Abstract
During the last decade, episodes of sepsis have increased and Escherichia coli has remained the most frequent clinical isolate. Lipopolysaccharides (LPS; endotoxin) are the major toxic and antigenic components of gram-negative bacteria and qualify as targets for therapeutic interventions. Molecules that neutralize the toxic effects of LPS are actively investigated. In this paper, we describe a murine monoclonal antibody (MAb; WN1 222-5), broadly cross-reactive and cross-protective for smooth (S)-form and rough (R)-form LPS. As shown in enzyme-linked immunosorbent assay and the passive hemolysis assay, WN1 222-5 binds to the five known E. coli core chemotypes, to Salmonella core, and to S-form LPS having these core structures. In immunoblots, it is shown to react with both the nonsubstituted core LPS and with LPS carrying O-side chains, indicating the exposure of the epitope in both S-form and R-form LPS. This MAb of the immunoglobulin G2a class is not lipid A reactive but binds to E. coli J5, an RcP+ mutant which carries an inner core structure common to many members of the family Enterobacteriaceae. Phosphate groups present in the inner core contribute to the epitope but are not essential for the binding of WN1 222-5 to complete core LPS. Cross-reactivity for clinical bacterial isolates is broad. WN1 222-5 binds to all E. coli clinical isolates tested so far (79 blood isolates, 80 urinary isolates, and 21 fecal isolates) and to some Citrobacter, Enterobacter, and Klebsiella isolates. This pattern of reactivity indicates that its binding epitope is widespread among members of the Enterobacteriaceae. WN1 222-5 exhibits biologically relevant activities. In vitro, it inhibits the Limulus amoebocyte lysate assay activity of S-form and R-form LPS in a dose-dependent manner and it neutralizes the LPS-induced release of clinically relevant monokines (interleukin 6 and tumor necrosis factor). In vivo, WN1 222-5 blocks endotoxin-induced pyrogenicity in rabbits and lethality in galactosamine-sensitized mice. The discovery of WN1 222-5 settles the long-lasting controversy over the existence of anti-core LPS MAbs with both cross-reactive and cross-protective activity, opening new possibilities for the immunotherapy of sepsis caused by gram-negative bacteria.
Full text
PDF



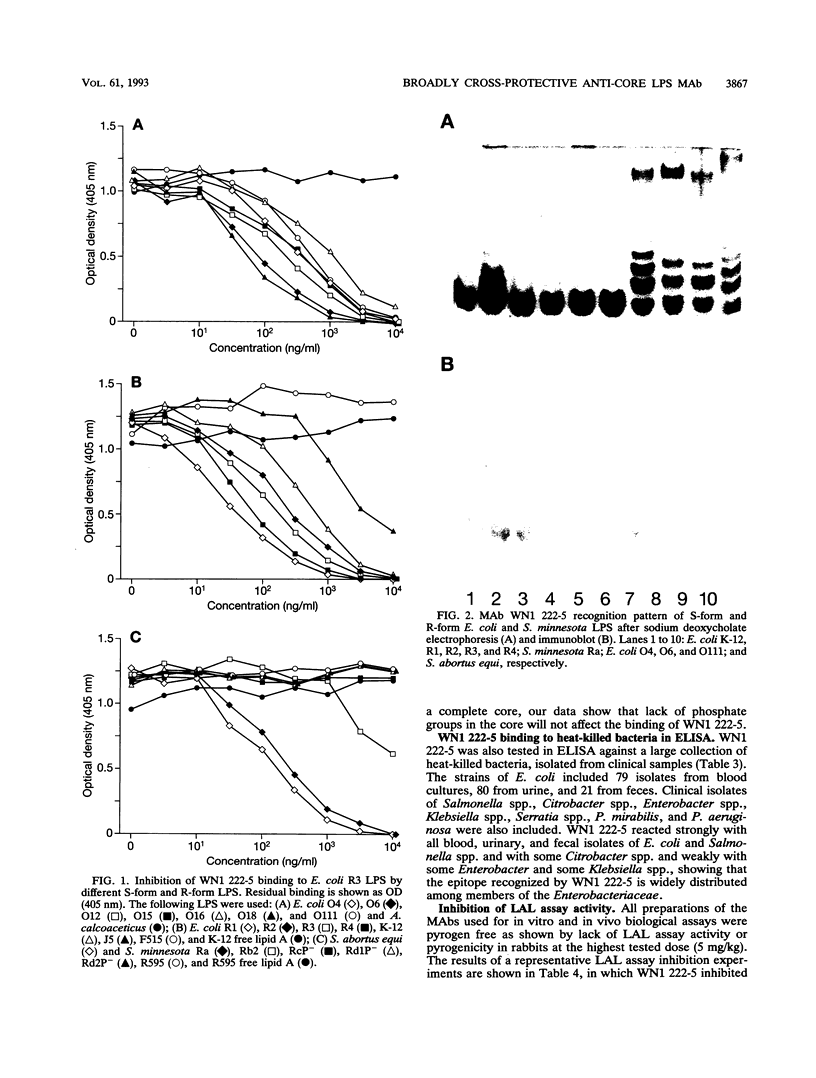

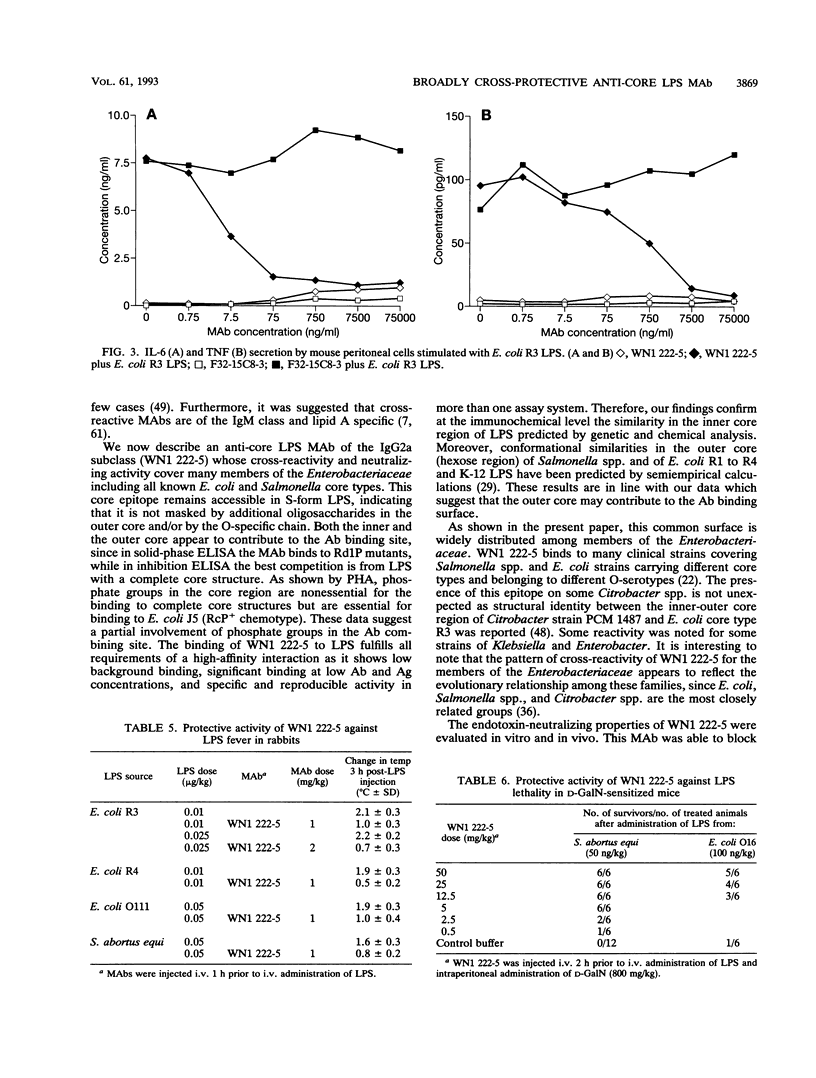



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelmelk B. J., Verweij-van Vught A. M., Maaskant J. J., Schouten W. F., De Jonge A. J., Thijs L. G., Maclaren D. M. Production and characterisation of mouse monoclonal antibodies reacting with the lipopolysaccharide core region of gram-negative bacilli. J Med Microbiol. 1988 Jun;26(2):107–114. doi: 10.1099/00222615-26-2-107. [DOI] [PubMed] [Google Scholar]
- Aydintug M. K., Inzana T. J., Letonja T., Davis W. C., Corbeil L. B. Cross-reactivity of monoclonal antibodies to Escherichia coli J5 with heterologous gram-negative bacteria and extracted lipopolysaccharides. J Infect Dis. 1989 Nov;160(5):846–857. doi: 10.1093/infdis/160.5.846. [DOI] [PubMed] [Google Scholar]
- Baumgartner J. D., Heumann D., Calandra T., Glauser M. P. Antibodies to lipopolysaccharides after immunization of humans with the rough mutant Escherichia coli J5. J Infect Dis. 1991 Apr;163(4):769–772. doi: 10.1093/infdis/163.4.769. [DOI] [PubMed] [Google Scholar]
- Baumgartner J. D., Heumann D., Gerain J., Weinbreck P., Grau G. E., Glauser M. P. Association between protective efficacy of anti-lipopolysaccharide (LPS) antibodies and suppression of LPS-induced tumor necrosis factor alpha and interleukin 6. Comparison of O side chain-specific antibodies with core LPS antibodies. J Exp Med. 1990 Mar 1;171(3):889–896. doi: 10.1084/jem.171.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. The endogenous mediator of endotoxic shock. Clin Res. 1987 Apr;35(3):192–197. [PubMed] [Google Scholar]
- Bogard W. C., Jr, Dunn D. L., Abernethy K., Kilgarriff C., Kung P. C. Isolation and characterization of murine monoclonal antibodies specific for gram-negative bacterial lipopolysaccharide: association of cross-genus reactivity with lipid A specificity. Infect Immun. 1987 Apr;55(4):899–908. doi: 10.1128/iai.55.4.899-908.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bone R. C., Fisher C. J., Jr, Clemmer T. P., Slotman G. J., Metz C. A., Balk R. A. A controlled clinical trial of high-dose methylprednisolone in the treatment of severe sepsis and septic shock. N Engl J Med. 1987 Sep 10;317(11):653–658. doi: 10.1056/NEJM198709103171101. [DOI] [PubMed] [Google Scholar]
- Brade L., Holst O., Kosma P., Zhang Y. X., Paulsen H., Krausse R., Brade H. Characterization of murine monoclonal and murine, rabbit, and human polyclonal antibodies against chlamydial lipopolysaccharide. Infect Immun. 1990 Jan;58(1):205–213. doi: 10.1128/iai.58.1.205-213.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braude A. I., Douglas H. Passive immunization against the local Shwartzman reaction. J Immunol. 1972 Feb;108(2):505–512. [PubMed] [Google Scholar]
- Chamberland S., L'Ecuyer J., Lessard C., Bernier M., Provencher P., Bergeron M. G. Antibiotic susceptibility profiles of 941 gram-negative bacteria isolated from septicemic patients throughout Canada. The Canadian Study Group. Clin Infect Dis. 1992 Oct;15(4):615–628. doi: 10.1093/clind/15.4.615. [DOI] [PubMed] [Google Scholar]
- Chedid L., Parant M., Parant F., Boyer F. A proposed mechanism for natural immunity to enterobacterial pathogens. J Immunol. 1968 Feb;100(2):292–306. [PubMed] [Google Scholar]
- Chia J. K., Pollack M., Guelde G., Koles N. L., Miller M., Evans M. E. Lipopolysaccharide (LPS)-reactive monoclonal antibodies fail to inhibit LPS-induced tumor necrosis factor secretion by mouse-derived macrophages. J Infect Dis. 1989 May;159(5):872–880. doi: 10.1093/infdis/159.5.872. [DOI] [PubMed] [Google Scholar]
- Chong K. T., Huston M. Implications of endotoxin contamination in the evaluation of antibodies to lipopolysaccharides in a murine model of gram-negative sepsis. J Infect Dis. 1987 Nov;156(5):713–719. doi: 10.1093/infdis/156.5.713. [DOI] [PubMed] [Google Scholar]
- Di Padova F., Pozzi C., Tondre M. J., Tritapepe R. Selective and early increase of IL-1 inhibitors, IL-6 and cortisol after elective surgery. Clin Exp Immunol. 1991 Jul;85(1):137–142. doi: 10.1111/j.1365-2249.1991.tb05694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. Preparation and properties of antisera against the lipid-A component of bacterial lipopolysaccharides. Eur J Biochem. 1971 Dec 22;24(1):116–122. doi: 10.1111/j.1432-1033.1971.tb19661.x. [DOI] [PubMed] [Google Scholar]
- Gibb A. P., Barclay G. R., Poxton I. R., di Padova F. Frequencies of lipopolysaccharide core types among clinical isolates of Escherichia coli defined with monoclonal antibodies. J Infect Dis. 1992 Nov;166(5):1051–1057. doi: 10.1093/infdis/166.5.1051. [DOI] [PubMed] [Google Scholar]
- Gigliotti F., Shenep J. L. Failure of monoclonal antibodies to core glycolipid to bind intact smooth strains of Escherichia coli. J Infect Dis. 1985 Jun;151(6):1005–1011. doi: 10.1093/infdis/151.6.1005. [DOI] [PubMed] [Google Scholar]
- Greenman R. L., Schein R. M., Martin M. A., Wenzel R. P., MacIntyre N. R., Emmanuel G., Chmel H., Kohler R. B., McCarthy M., Plouffe J. A controlled clinical trial of E5 murine monoclonal IgM antibody to endotoxin in the treatment of gram-negative sepsis. The XOMA Sepsis Study Group. JAMA. 1991 Aug 28;266(8):1097–1102. [PubMed] [Google Scholar]
- Greisman S. E., Johnston C. A. Failure of antisera to J5 and R595 rough mutants to reduce endotoxemic lethality. J Infect Dis. 1988 Jan;157(1):54–64. doi: 10.1093/infdis/157.1.54. [DOI] [PubMed] [Google Scholar]
- Holst O., Brade H. Structural studies of the core region of the lipopolysaccharide from Salmonella minnesota strain R7 (rough mutant chemotype Rd1). Carbohydr Res. 1991 Oct 14;219:247–251. doi: 10.1016/0008-6215(91)89058-n. [DOI] [PubMed] [Google Scholar]
- Jansson P. E., Lindberg A. A., Lindberg B., Wollin R. Structural studies on the hexose region of the core in lipopolysaccharides from Enterobacteriaceae. Eur J Biochem. 1981 Apr;115(3):571–577. doi: 10.1111/j.1432-1033.1981.tb06241.x. [DOI] [PubMed] [Google Scholar]
- Jansson P. E., Wollin R., Bruse G. W., Lindberg A. A. The conformation of core oligosaccharides from Escherichia coli and Salmonella typhimurium lipopolysaccharides as predicted by semi-empirical calculations. J Mol Recognit. 1989 Jul;2(1):25–36. doi: 10.1002/jmr.300020105. [DOI] [PubMed] [Google Scholar]
- Kemeny D. M., Urbanek R., Richards D., Greenall C. Development of a semi-quantitative enzyme-linked immunosorbent assay (ELISA) for detection of human IgG subclass antibodies. J Immunol Methods. 1987 Jan 26;96(1):47–56. doi: 10.1016/0022-1759(87)90366-8. [DOI] [PubMed] [Google Scholar]
- Kirkland T. N., Colwell D. E., Michalek S. M., McGhee J. R., Ziegler E. J. Analysis of the fine specificity and cross-reactivity of monoclonal anti-lipid A antibodies. J Immunol. 1986 Dec 1;137(11):3614–3619. [PubMed] [Google Scholar]
- Kirkland T. N., Ziegler E. J. An immunoprotective monoclonal antibody to lipopolysaccharide. J Immunol. 1984 May;132(5):2590–2592. [PubMed] [Google Scholar]
- Komuro T., Yomota C., Isaka H. Sodium deoxycholate-polyacrylamide gel electrophoresis of lipopolysaccharides at low temperature. Chem Pharm Bull (Tokyo) 1988 Mar;36(3):1218–1222. doi: 10.1248/cpb.36.1218. [DOI] [PubMed] [Google Scholar]
- Kreger B. E., Craven D. E., Carling P. C., McCabe W. R. Gram-negative bacteremia. III. Reassessment of etiology, epidemiology and ecology in 612 patients. Am J Med. 1980 Mar;68(3):332–343. doi: 10.1016/0002-9343(80)90101-1. [DOI] [PubMed] [Google Scholar]
- Kuhn H. M., Brade L., Appelmelk B. J., Kusumoto S., Rietschel E. T., Brade H. Characterization of the epitope specificity of murine monoclonal antibodies directed against lipid A. Infect Immun. 1992 Jun;60(6):2201–2210. doi: 10.1128/iai.60.6.2201-2210.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. G., Ochman H., Hartl D. L. Molecular and evolutionary relationships among enteric bacteria. J Gen Microbiol. 1991 Aug;137(8):1911–1921. doi: 10.1099/00221287-137-8-1911. [DOI] [PubMed] [Google Scholar]
- Loppnow H., Brade H., Dürrbaum I., Dinarello C. A., Kusumoto S., Rietschel E. T., Flad H. D. IL-1 induction-capacity of defined lipopolysaccharide partial structures. J Immunol. 1989 May 1;142(9):3229–3238. [PubMed] [Google Scholar]
- McCabe W. R. Immunization with R mutants of S. Minnesota. I. Protection against challenge with heterologous gram-negative bacilli. J Immunol. 1972 Mar;108(3):601–610. [PubMed] [Google Scholar]
- McConnell J. S., Appelmelk B. J., Cohen J. Dissociation between Limulus neutralisation and in vivo protection in monoclonal antibodies directed against endotoxin core structures. Microb Pathog. 1990 Jul;9(1):55–59. doi: 10.1016/0882-4010(90)90040-w. [DOI] [PubMed] [Google Scholar]
- Miner K. M., Manyak C. L., Williams E., Jackson J., Jewell M., Gammon M. T., Ehrenfreund C., Hayes E., Callahan L. T., 3rd, Zweerink H. Characterization of murine monoclonal antibodies to Escherichia coli J5. Infect Immun. 1986 Apr;52(1):56–62. doi: 10.1128/iai.52.1.56-62.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutharia L. M., Crockford G., Bogard W. C., Jr, Hancock R. E. Monoclonal antibodies specific for Escherichia coli J5 lipopolysaccharide: cross-reaction with other gram-negative bacterial species. Infect Immun. 1984 Sep;45(3):631–636. doi: 10.1128/iai.45.3.631-636.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelles M. J., Niswander C. A. Mouse monoclonal antibodies reactive with J5 lipopolysaccharide exhibit extensive serological cross-reactivity with a variety of gram-negative bacteria. Infect Immun. 1984 Dec;46(3):677–681. doi: 10.1128/iai.46.3.677-681.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnalue N. A., Lind S. M., Lindberg A. A. The disaccharide L-alpha-D-heptose1-->7-L-alpha-D-heptose1-->of the inner core domain of Salmonella lipopolysaccharide is accessible to antibody and is the epitope of a broadly reactive monoclonal antibody. J Immunol. 1992 Oct 15;149(8):2722–2728. [PubMed] [Google Scholar]
- Oishi K., Koles N. L., Guelde G., Pollack M. Antibacterial and protective properties of monoclonal antibodies reactive with Escherichia coli O111:B4 lipopolysaccharide: relation to antibody isotype and complement-fixing activity. J Infect Dis. 1992 Jan;165(1):34–45. doi: 10.1093/infdis/165.1.34. [DOI] [PubMed] [Google Scholar]
- Pollack M., Chia J. K., Koles N. L., Miller M., Guelde G. Specificity and cross-reactivity of monoclonal antibodies reactive with the core and lipid A regions of bacterial lipopolysaccharide. J Infect Dis. 1989 Feb;159(2):168–188. doi: 10.1093/infdis/159.2.168. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Galanos C. Lipid A antiserum-mediated protection against lipopolysaccharide- and lipid A-induced fever and skin necrosis. Infect Immun. 1977 Jan;15(1):34–49. doi: 10.1128/iai.15.1.34-49.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel E. T., Seydel U., Zähringer U., Schade U. F., Brade L., Loppnow H., Feist W., Wang M. H., Ulmer A. J., Flad H. D. Bacterial endotoxin: molecular relationships between structure and activity. Infect Dis Clin North Am. 1991 Dec;5(4):753–779. [PubMed] [Google Scholar]
- Romanowska E., Gamian A., Dabrowski J. Core region of Citrobacter lipopolysaccharide from strain PCM 1487. Structure elucidation by two-dimensional 1H-NMR spectroscopy at 500 MHz and methylation analysis/mass spectrometry. Eur J Biochem. 1986 Dec 15;161(3):557–564. doi: 10.1111/j.1432-1033.1986.tb10478.x. [DOI] [PubMed] [Google Scholar]
- Salles M. F., Mandine E., Zalisz R., Guenounou M., Smets P. Protective effects of murine monoclonal antibodies in experimental septicemia: E. coli antibodies protect against different serotypes of E. coli. J Infect Dis. 1989 Apr;159(4):641–647. doi: 10.1093/infdis/159.4.641. [DOI] [PubMed] [Google Scholar]
- Stähli C., Staehelin T., Miggiano V., Schmidt J., Häring P. High frequencies of antigen-specific hybridomas: dependence on immunization parameters and prediction by spleen cell analysis. J Immunol Methods. 1980;32(3):297–304. doi: 10.1016/0022-1759(80)90194-5. [DOI] [PubMed] [Google Scholar]
- Takada H., Kotani S., Tanaka S., Ogawa T., Takahashi I., Tsujimoto M., Komuro T., Shiba T., Kusumoto S., Kusunose N. Structural requirements of lipid A species in activation of clotting enzymes from the horseshoe crab, and the human complement cascade. Eur J Biochem. 1988 Aug 15;175(3):573–580. doi: 10.1111/j.1432-1033.1988.tb14230.x. [DOI] [PubMed] [Google Scholar]
- Teng N. N., Kaplan H. S., Hebert J. M., Moore C., Douglas H., Wunderlich A., Braude A. I. Protection against gram-negative bacteremia and endotoxemia with human monoclonal IgM antibodies. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1790–1794. doi: 10.1073/pnas.82.6.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Vacheron F., Mandine E., Lenaour R., Smets P., Zalisz R., Guenounou M. Inhibition of production of tumor necrosis factor by monoclonal antibodies to lipopolysaccharides. J Infect Dis. 1992 May;165(5):873–878. doi: 10.1093/infdis/165.5.873. [DOI] [PubMed] [Google Scholar]
- Warren H. S., Amato S. F., Fitting C., Black K. M., Loiselle P. M., Pasternack M. S., Cavaillon J. M. Assessment of ability of murine and human anti-lipid A monoclonal antibodies to bind and neutralize lipopolysaccharide. J Exp Med. 1993 Jan 1;177(1):89–97. doi: 10.1084/jem.177.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein M. P., Reller L. B., Murphy J. R., Lichtenstein K. A. The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. I. Laboratory and epidemiologic observations. Rev Infect Dis. 1983 Jan-Feb;5(1):35–53. doi: 10.1093/clinids/5.1.35. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J., Fisher C. J., Jr, Sprung C. L., Straube R. C., Sadoff J. C., Foulke G. E., Wortel C. H., Fink M. P., Dellinger R. P., Teng N. N. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N Engl J Med. 1991 Feb 14;324(7):429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J., McCutchan J. A., Fierer J., Glauser M. P., Sadoff J. C., Douglas H., Braude A. I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982 Nov 11;307(20):1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]
- van Deventer S. J., Buller H. R., ten Cate J. W., Sturk A., Pauw W. Endotoxaemia: an early predictor of septicaemia in febrile patients. Lancet. 1988 Mar 19;1(8586):605–609. doi: 10.1016/s0140-6736(88)91412-2. [DOI] [PubMed] [Google Scholar]