Abstract
Objective
To determine the response of dysplasia, carcinoma in situ (CIS), and T1 carcinoma of the oral cavity and larynx to photodynamic therapy with porfimer sodium.
Design
Prospective trial.
Setting
A National Cancer Institute–designated cancer institute.
Patients
Patients with primary or recurrent moderate to severe oral or laryngeal dysplasia, CIS, or T1N0 carcinoma.
Intervention
Porfimer sodium, 2 mg/kg of body weight, was injected intravenously 48 hours before treatment. Light at 630 nm for photosensitizer activation was delivered from an argon laser or diode laser using lens or cylindrical diffuser fibers. The light dose was 50 J/cm2 for dysplasia and CIS and 75 J/cm2 for carcinoma.
Main Outcome Measures
Response was evaluated at 1 week and at 1 month and then at 3-month intervals thereafter. Response options were complete (CR), partial (PR), and no (NR) response. Posttreatment biopsies were performed in all patients with persistent and recurrent visible lesions.
Results
Thirty patients were enrolled, and 26 were evaluable. Mean follow-up was 15 months (range, 7–52 months). Twenty-four patients had a CR, 1 had a PR, and 1 had NR. Three patients with oral dysplasia with an initial CR experienced recurrence in the treatment field. All the patients with NR, a PR, or recurrence after an initial CR underwent salvage treatment. Temporary morbidities included edema, pain, hoarseness, and skin phototoxicity.
Conclusion
Photodynamic therapy with porfimer sodium is an effective treatment alternative, with no permanent sequelae, for oral and laryngeal dysplasia and early carcinoma.
T1 squamous cell carcinoma of the larynx and oral cavity may be treated effectively with single-modality therapy. Because radiotherapy and limited surgical resection yield excellent tumor control, the choice of therapy in early-stage laryngeal and oral cancers is often dictated by functional treatment outcomes, such as voice quality and swallowing function. The preferred treatment modality is surgery for early-stage oral cavity cancer1 and radiotherapy for early laryngeal cancer.2 However, irradiation and surgery may result in long-term morbidity. The limitation of surgical resection in the oral cavity and larynx is the necessity to remove vital functional tissue, such as part of the tongue in the oral cavity, which may affect speech and swallowing. Radiotherapy to the oral cavity often results in long-term morbidities, such as xerostomia, dysphagia, loss of dentition, and risk of osteoradionecrosis.3 Endolaryngeal laser surgery is also an effective treatment for early-stage laryngeal cancers, but it requires considerable expertise and technology.4 The reported long-term results of these treatment modalities are variable.4–7 An optimal treatment for moderate to severe dysplasia and early carcinomas of the oral cavity and larynx would be one that is safe, effective, repeatable, minimally invasive, and devoid of permanent sequelae. Photodynamic therapy (PDT), a nonsurgical, minimally invasive treatment that uses light of a specific wavelength to activate a photosensitizing agent in the tumor and its microenvironment, offers some of these advantages.
To date, PDT with porfimer sodium (Photofrin; Axcan Pharma Inc, Birmingham, Alabama) has been approved by the US Food and Drug Administration and other health agencies to treat early- and late-stage endobronchial tumors and superficial, minimally invasive esophageal adenocarcinomas and high-grade dysplasia associated with Barrett esophagus.8 In particular, the use of PDT for early carcinomas of the head and neck has been promising.9–12 We report on the use of PDT with porfimer sodium in the treatment of laryngeal and oral cavity dysplasia and carcinoma in situ (CIS) and T1 squamous cell carcinoma.
METHODS
This is a nonrandomized prospective clinical trial to determine the response to and toxic effects of porfimer sodium PDT in head and neck premalignant and malignant disease. This study was conducted for 53 months (March 1, 2004, to July 31, 2008) in patients attending the Head and Neck Service at Roswell Park Cancer Institute, Buffalo, New York, whose institutional review board approved all of the procedures; informed consent was obtained from all of the patients.
The inclusion criteria for the trial were as follows: (1) patients with moderate to severe dysplasia or squamous CIS of the oral cavity or larynx; (2) patients with stage I (T1N0) squamous cell carcinoma of the oral cavity or larynx; (3) diagnosis confirmed by biopsy; (4) previous therapy of any type; (5) male or female patients must be at least 18 years old, and female patients must not be pregnant and must be practicing a medically acceptable form of birth control, be sterile, or be post-menopausal; (6) patients must have an Eastern Cooperative Oncology Group scale score of 0 to 2; (7) patients must sign an informed consent form according to US Food and Drug Administration guidelines acceptable to the Roswell Park Cancer Institute institutional review board; and (8) patients must have recurrent lesions and T1 lesions. Regarding T1 carcinomas, only lesions less than 3 mm in depth were included based on pre-treatment biopsy and clinical examination findings.
We established certain exclusion criteria. They were as follows: (1) patients with stage II or more advanced squamous cell carcinoma; (2) patients with porphyria or hypersensitivity to porphyrin or porphyrinlike compounds; (3) a white blood cell count less than 4000/μL or a platelet count less than 100×103/μL; (4) patients with impaired hepatic function (total serum bilirubin level >2.0 mg/dL [to convert to micromoles per liter, multiply by 17.104]), minimally impaired renal function (serum creatinine level >2.0 mg/dL [to convert to micromoles per liter, multiply by 88.4]), or alkaline phosphatase (hepatic) or aspartate aminotransferase levels greater than 3 times the upper normal limits; and (5) patients undergoing concurrent chemotherapy or radiotherapy for whom it has been less than 4 weeks since the last dose of chemotherapy or radiotherapy.
Pretreatment patient evaluation included a history, general and head and neck physical examinations, routine laboratory evaluation, and tumor mapping with photographic documentation. Patients with laryngeal lesions underwent videolaryngoscopy documentation. The PDT treatment consisted of porfimer sodium, 2 mg/kg of body weight, injected intravenously 48 hours before light activation. Patients with laryngeal lesions underwent suspension microlaryngoscopy and light activation of the tumor field in the operating room. Oral lesions were treated in either the operating room or the clinic according to the treating surgeon’s discretion. Most anterior oral cavity lesions were treated in the clinic without any need for topical or local anesthesia. Photosensitizer activation was achieved using a light with a 630-nm wavelength derived from an argon-pumped dye laser or a diode laser and delivered to the involved site using lens or cylindrical diffuser fiberoptic fibers. Light doses consisted of 50 J/cm2 for dysplasia and CIS and 75 J/cm2 for carcinoma. The mean (SE) surface area treated was 7.03 (0.8) cm2 (median, 6.61 cm2; range, 0.79–19.63 cm2). Seven patients had more than 1 lesion treated per session. The mean number of lesions per patient treated per session was 1.77 (median, 1.0; range, 1–3). The lesions were not stripped or biopsied again before light therapy. No patient received a second light treatment within 72 hours of the first. All the patients were prescribed oral corticosteroids, topical lidocaine hydrochloride (Xylocaine) in oral cavity lesions, and oral narcotics for pain control after completion of the light activation treatment.
Disease response was evaluated by means of physical examination at 1 week and at 1 month and then at 3-month intervals thereafter. Using the reports from esophageal and lung PDT,13,14 treatment response was recorded as a complete response (CR), a partial response (PR), or no response (NR). A PR was defined as a reduction by 50% or more in the maximum size of the initial lesion. No response was defined as a response less than a PR. Progressive disease was defined as increasing size of the treated lesion. The decision to perform a posttreatment biopsy was made by the treating surgeon. Post-treatment biopsies were performed in all patients with persistent and recurrent visible lesions at least 3 months after PDT. All of the patients were cautioned regarding phototoxicity and were advised to avoid direct sunlight and to wear protective clothing when outdoors for 6 to 8 weeks after injection of the photosensitizer. At each follow-up visit, any adverse reactions, including pain, edema, dysphagia, skin phototoxic reaction, and other unexpected symptoms, were recorded.
RESULTS
Thirty patients were enrolled in the study, and 26 were evaluable at the time of this analysis. One patient withdrew from the study and 3 patients were lost to follow-up. The cohort included 19 men and 7 women. The demographic and histopathologic characteristics by site of the lesions are listed in Table 1. Twelve patients (46%) had either persistent or recurrent disease after previous surgery or radiotherapy. The remaining 14 patients had primary disease. Five of 6 patients with dysplasia had previous surgical excision that failed. There were 2 patient cohorts based on histopathologic features, one composed of moderate to severe dysplasias and CIS and the other exclusively of carcinomas. Both study groups included lesions of the larynx and oral cavity. Mean follow-up was 15 months (range, 7–52 months). The details of treatment response according to site and histopathologic features of the lesion are given in Table 2. Overall, 24 patients had a CR, 1 had a PR, and 1 did not respond to therapy. Three patients with oral dysplasia had recurrence in the treatment field within 90 days of achieving an initial CR. The patient with a primary T1 laryngeal cancer with NR to PDT also did not respond to radiotherapy and required total laryngectomy. After PDT, 1 patient developed a second (metachronous) invasive primary cancer outside the treatment field.
Table 1.
Demographic and Histopathologic Characteristics by Site
| Characteristic | Oral Cavity (n=20) | Larynx (n=6) |
|---|---|---|
| Age, mean (range), y | 61.2 (36–85) | 65.1 (60–73) |
| Sex, M/F, No. | 14/6 | 5/1 |
| Lesions, No. | ||
| Dysplasias and CIS | 9 | 3 |
| T1 carcinoma | 11 | 3 |
| Recurrent disease (n=12/26) | 11 | 1 |
Abbreviation: CIS, carcinoma in situ.
Table 2.
Response to PDT by Lesion Distribution and Site
| Lesion and Response | Oral Cavity (n=20) | Larynx (n=6) |
|---|---|---|
| Dysplasias, No. | ||
| CR | 9a | 3 |
| PR | 0 | 0 |
| NR | 0 | 0 |
| T1 carcinoma, No. | ||
| CR | 10b | 2 |
| PR | 1 | 0 |
| NR | 0 | 1 |
Abbreviations: CR, complete response; NR, no response; PDT, photodynamic therapy; PR, partial response.
Three patients had recurrence after a CR.
One patient developed a second primary cancer after a CR.
There was no difference in response rates between primary and recurrent disease at both treatment sites. Patients with NR, a PR, or recurrence after a CR were treated with surgery, laser excision, or radiotherapy according to the type of lesion, resulting in 100% salvage.
Morbidity data are summarized in Table 3. Transient local edema, pain, and phototoxic reaction were expected PDT sequelae (Figure 1). However, 2 patients had severe edema of the floor of the mouth that resolved within 2 weeks. One patient had a severe phototoxic reaction of the hands due to noncompliance (not wearing gloves while driving a long distance). All the patients who underwent laryngeal PDT experienced transient mild hoarseness. None of these patients experienced dysphagia or odynophagia after treatment. In contrast, almost all of the patients with oral cavity PDT had transient odynophagia and continued a full liquid diet for approximately 7 to 10 days after treatment. No patient in either cohort required airway intervention as a result of treatment. All complications resolved without any permanent sequelae (Figure 2).
Table 3.
Distribution of Morbidity by Site
| Morbidity | Oral Cavity (n=20) | Larynx (n=6) |
|---|---|---|
| Pain, No. | ||
| Mild | 15 | 1 |
| Moderate | 2 | 1 |
| Severe | 0 | 0 |
| Edema, No. | ||
| Mild | 14 | 0 |
| Moderate | 1 | 0 |
| Severe | 2 | 0 |
| Phototoxicity, No. | ||
| Mild | 7 | 3 |
| Moderate | 3 | 1 |
| Severe | 1 | 0 |
| Itching, No. | 2 | 0 |
| Weight loss, No. | 1 | 0 |
| Transient hoarseness, No.a | NA | 6 |
Abbreviation: NA, not applicable.
The hoarseness in laryngeal patients was transient. On follow-up, all the patients had significant improvement in voice quality compared with the pretreatment status.
Figure 1.
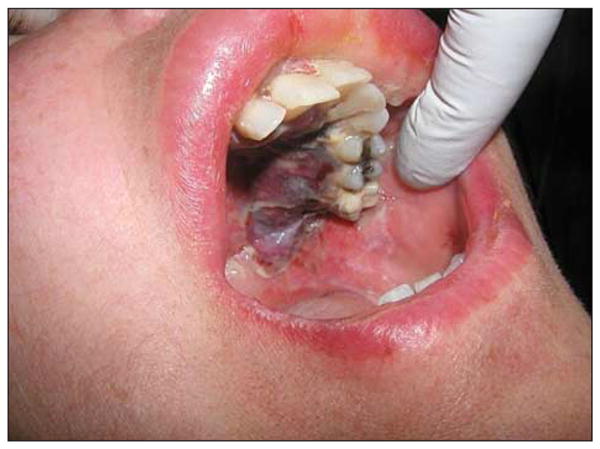
One week after photodynamic therapy for recurrent squamous cell carcinoma and osteoradionecrosis.
Figure 2.
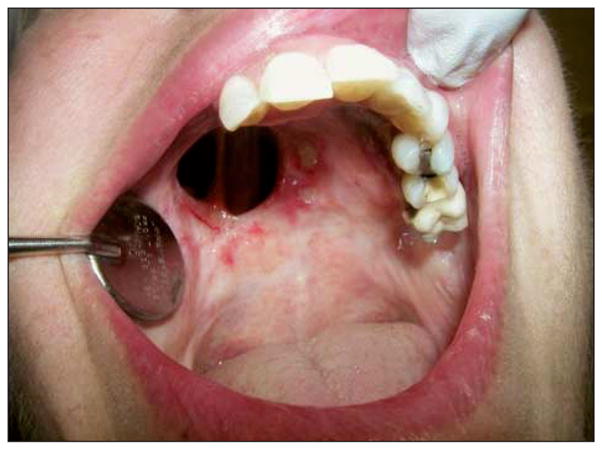
Six months after photodynamic therapy.
COMMENT
This study was performed to acquire experience in the use of PDT with porfimer sodium for the treatment of dysplasia, CIS, and early carcinoma of the larynx and oral cavity, an approach described as highly promising by Biel.9,10,12 The present prospective trial included 2 cohorts, the first consisting of moderate to severe dysplasias and CIS and the second of T1 carcinomas. Both cohorts included patients with oral and laryngeal primary and recurrent disease.
Current accepted treatments for dysplasia and stage I laryngeal carcinoma include endolaryngeal laser or cold instrument excision, open partial laryngectomy, and radiotherapy.4,15 To our knowledge, no prospective randomized trials compare the outcomes of these treatment modalities. A retrospective review by Mendenhall et al16 concluded that local control, laryngeal preservation, and survival rates in patients were similar after transoral laser resection, open partial laryngectomy, and radiotherapy for early-stage laryngeal cancer. In a recent meta-analysis on the management of laryngeal dysplasias, Sadri et al17 reported a superior local control rate with radiotherapy compared with other standard methods of dysplasia management. Generally, voice quality depends on the extent of surgical laryngeal resection. Results for patients undergoing laser resection for limited glottic lesions have been comparable with those of patients receiving radiotherapy, whereas open partial laryngectomy consistently yielded poorer voice quality.16 Radiotherapy has the advantage of preserving the anatomical integrity of the larynx, resulting in relatively good voice quality. However, there are constraints in repeating radiotherapy in the event of a recurrence or a second primary tumor. Peeters et al18 reported on health status and voice quality outcomes after treatment for T1a glottic carcinoma with either endoscopic laser surgery or radiotherapy. Fifty-eight percent of patients who underwent radiotherapy and 40% of patients who had endoscopic excision had abnormal Voice Handicap Index scores.18
Photodynamic therapy has been used in head and neck cancer with either porfimer sodium or certain other photosensitizers with favorable outcomes.9–12,19–22 Biel12 published the results of the largest cohort treated with porfimer sodium PDT. Of 115 patients with T1and T2 laryngeal cancers, there was a CR in 105 (91.3%) after a single treatment. Although post-PDT voice quality was evaluated subjectively in Biel’s laryngeal cancer cohort, it was reported to be excellent in most patients.12 Porfimer sodium PDT has also been used successfully to treat oral cavity carcinoma. The most robust series12 reports on 113 patients with early carcinomas (Tis, T1, and T2) of the oral cavity and 48 patients with superficial T2 and T3 carcinomas of the oral cavity. The maximum depth of these tumors was clinically less than 1 cm. With mean follow-up of 90 months, 106 of 113 patients (93.8%) with Tis, T1, and T2 carcinomas remained free of disease. Forty-three of 48 patients (89.6%) with superficial T2 and T3 oral carcinomas remained free of disease after a single PDT treatment, with follow-up ranging from 6 to 28 months. Two patients had recurrence at the margins of the treatment field and underwent salvage with laser excision.12
In this study, 6 patients had laryngeal pathologic abnormalities. All 3 laryngeal dysplasias exhibited a sustained CR. Two of 3 T1 laryngeal carcinomas had a CR. One patient with NR had a primary glottic cancer that progressed locally during radiotherapy and that was salvaged by means of total laryngectomy. This limited number of laryngeal patients does not allow for significant comparison with studies that contain more patients. However, the CR exhibited by all 3 patients with dysplasia is encouraging. No airway was compromised by the use of PDT, and all of the patients subjectively reported voice quality improvement compared with their pretreatment status.
The main advantage of PDT for dysplasia and early carcinoma of the larynx is the ability to preserve normal endolaryngeal tissue while effectively treating the lesion. This results in preservation of laryngeal function and voice quality.9 Furthermore, PDT may be performed in an outpatient setting using a single noninvasive light activation treatment, requiring a short duration of therapy. For selected recurrent carcinomas of the larynx that have failed conventional radiotherapy, PDT allows for voice preservation and may eliminate the need for salvage surgery. Importantly, PDT can be repeated without the additional permanent functional laryngeal impairment that can occur with repeated conventional laser surgery or cordectomy. Finally, PDT for dysplasia and CIS and primary T1 laryngeal carcinomas reserves radiotherapy for the treatment of recurrences and second head and neck primary cancers that may occur in this high-risk patient population.23
Dysplasias of the oral cavity are usually multicentric, and field cancerization has a role in the pathogenesis of this disease entity. Disease management remains controversial, polarized between active surgical excision to try to prevent malignant change and more conservative medical or observational techniques.6 Thomson and Wylie7 described the effectiveness of laser surgery in the management of these lesions. Early squamous cell carcinoma of the oral cavity can be treated by means of surgical or laser excision with good local control rates.1 Treatment disadvantages include limitations of speech and swallowing function depending on the extent of tissue resection. Radiotherapy in the form of external beam therapy or brachytherapy is also a current treatment option. External beam radiotherapy causes long-term problems, such as xerostomia, dental decay, dysphagia, and, occasionally, osteoradionecrosis. Although radiotherapy cure rates for early-stage disease in the oral cavity are excellent, the long-term sequelae have a substantially negative influence on quality of life.3
The present study is the first, to our knowledge, to include the PDT treatment of oral cavity dysplasias. In these patients, we observed 3 recurrences in the treatment field early after an initial clinical CR. This result is counterintuitive given the limited tissue depth of these lesions, and it merits further study. Possible causes of these recurrences include poor photosensitizer uptake by dysplastic tissues and mechanical blockage or reflectance of light delivery by the hyperkeratosis overlying the lesion.
In the present study, 10 of 11 oral cavity primary and recurrent carcinomas maintained a CR, and 1 had a PR. Notably, this series included recurrent lesions. Six of 9 oral cancers and 6 of 11 oral dysplasias and CIS were previously treated. These recurrent lesions had been treated multiple times with surgery and radiotherapy. In fact, all patients with radiotherapy failure and osteoradionecrosis in the treatment field exhibited a CR with PDT. Standard therapy for recurrent oral cancer in this patient population would have included extensive ablative surgery.
Overall, PDT has several advantages over traditional head and neck cancer treatments, such as surgical resection and radiotherapy. Patients have fewer, if any, long-term adverse effects, such as impaired swallowing and speech. Treatment with PDT is noninvasive, can be provided in an outpatient setting, and, with appropriate shielding of normal tissues, can be precisely targeted. Limited light penetration protects tissue immediately below and adjacent to tumors from phototoxic effects. Also, PDT spares the tissue architecture, providing a matrix for regeneration of normal tissue by leaving subepithelial collagen and elastin intact, and spares noncellular supporting elements.24,25 A further important positive aspect of PDT is that it can be repeated. This attribute is significant because patients with head and neck cancer have an increased lifelong rate of development of second primary cancers in the upper aerodigestive tract. The reported annual rate of second primary cancers varies between 3% and 10%.23 Use of PDT does not preclude other treatment modalities, such as radiotherapy, surgery, and chemotherapy, whether they are administered before, combined with, or given after PDT. Also note that primary and recurrent disease in the larynx and oral cavity resulted in similar and favorable response rates in these small patient cohorts.
The major general adverse effect of porfimer sodium PDT is prolonged photosensitivity, which makes strict avoidance of direct sunlight imperative. Incidences of mild to moderate skin phototoxic reactions in this study were frequent despite intensive patient education, with 1 severe phototoxic reaction due to noncompliance. Another PDT sequela was moderate to severe pain if the treatment field was in the oral cavity. Transient hoarseness was experienced by all patients who received laryngeal PDT. However, there were no long-term complications, such as xerostomia, chronic dental decay, dysphagia, and worsening of preexisting osteoradionecrosis. Voice quality improved in all patients with laryngeal lesions compared with their pretreatment status.
In conclusion, PDT with porfimer sodium is an effective alternative to the conventional modalities of treatment in dysplasias, CIS, and early carcinomas of the larynx and the oral cavity. Oral cavity dysplasias seemed to respond less well than did T1 carcinomas. Morbidity included transient treatment field edema, pain, and hoarseness. Phototoxic reactions were problematic in noncompliant patients.
Acknowledgments
Funding/Support: Photofrin was provided free by Axcan Pharma.
Footnotes
Trial Registration: clinicaltrials.gov Identifier: NCT00530088
Financial Disclosure: None reported.
Previous Presentation: This work was presented at the Seventh International Conference on Head and Neck Cancer; July 19, 2008; San Francisco, California.
Additional Contributions: David A. Bellnier, PhD, assisted in the preparation of the manuscript.
Author Contributions: Dr Rigual had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Rigual, Dougherty, and Biel. Acquisition of data: Rigual, Cooper, Sullivan, Popat, and Loree. Analysis and interpretation of data: Rigual, Thankappan, Cooper, Dougherty, and Henderson. Drafting of the manuscript: Rigual, Thankappan, Cooper, and Henderson. Critical revision of the manuscript for important intellectual content: Rigual, Thankappan, Sullivan, Dougherty, Popat, Loree, Biel, and Henderson. Statistical analysis: Cooper. Obtained funding: Sullivan. Administrative, technical, and material support: Rigual, Thankappan, Cooper, Sullivan, Dougherty, Popat, Loree, and Henderson. Study supervision: Rigual, Dougherty, and Henderson.
References
- 1.Shah JP, Gil Z. Current concepts in management of oral cancer: surgery. Oral Oncol. 2009;45(4–5):394–401. doi: 10.1016/j.oraloncology.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hinerman RW, Mendenhall WM, Amdur RJ, Villaret DB, Robbins KT. Early laryngeal cancer. Curr Treat Options Oncol. 2002;3(1):3–9. doi: 10.1007/s11864-002-0036-x. [DOI] [PubMed] [Google Scholar]
- 3.Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14(3):199–212. doi: 10.1177/154411130301400305. [DOI] [PubMed] [Google Scholar]
- 4.Steiner W. Results of curative laser microsurgery of laryngeal carcinomas. Am J Otolaryngol. 1993;14(2):116–121. doi: 10.1016/0196-0709(93)90050-h. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Serra A, Hinerman RW, Amdur RJ, Morris CG, Mendenhall WM. Radiotherapy for carcinoma in situ of the true vocal cords. Head Neck. 2002;24(4):390–394. doi: 10.1002/hed.10071. [DOI] [PubMed] [Google Scholar]
- 6.Vedtofte P, Holmstrup P, Hjørting-Hansen E, Pindborg JJ. Surgical treatment of premalignant lesions of the oral mucosa. Int J Oral Maxillofac Surg. 1987;16 (6):656–664. doi: 10.1016/s0901-5027(87)80049-8. [DOI] [PubMed] [Google Scholar]
- 7.Thomson PJ, Wylie J. Interventional laser surgery: an effective surgical and diagnostic tool in oral precancer management. Int J Oral Maxillofac Surg. 2002;31(2):145–153. doi: 10.1054/ijom.2001.0189. [DOI] [PubMed] [Google Scholar]
- 8.Dougherty TJ. An update on photodynamic therapy applications. J Clin Laser Med Surg. 2002;20(1):3–7. doi: 10.1089/104454702753474931. [DOI] [PubMed] [Google Scholar]
- 9.Biel MA. Photodynamic therapy and the treatment of neoplastic diseases of the larynx. Laryngoscope. 1994;104(4):399–403. doi: 10.1288/00005537-199404000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Biel MA. Photodynamic therapy and the treatment of head and neck neoplasia. Laryngoscope. 1998;108(9):1259–1268. doi: 10.1097/00005537-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Kulapaditharom B, Boonkitticharoen V. Photodynamic therapy in management of head and neck cancers and precancerous lesions. J Med Assoc Thai. 2000;83(3):249–258. [PubMed] [Google Scholar]
- 12.Biel MA. Photodynamic therapy treatment of early oral and laryngeal cancers. Photochem Photobiol. 2007;83(5):1063–1068. doi: 10.1111/j.1751-1097.2007.00153.x. [DOI] [PubMed] [Google Scholar]
- 13.Cortese DA, Edell ES, Kinsey JH. Photodynamic therapy for early stage squamous cell carcinoma of the lung. Mayo Clin Proc. 1997;72(7):595–602. doi: 10.1016/S0025-6196(11)63563-5. [DOI] [PubMed] [Google Scholar]
- 14.McCaughan JS, Jr, Ellison EC, Guy JT, et al. Photodynamic therapy for esophageal malignancy: a prospective twelve-year study. Ann Thorac Surg. 1996;62(4):1005–1009. doi: 10.1016/0003-4975(96)00563-2. [DOI] [PubMed] [Google Scholar]
- 15.Minni A, Barbaro M, Rispoli G, Diaferia F, Bernardeschi D, Filipo R. Treatment with laser CO2 cordectomy and clinical implications in management of mild and moderate laryngeal precancerosis. Eur Arch Otorhinolaryngol. 2008;265(2):189–193. doi: 10.1007/s00405-007-0480-0. [DOI] [PubMed] [Google Scholar]
- 16.Mendenhall WM, Werning JW, Hinerman RW, Amdur RJ, Villaret DB. Management of T1-T2 glottic carcinomas. Cancer. 2004;100(9):1786–1792. doi: 10.1002/cncr.20181. [DOI] [PubMed] [Google Scholar]
- 17.Sadri M, McMahon J, Parker A. Management of laryngeal dysplasia: a review. Eur Arch Otorhinolaryngol. 2006;263(9):843–852. doi: 10.1007/s00405-006-0078-y. [DOI] [PubMed] [Google Scholar]
- 18.Peeters AJ, van Gogh CD, Goor KM, Verdonckde Leeuw IM, Langendijk JA, Mahieu HF. Health status and voice outcome after treatment for T1a glottic carcinoma. Eur Arch Otorhinolaryngol. 2004;261(10):534–540. doi: 10.1007/s00405-003-0697-5. [DOI] [PubMed] [Google Scholar]
- 19.Wenig BL, Kurtzman DM, Grossweiner L, et al. Photodynamic therapy in the treatment of squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1990;116(11):1267–1270. doi: 10.1001/archotol.1990.01870110039003. [DOI] [PubMed] [Google Scholar]
- 20.Freche C, DeCorbiere S. Use of photodynamic therapy in the treatment of vocal cord carcinoma. J Photochem Photobiol B. 1990;6(3):291–296. doi: 10.1016/1011-1344(90)85099-i. [DOI] [PubMed] [Google Scholar]
- 21.Zhao SP, Tao ZD, Xiao JY, et al. Clinical use of hematoporphyrin derivative and photoradiation therapy in nasopharyngeal carcinoma. Chin Med J (Engl) 1988;101(2):86–91. [PubMed] [Google Scholar]
- 22.Gayl Schweitzer V. Photofrin-mediated photodynamic therapy for treatment of aggressive head and neck nonmelanomatous skin tumors in elderly patients. Laryngoscope. 2001;111(6):1091–1098. doi: 10.1097/00005537-200106000-00030. [DOI] [PubMed] [Google Scholar]
- 23.Hong WK, Lippman SM, Itri LM, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990;323(12):795–801. doi: 10.1056/NEJM199009203231205. [DOI] [PubMed] [Google Scholar]
- 24.Grant WE, Speight PM, Hopper C, Bown SG. Photodynamic therapy: an effective, but non-selective treatment for superficial cancers of the oral cavity. Int J Cancer. 1997;71(6):937–942. doi: 10.1002/(sici)1097-0215(19970611)71:6<937::aid-ijc4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 25.Triesscheijn M, Baas P, Schellens JH, Stewart FA. Photodynamic therapy in oncology. Oncologist. 2006;11(9):1034–1044. doi: 10.1634/theoncologist.11-9-1034. [DOI] [PubMed] [Google Scholar]


