulting from the attack of proteins by peroxynitrite, a strong oxidant generated by the reaction of nitric oxide with superoxide anion. With our light exposure paradigm at the time of analysis after exposure, we could not detect any differences in nitrotyrosine levels in covered and light-exposed eyes of control animals (Fig. 3). Interestingly, the retinas of curcumin-fed animals showed significant reduction in the basal level of nitrated proteins. Following exposure to light stress, nitrotyrosine levels increased but remained below the levels of controls (Fig. 3). At this time point of analysis, nitrosylation of proteins does not seem to be involved in retinal degeneration process, but reduction on supplementation of curcumin suggests an inherent property of curcumin to reduce basal level of protein nitrosylation. Taken together, these data indicate that curcumin plays a role in reducing the basal level of inflammatory and oxidative stress markers in the retina, which may be related to the underlying mechanism of curcumin-mediated protection.
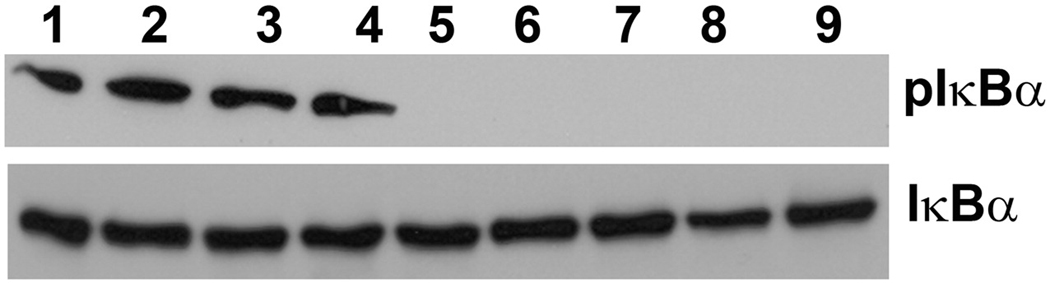
Curcumin Suppresses NF-κB Activation in the Retina
Curcumin is known to inhibit NF-κB activation [10]. NF-κB is activated after inhibitor κBα (IκBα) is phosphorylated and removed from the NF-κB complex. By Western blot analysis, we observed inhibition of phosphorylation of IκBα (pIκBα) in all the curcumin-fed rat retinas either exposed or not exposed to light (Fig. 4; upper panel, Lane 5–9). The level of total IκBα served as control (Fig. 4; lower panel).
FIGURE 4.
Western blot analysis of rat retina for NF-κB signaling. Retinas were harvested from curcumin and control diet-fed-rats after 3h of light exposure followed by 3h in darkness. Retinal protein extracted and subjected to western blotting with anti- IκBα and anti-phosphorylated-IκBα (pIκBα) antibodies. Lanes, 1–2: Control-diet, Capped eye; 3–4: Control-diet, Light-exposed eye; 5–6: Curcumin-diet, Capped eye; and 7–9: Curcumin-diet, Light-exposed eye.
Curcumin Protects Retinal Cells from Oxidative-stress Mediated Death
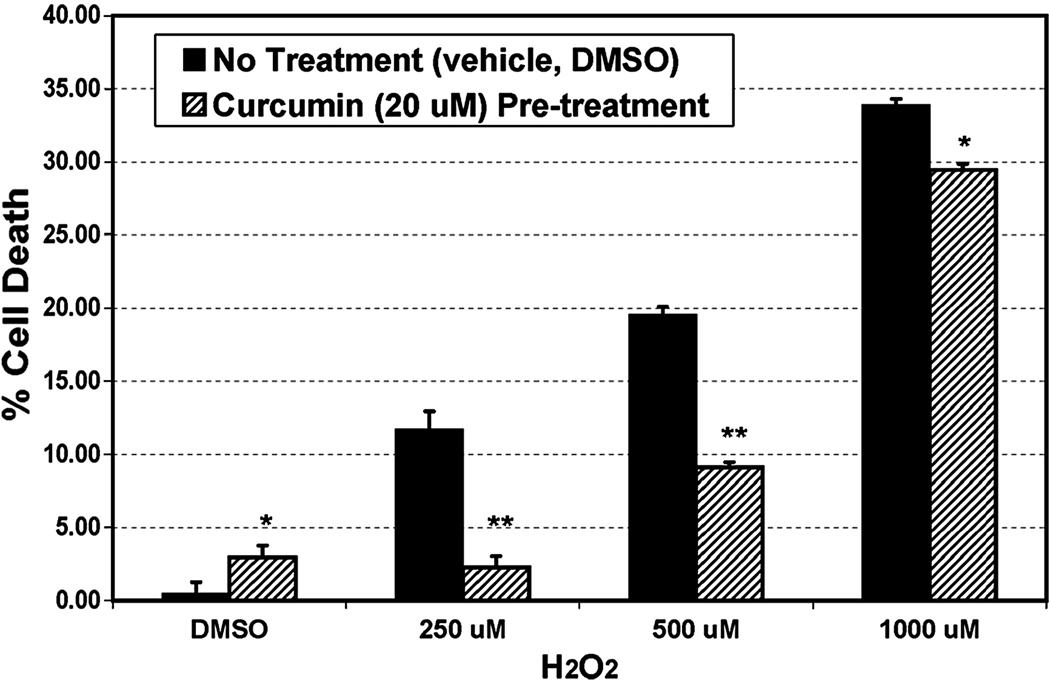
Curcumin is a hormetic compound [38]; high doses can kill cells but lower doses can protect. Pre-incubation of mouse photoreceptor-derived 661W cells or human RPE-derived ARPE-19 cells (data not shown) with 10 or 20 µM curcumin for 3 h protected cells from H2O2-mediated cell death as measured by LDH release (Fig. 5). Protection remained significant even when cells were stressed with H2O2 3 h after washing curcumin off from culture dishes (not shown). This indicates that curcumin up-regulates a cellular protective system rather than directly quenching H2O2 by stabilizing the superoxide anions.
FIGURE 5.
Cell viability assay. Freshly grown 661W cells in 10 cm plate were pre-treated in situ with 20 µM curcumin or with the vehicle (DMSO) for 3h. After thorough washing the cells were exposed to different doses of H2O2 for 3h. Cell death was indirectly measured by measuring the release of lactate dehydrogenase (LDH) in the media in which a plate containing 2% Triton served as 100% cell death (n = 4 plate × 4 replication assay). *P < 0.01, and **P < 0.001, by Student’s t-test.
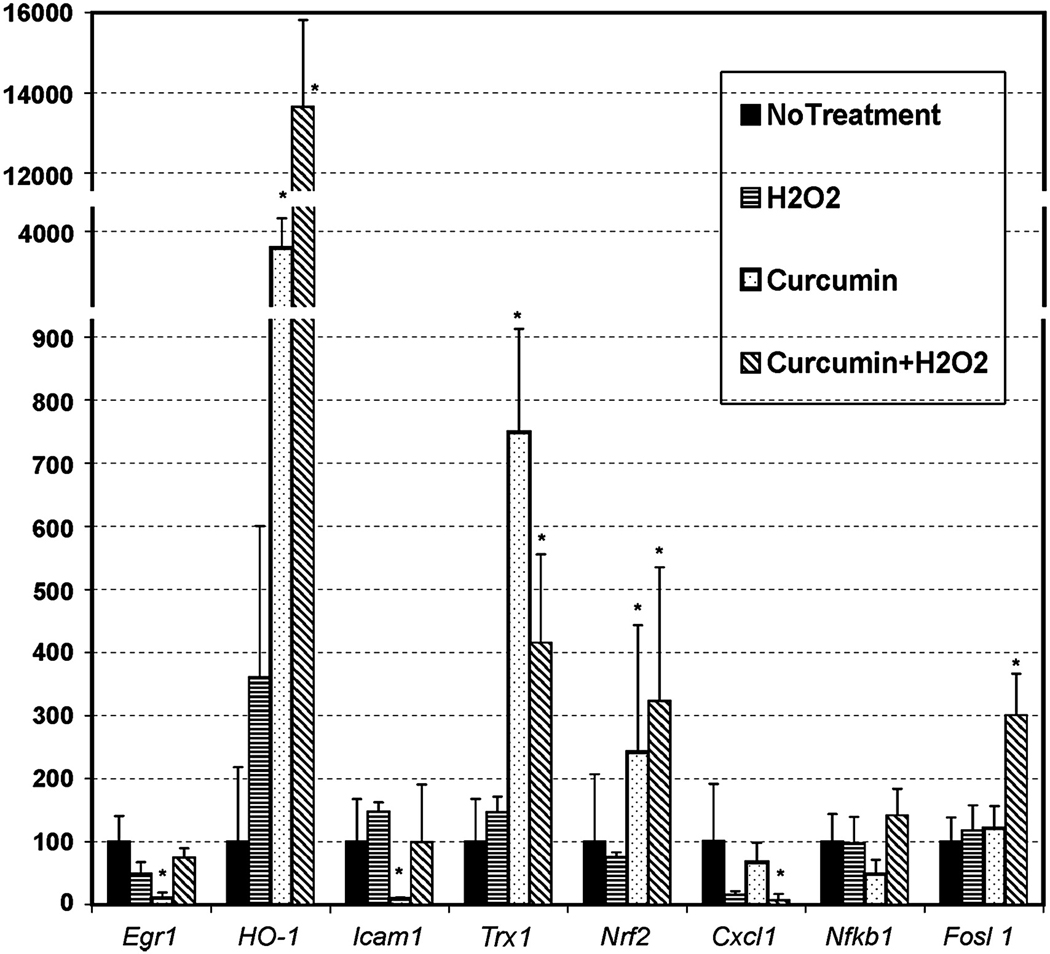
As the effect of curcumin in 661W cells was pronounced and experimentally-controllable, we studied the molecular mechanism of curcumin-mediated protection by analyzing the expression of some selected genes which are implicated in cell death and survival pathways. Treatment with 0.5 mM H2O2 for 3 h slightly induced the expression of Ho-1, Icam1, and Trx1 gene and repressed Egr1 and Cxcl1 as measured by qRT-PCR (Fig. 6). Interestingly, pretreatment with 20 µM curcumin significantly repressed the expression of Egr1 and Icam1 and induced the expression of Ho-1, Trx1, and Nrf2 (Fig. 6). When these pre-treated cells were exposed to H2O2 for 3 h, expression of Egr1 and Icam1 reverted, but higher levels of Ho-1, Trx1, and Nrf2 were maintained; expression of Fosl1 was increased and the expression of Cxcl1 was repressed (Fig. 6). Together with the retinal gene expression data in Table 1, this information supports the notion that curcumin acts as an immunomodulatory compound which can alter the expression of major inflammatory genes. The observed differences in the gene expression patterns of in vivo and in vitro models are probably due to the inherent difference between the models themselves (e.g., complex tissue vs. pure cell lines, dietary supplementation vs. direct uptake from media, etc.) which will be discussed later. Interestingly, the in vitro studies were more informative on curcumin-mediated protection pathways in retinal cells such as up-regulation of phase II detoxifying enzymes such as HO-1 and Trx-1, possibly via the NRF2 transcription factor.
FIGURE 6.
Expression assay of some selected genes in 661W cells pre-treated with curcumin followed by exposing to H2O2. Pre-treatment with curcumin was for 3h followed by thorough washing off the media and after 3h of washing the cells were exposed to 0.5 mM of H2O2. Cells were harvested after 3h of H2O2 treatment and RNA isolated for quantitative RT-PCR. The expression data was analyzed by comparative Ct value method after normalizing against house keeping gene, Hprt. Expression values (±SD) are presented against no-treatment value which is 100% (n = 4 sample × 3 replication assay per sample). *P < 0.001, by Student’s t-test.
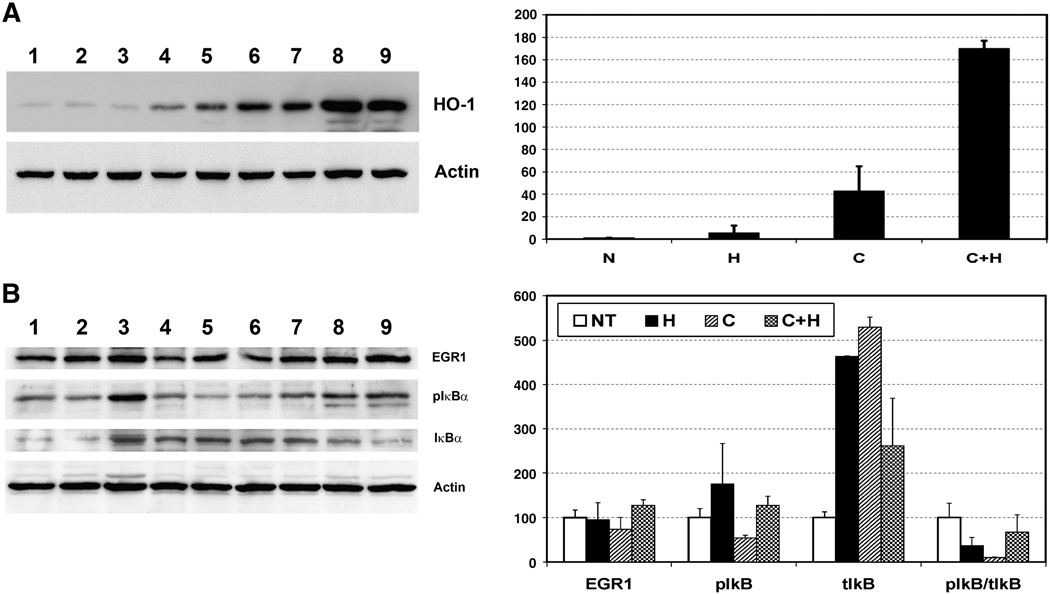
Up-regulation of HO-1 by curcumin treatment was confirmed at the protein level by Western blot analysis (Fig. 7a). The HO-1 protein level was increased 3-fold with 0.5 mM H2O2, 40-fold with 20 µM curcumin and 150-fold when curcumin pre-treated cells were further challenged with H2O2, which are very similar to the mRNA expression pattern shown in Fig 6. Consistent with its mRNA expression, EGR1 protein levels were also decreased by curcumin treatment, although this decrease was not statistically significant (Fig. 7b). As observed in retinal samples, the level of phosphorylated IκBα was reduced with curcumin treatment in 661W cells (Fig. 7b). The total IκBα level increased with H2O2 and curcumin treatment, but the ratio of pIκBα/IκBα was reduced significantly (Fig. 7b), indicating higher level of non-phosphorylated IκBα protein in curcumin treated cells which in turn indicate inactivation of NF-κB signaling. In summary, the mechanism by which curcumin mediates protection of retinal cells from oxidative stress involves up-regulation of cellular protective enzymes and inhibition of NF-κB activation.
FIGURE 7.
FIGURE 7a. Left Panel: Western blot analysis of 661W cells pre-treated with curcumin followed by exposing to H2O2. An aliquot of each sample used for RNA study has been used for protein analysis. Rat monoclonal anti-Heme Oxygenase-1(1:1000) and mouse monoclonal anti-Actin (1:2000) were used as primary antibodies. Lanes, 1–2: No treatment; 3–4: 0.5 mM H2O2; 5–7: 20 µM curcumin; and 8–9: 20 µM curcumin followed by 0.5 mM H2O2. Right Panel: Quantitation of HO-1 protein by densiometric analysis. N, No treatment; H, 0.5 mM H2O2 ; C, µM curcumin; C+H, µM curcumin followed by 0.5 mM H2O2.
FIGURE 7b. Left Panel: Western blot analysis of 661W cells pre-treated with curcumin followed by exposing to H2O2 as above. Rabbit polyclonal anti-EGR1 (1:1000), mouse monoclonal anti-pIκBα (1:1000), rabbit polyclonal anti-total-IκBα (1:1000) and mouse monoclonal anti-Actin (1:2000) were used as primary antibodies. Lanes, 1–2: No treatment; 3–4: 0.5 mM H2O2; 5–6: 20 µM curcumin; and 7–9: 20 µM curcumin followed by 0.5 mM H2O2. Right Panel: Quantitation of EGR1, pIκBα, total-IκBα and ratio of pIκBα / IκBα, as obtained by densiometric analysis and normalized by Actin. N, No treatment; H, 0.5 mM H2O2; C, µM curcumin; C+H, µM curcumin followed by 0.5 mM H2O2.
Discussion
In this study, dietary supplementation of curcumin provided both functional and structural protection of photoreceptor cells against acute light-induced damage in Wistar albino rats. Previously, dietary supplementation of curcumin was shown to be effective against STZ-induced diabetic retinopathy in Lewis rats [20]. In another study, feeding curcumin was shown to inhibit retinal VEGF up-regulation due to STZ-induced diabetes [39]. Curcumin protected cultured rat retinal neurons from NMDA (N-methyl-D-aspartic acid)-mediated excitotoxicity [40]. Our study is the first to demonstrate a protective effect of dietary supplementation of curcumin on retinal structure and function in an in vivo model of light-induced retinal degeneration. We found significant alterations in expression of inflammatory genes in curcumin-fed retinas and suppression of NF-κB activation, which may underlie the mechanism of curcumin-mediated protection of retinal cells. Curcumin also protected retina-derived 661W cells and RPE-derived ARPE-19 cells from oxidative stress-mediated damage and up-regulated expression of phase II enzymes such as HO-1 and TRX1 that are mediated by the NRF2 transcription factor. These results suggest that curcumin-mediated protection may be through regulation of NRF2 activity.
The mechanism of retinal photic injury involves significant oxidative-stress, and the involvement of inflammatory processes has been established [41]. Induction of superoxide dismutase (SOD) was observed in the rat retina after exposure to light [42] and mice expressing mutant SOD1 are highly susceptible to environmental light-induced retinal damage [43], as well as degenerate with age [44]. In addition, iron-derived hydroxyl radicals produced by the Fenton reaction may be important mediators of retinal photic injury as systemic administration of the iron chelating reagent desferrioxamine attenuated light-induced damage in rat retinas [45]. We have observed that Wistar rats are more sensitive to light-damage than Sprague Dawley rats (unpublished results) hence we have selected a light dose that causes ~ 50% reduction in retinal function (i.e., a 50% reduction in ERG). At this light dose, we observed induction of expression of a group of pro-inflammatory genes (Table 1). Reduced activation of the complement cascade decreases retinal light damage susceptibility, indicating that inflammation plays an important role in the pathogenesis of this process [46].
Activation and nuclear localization of the transcription factor Nuclear factor kappa B (NF-κB) by exposure to damaging light has been reported in mouse retina and may play a role in light-induced photoreceptor degeneration [47, 48]. NF-κB is a master regulator for expression of many genes and its activation may induce the apoptotic degenerative process. At the same time, NF-κB may be required for the survival of central neurons [49]. In the cytosol, NF-κB remains inactive by forming a complex with inhibitory IκB proteins. In response to stimuli, IκB kinases (IKKs) mediate IκB phosphorylation, dissociation of the NF-κB inhibitory complex and activation of NF-κB which then translocates to the nucleus [50, 51]. We observed dramatically reduced IκBα phosphorylation in curcumin-fed retinas suggesting a reduction in activity of NF-κB. This observation is consistent with previous studies that curcumin can abolish the phosphorylation and degradation of IκB thereby inhibiting NF-κB activation [10].
Curcumin is a hormetic compound, i.e., at higher doses it is cytotoxic but at lower doses it can exert adaptive stress responses [38, 52]. The dose we have used (20 µM) can induce cell death when treated for more than 24h [33, 53]. In our cell culture system, we have used doses of 10 and 20 µM curcumin for only 3h, which is below cytotoxic levels. Twenty micro-molar curcumin exerts significant protection against increasing doses of H2O2, but may also be stressful to the cell by itself as we observed a 3% increase in cell death in curcumin-treated cells over the vehicle (DMSO)-treated cells (Fig. 5). We also observed significant up-regulation of HO-1, Trx-1, and Nrf2 with curcumin treatment. This argues favorably for the idea of curcumin-mediated neuro-hormesis, i.e., it acts as a mild stressor to induce adaptive expression of stress-resistance genes such as those encoding antioxidant enzymes. Curcumin-mediated activation of the hormetic NRF2 pathway, resulting in the production of HO-1, a redox stress-inducible protein known to protect cells against various types of stress, has been shown in renal epithelial cells [14]. The same molecular mechanism has been observed in adaptive retinal neuroprotection from damaging light (3000 lux) by rearing rats under bright cyclic light (400 lux) and in 661W cells stressed with sub-lethal amounts of 4-HNE [54].
Curcumin down-regulated the expression Egr1 and Icam1 in 661W cells and Egr1 in rat retina with light-damage. EGR1, a Zn2+ finger containing transcription factor is known to be a mediator of oxidative stress-induced tissue damage and controls expression of many inflammatory genes including Icam1[55, 56]. Down-regulation of Egr1 and Icam1 in 661W cells indicates anti-inflammatory activity of curcumin. Suppression of EGR1 expression and transactivation by curcumin has been demonstrated for suppressing inflammation [57–59]. We demonstrated that our model of LIRD has significant involvement of inflammation and curcumin could modulate the process. Thus, the curcumin mediated immunomodulation in the retina could be through EGR1 protein.
The effect of curcumin on 661W cells is more pronounced than on the retina in vivo as evident from its protection from H2O2-mediated cell death and modulation of the protective and stress-related genes and proteins. This probably results from the direct access and increased uptake of the compound by 661W cells compared to the rat retina where curcumin is supplied through diet. Curcumin has long been criticized as being poorly accessible to target tissues because of its relative insolubility in biological fluids and poor absorption in the gut [60, 61]. On the other hand, curcumin is not toxic even at very high doses in in vivo models and human clinical trials [62], which may be seen as an advantage of poor adsorption. We do not know how much of the diet-supplemented curcumin reaches the retina and this is the focus of our future investigation. But curcumin is known to pass through the blood-brain barrier and dietary curcumin was found to be effective against forebrain ischemia in gerbils [63]. Dietary curcumin also reduced brain damage from traumatic injury in rats [64] and decreased accumulation of amyloid-b peptide, and reduced expression of markers of oxidative stress and inflammation in the cerebral cortex in a transgenic mouse model of Alzheimer’s disease (APPSw Tg2576 mice) [65], which argues for its usefulness in other neuronal tissues such as the retina. Our data put forward supporting evidence that curcumin could suppress the basal level of inflammation and oxidative stress and protect the retina from light-induced damage.
Curcumin has pleiotropic effects on cell death and survival. It can modulate the expression and activation of cellular regulatory system such as NF-κB, AKT, AP-1, NRF2 and growth factors, which in turn inhibit cellular inflammatory responses and protect cells. On the other hand, AMD is a complex disease, the pathology of which involves significant oxidative stress and inflammation with age. With our demonstration of reduction of retinal oxidative and inflammatory stress and up-regulation of cytoprotective machinery, and protection of the retina from intense apoptotic insult, we hypothesize that curcumin could be an effective nutraceutical compound for preventive and augmentative therapy of AMD. Although light-damage models are being recognized as a closer model of dry-atrophic AMD [41], investigation in other mouse models of macular degeneration would pave the way for clinical trial of curcumin for delaying or preventing the onset of AMD.
Supplementary Material
Acknowledgements
The authors are grateful to Mark Dittmar and Alicia Avila (Dean A. McGee Eye Institute, Oklahoma City, OK) for their assistance with animal breeding and feeding; and Louisa J. Williams and Linda S. Boone (Dean A. McGee Eye Institute, Oklahoma City, OK) for assistance in histology. Financial support from Knight’s Templar Eye Foundation (MNAM), OU College of medicine Alumni Association (MNAM), Foundation Fighting Blindness (REA), Research to Prevent Blindness, Inc., National Eye Institute (EY12190, EY04149, and EY00871 to REA), National Center for Research Resources Grant RR17703 (MNAM, REA) and National Cancer Institute (109247, CVR) are duly acknowledged.
List of Abbreviations
- AMD
age-related macular degeneration
- LIRD
light-induced retinal degeneration
- NF-κB
nuclear factor kappa B
- H2O2
hydrogen peroxide
- HO-1
Heme oxygenase-1
- AKT
v-akt murine thymoma viral oncogene homolog 1
- NRF2
nuclear factor (erythroid-derived 2)-like 2
- DR
diabetic retinopathy
- STZ
streptozotocin
- ERG
Electroretinography
- RNA
ribo nucleic acid
- ONL
outer nuclear layer
- DMEM
Dulbecco’s modified Eagle’s medium
- LDH
lactate dehydrogenase
- PBS
phosphate buffered-saline
- qRT-PCR
quantitative reverse-transcriptase PCR
- Ct
threshold cycle
- 4-HNE
4-Hydroxynonenal
- Mmp3
Matrix metalloproteinase 3
- Fosl1
FOS-like antigen 1
- Egr1
early growth response 1
- Cxcl1
chemokine (C-X-C motif) ligand 1
- Icam1
intercellular adhesion molecule 1
- Timp1
tissue inhibitor of metalloproteinase 1
- Lox12
lipoxygenase 12
- Ccl2
chemokine (C-C motif) ligand 2
- Cxcl10
chemokine (C-X-C motif) ligand 10
- Tnf-a
tumor necrosis factor a
- Trx1
Thioredoxin 1
- Con-NLD
control-diet no-light-damaged
- Con-LD
control-diet light-damaged
- Cur-NLD
curcumin-diet no-light-damaged
- Cur-LD
curcumin-diet light-damaged
- IκBα
inhibitor κBα
- pIκBα
phosphorylated IκBα
- VEGF
vascular endothelial growth factor
- NMDA
N-methyl-D-aspartic acid
- AP-1
activator protein 1
- SOD
superoxide dismutase
- DMSO
dimetyl sulfoxide
References
- 1.Mukherjee PK, Wahile A. Integrated approaches towards drug development from Ayurveda and other Indian system of medicines. Journal of ethnopharmacology. 2006;103:25–35. doi: 10.1016/j.jep.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 2.Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta medica. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 3.Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as "Curecumin": from kitchen to clinic. Biochemical pharmacology. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Rao CV. Regulation of COX and LOX by curcumin. Advances in experimental medicine and biology. 2007;595:213–226. doi: 10.1007/978-0-387-46401-5_9. [DOI] [PubMed] [Google Scholar]
- 6.Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Annals of the New York Academy of Sciences. 2005;1056:206–217. doi: 10.1196/annals.1352.010. [DOI] [PubMed] [Google Scholar]
- 7.Abe Y, Hashimoto S, Horie T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol Res. 1999;39:41–47. doi: 10.1006/phrs.1998.0404. [DOI] [PubMed] [Google Scholar]
- 8.Reddy AC, Lokesh BR. Studies on spice principles as antioxidants in the inhibition of lipid peroxidation of rat liver microsomes. Molecular and cellular biochemistry. 1992;111:117–124. doi: 10.1007/BF00229582. [DOI] [PubMed] [Google Scholar]
- 9.Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, Conney AH. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer research. 1991;51:813–819. [PubMed] [Google Scholar]
- 10.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] The Journal of biological chemistry. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 11.Woo JH, Kim YH, Choi YJ, Kim DG, Lee KS, Bae JH, Min DS, Chang JS, Jeong YJ, Lee YH, Park JW, Kwon TK. Molecular mechanisms of curcumin-induced cytotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis. 2003;24:1199–1208. doi: 10.1093/carcin/bgg082. [DOI] [PubMed] [Google Scholar]
- 12.Susan M, Rao MN. Induction of glutathione S-transferase activity by curcumin in mice. Arzneimittel-Forschung. 1992;42:962–964. [PubMed] [Google Scholar]
- 13.Scapagnini G, Colombrita C, Amadio M, D'Agata V, Arcelli E, Sapienza M, Quattrone A, Calabrese V. Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxidants & redox signaling. 2006;8:395–403. doi: 10.1089/ars.2006.8.395. [DOI] [PubMed] [Google Scholar]
- 14.Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. The Biochemical journal. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nature medicine. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donoso LA, Kim D, Frost A, Callahan A, Hageman G. The role of inflammation in the pathogenesis of age-related macular degeneration. Survey of ophthalmology. 2006;51:137–152. doi: 10.1016/j.survophthal.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen JK, Dong A, Hackett SF, Bell WR, Green WR, Campochiaro PA. Oxidative damage in age-related macular degeneration. Histology and histopathology. 2007;22:1301–1308. doi: 10.14670/HH-22.1301. [DOI] [PubMed] [Google Scholar]
- 18.Madsen-Bouterse SA, Kowluru RA. Oxidative stress and diabetic retinopathy: Pathophysiological mechanisms and treatment perspectives. Reviews in endocrine & metabolic disorders. 2008 doi: 10.1007/s11154-008-9090-4. [DOI] [PubMed] [Google Scholar]
- 19.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Experimental diabetes research. 2007;2007:95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kowluru RA, Kanwar M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutrition & metabolism. 2007;4:8. doi: 10.1186/1743-7075-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demontis GC, Longoni B, Marchiafava PL. Molecular steps involved in light-induced oxidative damage to retinal rods. Investigative ophthalmology & visual science. 2002;43:2421–2427. [PubMed] [Google Scholar]
- 22.Wiegand RD, Giusto NM, Rapp LM, Anderson RE. Evidence for rod outer segment lipid peroxidation following constant illumination of the rat retina. Investigative ophthalmology & visual science. 1983;24:1433–1435. [PubMed] [Google Scholar]
- 23.Specht S, Leffak M, Darrow RM, Organisciak DT. Damage to rat retinal DNA induced in vivo by visible light. Photochemistry and photobiology. 1999;69:91–98. [PubMed] [Google Scholar]
- 24.Tanito M, Elliott MH, Kotake Y, Anderson RE. Protein modifications by 4-hydroxynonenal and 4-hydroxyhexenal in light-exposed rat retina. Investigative ophthalmology & visual science. 2005;46:3859–3868. doi: 10.1167/iovs.05-0672. [DOI] [PubMed] [Google Scholar]
- 25.Tanito M, Brush RS, Elliott MH, Wicker LD, Henry KR, Anderson RE. High levels of retinal membrane docosahexaenoic acid increase susceptibility to stress-induced degeneration. Journal of lipid research. 2008 doi: 10.1194/jlr.M800170-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Organisciak DT, Wang HM, Li ZY, Tso MO. The protective effect of ascorbate in retinal light damage of rats. Investigative ophthalmology & visual science. 1985;26:1580–1588. [PubMed] [Google Scholar]
- 27.Organisciak DT, Darrow RM, Jiang YI, Marak GE, Blanks JC. Protection by dimethylthiourea against retinal light damage in rats. Investigative ophthalmology & visual science. 1992;33:1599–1609. [PubMed] [Google Scholar]
- 28.Tanito M, Masutani H, Nakamura H, Ohira A, Yodoi J. Cytoprotective effect of thioredoxin against retinal photic injury in mice. Investigative ophthalmology & visual science. 2002;43:1162–1167. [PubMed] [Google Scholar]
- 29.Kaldi I, Dittmar M, Pierce P, Anderson RE. L-NAME protects against acute light damage in albino rats, but not against retinal degeneration in P23H and S334ter transgenic rats. Experimental eye research. 2003;76:453–461. doi: 10.1016/s0014-4835(02)00334-2. [DOI] [PubMed] [Google Scholar]
- 30.Ranchon I, LaVail MM, Kotake Y, Anderson RE. Free radical trap phenyl-N-tert-butylnitrone protects against light damage but does not rescue P23H and S334ter rhodopsin transgenic rats from inherited retinal degeneration. J Neurosci. 2003;23:6050–6057. doi: 10.1523/JNEUROSCI.23-14-06050.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanito M, Li F, Elliott MH, Dittmar M, Anderson RE. Protective effect of TEMPOL derivatives against light-induced retinal damage in rats. Investigative ophthalmology & visual science. 2007;48:1900–1905. doi: 10.1167/iovs.06-1057. [DOI] [PubMed] [Google Scholar]
- 32.Tan E, Ding XQ, Saadi A, Agarwal N, Naash MI, Al-Ubaidi MR. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Investigative ophthalmology & visual science. 2004;45:764–768. doi: 10.1167/iovs.03-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaiswal AS, Marlow BP, Gupta N, Narayan S. Beta-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002;21:8414–8427. doi: 10.1038/sj.onc.1205947. [DOI] [PubMed] [Google Scholar]
- 34.Mandal MN, Vasireddy V, Jablonski MM, Wang X, Heckenlively JR, Hughes BA, Reddy GB, Ayyagari R. Spatial and temporal expression of MFRP and its interaction with CTRP5. Investigative ophthalmology & visual science. 2006;47:5514–5521. doi: 10.1167/iovs.06-0449. [DOI] [PubMed] [Google Scholar]
- 35.Mandal MN, Vasireddy V, Reddy GB, Wang X, Moroi SE, Pattnaik BR, Hughes BA, Heckenlively JR, Hitchcock PF, Jablonski MM, Ayyagari R. CTRP5 is a membrane-associated and secretory protein in the RPE and ciliary body and the S163R mutation of CTRP5 impairs its secretion. Investigative ophthalmology & visual science. 2006;47:5505–5513. doi: 10.1167/iovs.06-0312. [DOI] [PubMed] [Google Scholar]
- 36.Tanito M, Kaidzu S, Ohira A, Anderson RE. Topography of retinal damage in light-exposed albino rats. Experimental eye research. 2008;87:292–295. doi: 10.1016/j.exer.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Catala A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chemistry and physics of lipids. 2008 doi: 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Mattson MP. Dietary factors, hormesis and health. Ageing research reviews. 2008;7:43–48. doi: 10.1016/j.arr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mrudula T, Suryanarayana P, Srinivas PN, Reddy GB. Effect of curcumin on hyperglycemia-induced vascular endothelial growth factor expression in streptozotocin-induced diabetic rat retina. Biochemical and biophysical research communications. 2007;361:528–532. doi: 10.1016/j.bbrc.2007.07.059. [DOI] [PubMed] [Google Scholar]
- 40.Matteucci A, Frank C, Domenici MR, Balduzzi M, Paradisi S, Carnovale-Scalzo G, Scorcia G, Malchiodi-Albedi F. Curcumin treatment protects rat retinal neurons against excitotoxicity: effect on N-methyl-D: -aspartate-induced intracellular Ca(2+) increase. Experimental brain research. Experimentelle Hirnforschung. 2005;167:641–648. doi: 10.1007/s00221-005-0068-0. [DOI] [PubMed] [Google Scholar]
- 41.Marc RE, Jones BW, Watt CB, Vazquez-Chona F, Vaughan DK, Organisciak DT. Extreme retinal remodeling triggered by light damage: implications for age related macular degeneration. Molecular vision. 2008;14:782–806. [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto M, Lidia K, Gong H, Onitsuka S, Kotani T, Ohira A. Changes in manganese superoxide dismutase expression after exposure of the retina to intense light. The Histochemical journal. 1999;31:81–87. doi: 10.1023/a:1003510719302. [DOI] [PubMed] [Google Scholar]
- 43.Mittag TW, Bayer AU, La VM. Light-induced retinal damage in mice carrying a mutated SOD I gene. Experimental eye research. 1999;69:677–683. doi: 10.1006/exer.1999.0748. [DOI] [PubMed] [Google Scholar]
- 44.Hashizume K, Hirasawa M, Imamura Y, Noda S, Shimizu T, Shinoda K, Kurihara T, Noda K, Ozawa Y, Ishida S, Miyake Y, Shirasawa T, Tsubota K. Retinal dysfunction and progressive retinal cell death in SOD1-deficient mice. The American journal of pathology. 2008;172:1325–1331. doi: 10.2353/ajpath.2008.070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li ZL, Lam S, Tso MO. Desferrioxamine ameliorates retinal photic injury in albino rats. Current eye research. 1991;10:133–144. doi: 10.3109/02713689109001741. [DOI] [PubMed] [Google Scholar]
- 46.Rohrer B, Guo Y, Kunchithapautham K, Gilkeson GS. Eliminating complement factor D reduces photoreceptor susceptibility to light-induced damage. Investigative ophthalmology & visual science. 2007;48:5282–5289. doi: 10.1167/iovs.07-0282. [DOI] [PubMed] [Google Scholar]
- 47.Tanito M, Nishiyama A, Tanaka T, Masutani H, Nakamura H, Yodoi J, Ohira A. Change of redox status and modulation by thiol replenishment in retinal photooxidative damage. Investigative ophthalmology & visual science. 2002;43:2392–2400. [PubMed] [Google Scholar]
- 48.Wu T, Chen Y, Chiang SK, Tso MO. NF-kappaB activation in light-induced retinal degeneration in a mouse model. Investigative ophthalmology & visual science. 2002;43:2834–2840. [PubMed] [Google Scholar]
- 49.Bhakar AL, Tannis LL, Zeindler C, Russo MP, Jobin C, Park DS, MacPherson S, Barker PA. Constitutive nuclear factor-kappa B activity is required for central neuron survival. J Neurosci. 2002;22:8466–8475. doi: 10.1523/JNEUROSCI.22-19-08466.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Seminars in immunology. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- 51.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annual review of immunology. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 52.Mattson MP, Cheng A. Neurohormetic phytochemicals: Low-dose toxins that induce adaptive neuronal stress responses. Trends in neurosciences. 2006;29:632–639. doi: 10.1016/j.tins.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Watson JL, Hill R, Lee PW, Giacomantonio CA, Hoskin DW. Curcumin induces apoptosis in HCT-116 human colon cancer cells in a p21-independent manner. Experimental and molecular pathology. 2008;84:230–233. doi: 10.1016/j.yexmp.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 54.Tanito M, Agbaga MP, Anderson RE. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free radical biology & medicine. 2007;42:1838–1850. doi: 10.1016/j.freeradbiomed.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 55.Maltzman JS, Carmen JA, Monroe JG. Transcriptional regulation of the Icam-1 gene in antigen receptor- and phorbol ester-stimulated B lymphocytes: role for transcription factor EGR1. The Journal of experimental medicine. 1996;183:1747–1759. doi: 10.1084/jem.183.4.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Progress in neurobiology. 2004;74:183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Pendurthi UR, Rao LV. Suppression of transcription factor Egr-1 by curcumin. Thrombosis research. 2000;97:179–189. doi: 10.1016/s0049-3848(99)00148-6. [DOI] [PubMed] [Google Scholar]
- 58.Giri RK, Rajagopal V, Kalra VK. Curcumin, the active constituent of turmeric, inhibits amyloid peptide-induced cytochemokine gene expression and CCR5-mediated chemotaxis of THP-1 monocytes by modulating early growth response-1 transcription factor. Journal of neurochemistry. 2004;91:1199–1210. doi: 10.1111/j.1471-4159.2004.02800.x. [DOI] [PubMed] [Google Scholar]
- 59.Chen A, Xu J, Johnson AC. Curcumin inhibits human colon cancer cell growth by suppressing gene expression of epidermal growth factor receptor through reducing the activity of the transcription factor Egr-1. Oncogene. 2006;25:278–287. doi: 10.1038/sj.onc.1209019. [DOI] [PubMed] [Google Scholar]
- 60.Ravindranath V, Chandrasekhara N. Absorption and tissue distribution of curcumin in rats. Toxicology. 1980;16:259–265. doi: 10.1016/0300-483x(80)90122-5. [DOI] [PubMed] [Google Scholar]
- 61.Ravindranath V, Chandrasekhara N. Metabolism of curcumin--studies with [3H]curcumin. Toxicology. 1981;22:337–344. doi: 10.1016/0300-483x(81)90027-5. [DOI] [PubMed] [Google Scholar]
- 62.Shankar TN, Shantha NV, Ramesh HP, Murthy IA, Murthy VS. Toxicity studies on turmeric (Curcuma longa): acute toxicity studies in rats, guineapigs & monkeys. Indian journal of experimental biology. 1980;18:73–75. [PubMed] [Google Scholar]
- 63.Wang Q, Sun AY, Simonyi A, Jensen MD, Shelat PB, Rottinghaus GE, MacDonald RS, Miller DK, Lubahn DE, Weisman GA, Sun GY. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. Journal of neuroscience research. 2005;82:138–148. doi: 10.1002/jnr.20610. [DOI] [PubMed] [Google Scholar]
- 64.Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Experimental neurology. 2006;197:309–317. doi: 10.1016/j.expneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 65.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.