Abstract
CD44 plays an important role in inflammation and healing. Previous studies have investigated its role in inflammatory diseases and skin wounds; however, the role of CD44 in tendon healing is unknown. Therefore, the aim of this study was to investigate the effect of CD44 in the healing of the patellar tendon in a knockout mouse model. It was hypothesized that in comparison to wild type counterparts, CD44 knockout mice would have decreased material parameters, increased organization, decreased expression of proinflammatory cytokines and increased expression of matrix components during healing. These hypotheses were tested through an in vivo surgical model and mechanical, organizational, and gene expression analyses. Material strength and tissue organization were significantly improved in the CD44 knockout mouse. This could be attributed to increased expression of cytokines and matrix components that are elevated in regenerative healing. This study shows that the absence of CD44 in a mouse patellar tendon injury creates an environment that is conducive to regenerative healing through altered gene expression, resulting in superior material properties and reduced cross-sectional area. Therefore, this study indicates that limiting the role of CD44 may improve healing parameters in adult tendon injury.
Keywords: CD44, tendon, healing, mechanics, hyaluronic acid, scar formation
INTRODUCTION
Tendon injuries affect a large number of individuals and account for enormous associated costs1. The number of tendon injuries will continue to increase as our population continues to age and remain active. A complex cascade of events occurs during healing which results in a fibrotic scar. Scar tissue is characterized by altered structure and composition and decreased tendon function. Despite advances in surgical techniques and postoperative rehabilitation protocols, adhesion formation, reruptures and decreased function due to scaring are still common problems; therefore, decreasing the formation of the fibrotic scar may lead to improved healing properties.
CD44, a polymorphic type I transmembrane glycoprotein, is a key mediator during fibrotic healing. CD44 is involved in cell-cell and cell-matrix interactions, leukocyte homing and activation, cellular migration, extracellular matrix assembly and cytokine binding and activation2, 3. However, its main role is mediating the uptake and clearance of hyaluronic acid (HA)4. HA is a glycosaminoglycan that is abundant in tendons and is associated with embryogenesis, inflammation and healing5–7. During adult tendon healing, CD44 and HA levels are both elevated8, 9. However, during scarless regenerative fetal healing, CD44 expression is down regulated8 and HA levels surpass those of healing adult tendons10, 11. Studies attempting to recreate regenerative wound environments have demonstrated that increased levels of HA are indeed beneficial12. Therefore, since HA is degraded primarily through CD44, strategies to decrease CD44 expression or activation may reduce scar formation.
A CD44 knockout mouse has been created to study the effects of a complete absence of CD4413, 14. These mice had decreased macrophage recruitment and decreased expression of proinflammatory cytokines at the site of lung injury15. When examining inflammatory disease, CD44 knockout mice had greater HA accumulation when compared to their wild type counterparts16; however, increased signaling through RHAMM, another HA receptor, may have exacerbated inflammation in this disease model17. Lastly, the absence of CD44 in skin wounds delayed early wound closure18. These studies collectively demonstrate that CD44 plays a crucial role in wound healing and inflammation; however, to date, the role of CD44 in tendon healing has not been evaluated.
The purpose of this study was to investigate the effect of CD44 in the healing of the patellar tendon. We hypothesize that CD44 knockout mice14 will have decreased material properties and increased organization of the healing patellar tendon. In addition, we hypothesize that proinflammatory cytokines will be down regulated and extracellular matrix proteins will be up regulated during early stages of healing.
MATERIALS AND METHODS
Specimens
This study was approved by the University of Pennsylvania IACUC. Forty two male mice from both C57Bl/6 wild type (WT) and CD44 knockout (KO) in a C57Bl/6 background were included in this study. Thirty two mice from each group underwent surgery at 12 weeks of age using an established tendon injury model in our laboratory as previously described19. Briefly, mice were prepared under normal sterile conditions and both hind limbs were shaved. Following a skin incision, two cuts parallel to the tendon were made in the retinaculum on each side, and a plastic coated blade was then placed underneath the patellar tendon. With the coated blade serving as support, a 0.75mm diameter biopsy punch (Shoney Scientific, Waukesha, WI) was used to create a full thickness partial transection in the patellar tendon (Figure 1). Marginal fibers were left intact, circumventing the need for suture repair. An injury was created in each leg. Skin wounds were closed with suture and the mice were allowed to resume normal cage activity until they were sacrificed at 1 day (n=4), 3 days (n=4), 7 days (n=4), 3 weeks (n=10), and 6 weeks (n=10) post surgery. An additional ten mice per genotype were sacrificed without injury to serve as controls.
Figure 1.
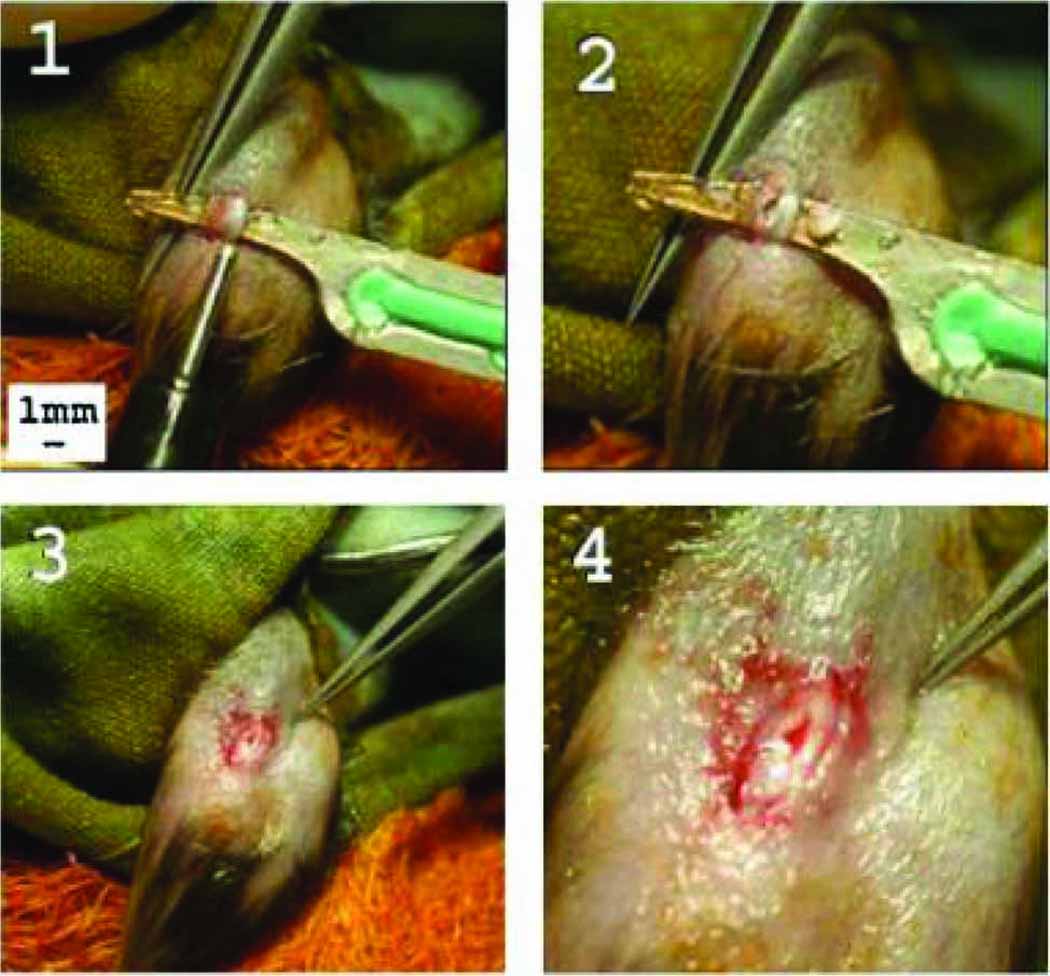
Photos that depict the partial transection created in the mouse patellar tendon. A plastic coated backing is placed underneath the tendon to provide support (Fig. 1(1)), and a biopsy punch is used to create a full thickness partial transection. The backing is removed, leaving a distinct and reproducible injury (Figs. 1(2)).
Biomechanical Analysis
To determine the mechanical properties of the healing patellar tendon, one patellar tendon from each of twenty-six mice of each genotype was used. Ten mice were sacrificed at both 3 and 6 weeks post injury while six uninjured mice were sacrificed to serve as controls. As described previously19, the patellar tendons were dissected and cleaned, leaving only the patella, patellar tendon, and tibia as one unit. Tendon width and thickness were then quantified, and cross-sectional area was measured using a custom built device consisting of LVDTs, a CCD laser, and translation stages20. Each tendon was dumbbell stamped to remove the uninjured fibers on either side of the injury. The cross-sectional area was then measured again for material property calculations. The tibia was then embedded in polymethylmethacrylate in a custom-designed fixture. The patella was held in place with a custom-designed cone-shaped fixture. Each tendon specimen underwent the following protocol while immersed in a 37°C saline bath—preloaded to 0.02 N, preconditioned for 10 cycles from 0.02 to 0.04N at a rate of 0.1%/s (0.003 mm/s), and held for 300 s. Immediately following, a stress relaxation experiment was performed by elongating the tendon to a strain of 5% (0.15 mm) at a rate of 5%/s (0.15 mm/s), followed by a relaxation for 600s. The tendon was returned to pre-stress relaxation elongation (−0.15mm) for 60s and finally, a ramp to failure was applied at a rate of 0.1%/s (0.003 mm/s). Local tissue strain was measured optically as described previously21. From these tests, maximum stress and failure strain were determined, modulus was calculated using linear regression from the near-linear region of the stress–strain curve and strain energy density was calculated as the area under the stress-strain curve until failure. Failure strain was defined as the strain at the point of maximum stress.
Histological Analysis
To determine the organization of the healing patellar tendon, the contralateral legs used in the biomechanical analysis were evaluated using a quantitative polarized light microscopy method as described previously22. Briefly, the hind limb was disarticulated, the skin dissected off, and processed with standard histological techniques. 7 µm sagital sections were cut parallel to the tendon fibers, stained with hematoxylin and eosin, and analyzed using a quantitative polarized light microscopy method. Using circular statistics (Oriana, Kovach Computing Services, Wales, UK), the angular deviation, a measure of collagen fiber orientation, was determined.
Gene Expression
To determine the expression of matrix components and cytokines (Table 1), one patellar tendon from each of sixteen mice of each genotype was used. Four mice were sacrificed at each of 1, 3 and 7 days post injury while four uninjured mice were sacrificed to serve as controls. Individual patellar tendons were mechanically homogenized with a mortar and pestle under liquid nitrogen and RNase-free conditions. RNA was extracted using the TRIZOL isolation system (Invitrogen, Carlsbad, CA) and RNeasy Mini Kit (Qiagene Inc., Valencia, CA) as described by the manufacturers. cDNA was produced by reverse transcription-polymerase chain reaction (RT-PCR) from 100 ng of total RNA using the Superscript first-strand synthesis system for RT-PCR as described by the manufacturer (Invitrogen). 1 µL aliquots of the cDNA were amplified by real time quantitative polymerase chain reaction (Q-PCR) in a 25 µL reaction volume in a ABI Prism 7300 Sequence Detection System (Perkin-Elmer; Applied Biosystems, Warrington, UK) with a SYBR Green PCR Master Mix (Applied Biosystems). Mouse specific primers were used for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), collagen type I (COL1A2), collagen type III (COL3A1), decorin (DEC), biglycan (BIG), hyaluronan synthase 2 (HAS2), interleukin-1 beta (IL1β), transforming growth factor beta 1 (TGFβ1), transforming growth factor beta 3 (TGFβ3), platelet derived growth factor B (PDGFB) and basic fibroblast growth factor (bFGF) (Table 2). Each replicate was performed in triplicate. The relative quantity and range of mRNA for each gene of interest was computed using the comparative 2−ΔΔCT method (ABI; Sequence Detection System User Bulletin #2) relative first to GAPDH within each specimen and then to the uninjured tendons within each genotype.
Table 1.
Brief description of the growth factors and matrix components examined.
| Cytokine | Function |
|---|---|
| bFGF | Cytokine that stimulates angiogenesis, cellular migration and proliferation |
| IL1β | Cytokine that initiates multiple proinflammatory cascades |
| PDGFB | Cytokine that influences extracellular matrix remodeling and DNA synthesis |
| TGFβ1 | Cytokine that plays a role in the initial inflammatory response and thought to increase fibrosis |
| TGFβ3 | Cytokine involved in inflammation and is increased in scarless healing |
| COL1A2 | Fibrillar collagen that is the main component of tendon |
| COL3A1 | Fibrillar collagen involved in development and healing |
| DEC | Proteoglycan that regulates lateral fusion of fibrils |
| BIG | Proteoglycan that promotes fibril diameter growth |
| HAS2 | Main synthase of hyaluronic acid |
| GAPDH | Housekeeping gene |
Table 2.
Q-PCR Primers. Forward and reverse primers and the base pair length of the amplicon (BP) used in Q-PCR.
| Gene | Forward Primer | Reverse Primer | BP |
|---|---|---|---|
| bFGF | AGCGACCCACACGTCAAACT | CGTCCATCTTCCTTCATAGCAA | 103 |
| BIG | CCTTCCGCTGCGTTACTGA | GCAACCACTGCCTCTACTTCTTATAA | 68 |
| COL1A2 | AGGCTGACACGAACTGAGGT | ATGCACATCAATGTGGAGGA | 137 |
| COL3A1 | CCCTGGTCCACAAGGATTACA | CTCCAGGTGCACCAGAATCA | 142 |
| DEC | GCTGCGGAAATCCGACTTC | TTGCCGCCCAGTTCTATGAC | 139 |
| GAPDH | CTCGTCCCGRAGACAAAATGG | GTGACCAGGCGCCCAATA | 66 |
| HAS2 | CATTCCCAGAGG ACC GCT TAT | AAGACCCTATGGTTGGAGGTGTT | 166 |
| IL1β | TTGACGGACCCCAAAAGATG | TGGACAGCCCAGGTCAAAG | 54 |
| PDGFB | CGCCTGCAAGTGTGAGACAAT | CGAATGGTCACCCGAGCTT | 104 |
| TGFβ1 | TCGACATGGAGCTGGTGAAA | GAGCCTTAGTTTGGACAGGATCTG | 70 |
| TGFβ3 | GTCCTCAGTGGAGAAAAATGGAA | TTGGGCACCCGCAAGA | 65 |
Statistical Analysis
Prior to initiating the study, a power analysis was performed to ensure sufficient power for the experiments proposed (80% power, with p<0.05). All material, structural and organizational parameters were statistically analyzed by 2-way ANOVA across time post injury and genotype. An LSD post-hoc test was used to evaluate differences across time and between genotypes. Statistical significance was set at p ≤0.05; and a trend was defined at p ≤ 0.1.
RESULTS
Biomechanical Analysis
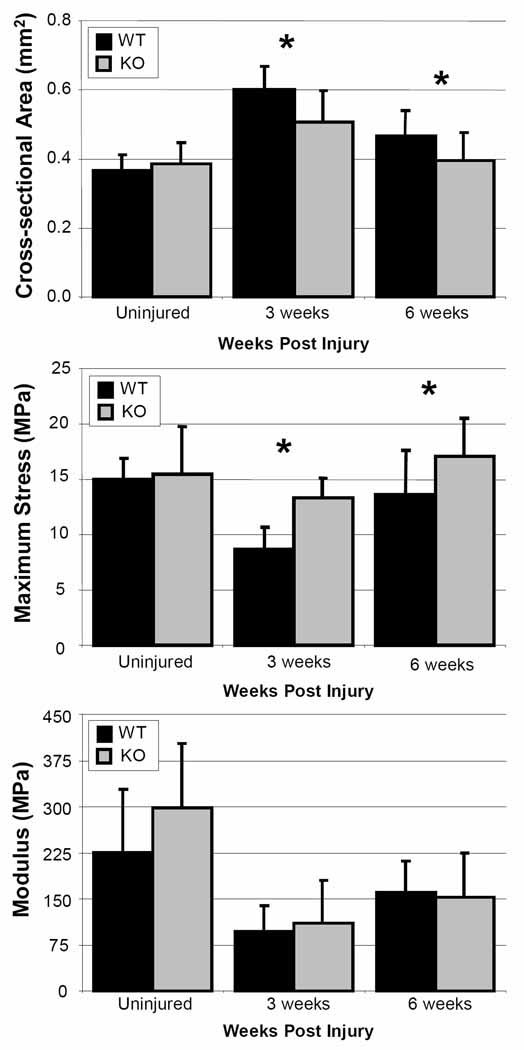
There were no differences among groups based on gross inspection at any time point post surgery. In addition, no significant difference in cross sectional area was found across genotype in the uninjured tendons (Figure 2). However, the cross-sectional area of the CD44 knockout tendons was significantly reduced at three (p = 0.008) and six weeks (p = 0.05) post injury when compared to wild type. Similarly, no significant differences across genotype were seen in maximum stress, failure strain or strain energy density in the uninjured tendons (Figure 3); however, the maximum stress in the knockout animals was significantly greater than in the wild type at three (p = 0.003) and six (p = 0.02) weeks post injury. The strain energy density was also significantly increased at three (p = 0.02) and six (p = 0.03) weeks post injury. The knockout animals demonstrated a trend toward increased failure strain at both three (p = 0.1) and six (p = 0.09) weeks post injury. No differences across genotype were observed for modulus.
Figure 2.
Cross-sectional area compared across genotype. Cross-sectional area was significantly reduced in knockouts (KO) compared to wild types (WT) at both 3 and 6 weeks post injury.
Figure 3.
Material properties compared across genotype. Maximum stress and strain energy density were significantly increased in knockouts (KO) compared to wild type (WT) at both 3 and 6 weeks post injury. Similarly, knockout animals demonstrated at trend toward greater failure strain compared to wildtype. No significant changes were seen across genotype in modulus. *: p ≤ 0.05; #: p ≤ 0.1.
Histological Analysis
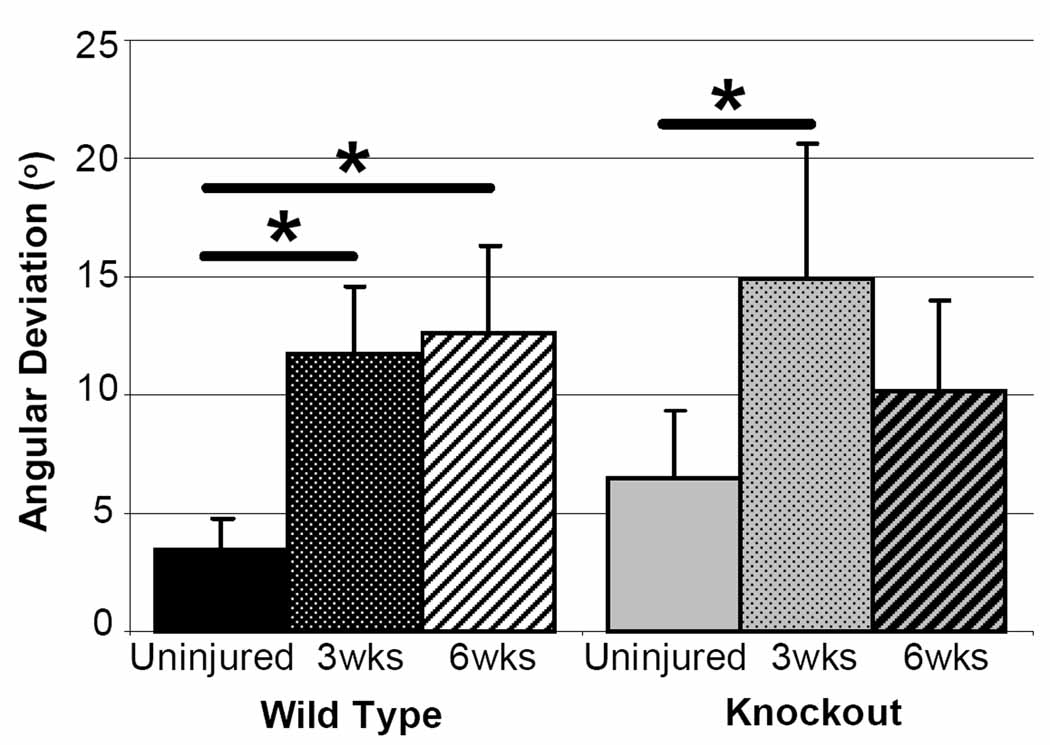
Qualitative observations of hematoxylin and eosin stained sections showed no differences across genotype at any time point. Injured tendons in both genotypes and at both time points post injury had increased cellularity and an obvious injury site when compared to uninjured tendons. No difference in angular deviation between CD44 knockout and wild type tendons was seen at any time point when comparing across genotype. When examining across time, in contrast to comparing knockout to wild type, angular deviation showed an appreciable difference. The wild type tendons remained disorganized up to 6 weeks post injury; however, the knockout tendons approached uninjured values at 6 weeks post injury (Figure 4).
Figure 4.
Angular deviation compared across time. Appreciable differences were seen in the knockout (KO) tendons across time. While the wild type (WT) tendons remain disorganized, the KO tendons approach uninjured values at 6 weeks post injury.
Gene Expression
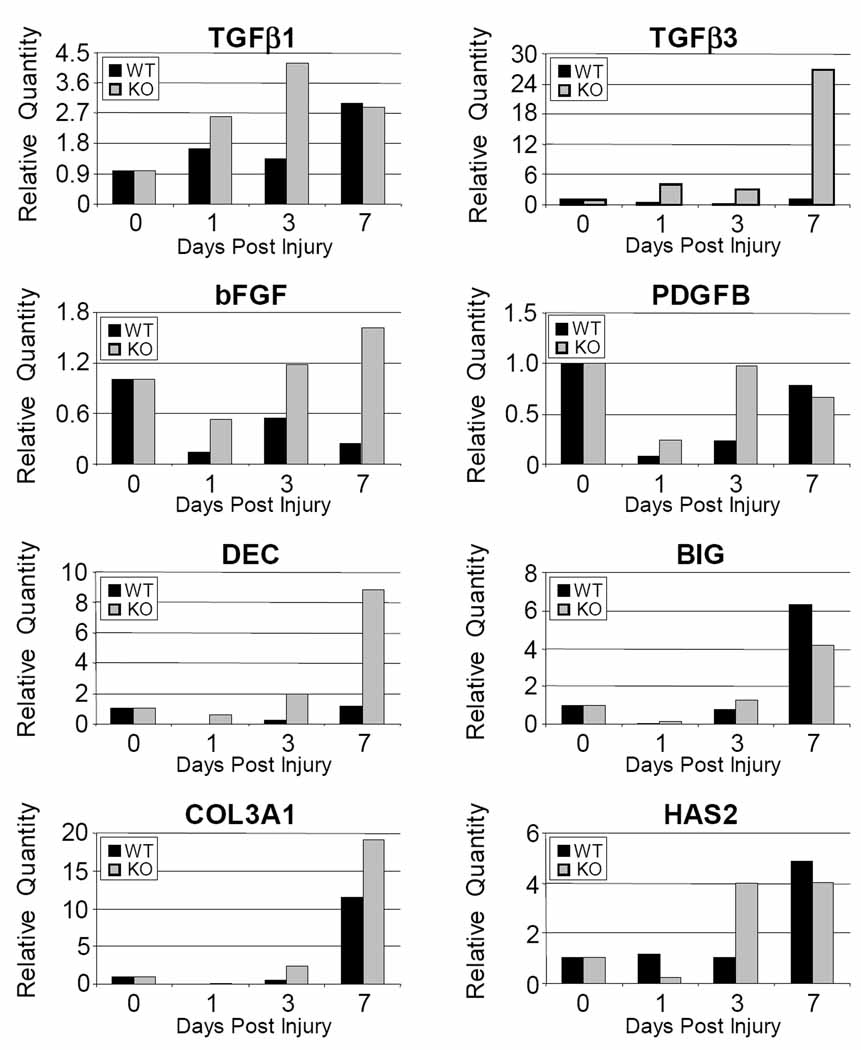
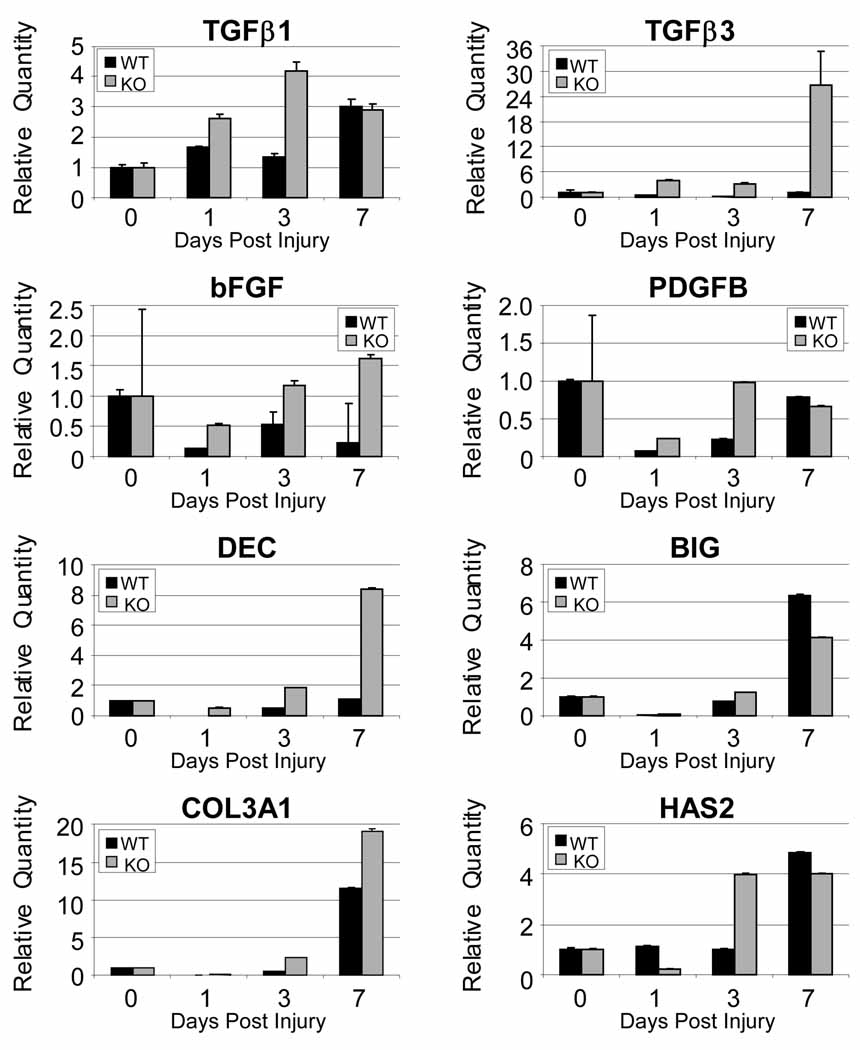
The relative quantity of TGFβ3, bFGF and DCN was increased at least two fold at 1, 3 and 7 days post injury in the CD44 knockout when compared to wild type (Figure 5). Similarly, the relative expression of COL3A1 and PDGFB was increased at least two fold at 1 and 3 days post injury. Relative expression was also increased at least two fold for BGN at 1 day post injury and for TGFβ1 at 3 days post injury in the CD44 knockout. HAS2 was initially decreased at 1 day post injury in knockout animals but by 3 days was increased to at least 2 fold. There were no appreciable differences in COL1A2 and IL1β (data not shown).
Figure 5.
Gene expression in a patellar tendon injury in wild type (WT) and CD44 knockout (KO) represented as relative quantity fold changes (2−ΔΔCT) with error bars representing 2(−ΔΔCT-s) where s is the standard deviation of the ΔΔCT value. All post injury time points are normalized to uninjured tendons (day 0) which is represented by a value of one.
DISCUSSION
This study demonstrated that the absence of CD44 led to improved healing in a patellar tendon injury. Alternative to our hypothesis, the material properties of the healing CD44 knockout patellar tendon were superior when compared to wild type tendons at both three and six weeks post injury. We initially hypothesized that CD44 knockout mice would have decreased material properties based on delayed wound healing in skin18. However, in the current study, we demonstrated that extracellular matrix components were upregulated while some of the proinflammatory cytokines showed little change. The gene expression data indicates that matrix components and cytokines that are beneficial to superior healing were up regulated in CD44 knockout tendons. These findings are in agreement with previous studies that showed improved healing through decreased inflammation and an accumulation of HA in the absence of CD4415, 16. Therefore, the current study demonstrated that the absence of CD44 in tendon healing leads to improved material properties and reduced cross-sectional area through superior extracellular matrix expression.
CD44 and HA are important mediators in healing and numerous studies have shown beneficial effects from decreased CD44 expression and elevated levels of HA. It has previously been shown, by our laboratory and others, that this environment occurs naturally during fetal wound healing8, 11. Unlike reparative healing in adult tissue, fetal tissue is well known to heal through a scarless regenerative process11, 23. It is believed that recapitulating a regenerative environment during adult healing will reduce adhesion formation and the rate of reruptures8. In the current study HAS2 expression, the main HA synthase, was elevated in concurrence with a previous CD44 knockout study15. Interestingly, in a prior in vitro study, HA-treated cells stimulated type III collagen and TGFβ3 expression24, both of which are indicative of regenerative healing. TGFβ1, which is indicative of fibrotic healing, was also increased by HA; however, this effect was no longer present when cells were pretreated with CD44 silencing RNA. Similarly, this study also showed an increase in type I collagen expression only when low molecular weight HA was added but not with high molecular weight or native HA. Conversely, type III collagen expression was increased with all three forms of HA. Even more importantly is that the increased type I expression by low molecular weight HA was reserved with the application of CD44 silencing RNA. Therefore, in agreement with this study, we demonstrated increased HAS2, an increased expression of TGFβ3 and type III collagen with only a mild up regulation in TGFβ1 and no changes in type I collagen. These factors combined indicate that the absence of CD44 created an environment that is conducive to regenerative healing.
The absence of CD44 in this study altered the expression of multiple extracellular matrix components and cytokines. The effects of cytokines and matrix components on cells are interdependent. Cytokines stimulate extracellular matrix production; however, the interaction between extracellular matrix molecules and cellular receptors, like CD44, lead to the upregulation of certain cytokines. For example, administration of bFGF to injured tendon increased expression of type III collagen25. Similarly, application of TGFβ3 to skin wounds reduced scar formation26. Conversely, decorin is closely related to both collagen metabolism and TGFβ activity 27. Similar results were demonstrated in the current study, indicating that these synergistic effects helped create an improved tendon healing environment.
Previous studies showed that CD44 was over expressed in fibrotic healing10 yet had decreased expression in scarless healing. This suggests that CD44 is detrimental to healing and subsequently led to methods that decreased CD44 expression or activation. One strategy was the application of anti-CD44 blocking antibodies in a healing environment. Blocking antibodies decreased cellular migration28 and tissue damage29, but interpretation of these results warrant some caution due to poor understanding of the exact function of the antibodies. Studies have also previously been conducted in the CD44 knockout mouse model. While most CD44 knockout studies examined inflammatory disease17, one group demonstrated a delay in skin wound closure at 1 and 3 days post injury which recovered by 7 days18. Unlike the previous study, in the current study tendon injury was examined through multiple parameters of healing at both early and late stages of healing. While we did not measure healing directly during early stages, we demonstrated improved extracellular matrix gene expression. An improved environment early in healing resulted in improved material properties during late stages. Therefore, the current study offers a more complete understanding of how the absence of CD44 affects wound healing at both early and late stages of healing.
This study has several limitations. First, mRNA expression may not completely mirror the protein levels since there is post-transcriptional regulation of protein synthesis. Nevertheless, our findings are consistent with those reported by other investigators. Second, only male mice were examined in this study. It was recently demonstrated that collagen synthesis in human females is significantly less than in males30; therefore, the results of this study may not be translatable to female mice. However, since we did not show a change in type I collagen expression, the overall conclusions are likely still applicable to both sexes. Third, all the uninjured control tendons were harvested at 12 weeks of age, even though they were compared to injured mice as up to 18 weeks of age. Somerville et al 31demonstrated that most material and mechanical properties of cortical bone had reached their maximum value around 3 months of age. Therefore, it is unlikely that an additional 6 weeks would have significantly affected results due to growth on the material properties of the tendons. Fourth, as with all knockout studies, there is a possibility of compensation by other molecules. The most likely candidate is RHAMM, another HA receptor. Expression of RHAMM was not increased in the CD44 knockout under normal or arthritic conditions in a previous study 17. However, prolonged RHAMM signaling was seen and could be attributed to the accumulation of HA. Finally, we did not directly examine the inflammatory response or RHAMM expression and signaling. Nevertheless, expression of IL1β, a main down stream effecter of RHAMM, was not increased in this study; therefore, it is unlikely that RHAMM signaling increased in this study.
In summary, we have demonstrated that the absence of CD44 in a mouse patellar tendon injury creates an environment that is conducive to regenerative healing through altered gene expression, resulting in superior material properties and reduced cross-sectional area. We have also shown that cytokines and matrix molecules that are characteristic of regenerative healing are elevated in the CD44 knockout animals during healing. The application of these and similar cytokines to improve tendon healing is commonly studied; however, varying concentrations and time courses of application make the results difficult to interpret and implement in a clinical setting. In this study, we demonstrated that the elimination of CD44 during wound healing gave rise to beneficial changes in cytokines and extracellular matrix gene expression. Therefore, by altering the activities of this single cellular receptor, multiple advantageous downstream effects are initiated that lead to superior tendon healing. This study indicates that limiting the role of CD44 may improve healing parameters in adult tendon injury.
ACKNOWLEDGEMENTS
This study was supported by NIH/NIAMS and the NIH/NIAMS sponsored Penn Center for Musculoskeletal Disorders. The authors would like to thank Drs. Rick Assoian and Ellen Pure for the mice14.
REFERENCES
- 1.Praemer A, Furner S, Rice DP. Musculoskeletal conditions in the United States. 2nd ed. Park Ridge, Ill: American Academy of Orthopaedic Surgeons; 1999. American Academy of Orthopaedic Surgeons. [Google Scholar]
- 2.Noble PW, Lake FR, Henson PM, Riches DW. Hyaluronate activation of CD44 induces insulin-like growth factor-1 expression by a tumor necrosis factor-alpha-dependent mechanism in murine macrophages. J Clin Invest. 1993;91:2368–2377. doi: 10.1172/JCI116469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pure E, Cuff CA. A crucial role for CD44 in inflammation. Trends Mol Med. 2001;7:213–221. doi: 10.1016/s1471-4914(01)01963-3. [DOI] [PubMed] [Google Scholar]
- 4.Aruffo A, Stamenkovic I, Melnick M, et al. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 5.Tammi R, Pasonen-Seppanen S, Kolehmainen E, Tammi M. Hyaluronan synthase induction and hyaluronan accumulation in mouse epidermis following skin injury. J Invest Dermatol. 2005;124:898–905. doi: 10.1111/j.0022-202X.2005.23697.x. [DOI] [PubMed] [Google Scholar]
- 6.Noble PW. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002;21:25–29. doi: 10.1016/s0945-053x(01)00184-6. [DOI] [PubMed] [Google Scholar]
- 7.Laurent TC, Fraser JR. Hyaluronan. Faseb J. 1992;6:2397–2404. [PubMed] [Google Scholar]
- 8.Favata M, Beredjiklian PK, Zgonis MH, et al. Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment. J Orthop Res. 2006;24:2124–2132. doi: 10.1002/jor.20271. [DOI] [PubMed] [Google Scholar]
- 9.Berglund M, Hart DA, Wiig M. The inflammatory response and hyaluronan synthases in the rabbit flexor tendon and tendon sheath following injury. J Hand Surg Eur Vol. 2007;32:581–587. doi: 10.1016/J.JHSE.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Lovvorn HN, 3rd, Cass DL, Sylvester KG, et al. Hyaluronan receptor expression increases in fetal excisional skin wounds and correlates with fibroplasia. J Pediatr Surg. 1998;33:1062–1069. doi: 10.1016/s0022-3468(98)90532-2. discussion 1069–1070. [DOI] [PubMed] [Google Scholar]
- 11.Adzick NS, Longaker MT. Scarless fetal healing. Therapeutic implications. Ann Surg. 1992;215:3–7. doi: 10.1097/00000658-199201000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yagishita K, Sekiya I, Sakaguchi Y, et al. The effect of hyaluronan on tendon healing in rabbits. Arthroscopy. 2005;21:1330–1336. doi: 10.1016/j.arthro.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Protin U, Schweighoffer T, Jochum W, Hilberg F. CD44-deficient mice develop normally with changes in subpopulations and recirculation of lymphocyte subsets. J Immunol. 1999;163:4917–4923. [PubMed] [Google Scholar]
- 14.Schmits R, Filmus J, Gerwin N, et al. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood. 1997;90:2217–2233. [PubMed] [Google Scholar]
- 15.Hollingsworth JW, Li Z, Brass DM, et al. CD44 Regulates Macrophage Recruitment to the Lung in Lipopolysaccharide-Induced Airway Disease. Am J Respir Cell Mol Biol. 2007;37:248–253. doi: 10.1165/rcmb.2006-0363OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoop R, Kotani H, McNeish JD, et al. Increased resistance to collagen-induced arthritis in CD44-deficient DBA/1 mice. Arthritis Rheum. 2001;44:2922–2931. doi: 10.1002/1529-0131(200112)44:12<2922::aid-art480>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Nedvetzki S, Gonen E, Assayag N, et al. RHAMM, a receptor for hyaluronan-mediated motility, compensates for CD44 in inflamed CD44-knockout mice: a different interpretation of redundancy. Proc Natl Acad Sci U S A. 2004;101:18081–18086. doi: 10.1073/pnas.0407378102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao G, Savani RC, Fehrenbach M, et al. Involvement of endothelial CD44 during in vivo angiogenesis. Am J Pathol. 2006;169:325–336. doi: 10.2353/ajpath.2006.060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin TW, Cardenas L, Glaser DL, Soslowsky LJ. Tendon healing in interleukin-4 and interleukin-6 knockout mice. J Biomech. 2006;39:61–69. doi: 10.1016/j.jbiomech.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Favata M. Bioengineering. Philadelphia: University of Pennsylvania; 2006. Scarless healing in the fetus: Implications and strategies for postnatal tendon repair; p. 216. Edited by, 216. [Google Scholar]
- 21.Derwin KA, Soslowsky LJ, Green WD, Elder SH. A new optical system for the determination of deformations and strains: calibration characteristics and experimental results. J Biomech. 1994;27:1277–1285. doi: 10.1016/0021-9290(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 22.Thomopoulos S, Williams GR, Gimbel JA, et al. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res. 2003;21:413–419. doi: 10.1016/S0736-0266(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 23.Beredjiklian PK, Favata M, Cartmell JS, et al. Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Ann Biomed Eng. 2003;31:1143–1152. doi: 10.1114/1.1616931. [DOI] [PubMed] [Google Scholar]
- 24.David-Raoudi M, Tranchepain F, Deschrevel B, et al. Differential effects of hyaluronan and its fragments on fibroblasts: relation to wound healing. Wound Repair Regen. 2008;16:274–287. doi: 10.1111/j.1524-475X.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- 25.Chan BP, Fu S, Qin L, et al. Effects of basic fibroblast growth factor (bFGF) on early stages of tendon healing: a rat patellar tendon model. Acta Orthop Scand. 2000;71:513–518. doi: 10.1080/000164700317381234. [DOI] [PubMed] [Google Scholar]
- 26.Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108(Pt 3):985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- 27.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 28.Savani RC, Cao G, Pooler PM, et al. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J Biol Chem. 2001;276:36770–36778. doi: 10.1074/jbc.M102273200. [DOI] [PubMed] [Google Scholar]
- 29.Nedvetzki S, Walmsley M, Alpert E, et al. CD44 involvement in experimental collagen-induced arthritis (CIA) J Autoimmun. 1999;13:39–47. doi: 10.1006/jaut.1999.0294. [DOI] [PubMed] [Google Scholar]
- 30.Miller BF, Hansen M, Olesen JL, et al. Tendon collagen synthesis at rest and after exercise in women. J Appl Physiol. 2007;102:541–546. doi: 10.1152/japplphysiol.00797.2006. [DOI] [PubMed] [Google Scholar]
- 31.Somerville JM, Aspden RM, Armour KE, et al. Growth of C57BL/6 mice and the material and mechanical properties of cortical bone from the tibia. Calcif Tissue Int. 2004;74:469–475. doi: 10.1007/s00223-003-0101-x. [DOI] [PubMed] [Google Scholar]