Abstract
We analyzed outcomes for 668 patients who had systemic treatment for chronic graft-versus-host disease (GVHD) to assess the utility of early treatment change for exacerbation of chronic GVHD as a surrogate for survival endpoints in clinical trials. Fifty-six percent of patients had treatment change within 2 years after diagnosis of chronic GVHD. The median onset of treatment change was 4.4 months (range, 0.3 – 50 months). The cumulative incidence of non-relapse mortality (NRM) at 2 years was 16%, and overall survival at 2 years was 74%. In time-dependent Cox models, treatment change was associated with an increase in risk of NRM (hazard ratio, 2.53; 95% CI, 1.7-3.7; p < .0001). The hazard ratio was attenuated by 6% per month of delay in treatment change. Our results confirm that exacerbation of chronic GVHD is associated with an increased risk of NRM and with decreased survival, but the strength of this association is not large enough to allow the use of early exacerbation as a surrogate for survival endpoints in clinical trials. Other measures of clinical benefit, such as response, will need to be developed as endpoints in phase II trials for patients with chronic GVHD.
Introduction
Chronic graft-versus-host disease (GVHD) is the major determinant of late non-relapse morbidity and mortality after allogeneic hematopoietic cell transplantation (HCT). 1-3 In addition to increased risk of nonrelapse mortality, patients with chronic GVHD have decreased performance, impaired quality of life and delayed immune reconstitution. 4-6 In most cases, resolution of chronic GVHD requires prolonged systemic immunosuppressive treatment. 7
Clinical trials of treatment for chronic GVHD have been hampered by the lack of easily documented early indicators of clinical benefit. Demonstration of prolonged survival or a shorter time to resolution of chronic GVHD may be feasible for pivotal phase III studies, but ascertainment of these endpoints occurs too late to be useful for phase II studies. 8,9 An NIH consensus development project recommended an approach that could be used to measure response in clinical trials for treatment of chronic GVHD, but these recommendations have not yet been validated. 10 Implementation of these recommendations has been difficult, because they require a much larger burden of documentation than would ordinarily be required for clinical practice. In addition, certain features of chronic GVHD have important qualitative characteristics such as sclerosis and intensity of erythema that are not readily amenable to quantitative measurement.
Identification of more accessible validated endpoints indicating clinical benefit could facilitate the conduct of clinical trials in chronic GVHD. Kim et al. 11 recently proposed that an early flare or exacerbation of chronic GVHD could be used as an endpoint in clinical trials. Early flare was strongly associated with decreased GVHD-specific survival, i.e., increased non-relapse mortality. GVHD-specific survival at 2 years was 96% among 27 patients who had no flare, 65% among 34 patients with a first flare more than 78 days after diagnosis of chronic GVHD, and 33% among 35 patients who had a first flare within 78 days after diagnosis. 11 Prompted by this report, we reviewed outcomes among patients with chronic GVHD at our center in an effort to validate these results and to assess the utility of early flare as a surrogate for survival in clinical trials.
Patient and methods
We reviewed data collected in real time for 668 consecutive patients who were given initial systemic treatment for chronic GVHD diagnosed after May 1, 2001 following a first allogeneic HCT after myeloablative or non-myeloablative conditioning regimens. Patients who had recurrent malignancy before the diagnosis of chronic GVHD were excluded. NIH criteria were used for the diagnosis of classic chronic GVHD, except that an ocular clinical score of 2 or 3 without Schirmer's test was accepted as sufficient to define ocular involvement. 12 The cohort did not include patients with persistent, recurrent or delayed-onset acute GVHD. 12 We used the first treatment change as a surrogate for exacerbation of chronic GVHD, and we evaluated the association of treatment change with non-relapse mortality and overall survival.
Change of treatment
Dates and the reasons for change of treatment after the initial systemic treatment for chronic GVHD have been prospectively recorded at our center since May, 2001. Change of treatment was defined as 1) addition of a new systemic medication to control chronic GVHD, 2) increase in the dose of prednisone to at least 1 mg/kg every other day for control of chronic GVHD, or 3) addition of a topical treatment for chronic GVHD involving a previously unaffected site. Change of treatment because of toxicity alone was not included, since the focus of the study was to use change of treatment as a surrogate for “flare” or inadequate control of chronic GVHD.
Statistical methods
Results were analyzed according to information available as of May 2008. Cox proportional hazards regression was used to identify risk factors for treatment change, and time-dependent Cox proportional hazards regression was used to evaluate the association of treatment change with mortality. Cumulative incidence estimates and confidence intervals (CIs) were evaluated as previously described 13. Factors evaluated for association with treatment change included age of the patient at time of transplant, recipient HLA-mismatching (i.e., any graft-versus-host mismatching at HLA A, B, C, DRB1 or DQB1), donor type (i.e., unrelated and related), recipient and donor gender, type of graft (i.e., marrow or mobilized blood), type of preparative regimen (i.e., nonmyelobative, myeloablative with and without total body irradiation), onset of chronic GVHD during systemic glucocorticoid treatment, type of onset of chronic GVHD (i.e., progressive, de novo, quiescent), platelet count less than 100,000/μL at onset of chronic GVHD, and sites involved at the onset of chronic GVHD. 7,14
Results
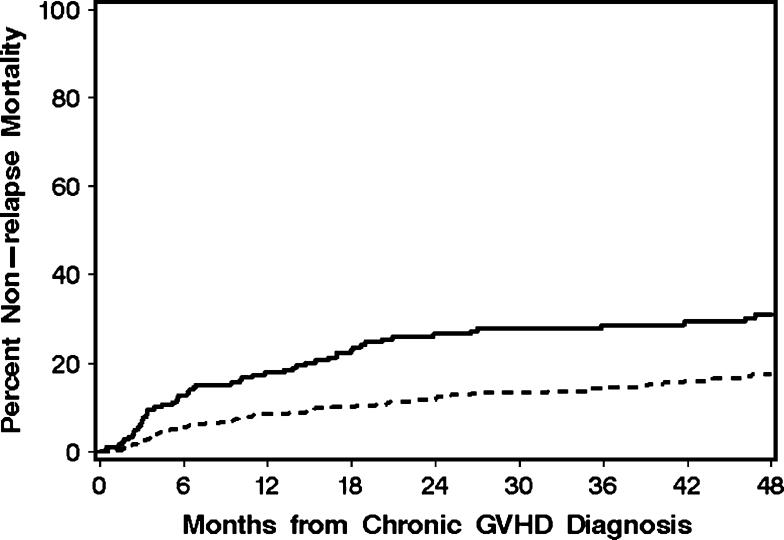
The median patient age at transplant of the cohort was 46.7 years (range, 0.5 – 73 years). Four hundred and forty-six patients (67%) received a myeloablative conditioning regimen pretransplant. Other demographic and graft characteristics for the 668 patients are summarized in Table 1 and Figure 1. Chronic GVHD was diagnosed during steroid treatment in 37% of the patients, and 30% had a platelet count <100,000/μL at the onset of chronic GVHD. Overall, 49% of patients had at least one of these risk factors as an indicator or poor prognosis. The distribution of organ involvement was typical for patients with chronic GVHD. Among the 668 patients, 56% had a treatment change within 2 years after beginning systemic treatment for chronic GVHD, and the median interval time from initial systemic treatment to the first treatment change was 4.4 months (range, 0.3 - 54 months) (Figure 2A). Treatment change was associated with two risk factors: skin involvement at the onset of chronic GVHD and use of a female donor for male recipients (Table 2). The cumulative incidence of non-relapse mortality at 2 years was 16% (95% CI, 13%-19%) (Figure 2B), and overall survival at 2 years was 74% (95% CI, 70%-77%). As shown in Figure 3, the cumulative incidence of non-relapse mortality was higher for patients who had a treatment change within 4 months after the initiation of systemic treatment for chronic GVHD than for those who did not have a treatment change during the first 4 months (27% versus 12%, respectively).
Table 1.
Characteristics of the study cohort (n = 668)
| Characteristic | N (%) |
|---|---|
| Demographic and graft characteristics | |
| Patient age ≥ 50 years | 271 (41) |
| Unrelated donor | 354 (53) |
| HLA-mismatched recipient | 105 (16) |
| Female donor, male recipient | 187 (28) |
| Mobilized blood cell graft | 573 (86) |
| Cord blood | 11 (2) |
| Nonmyeloablative conditioning regimens | 222 (33) |
| Myeloablative conditioning regimens: | |
| - with total body irradiation | 186 (28) |
| - without total body irradiation | 260 (39) |
| Chronic GVHD characteristics at onset | |
| Diagnosis during steroid treatment | 244 (37) |
| Progressive | 77 (12) |
| Quiescent | 438 (66) |
| De novo | 153 (23) |
| Platelet count <100,000/uL | 201 (30) |
Figure 1.
Sites affected by chronic GVHD at time of diagnosis. Columns show the proportions of patients with organs affected by chronic GVHD at diagnosis.
Figure 2.
A. Cumulative incidence of first change in treatment after initial systemic treatment for chronic GVHD. B. Cumulative incidence of non-relapse mortality after the initial diagnosis of chronic GVHD.
Table 2.
Risk factors for treatment change
| Characteristic | Univariate HR (95% CI) | P | Multivariate HR (95% CI) | P |
|---|---|---|---|---|
| Female donor, male recipient | 1.31 (1.1-1.6) | .02 | 1.31 (1.0-1.6) | .01 |
| Skin involved at onset | 1.26 (1.0-1.6) | .04 | 1.26 (1.0-1.6) | .04 |
Figure 3.
The cumulative incidence of non-relapse mortality was higher for patients who had a treatment change within 4 months after initial systemic treatment for chronic GVHD (solid line) than for those who did not have a treatment change during the first 4 months (dashed line).
As expected, time-dependent analysis showed that treatment change after initial systemic treatment for chronic GVHD was associated with an increased risk of non-relapse mortality (Table 3). Since the median time of treatment change was 4.3 months after initial systemic treatment for chronic GVHD, we evaluated results for patients who had a treatment change before 4 months as compared to change after 4 months. As shown in Table 3, the association with non-relapse mortality was stronger for early treatment change than for late change, although the difference between early and late change was not statistically significant (p = .06). A similar trend was found when we evaluated results for treatment change before 6 months as compared to treatment change after 6 months (p = .09). When time of change was analyzed continuously, change at 4 months was associated with a 2.5-fold increase in non-relapse mortality, and for each month of delay in treatment change, this effect on non-relapse mortality was attenuated by a factor of 0.94, equivalent to a 6% reduction per month (Table 3). An association between treatment change and overall mortality was also noted, but the hazard ratios were lower (data not shown). For each month of delay in treatment change, the effect on overall mortality was attenuated by a factor of 0.93.
Table 3.
Factors associated with non-relapse mortality
| Characteristic | HR (95% CI) | P |
|---|---|---|
| Treatment change at any time vs. none | 2.53 (1.7-3.7) | <0.0001 |
| Treatment change before 4 mos. vs. none | 2.84 (1.9-4.2) | |
| Treatment change after 4 mos. vs. none | 1.84 (1.1-3.1) | |
| Effect after vs. before 4 mos. | .06 | |
| Treatment change before 6 mos. vs. none | 2.72 (1.8-4.0) | |
| Treatment change after 6 mos. vs. none | 1.77 (1.0-3.2) | |
| Effect after vs. before 6 mos. | .09 | |
| Treatment change at 4 mos. vs. none | 2.45 (1.7-3.6) | <0.0001 |
| Relative effect per month | 0.94 (0.89-0.99) | .02 |
Discussion
Most phases II studies have relied on “response” as the primary endpoint in clinical trials for chronic GVHD. While “response” is easily recognized in clinical practice, documentation of response for purposes of clinical trials (i.e., convincing other people who cannot interview or examine the patient) is much more difficult and burdensome. Disease characteristics such as the severity of sclerosis or fasciitis and the intensity of cutaneous or mucosal erythema do not lend themselves to quantitative measurement, which makes it difficult to document the magnitude of change from baseline. The difficulty of documenting response made it attractive to consider change of treatment as an alternative endpoint for clinical trials, since this endpoint is objective and easy to document, although it measures failure rather than success and would be susceptible to bias in open-label studies.
Our results confirmed those reported by Kim et al 11 in demonstrating that exacerbation of chronic GVHD is associated with an increased risk of non-relapse mortality and with decreased overall survival. As in the Korean study, the associations with non-relapse mortality and survival endpoints are stronger for early exacerbation than for late exacerbation. Skin involvement at onset of chronic GVHD and the use of female donor for male recipients were the only risk factors associated with treatment change. Because accrual of the cohort antedated the NIH consensus definition of severity scores for chronic GVHD12, these factors could not be analyzed as risk factors for change of therapy and nonrelapse survival in our study.
We evaluated whether early treatment change could be used as a surrogate for non-relapse mortality in clinical trials (see supplemental Table). In our cohort, 27% of the patients had a treatment change within 4 months after diagnosis of chronic GVHD, and the risk of non-relapse mortality at 2 years for these patients was 27%. The contribution to non-relapse mortality at 2 years among these patients is 0.27 × 0.27, or 7%. Among the remaining 73% of patients who did not have a treatment change during the first 4 months, the risk of non-relapse mortality was 12%, and the contribution to non-relapse mortality at 2 years among these patients is 9%. The total non-relapse mortality for both groups at 2 years is 16%. If we had a therapy that could eliminate the need for any treatment change during the first 4 months after diagnosis, the expected non-relapse mortality at 2 years would be 12%, only 4 percentage points lower than historical results. Under the same assumptions, the difference in overall mortality would be only 2 percentage points. When the same analysis was repeated using 1 year (by which time 47% of patients had a treatment change) instead of 4 months as the time point for assessment of treatment change, the difference in NRM at 2 years was only 3 percentage points.
Our results indicate that differences in the strength of associations between early change of treatment and non-relapse mortality or survival are not large enough to allow the use of early exacerbation as a surrogate endpoint for non-relapse mortality or survival in clinical trials for treatment of chronic GVHD. In evaluating our results, it should be noted that the acuity of chronic GVHD was higher in the Korean cohort11 than in our cohort, as indicated by a higher incidence of progressive onset (38% versus 12%) and thrombocytopenia (49% versus 30%), a higher incidence of exacerbation at 2 years (70% versus 56%), a shorter time to exacerbation, (2.4 versus 4.4 months) and a higher incidence of non-relapse mortality at 2 years (39% versus 16%), respectively. In addition, the previous report included patients with late, persistent or recurrent acute GVHD while our cohort included only patients with classic chronic GVHD and overlap syndrome12. These differences might account for the stronger association between early change of treatment and non-relapse mortality in the Korean study than in our study. We conclude that other measures of clinical benefit, such as response, will need to be developed as endpoints in phase II trials of treatment for chronic GVHD.
Acknowledgement
We thank the staff of the Long-Term Follow-Up for invaluable assistance with data collection including Judy Campbell, Colleen McKinnon, Catherine Baker, Gina Brooks and Linda Guerrero. We are also very grateful to our patients for their participation in clinical trials and the referring physicians and medical staff for their collaborative efforts in the excellent care provided to patients and families.
Supported in part by grants CA 118953-01A1 and CA78902 from the National Institutes of Health, Department of Health and Human Services.
Footnotes
Authorship
Contribution: Mary ED Flowers: study design, analyzed data and wrote the paper; Paul Martin: analyzed the data and wrote the paper, Barry Storer performed the statistical analysis, Paul Carpenter, Afonso Vigorito, Paulo Campregher, Andrew Rezvani, Carina Moravec, Hans-Peter Kiem, Fero Matthew, George Georges, Edus Warren and Stephanie Lee and Jean Sanders: collected the data for the study. Fred Appelbaum: helped to write the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Duell T, Van Lint MT, Ljungman P, et al. Health and functional status of long-term survivors of bone marrow transplantation. EBMT Working Party on Late Effects and EULEP Study Group on Late Effects. Ann Intern Med. 1997;126:184–192. doi: 10.7326/0003-4819-126-3-199702010-00002. [DOI] [PubMed] [Google Scholar]
- 2.Socie G, Stone JV, Wingard JR, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341:14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 3.Syrjala KL, Chapko MK, Vitaliano PP, Cummings C, Sullivan KM. Recovery after allogeneic marrow transplantation: prospective study of predictors of long-term physical and psychosocial functioning. Bone Marrow Transplant. 1993;11:319–327. [PubMed] [Google Scholar]
- 4.Goerner M, Gooley T, Flowers MED, et al. Morbidity and mortality of chronic GVHD after hematopoietic stem cell transplantation from HLA-identical siblings for patients with aplastic or refractory anemias. Biol Blood Marrow Transplant. 2002;8:47–56. doi: 10.1053/bbmt.2002.v8.pm11858190. [DOI] [PubMed] [Google Scholar]
- 5.Lee SJ, Vogelsang G, Flowers MED. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 6.Storek J, Dawson MA, Storer B, et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood. 2001;97:3380–3389. doi: 10.1182/blood.v97.11.3380. [DOI] [PubMed] [Google Scholar]
- 7.Stewart BL, Storer B, Storek J, et al. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood. 2004;104:3501–3506. doi: 10.1182/blood-2004-01-0200. [DOI] [PubMed] [Google Scholar]
- 8.Martin PJ. Study design and endpoints in graft-versus-host disease. Best Practice & Research Clinical Haematology. 2008;21:357–372. doi: 10.1016/j.beha.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin PJ, Weisdorf D, Przepiorka D, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: VI. Design of Clinical Trials Working Group report. Biol Blood Marrow Transplant. 2006;12:491–505. doi: 10.1016/j.bbmt.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Pavletic S, Martin P, Lee SJ, et al. Measuring therapeutic response in chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. Response Criteria Working Group report. Biol Blood Marrow Transplant. 2006;12:252–266. doi: 10.1016/j.bbmt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Kim DH, Sohn SK, Baek JH, et al. Time to first flare-up episode of GVHD can stratify patients according to their prognosis during clinical course of progressive- or quiescent-type chronic GVHD. Bone Marrow Transplant. 2007;40:779–784. doi: 10.1038/sj.bmt.1705806. [DOI] [PubMed] [Google Scholar]
- 12.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Lee JH, Choi SJ, et al. Graft-versus-host disease (GVHD)-specific survival and duration of systemic immunosuppressive treatment in patients who developed chronic GVHD following allogeneic haematopoietic cell transplantation. Br J Haematol. 2003;122:637–644. doi: 10.1046/j.1365-2141.2003.04472.x. [DOI] [PubMed] [Google Scholar]