Abstract
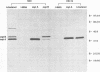
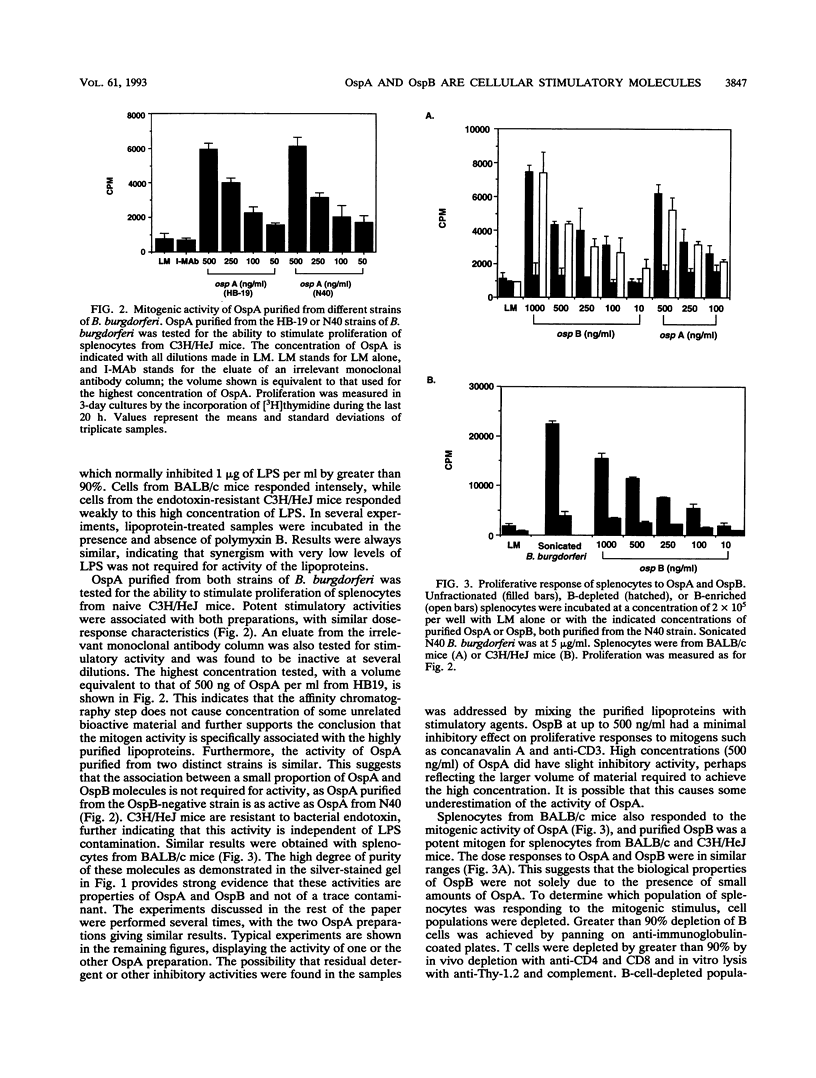
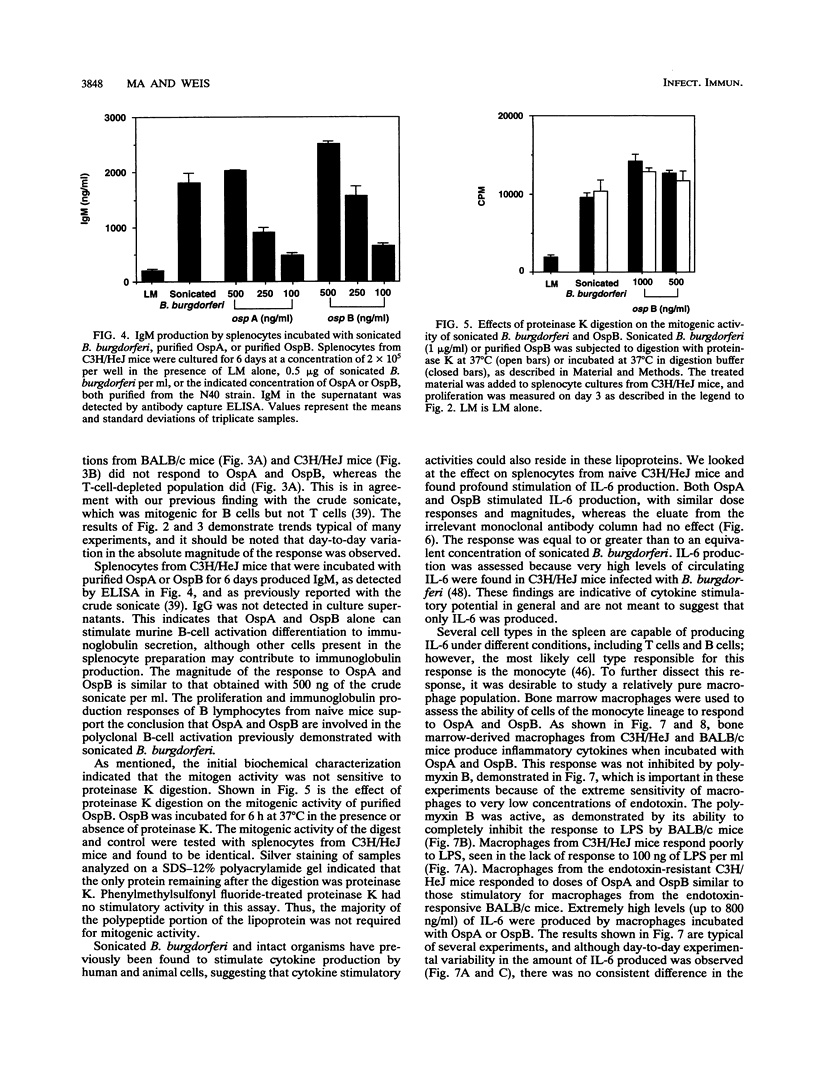
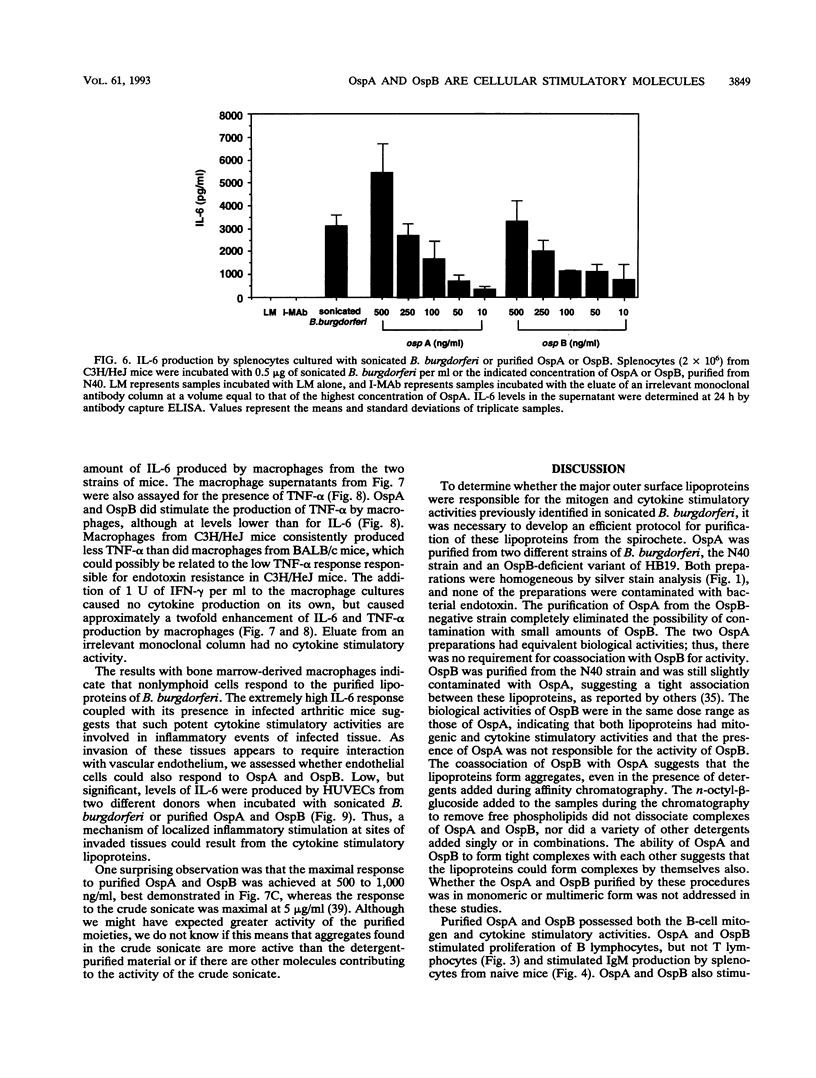
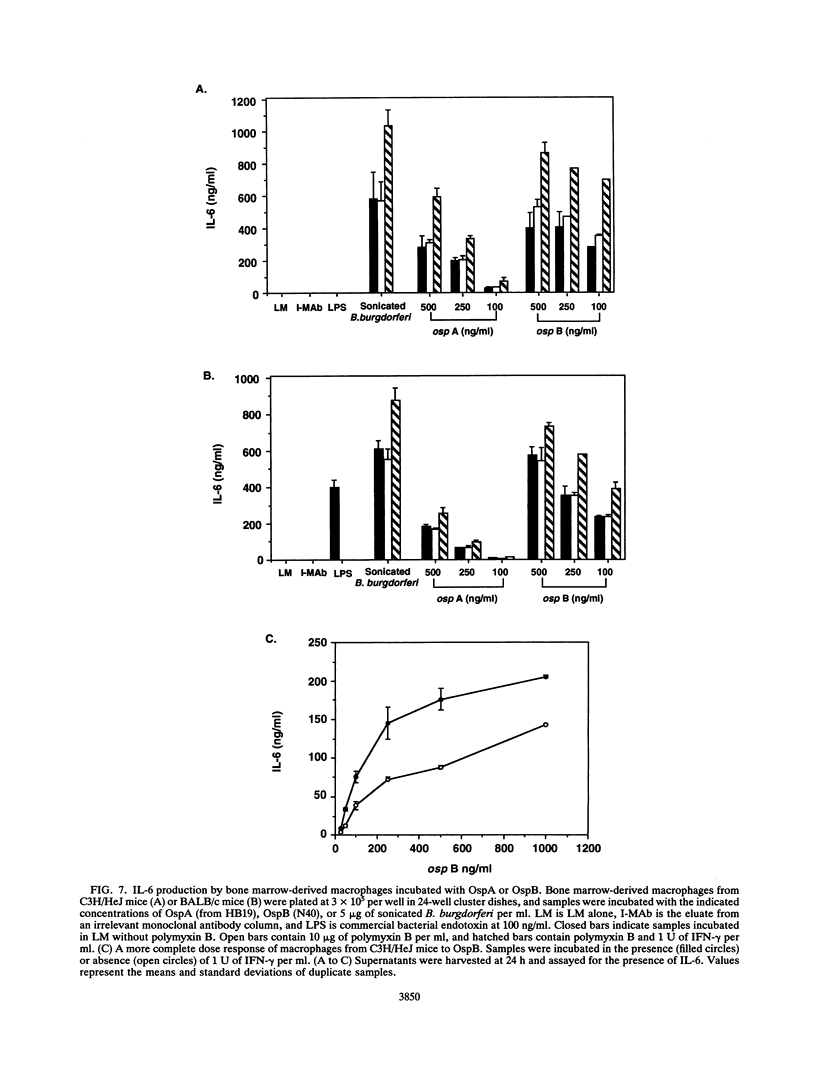
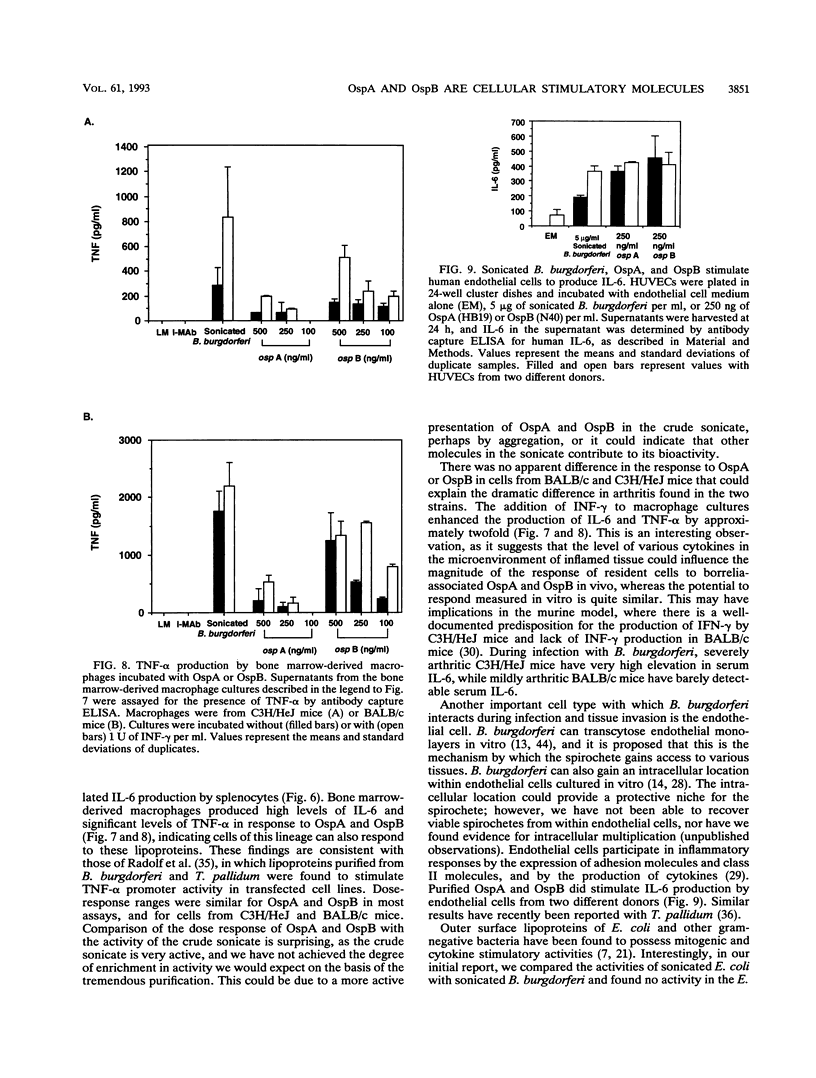
Sonicated Borrelia burgdorferi was previously reported to possess both B-cell mitogenic and interleukin-6 (IL-6) stimulatory activities. In this report, two outer surface lipoproteins, OspA and OspB, were purified from B. burgdorferi and assessed for the presence of these functions. OspA was purified from two strains, an OspB-deficient variant of HB19 and N40, while OspB was purified from the N40 strain. All lipoprotein preparations were free of endotoxin contamination, and polymyxin B failed to inhibit responses, indicating that media contamination was not contributing to biological assays. All three preparations were able to stimulate proliferation of mononuclear cells from naive C3H/HeJ and BALB/c mice. Depletion experiments indicated that the responding cells were B lymphocytes and not T lymphocytes. Purified OspA and OspB stimulated immunoglobulin M production by splenocyte cultures from naive mice, a property also previously attributed to sonicated B. burgdorferi. OspA and OspB also stimulated the production of IL-6 and tumor necrosis factor alpha by bone marrow-derived macrophages from BALB/c and C3H/HeJ mice. Cytokine production was enhanced by the presence of gamma interferon in the cultures, indicating that the magnitude of responses to these lipoproteins may be modulated by cytokines in the microenvironment of infected tissues. Human endothelial cells produced IL-6 when incubated with OspA and OspB, indicating that non-hematopoietic lineage cells can respond to the lipoproteins. Purified OspA and OspB had approximately equal activity, with responses detected in the range of 10 ng of lipoprotein per ml to 1 microgram of lipoprotein per ml. Comparison with published dose responses for lipoproteins purified from Escherichia coli indicates that OspA and OspB purified from B. burgdorferi are much more potent. The high potency of the B. burgdorferi lipoproteins and the ability of the spirochete to invade tissues and persist argue that they could be important in the localized events contributing to the pathology of Lyme disease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Todd W. J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983 Aug;41(2):795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold S. W., Beck D. S., Hansen G. M., Terwilliger G. A., Moody K. D. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990 Jul;162(1):133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- Barthold S. W., Persing D. H., Armstrong A. L., Peeples R. A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991 Aug;139(2):263–273. [PMC free article] [PubMed] [Google Scholar]
- Beck G., Benach J. L., Habicht G. S. Isolation of interleukin 1 from joint fluids of patients with Lyme disease. J Rheumatol. 1989 Jun;16(6):800–806. [PubMed] [Google Scholar]
- Bergström S., Bundoc V. G., Barbour A. G. Molecular analysis of linear plasmid-encoded major surface proteins, OspA and OspB, of the Lyme disease spirochaete Borrelia burgdorferi. Mol Microbiol. 1989 Apr;3(4):479–486. doi: 10.1111/j.1365-2958.1989.tb00194.x. [DOI] [PubMed] [Google Scholar]
- Bessler W. G., Cox M., Lex A., Suhr B., Wiesmüller K. H., Jung G. Synthetic lipopeptide analogs of bacterial lipoprotein are potent polyclonal activators for murine B lymphocytes. J Immunol. 1985 Sep;135(3):1900–1905. [PubMed] [Google Scholar]
- Brandt M. E., Riley B. S., Radolf J. D., Norgard M. V. Immunogenic integral membrane proteins of Borrelia burgdorferi are lipoproteins. Infect Immun. 1990 Apr;58(4):983–991. doi: 10.1128/iai.58.4.983-991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundoc V. G., Barbour A. G. Clonal polymorphisms of outer membrane protein OspB of Borrelia burgdorferi. Infect Immun. 1989 Sep;57(9):2733–2741. doi: 10.1128/iai.57.9.2733-2741.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Comstock L. E., Thomas D. D. Characterization of Borrelia burgdorferi invasion of cultured endothelial cells. Microb Pathog. 1991 Feb;10(2):137–148. doi: 10.1016/0882-4010(91)90074-k. [DOI] [PubMed] [Google Scholar]
- Comstock L. E., Thomas D. D. Penetration of endothelial cell monolayers by Borrelia burgdorferi. Infect Immun. 1989 May;57(5):1626–1628. doi: 10.1128/iai.57.5.1626-1628.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defosse D. L., Johnson R. C. In vitro and in vivo induction of tumor necrosis factor alpha by Borrelia burgdorferi. Infect Immun. 1992 Mar;60(3):1109–1113. doi: 10.1128/iai.60.3.1109-1113.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Erdile L. F., Brandt M. A., Warakomski D. J., Westrack G. J., Sadziene A., Barbour A. G., Mays J. P. Role of attached lipid in immunogenicity of Borrelia burgdorferi OspA. Infect Immun. 1993 Jan;61(1):81–90. doi: 10.1128/iai.61.1.81-90.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondolf K. B., Batsford S. R., Vogt A. Isolation of an outer membrane protein complex from Borrelia burgdorferi by n-butanol extraction and high-performance ion-exchange chromatography. J Chromatogr. 1990 Nov 23;521(2):325–334. doi: 10.1016/0021-9673(90)85056-2. [DOI] [PubMed] [Google Scholar]
- Habicht G. S., Katona L. I., Benach J. L. Cytokines and the pathogenesis of neuroborreliosis: Borrelia burgdorferi induces glioma cells to secrete interleukin-6. J Infect Dis. 1991 Sep;164(3):568–574. doi: 10.1093/infdis/164.3.568. [DOI] [PubMed] [Google Scholar]
- Hauschildt S., Hoffmann P., Beuscher H. U., Dufhues G., Heinrich P., Wiesmüller K. H., Jung G., Bessler W. G. Activation of bone marrow-derived mouse macrophages by bacterial lipopeptide: cytokine production, phagocytosis and Ia expression. Eur J Immunol. 1990 Jan;20(1):63–68. doi: 10.1002/eji.1830200110. [DOI] [PubMed] [Google Scholar]
- Hoffmann P., Heinle S., Schade U. F., Loppnow H., Ulmer A. J., Flad H. D., Jung G., Bessler W. G. Stimulation of human and murine adherent cells by bacterial lipoprotein and synthetic lipopeptide analogues. Immunobiology. 1988 May;177(2):158–170. doi: 10.1016/S0171-2985(88)80036-6. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenefick K. B., Lederer J. A., Schell R. F., Czuprynski C. J. Borrelia burgdorferi stimulates release of interleukin-1 activity from bovine peripheral blood monocytes. Infect Immun. 1992 Sep;60(9):3630–3634. doi: 10.1128/iai.60.9.3630-3634.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Ma Y., Sturrock A., Weis J. J. Intracellular localization of Borrelia burgdorferi within human endothelial cells. Infect Immun. 1991 Feb;59(2):671–678. doi: 10.1128/iai.59.2.671-678.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Dejana E. Cytokines as communication signals between leukocytes and endothelial cells. Immunol Today. 1989 Nov;10(11):370–375. doi: 10.1016/0167-5699(89)90270-3. [DOI] [PubMed] [Google Scholar]
- Meerpohl H. G., Lohmann-Matthes M. L., Fischer H. Studies on the activation of mouse bone marrow-derived macrophages by the macrophage cytotoxicity factor (MCF). Eur J Immunol. 1976 Mar;6(3):213–217. doi: 10.1002/eji.1830060313. [DOI] [PubMed] [Google Scholar]
- Melchers F., Braun V., Galanos C. The lipoprotein of the outer membrane of Escherichia coli: a B-lymphocyte mitogen. J Exp Med. 1975 Aug 1;142(2):473–482. doi: 10.1084/jem.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. C., Isa S., Vannier E., Georgilis K., Steere A. C., Dinarello C. A. Live Borrelia burgdorferi preferentially activate interleukin-1 beta gene expression and protein synthesis over the interleukin-1 receptor antagonist. J Clin Invest. 1992 Sep;90(3):906–912. doi: 10.1172/JCI115966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Jacobs D. M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976 Oct;13(10):813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- Radolf J. D., Norgard M. V., Brandt M. E., Isaacs R. D., Thompson P. A., Beutler B. Lipoproteins of Borrelia burgdorferi and Treponema pallidum activate cachectin/tumor necrosis factor synthesis. Analysis using a CAT reporter construct. J Immunol. 1991 Sep 15;147(6):1968–1974. [PubMed] [Google Scholar]
- Riley B. S., Oppenheimer-Marks N., Hansen E. J., Radolf J. D., Norgard M. V. Virulent Treponema pallidum activates human vascular endothelial cells. J Infect Dis. 1992 Mar;165(3):484–493. doi: 10.1093/infdis/165.3.484. [DOI] [PubMed] [Google Scholar]
- Rosa P. A., Schwan T., Hogan D. Recombination between genes encoding major outer surface proteins A and B of Borrelia burgdorferi. Mol Microbiol. 1992 Oct;6(20):3031–3040. doi: 10.1111/j.1365-2958.1992.tb01761.x. [DOI] [PubMed] [Google Scholar]
- Rose N. R. The discovery of thyroid autoimmunity. Immunol Today. 1991 May;12(5):167–168. doi: 10.1016/S0167-5699(05)80047-7. [DOI] [PubMed] [Google Scholar]
- Schoenfeld R., Araneo B., Ma Y., Yang L. M., Weis J. J. Demonstration of a B-lymphocyte mitogen produced by the Lyme disease pathogen, Borrelia burgdorferi. Infect Immun. 1992 Feb;60(2):455–464. doi: 10.1128/iai.60.2.455-464.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal L. H. Lyme disease, 1988: immunologic manifestations and possible immunopathogenetic mechanisms. Semin Arthritis Rheum. 1989 Feb;18(3):151–167. doi: 10.1016/0049-0172(89)90058-9. [DOI] [PubMed] [Google Scholar]
- Sigal L. H., Steere A. C., Dwyer J. M. In vivo and in vitro evidence of B cell hyperactivity during Lyme disease. J Rheumatol. 1988 Apr;15(4):648–654. [PubMed] [Google Scholar]
- Starnes H. F., Jr, Pearce M. K., Tewari A., Yim J. H., Zou J. C., Abrams J. S. Anti-IL-6 monoclonal antibodies protect against lethal Escherichia coli infection and lethal tumor necrosis factor-alpha challenge in mice. J Immunol. 1990 Dec 15;145(12):4185–4191. [PubMed] [Google Scholar]
- Steere A. C. Lyme disease. N Engl J Med. 1989 Aug 31;321(9):586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- Szczepanski A., Furie M. B., Benach J. L., Lane B. P., Fleit H. B. Interaction between Borrelia burgdorferi and endothelium in vitro. J Clin Invest. 1990 May;85(5):1637–1647. doi: 10.1172/JCI114615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sădziene A., Rosa P. A., Thompson P. A., Hogan D. M., Barbour A. G. Antibody-resistant mutants of Borrelia burgdorferi: in vitro selection and characterization. J Exp Med. 1992 Sep 1;176(3):799–809. doi: 10.1084/jem.176.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder T. F., Clement L. T., Cooper M. D. Expression of C3d receptors during human B cell differentiation: immunofluorescence analysis with the HB-5 monoclonal antibody. J Immunol. 1984 Aug;133(2):678–683. [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Whitmire W. M., Garon C. F. Specific and nonspecific responses of murine B cells to membrane blebs of Borrelia burgdorferi. Infect Immun. 1993 Apr;61(4):1460–1467. doi: 10.1128/iai.61.4.1460-1467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Ma Y., Schoenfeld R., Griffiths M., Eichwald E., Araneo B., Weis J. J. Evidence for B-lymphocyte mitogen activity in Borrelia burgdorferi-infected mice. Infect Immun. 1992 Aug;60(8):3033–3041. doi: 10.1128/iai.60.8.3033-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza M. S., Fikrig E., Smith A. L., Flavell R. A., Barthold S. W. Nonspecific proliferative responses of murine lymphocytes to Borrelia burgdorferi antigens. J Infect Dis. 1992 Mar;165(3):471–478. doi: 10.1093/infdis/165.3.471. [DOI] [PubMed] [Google Scholar]