Abstract
Vocal fold surface liquid homeostasis contributes to optimal vocal physiology. In this paper we review emerging evidence that vocal fold surface liquid is maintained in part by salt and water fluxes across the epithelium. Based on recent immunolocalization and electrophysiological findings, we describe a transcellular pathway as one mechanism for regulating superficial vocal fold hydration. We propose that the pathway includes the sodium-potassium pump, sodium-potassium-chloride cotransporter, epithelial sodium channels, cystic fibrosis transmembrane regulator chloride channels, and aquaporin water channels. By integrating knowledge of the regulating mechanisms underlying ion and fluid transport with observations from hydration challenges and treatments using in vitro and in vivo studies, we provide a theoretical basis for understanding how environmental and behavioral challenges and clinical interventions may modify vocal fold surface liquid composition. We present converging evidence that clinical protocols directed at facilitating vocal fold epithelial ion and fluid transport may benefit healthy speakers, those with voice disorders, and those at risk for voice disorders.
Introduction
Vocal folds are covered by a thin layer of liquid.1 This liquid serves as a physical and biochemical barrier that protects the underlying tissue from damage from inhaled particulates and pathogens.2 Presence of surface liquid is also posited to maintain optimal biomechanical characteristics of vocal fold mucosa, increase efficiency of vocal fold oscillation, and promote normal voice quality.1,3-14 This is consistent with the well-accepted clinical practice of recognizing the importance of vocal fold hydration in maintaining optimal vocal physiology. However, the source of surface liquid and mechanisms for maintaining liquid homeostasis are not fully understood.
In this paper we will present an overview of the current understanding of cellular mechanisms that participate in maintaining the composition and depth of the layer of liquid covering the vocal fold surface. This liquid layer constitutes a portion of airway surface liquid that lines the proximal and distal respiratory tract. The depth of airway surface liquid is maintained primarily by sodium ion (Na+) absorption and chloride ion (Cl−) secretion by epithelia of the lungs, bronchi, trachea, and nose.15 Here we will present emerging evidence that vocal fold surface liquid is similarly maintained in part by ion and water fluxes across vocal fold epithelia.
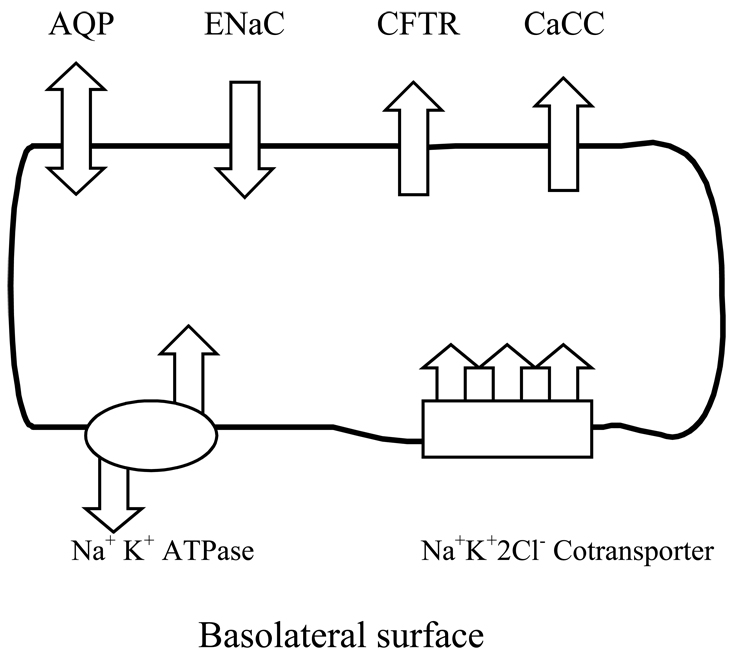
In 2001, Fisher and colleagues16 established a role for vocal fold epithelium in regulating vocal fold surface liquid. Epithelium was shown to be polarized and to demonstrate bidirectional transcellular water fluxes driven by active ion transport. The presence of transcellular water fluxes demonstrates that vocal epithelial cells, in addition to laryngeal gland secretions and mucociliary clearance, determine the depth and composition of surface liquid. Given that water fluxes can be manipulated pharmacologically, epithelial cells provide an important target for therapeutic interventions to regulate vocal fold surface liquid homeostasis. We will describe pathways for Na+, Cl−, and water fluxes across vocal fold epithelial cells that includes the sodium-potassium (Na+K+ATPase) pump, sodium-potassium-chloride (Na+K+2Cl−) cotransporter, epithelial sodium channels (ENaC), cystic fibrosis transmembrane regulator (CFTR) chloride channels, and aquaporin (AQP) water channels. We will outline the role of these transport proteins in maintaining homeostasis of vocal fold surface liquid by regulating transepithelial Na+, Cl−, and water fluxes. Based on recent investigations of transepithelial ion and water fluxes, a preliminary composite model of pathways for ion and water fluxes across epithelial cells will be proposed (Figure 1). An attempt will be made to integrate knowledge of cellular mechanisms underlying salt and water transport with observations of the effects of hydration challenges and treatments on vocal fold function in ex vivo and in vivo studies. The effectiveness of clinical hydration interventions in maintaining phonatory function in healthy speakers exposed to environmental challenges, and restoring voice function in individuals at risk for voice disorders and in speakers with vocal pathologies, will be reviewed.
Figure 1. Model of transport proteins underlying transcellular ion and water fluxes.
Legend: The sodium-potassium pump (Na+K+ATPase) was immunolocalized to the basolateral (serosal) plasma membrane where it establishes an electrochemical gradient creating a driving force for transcellular ion movement. The sodium-potassium-chloride (Na+K+2Cl−) cotransporter provides a pathway for Na+, K+ and Cl− entry into the cells. The Na+ also enter the cells via epithelial sodium channels (ENaC) located on the luminal (air-facing) surface. The Cl− exit cells via cystic fibrosis transmembrane regulator (CFTR) chloride channels and calcium-activated chloride channels (CaCC) also located on the luminal surface. Luminal aquaporins provide a pathway for bidirectional water fluxes across the cell membrane.
1. Transepithelial ion and water transport
Vocal fold surface hydration is subjected to persistent behavioral and environmental challenges.8,9,12,17 These challenges may compromise vocal fold defense and physiology. If optimal vocal fold function is to be maintained, it is necessary that there be an intrinsic mechanism for ensuring homeostasis of surface liquid in the face of these daily challenges. Based on observations of epithelial cell function in other airway epithelia,18 Fisher and colleagues16 hypothesized that the depth and ionic composition of vocal fold surface liquid is determined in part by active ion transport in vocal fold epithelial cells. Specifically, it was proposed that epithelial cells provide a pathway for Na+- and Cl−-coupled fluid fluxes.16,19,20 Recent studies have sought to identify the pathways and cellular mechanisms for maintaining local vocal fold surface hydration using three approaches: immunohistochemistry, electrophysiology, and measurement of water fluxes. Using these approaches, Na+, Cl−, and water transport proteins have been localized to vocal fold epithelial cells and net ion and water fluxes supported by these proteins have been quantified.
A. Immunolocalization of ion transport processes in vocal fold epithelia
Integral membrane transport proteins believed to support transepithelial ion and water fluxes have been localized to vocal fold epithelial cells (Table 1). Immunolocalization assays have revealed that the α-subunit of the sodium-potassium (Na+K+ATPase) pump protein is localized to the plasma membrane of the most luminal and most basal vocal fold epithelial cells.16 This Na+K+ATPase pump supports active ion transport across cells. By transporting three Na+ ions out of the cell and two potassium ions (K+) into the cell, the Na+K+ATPase pump creates an asymmetric distribution of ions across the cell wall, resulting in the build up of transepithelial electrochemical gradients21 and driving ion transport. Other proteins that play an important role in ion transport have also been localized to vocal fold epithelia. The carboxy (C)-terminus of the sodium-potassium-chloride cotransporter (Na+K+2Cl−) has been localized to the plasma membranes of vocal fold epithelial cells where it may provide a point of entry for sodium (Na+), potassium (K+), and chloride ions (Cl−) into epithelial cells.20 Two primary ion channels, the epithelial sodium channels (ENaC)19 and the cystic fibrosis transmembrane regulator (CFTR) chloride channels20 have also been localized to vocal fold epithelial cells. ENaC and CFTR provide a pathway for transmembrane Na+ and Cl− fluxes, respectively.19,20 ENaC is a heterotetramer composed of two α, one β, and one γ homologous subunits.22 The α- and β- subunits of ENaC have been localized to the plasma membrane of epithelial cells, with greatest density noted in the luminal cell layer. 19 Luminal sodium absorption occurs predominantly through these ENaC, however sodium ions may also enter the cell coupled with potassium and chloride ions via the Na+K+2Cl− cotransporter.20 A pathway for vocal fold epithelial Cl− secretion similar to that observed in other airway epithelia15 has also been proposed.19 Chloride ion entry into vocal fold epithelial cells is believed to occur via the Na+K+2Cl− cotransporter described above. Cl− secretion is thought to occur via the CFTR chloride channels. The carboxy (C)-terminus and regulatory (R)-domain of CFTR have been localized to the plasma membranes of the two most superficial vocal fold epithelial cell layers.20 Based on electrophysiological findings described below, a second Cl− specific channel, the calcium-activated chloride channel (CaCC) may also provide a pathway for Cl− secretion from the cell.20 Localization of CaCC to vocal fold epithelial cells is awaited.
Table 1.
Summary of immunolocalization studies of membrane transport proteins
| Ref | Transport Protein | Technique | Outcome |
|---|---|---|---|
| 16 | Sodium-potassium (NaKATPase) pump | Immunohistochemistry | Localized to the basal plasma membrane of epithelia cells. Most intense staining occurred in luminal cells layers |
| 19 | Epithelial sodium channels (ENaC) | Immunohistochemistry | Localized to the apical plasma membrane of epithelia cells. Most intense labeling occurred in luminal cell layers. |
| 20 | Cystic fibrosis transmembrane regulator (CFTR) chloride channels | Immunohistochemistry | Localized to the apical plasma membrane and cytoplasmic structures of epithelial cells. Most intense labeling occurred in luminal cell layers. |
| 20 | Sodium-potassium-chloride(Na+K+2Cl−) cotransporter | Immunohistochemistry | Localized to the apical and basolateral plasma membranes and cytoplasm of epithelial cells. |
| 24 | Aquaporin (AQP) water channels (AQP1, AQP2) | Immunohistochemistry | Localized to the apical plasma membrane and cytoplasmic structures of epithelial cells. Most intense labeling occurred in luminal cell layers. |
B. Pharmacological manipulation of transepithelial ion transport
Recent electrophysiological studies have revealed that the transport proteins localized to vocal fold epithelial cells support transepithelial ion fluxes. Short-circuit current (Isc) provides a measure of the net flow of ions across epithelium. To assess the extent to which each protein contributes to measures of the Isc, viable excised ovine and canine mucosae have been treated with pharmacological agents to selectively inhibit or stimulate protein function (Table 2). Changes in Isc capture the effects of stimulation or inhibition of transport protein activity on ion fluxes. For example, inhibition of Na+K+ATPase with acetylstrophanthidin reduced transepithelial Isc.16 This finding is consistent with speculation that functional Na+K+ATPase participates in active ion transport across epithelial cells. Inhibition of ENaC with amiloride, a known epithelial sodium channel (ENaC) inhibitor, reduced Isc consistent with decreased Na+ absorption.19 Inhibition of CFTR with diphenylamine-2-carboxylate (DPC), a broad-spectrum Cl− transport inhibitor, decreased Isc consistent with a reduction in transepithelial Cl− movement. Conversely, stimulation of CFTR with secretagogues, isobutymethylxanthine (IBMX) and uridine triphosphate (UTP), induced a Cl−-dependent increase in Isc consistent with an increase in Cl− movement across the epithelium.20 Closer examination of the kinetics of the Isc response to UTP revealed a biphasic response consistent with the presence of CaCC in vocal fold epithelial cells.
Table 2.
Summary of in vitro electrophysiolgical studies
| Ref | Transport Protein | Intervention | Outcome |
|---|---|---|---|
| 18 | Sodium-potassium (NaKATPase) pump |
Inhibition with acetylstrephanthidin | ↓ PD |
| ↓Isc | |||
| 16 | Epithelial sodium channel (ENaC) | Inhibition with amiloride | ↓PD |
| ↓Isc | |||
| 20 | Cystic fibrosis transmembrane regulator (CFTR) chloride channels |
Inhibition with diphenylamine-3 carboxylate (DPC) |
↓PD |
| ↓Isc | |||
| 20 | Cystic fibrosis transmembrane regulator (CFTR) chloride channels |
Stimulation with isobutylmethylxanthine (IBMX) |
↑PD |
| ↑Isc | |||
| 20 | ? Cystic fibrosis transmembrane regulator (CFTR) chloride channels |
Stimulation with 10−4M uridine triphosphate (UTP) |
↑ PD |
| ? Calcium activated chloride channels (CaCC) |
↑ Isc | ||
PD: Transepithelial potential difference; Is Short-circuit current; ↑ Increase; ↓ Decrease; ?: Suspected pathway
C. Transepithelial water transport
Bidirectional water fluxes across excised vocal fold epithelium have been quantified using a Transepithelial Water and Ion Measurement System (TWIMS; Bio-Tech Plex, San Marco, CA).16,19,23 Transepithelial ion movements provide the driving force for bidirectional water fluxes across vocal fold epithelium. The effects of ion transport inhibitors on the magnitude of water fluxes have been examined. Inhibition of Na+K+ATPase by acetylstrophantidin resulted in a reduction in both secretory and absorptive water fluxes.16,19 Similarly, a reduction in absorption of ENaC mediated ion fluxes across vocal fold mucosae following treatment with amiloride resulted in decreased absorptive water consistent with decreased Na+ absorption.19
The interaction between ENaC and CFTR may play an important role in dictating the net driving forces for water secretion and absorption across vocal fold epithelium. Airway epithelium can be both absorptive and secretory. The transport of Na+ and Cl− ions across the epithelium creates a local osmotic gradient that serves as a driving force for transepithelial water fluxes. These water fluxes may occur via specialized water transport proteins (aquaporin, AQP). Two members of the AQP family, AQP1 and AQP2, have been immunolocalized to the luminal plasma membrane and cytoplasmic structures of vocal folds.24 The interaction between ENaC and CFTR also determines whether the epithelial tissue is absorptive or secretory. At rest, airway tissue is absorptive.25 When stimulated, a net secretion of Cl− towards the surface occurs. Activation of CFTR provides a pathway for Cl− secretion towards the surface while reducing Na+ absorption through inhibition of ENaC activity.26 When CFTR are absent or mutated (as in airway diseases such as cystic fibrosis) Cl− flux towards the surface is reduced and the inhibitory effect on ENaC is absent.27 Consequently, Na+ absorption remains unchecked and epithelial dehydration ensues.28 The mechanism underlying CFTR-mediated inhibition of ENaC is not known.26 Future studies are warranted to identify the mechanisms underlying CFTR-mediated inhibition of ENaC and the impact of the interaction of CFTR and ENaC on relative movements of Na+ and Cl− and, therefore on vocal fold surface hydration.
A review of electrophysiological and immunohistochemical data suggests that vocal fold epithelium may participate in regulating and maintaining vocal fold surface liquid homeostasis via ion transport and bi-directional water fluxes. Based on these data, we propose a functional pathway for transcellular ion and water fluxes (Figure 1). This model provides a theoretical basis for understanding how epithelial cells may alter the depth and composition of surface liquid in response to behavioral and environmental challenges, clinical interventions, and pharmacological treatment. Since ion-coupled water fluxes can be manipulated through luminal application of drugs to the vocal fold surface suggests that bidirectional water fluxes that contribute to vocal fold surface hydration and function can be controlled pharmacologically.
An understanding of the mechanisms by which vocal fold epithelial cells regulate local hydration offers a theoretical framework for appreciating the effects of behavioral and environmental challenges on surface hydration and provides the knowledge base necessary for the development of effective clinical interventions to maintain superficial and systemic hydration.
2. Effects of hydration challenges and treatments on vocal fold function
A. Consequences of behavioral and environmental challenges on vocal fold physiology and voice quality
Drying of the vocal fold surface can occur due to environmental and behavioral challenges associated with mouth breathing, exercising, and inhaling poorly conditioned air (Table 3). 8,9,13,17 Vocal fold dehydration can also occur secondary to reduced systemic hydration,17,29–31 emotional factors,32 and the normal aging process.33,34 The relationship between dehydration and vocal fold physiology has been examined empirically in in vitro and in vivo studies.
Table 3.
Summary of in vivo human clinical studies
| Ref | Subjects | Challenges | Outcome |
|---|---|---|---|
| 4 | 8 vocally healthy individuals (equal male and female) |
Random administration of each of the following challenges:
|
↑jitter and shimmer after inhaling dry air in low ambient air condition |
| 8 | 20 vocally healthy females |
Exposure to one of the following challenges:
|
↑ PTP at 10% and 20% of pitch range with oral breathing |
| ↑ PPE in 60% of subjects after oral breathing | |||
| ↓ PTP at 10%, 20%, and 80% after nasal breathing | |||
| ↓ PPE in 70% of subjects after nasal breathing | |||
| 9 | 38 healthy females (20 vocally normal and 18 vocal attrition) |
Exposure to one of the following challenges:
|
↑ in PTP and PPE was greater after oral breathing in subjects with vocal attrition than in normal controls. |
| ↓ PTP with nasal breathing for all controls but only some participants with vocal attrition. | |||
| 10 | 4 untrained vocally healthy females |
2 hour loud reading on each of the following in counterbalanced order:
|
↑ PTP at 80% of pitch range after loud reading in low versus high hydration conditions in 75% of subjects. |
| 11 | 4 untrained vocally healthy men |
Similar design as Solomon et al. (2000)10 | ↑ PTP at 80% of pitch range after loud reading in low as compared to high hydration condition in 50% of subjects. |
| 7 | 18 vocally healthy untrained females |
2 ml of each of the following treatments in counterbalanced order:
|
↓ PTP at 80% of pitch range within 20 min of treatment with hyperosmotic mannitol. |
| ↔ PTP after other challenges | |||
| 12 | 60 vocally healthy females |
15 min laryngeal desiccation (<1% RH) followed by one of these challenges:
|
↑ PTP after laryngeal desiccation in all subjects |
| ↓ PPE after laryngeal desiccation | |||
| ↔ PTP or PPE after any treatment | |||
| 42 | 6 adult females with vocal nodules or vocal polyps |
5 days of both treatments in counterbalanced order:
|
↓ in PTP, PPE, and jitter was greater following hydration treatment as compared to placebo treatment |
| 43 | 80 untrained vocally healthy individuals (equal males and females) |
Three 45 min reading sessions followed by 45 min lunch break, followed by two 45 min reading sessions in either:
|
↑ symptoms of dry mouth and throat and |
| ↑ fatigue of neck, shoulders, and back in low versus high humidity. | |||
| 41 | 20 amateur karaoke singer (equal males and females) |
Exposure to one of the following during continuous karaoke singing:
|
↑ ability to sustain singing when provided vocal rest and hydration (102 min. versus 85 min.) |
| ↑ jitter after singing 10 songs when not provided vocal rest and hydration. | |||
| 13 | 6 healthy adults (equal male and female) |
Counterbalanced exposure to the following 4 hr challenges:
|
↓ PTP following hydration challenge. This decrease was greatest at high pitches. |
| 14 | 12 untrained vocally healthy subjects (9 females and 3 males) |
Counterbalanced exposure to the following 4 hr challenges:
|
↑ PTP at 10th, 20th, and 80th pitch following dehydration versus hydration challenge or placebo |
| ↑ PPE following dehydration versus hydration challenge or placebo | |||
| 27 | 4 vocally untrained healthy subjects (2 males and 2 females) |
Three challenges in couterbalanced order:
|
↑ PTP at high pitch following systemic dehydration but not secretory dehydration or placebo |
| ↔ PPE after any challenge | |||
| 25 | 2 females and 4 males with end- stage renal disease and 2 male controls |
Single-subject reversal design with voice measurements repeated at either 1.0 L or 0.5 L fluid removal intervals. |
↑ PTP at 30% of pitch range after 3–4% reduction in body fluid volume in 4/6 subjects. Reversal of PTP to baseline after rehydration. |
PTP: Phonation threshold pressure; PPE: Perceived phonatory effort; RH: Relative humidity; ↑ Increase; ↓ Decrease; ↔ No change
In vitro studies
Bench models have allowed study of the effects of hydration on the biomechanical and, consequently, phonatory characteristics of vocal folds (Table 4). Evaporative water loss from the airway surface due to dry air exposure can increase the stiffness and viscosity of ovine vocal fold mucosa.35 Adherent, viscous mucus on the vocal fold surface can also reduce vocal fold separation and increase vocal fold contact excised larynges,36 affecting vocal quality. Optimal viscoelastic properties of vocal folds are necessary to maintain ease of phonation,37,38 and the detrimental effects of surface dehydration on vocal fold viscoelasticity are consistent with the clinical adage to avoid dry environments that could adversely affect voice production.39 In excised larynges, dehydration of vocal folds raised phonation threshold pressure (PTP), the minimum subglottal pressure required to initiate and sustain vocal fold oscillation,5,40 and increased tissue stiffness.3
Table 4.
Summary of ex-vivo animal studies
| Ref | Tissue | Challenge | Outcome |
|---|---|---|---|
| 3 | Excised canine larynges | Dehydration of excised vocal folds by immersion in a 25% sucrose solution | ↑ Stiffness |
| ↑ Viscosity | |||
| ↑ Damping ratio | |||
| Rehydration of excised vocal folds through immersion in dH20 | ↓ Stiffness | ||
| ↓ Viscosity | |||
| ↓ Damping ratio | |||
| 5 | Excised canine larynges | Dehydration of vocal folds by exposure to dry air | ↑ PTP |
| ↑ Glottal airflow | |||
| ↓ (Slight) Sound intensity | |||
| ↓ Vocal efficiency | |||
| Rehydration of vocal folds by immersion in a saline solution | ↑ PTP | ||
| ↓ Glottal airflow | |||
| ↑ (Slight) Sound intensity | |||
| ↑ Vocal efficiency | |||
| 6 | Excised canine larynges | Dehydration of vocal folds by exposure to dry air | ↑ PTP |
| ↑ Glottal airflow | |||
| ↓ Sound intensity | |||
| ↓ Vocal efficiency | |||
| 31 | Excised ovine larynges | Dehydration of vocal folds by exposure to dry air (0% humidity) | ↑ Stiffness |
| ↑ Viscosity | |||
| Hydration of vocal folds by exposure to humid air (100% humidity). | ↑ Stiffness to a lesser extent | ||
| ↑ Viscosity to a lesser extent | |||
Clinical studies
The negative effects of dehydration on efficiency of vocal fold function in bench models are consistent with those observed in clinical trials. Challenges to systemic and superficial vocal fold dehydration compromise vocal quality and phonatory efficiency in vocally healthy and disordered participants (Table 3). Decreased systemic hydration increased PTP14,29 and compromised voice quality.41 A presumed reduction in systemic hydration following ingestion of a diuretic, Lasix, increased phonatory effort at high pitches in healthy participants.31 Increased superficial and systemic hydration through ingestion of water and a mucolytic expectorant resulted in an improvement in phonatory efficiency in vocally healthy participants13,14 and participants with vocal nodules or polyps.42 Fisher and colleagues29 demonstrated that phonatory effort increased temporarily in patients following dialysis. Measures of phonatory effort returned to baseline values in these patients following rehydration. Improved phonatory efficiency following interventions purported to increase systemic hydration have also been reported.41,42 For example, ingestion of water and mucolytic agents decreased PTP and perceived phonatory effort (PPE) in participants presenting with voice disorders.42 Drinking water in combination with vocal rest between demanding vocal tasks improved voice quality in healthy amateur singers.41 Behavioral, environmental, and medical treatments to increase superficial and systemic hydration appear to improve vocal function. Notwithstanding differences in the nature of challenges, attributes of participants, and measures of vocal fold function including efficiency of vocal fold oscillation, vocal quality, and perception, these clinical studies provide a rationale for inclusion of interventions to increase systemic and superficial hydration in vocal hygiene treatment. A meta-analysis of this growing body of literature is underway to assess the clinical effectiveness of treatment on vocal fold function.
Challenges to superficial vocal fold hydration result in decreased efficiency of vocal fold vibration and compromised voice quality. Inhaling desiccated air increased jitter and shimmer in vocally healthy individuals.4 Superficial vocal fold dehydration induced by short-term oral breathing increased PTP in healthy female speakers8 and individuals reporting symptoms of vocal fatigue. The detrimental phonatory effects of challenges to systemic and superficial hydration in combination with vocal loading have been documented in healthy adults.10,11,39 While the adverse phonatory effects of behavioral and environmental challenges that perturb vocal fold hydration are well-recognized, the mechanisms by which these challenges alter the state of vocal fold hydration is not completely understood. Understanding of the cellular mechanisms underlying transepithelial ion and water flux may shed light on the manner in which behavioral and environmental challenges affect vocal fold function.
B. Mechanisms by which hydration challenges impact transepithelial ion and fluid fluxes
Investigations of the cellular mechanisms governing salt and water transport across vocal fold epithelium outlined above suggest potential ion transport related mechanisms by which vocal folds may respond to dehydration challenges to the luminal surface. It has been demonstrated in other airway epithelia that drying of the respiratory surface increases the salt concentration and decreases the depth of airway surface liquid (ASL).44–48 These changes to the depth and volume of ASL are transient as epithelia lining the nose, trachea, and lungs detect the increased ionic and osmotic concentration49–50 and generate water fluxes to replenish surface hydration. The secretory water fluxes observed in response to threats to airway surface liquid homeostasis are predominantly associated with ion and water transport processes.51
Based on observed increases in ion-coupled water fluxes in other airway epithelia in response to hyperosmotic and ionic challenges in in vitro studies, Sivasankar and Fisher posited that vocal fold epithelium would respond to perturbations in the composition of vocal fold surface liquid in vivo. 23,52 While the beneficial effects of osmotic agents on superficial hydration have not been universally supported in clinical trials,11 Roy and colleagues demonstrated that a nebulized osmotic agent transiently lowered PTP in vocally healthy volunteers.7 This decrease in PTP is consistent with increased vocal fold surface hydration resulting from compensatory secretory ion-coupled water fluxes in response to a threat to local surface hydration. The manner by which epithelium detects changes in surface fluid composition and depth awaits further study. It has been posited that the peripheral nervous system plays an important role in detecting changes in the composition and depth of airway surface liquid in vivo.53–55 However, the mechanisms for detecting ionic and osmotic perturbations in vocal fold surface liquid in excised, de-innervated vocal folds has yet to be determined.
Improved superficial and systemic vocal fold hydration promote efficient voice production.1,3-14 An understanding of the relationship between superficial and systemic vocal fold hydration is emerging; however, the distinct roles of superficial and systemic hydration remain unknown. It has been traditionally suggested that superficial vocal fold surface liquid is maintained by glandular secretions and that internal vocal fold liquid is provided by local vasculature.56 However, based on the presence of transepithelial ion-coupled water fluxes, we suggest that superficial and internal vocal fold hydration are interdependent. We further posit that ion-coupled water fluxes towards the vocal fold surface may influence internal ion and water composition, potentially altering the biomechanical properties of the vocal folds. It has been demonstrated that the ionic and osmotic composition of airway surface liquid overlying the trachea impacts the ionic environment of underlying tissue.57 These effects are greater in the presence of epithelial cell damage (for example, in cystic fibrosis) in which airway epithelial cells are unable to regulate transepithelial ion and water fluxes.
Summary & Conclusions
Here we propose a model of cellular mechanisms by which vocal fold epithelium may contribute to maintaining vocal fold surface liquid homeostasis. The preliminary model outlines pathways for transcellular ion and water fluxes that may regulate the composition and depth of surface liquid in the face of challenges. For example, vocal fold epithelial cells may respond to dehydration through activation of transport proteins. Ionic and osmotic challenges to surface liquid as a result of vocal fold drying may induce increased transepithelial secretory ion and fluid fluxes to restore surface liquid homeostasis. The proposed model also provides a theoretical basis for understanding the changes in vocal fold surface liquid associated with clinical interventions. We posit that clinical protocols directed at facilitating vocal fold epithelial ion and water transport may benefit individuals who experience systemic and superficial dehydration. While the presence of functional ion and water transport proteins in vocal fold epithelial cells suggests a role for epithelial cells in regulating vocal fold hydration, other possible sources of hydration are recognized. Vocal fold hydration may also be regulated through paracellular ion-coupled water fluxes, laryngeal glandular secretions29 and mucociliary clearance.1 The relative contribution and mechanisms underlying these sources of vocal fold surface liquid await further study.
Acknowledgements
This work was supported by a grant from the National Institute of Deafness and Other Communication Disorders (K08-DC0068) awarded to the last author.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was presented in part at the 34th Annual Symposium of the Voice Foundation: Care of the Professional Voice, Philadelphia, PA, June2, 2005
Contributor Information
Ciara Leydon, Department of Speech Communication Arts and Sciences, Brooklyn College of The City University of New York.
Mahalakshmi Sivasankar, Department of Speech, Language, and Hearing Sciences, Purdue University.
Danielle Lodewyck Falciglia, Department of Otolaryngology, New York University School of Medicine.
Christopher Atkins, Department of Communication Sciences and Disorders, Northwestern University.
Kimberly V. Fisher, Department of Communication Sciences and Disorders, Northwestern University
References
- 1.Fukuda H, Kawaida M, Tatchara T, Ling E, Kita K, Ohki K, et al. A new concept of lubricating mechanisms of the larynx. In: Fujimara O, editor. Vocal Fold Physiology: Voice Production, Mechanisms, and Functions. New York, NY: Raven Press Ltd; 1988. pp. 83–92. [Google Scholar]
- 2.Mogi G, Watanabe N, Maeda S, Umehara T. Laryngeal secretions. An immunochemical and immunohistological study. Acta Otolaryngol. 1979;87:129–141. doi: 10.3109/00016487909126397. Otolaryngol Head Neck Surg. 2002;126:528-37. [DOI] [PubMed] [Google Scholar]
- 4.Hemler RJ, Wienke GH, Dejonckere PH. The effect of relative humidity of inhaled air on acoustic parameters of voice in normal subjects. J Voice. 1997;11:295–300. doi: 10.1016/s0892-1997(97)80007-0. [DOI] [PubMed] [Google Scholar]
- 5.Jiang J, Ng J, Hanson D. The effects of rehydration on phonation in excised canine larynges. J Voice. 1999;13:51–59. doi: 10.1016/s0892-1997(99)80061-7. [DOI] [PubMed] [Google Scholar]
- 6.Jiang J, Verdolini K, Ng J, Aquino B, Hanson D. Effects of dehydration on phonation in excised canine larynges. Ann Otol Rhinol Laryngol. 2000;190:568–575. doi: 10.1177/000348940010900607. [DOI] [PubMed] [Google Scholar]
- 7.Roy N, Tanner K, Gray S, Blomgren M, Fisher K. An evaluation of the effects of three laryngeal lubricants on phonation threshold pressure (PTP) J Voice. 2003;17:331–342. doi: 10.1067/s0892-1997(03)00078-x. [DOI] [PubMed] [Google Scholar]
- 8.Sivasankar M, Fisher KV. Oral breathing increases Pth and vocal effort by superficial drying of vocal fold mucosa. J Voice. 2002;16:172–181. doi: 10.1016/s0892-1997(02)00087-5. [DOI] [PubMed] [Google Scholar]
- 9.Sivasankar M, Fisher KV. Oral breathing challenges in participants with vocal attrition. J Speech Hear Res. 2003;46:1416–1427. doi: 10.1044/1092-4388(2003/110). [DOI] [PubMed] [Google Scholar]
- 10.Solomon N, DiMattia M. Effects of a vocally fatiguing task and systemic hydration on phonation threshold pressure. Journal of Voice. 2000;14:341–362. doi: 10.1016/s0892-1997(00)80080-6. [DOI] [PubMed] [Google Scholar]
- 11.Solomon NP, Glaze LE, Arnold RR, van Mersbergen M. Effects of a vocally fatiguing task and systemic hydration on men’s voices. J Voice. 2003;17:31–45. doi: 10.1016/s0892-1997(03)00029-8. [DOI] [PubMed] [Google Scholar]
- 12.Tanner K, Roy N, Merrill R, Elstad M. The effects of three nebulized osmotic agents in the dry larynx. J Speech Hear Res. 2007;50:635–646. doi: 10.1044/1092-4388(2007/045). [DOI] [PubMed] [Google Scholar]
- 13.Verdolini K, Titze IR, Druker DG. Changes in phonation threshold pressure with induced conditions of hydration. J Voice. 1990;4:142–151. [Google Scholar]
- 14.Verdolini K, Titze I, Fennel A. Dependence of phonatory effort on hydration level. Speech Hear Res. 1994;37:1001–1007. doi: 10.1044/jshr.3705.1001. [DOI] [PubMed] [Google Scholar]
- 15.Tarran R, Button B, Boucher RC. Regulation of normal and cystic fibrosis airway surface liquid by phasic shear stress. Annu Rev Physiol. 2006;68:543–561. doi: 10.1146/annurev.physiol.68.072304.112754. [DOI] [PubMed] [Google Scholar]
- 16.Fisher KV, Telser A, Phillips JE, Yeates DB. Regulation of vocal fold transepithelial water flux. J Appl Physiol. 2001;91:1401–1411. doi: 10.1152/jappl.2001.91.3.1401. [DOI] [PubMed] [Google Scholar]
- 17.Sataloff RT, Hawkshaw M, Rosen D. Medications: effects and side effects in professional voice users. In: Sataloff, editor. Professional Voice: The Science and Art of Clinical Care. San Diego, CA: Singular Publishing Group; 1997. pp. 457–470. [Google Scholar]
- 18.Boucher RC. Regulation of airway surface liquid volume by human airway epithelia. Pflugers Arch. 2003;445:495–498. doi: 10.1007/s00424-002-0955-1. [DOI] [PubMed] [Google Scholar]
- 19.Fisher KV, Lodewyck D, Menco B, Telser A, Yeates D. Sodium dependent transepithelial water fluxes of the vocal fold; Paper presented at: The International Conference on Voice Physiology and Biomechanics; September 2002; Denver, CO. [Google Scholar]
- 20.Leydon C. Communication sciences and disorders [Ph.D.] Evanston, IL: Northwestern University; 2005. Stimulating Chloride Ion Fluxes Across Vocal Fold Epithelium. [Google Scholar]
- 21.Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- 22.Canessa CM, Schild L, Buell G, Thorens I, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 23.Sivasankar M, Fisher KV. Vocal fold epithelial response to luminal osmotic perturbation. J Speech Lang Hear Res. 2007a;20:866–898. doi: 10.1044/1092-4388(2007/063). [DOI] [PubMed] [Google Scholar]
- 24.Lodewyck D, Menco BP, Fisher KV. Immunolocalization of aquaporins in vocal fold epithelia. Arch Otolaryngol Head Neck Surg. 2007;33:557–563. doi: 10.1001/archotol.133.6.557. [DOI] [PubMed] [Google Scholar]
- 25.Cotton CU, Lawson EE, Boucher RC, Gatzy JT. Bioelectric Properties and Ion Transport of Airways Excised from Adult and Fetal Sheep. J Appl Physiol. 1983;55:1542–1549. doi: 10.1152/jappl.1983.55.5.1542. [DOI] [PubMed] [Google Scholar]
- 26.Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. CFTR as a cAMP-Dependent Regulator of Sodium Channels. Science. 1995;269:847–855. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- 27.Boucher RC, Cotton CU, Gatzy JT, Knowles MR, Yankaskas JR. Evidence For Reduced Cl− and Increased Na+ Permeability in Cystic Fibrosis Primary Cell Cultures. J Physiol. 1988;405:77–103. doi: 10.1113/jphysiol.1988.sp017322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 29.Fisher KV, Ligon J, Sobecks JL, Roxe DM. Phonatory effects of body fluid removal. J Speech Lang Hear Res. 2001a;44:354–367. doi: 10.1044/1092-4388(2001/029). [DOI] [PubMed] [Google Scholar]
- 30.Spiegel JR, Hawkshaw M, Sataloff RT. Dysphonia related to medical treatment. Otolaryngol Clin North Am. 2000;33:771–784. doi: 10.1016/s0030-6665(05)70243-7. [DOI] [PubMed] [Google Scholar]
- 31.Verdolini K, Min Y, Titze I, Lemke JH, Brown K, VanMersbergen M, Jiang J, Fisher K. Biological mechanisms underlying voice changes due to dehydration. J Speech Lang Hear Res. 2002;45:268–282. doi: 10.1044/1092-4388(2002/021). [DOI] [PubMed] [Google Scholar]
- 32.Punt NA. Lubrication of the vocal mechanism. Folia Phoniatr. 1974;26:287–288. doi: 10.1159/000263787. [DOI] [PubMed] [Google Scholar]
- 33.Gracco C, Kahane J. Age-related changes in the vestibular folds of the human larynx: A histomotophometric study. J Voice. 1989;3:204–212. [Google Scholar]
- 34.Sato K, Hirano M. Age-related changes in the human laryngeal glands. Ann Otol Rhinol Laryngol. 1998;107:525–529. doi: 10.1177/000348949810700612. [DOI] [PubMed] [Google Scholar]
- 35.Hemler RJ, Wieneke GH, van Riel AM, Lebacq J, Dejonckere PH. A new method for measuring mechanical properties of laryngeal mucosa. Eur Arch Otorhinolaryngol. 2001;258:130–136. doi: 10.1007/s004050100320. [DOI] [PubMed] [Google Scholar]
- 36.Ayache S, Ouaknine M, Dejonckere P, Prindere P, Giovanni A. Experimental study of the effects of surface mucus viscosity on the glottic cycle. J Voice. 2004;18:107–115. doi: 10.1016/j.jvoice.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Chan R, Titze I. Viscoelastic shear properties of human vocal fold mucosa: Measurement methodology and empirical results. J Acoust Soc America. 1999;106:2008–2019. doi: 10.1121/1.427947. [DOI] [PubMed] [Google Scholar]
- 38.Titze I. Phonation threshold pressure: a missing link in glottal aerodynamics. J Acoust Soc America. 1992;91:2926–2935. doi: 10.1121/1.402928. [DOI] [PubMed] [Google Scholar]
- 39.Ayala KJ, Cruz KJ, Sivasankar M. Increased hydration is voice therapy: Is there support for its wide spread use? Texas J Speech-Language Pathology. 2007;1:47–57. [Google Scholar]
- 40.Finkelhor BK, Titze IR, Durham PL. The effects of viscosity changes in the vocal folds on the range of oscillation. J Voice. 1988;1:320–335. [Google Scholar]
- 41.Yiu E, Chan R. Effects of hydration and vocal rest on vocal fatigue in amateur singers. J Voice. 2003;17:216–227. doi: 10.1016/s0892-1997(03)00038-9. [DOI] [PubMed] [Google Scholar]
- 42.Verdolini-Marston K, Sandage M, Titze I. Effect of hydration treatment on laryngeal nodules and polyps and related voice measures. J Voice. 1994;8:30–47. doi: 10.1016/s0892-1997(05)80317-0. [DOI] [PubMed] [Google Scholar]
- 43.Vintturi J, Alku P, Sala E, Sihvo M, Vilkman E. Loading-related subjective symptoms during a vocal loading test with special reference to gender and some ergonomic factors. Folia Phoniatrica et Logopedica. 2003;55:55–69. doi: 10.1159/000070088. [DOI] [PubMed] [Google Scholar]
- 44.Boucher R, Stutts M, Bromberg P, Gatzy J. Regional differences in airway surface liquid composition. J Appl Physiol. 1981;50:610–620. doi: 10.1152/jappl.1981.50.3.613. [DOI] [PubMed] [Google Scholar]
- 45.Chen B, Yeates D. Ion transport and regulation of respiratory tract fluid output in dogs. J Appl Physiol. 2001;90:821–831. doi: 10.1152/jappl.2001.90.3.821. [DOI] [PubMed] [Google Scholar]
- 46.Freed A, Davis M. Hyperventilation with dry air increases airway surface fluid osmolality in canine peripheral airways. Am J Respir Crit Care Med. 1999;159:1101–1107. doi: 10.1164/ajrccm.159.4.9802072. [DOI] [PubMed] [Google Scholar]
- 47.Freed A, Omori C, Schofeild B, Mitzner W. Dry air induced mucosal cell injury and bronchovascular leakage in canine peripheral airways. Am J Respir Crit Care Med. 1994;11:724–732. doi: 10.1165/ajrcmb.11.6.7946400. [DOI] [PubMed] [Google Scholar]
- 48.Man SP, Adams GK, Proctor DF. Effect of temperature, relative humidity, and mode of breathing on canine airway secretion. J Appl Physiol. 1979;50:613–620. doi: 10.1152/jappl.1979.46.2.205. [DOI] [PubMed] [Google Scholar]
- 49.Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med. 2007;261:5–16. doi: 10.1111/j.1365-2796.2006.01744.x. [DOI] [PubMed] [Google Scholar]
- 50.Willumsen NJ, Boucher RC. Shunt resistance and ion permeabilities in normal and cystic fibrosis airway epithelia. Am J Physiol. 1989;256 doi: 10.1152/ajpcell.1989.256.5.C1054. C1054-C1053. [DOI] [PubMed] [Google Scholar]
- 51.Jayaraman S, Song Y, Verkman A. Airway surface liquid osmolality measures using fluorophore-encapsulated liposomes. J Gen Physiol. 2001;117:423–430. doi: 10.1085/jgp.117.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sivasankar M, Fisher KV. Vocal fold detect ionic perturbations on the luminal surface: an in vitro investigation. J Voice. 2007b doi: 10.1016/j.jvoice.2006.11.005. In press. [DOI] [PubMed] [Google Scholar]
- 53.Anderson JW, Sant’Ambrogio FB, Mathew OP, Sant’Ambrogio G. Water-responsive laryngeal receptors in the dog are not specialized endings. Respir Physiol. 1990;79:33–43. doi: 10.1016/0034-5687(90)90058-7. [DOI] [PubMed] [Google Scholar]
- 54.Kuna ST, Sant’Ambrogio FB, Sant’Ambrogio G. (). Effect of Airway Surface Liquid Composition on Laryngeal Muscle Activation. Sleep. 1996;19:S180–S183. doi: 10.1093/sleep/19.suppl_10.180. [DOI] [PubMed] [Google Scholar]
- 55.Sant’Ambrogio G, Anderson JW, Sant’Ambrogio FB, Mathew OP. Respir Med. 1991;85:S57–S60. doi: 10.1016/s0954-6111(06)80256-8. [DOI] [PubMed] [Google Scholar]
- 56.Gray SD, Hirano M, Sato M. Molecular and Cellular Structure of Vocal Fold Tissue. In: Titze IR, editor. Vocal Fold Physiology: Frontiers in Basic Science. San Diego, CA: Singular Publishing Group; 1993. pp. 1–23. [Google Scholar]
- 57.Relova AJ, Roomans GM. Effect of luminal osmolarity on ion content of connective tissue in rat trachea after epithelial damage. Eur Resp J. 2001;18:810–816. doi: 10.1183/09031936.01.00216201. [DOI] [PubMed] [Google Scholar]