Summary
Osteomyelitis (OM) from multidrug-resistant (MDR) Acinetobacter has emerged in >30% of combat-related injuries in Iraq and Afghanistan. While most of these strains are sensitive to colistin, the drug is not availible in bone void fillers for local high-dose delivery. To address this we developed a mouse model with MDR strains isolated from wounded military personnel. In contrast to S. aureus OM, which is osteolytic and characterized by biofilm in necrotic bone, A. baumannii OM results in blastic lesions that do not contain apparent biofilm. We also found that mice mount a specific IgG response against 3 proteins (40, 47 & 56KDa) regardless of the strain used, suggesting that these may be immunuo-dominant antigens. PCR for the A. baumannii specific parC gene confirmed a 100% infection rate with 75% of the MDR strains, and in vitro testing confirmed that all strains were sensitive to colistin. We also developed a real-time quantitative PCR (RTQ-PCR) assay that could detect as few as 10 copies of parC in a sample. To demonstrate the efficacy of colistin prophylaxis in this model, mice were treated with either parenteral colistin (0.2mg colistinmethate i.m. for 7 days), local colistin (PMMA bead impregnated with 1.0mg colistin sulfate), or an unloaded PMMA bead control. While the parenteral colistin failed to demonstrate any significant effects vs. the placebo, the colistin PMMA bead significantly reduced the infection rate such that only 29.2% of the mice had detectable levels of parC at 19 days (p<0.05 vs. i.m. colistin and placebo).
Keywords: Multi-Drug Resistant, Acinetobacter baumannii, Osteomyelitis, Colistin
Introduction
It has been well established from current combat-related injuries during US military operations in Iraq and Afghanistan that the ratio of serious injuries to fatal casualties exceeds that of previous conflicts.1 Orthopaedic trauma comprises the vast majority of these war wounds, as 70% of casualties involve the musculoskeletal system, 26% are fractures, and 82% of the fractures are open. 2–5 Thus, it is not surprising that the incidence of osteomyelitis (OM) in combat-related extremity injuries is between 2% to 15%, and is of great concern.6–8 Most alarming is the incidence of infections caused by multidrug-resistant (MDR) Acinetobacter species, which can be difficult to cure in some settings.9–11 Surprisingly this pathogen has been reported in less than 2% of nosocomial infections within the United States, but has emerged in over 30% of admitted deployed soldiers.10
In contrast to Staphylococcus, which is responsible for >80% of OM infections,12 Acinetobacter baumannii-calcoaceticus complex (ABC) are Gram-negative, non-fermentative, non-spore forming, strictly aerobic, oxidase-negative coccobacillary organisms. Additionally, infections caused by Acinetobacter appear to be hospital-acquired and not from an initial colonization of the injury. 13 Thus, one critical question involving Acinetobacter is whether or not they can produce osteolytic OM on their own, or if they are only present in super-infections with other microorganisms. Another important question is whether or not the MDR Acinetobacter OM can be effectively prevented with parenteral or local antibiotic therapy at the time of initial surgery. In support of this a clinical study has demonstrated that most MDR Acinetobacter strains are sensitive to colistin,10 and that colistin heteroresistance primarily occurs in patients treated with colistin.14 Thus, we aimed to test the hypotheses that: i) pure clinical isolates of MDR Acinetobacter can induce implant-associated OM, and ii) prophylactic colistin prevents these orthopaedic infections. To this end, we utilized a quantitative murine model of implant-associated OM, orginally developed for S. aureus,15 in which an insect pin is innoculated with cultured bacteria and transcortically implanted through the tibia metaphysis. Given the well-established use of antibiotic impregnated bone cement to deliver high doses of drug locally,16–18 we have developed colistin sulfate impregnated polymethylmethacrylate (PMMA) beads, since high dose parenteral administration of colistin is limited by nephrotoxicity and neurotoxicity.19 With this system, here we provide the first evidence that A. baumannii can induce OM in the absence of other pathogens, however these lesions are blastic rather than osteolytic and do not contain apparent biofilms in necrotic bone. Moreover, local but not parenteral pharmacological doses of colistin are capable of preventing the establishment of MDR Acinetobacter implant-associated OM.
Methods
Bacterial strains
Four clinical isolates of Acinetobacter baumannii with confirmed resistance to amikacin, ampicillin, aztraeonam, ceftriaxone, ciprofloxacin, gentamicin, imipenem, tobramycin and vancomycin were obtained from wounded soldiers treated at the Brooke Army Medical Center at Fort Sam Houston, San Antonio, under Institutional Review Board approved protocols. Bacterial strains were grown overnight at 250rpm and 37°C in Tryptic Soy broth (Sigma-Aldrich, St. Louis, MO). Strain sensitivity to colistin was determined by streaking overnight cultures on Trypic Soy agar plates containing 10µg/ml of colistinmethate (Paddock Laboratories, Inc, Minneapolis, MN).
DNA extraction, PCR cloning of parC and RTQ-PCR of Acinetobacter DNA
DNA was extracted from all four strains using a DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA). DNA primers (forward 5’-AAAAATCAGCGCGTACAGTG-3’ and reverse 5’-CGAGAGTTTGGCTTCGGTAT-3’) specific for the Acinetobacter topoisomerase gene parC, were used for PCR and RTQ-PCR as previously described.20 To confirm the purity of the clinical isolates, 5 individual colonies from each strain were isolated and the parC gene was amplified and sequenced using an i-cycle PCR machine (Bio-Rad, Hercules, CA). The amplification protocol was as follows: 95°C pre-melt for 15min followed by 40 cycles of 95°C for 30s, 54°C for 30s and 72°C for 30s. To generate a standard curve for RTQ-PCR, the 196bp parC fragment was cloned into the pTOPO2.1 cloning vector (Invitrogen, Carlsbad, CA), and 10-fold serial dilutions were used to perform Syber Green (Thermo Scientific) RTQ-PCR (Corbett Research, Sydney, AU). A similar curve was generated using the mouse β-actin primers (forward 5’-AGATGTGAATCAGCAAGCAG-3’ and reverse 5’-GCGCAAGTTAGGTTTTGTCA-3’), to control for sample integrity as we have previously described.15 In order to calculate the parC gene copies in a tibia sample, we first generated a standard curve with A. baumannii genomic DNA purified directly from an overnight culture. The standard curve was generated with 10-fold dilutions of the TOPO plasmid with parC insert. The mean of the 3 Ct values from each tibia sample were then plotted against this curve to extrapolate the number of parC genes (n= 24 mice/treatment group). This number was then normalized to β-actin and the data are presented as normalized parC gene copies per sample.
Colistin in vitro release kinetics
PMMA beads (Heraeus Medical GmbH, Wehrheim, Germany) were impregnated with 1.0mg or 2.0mg colistin sulphate (Alpharma, Copenhagen, Denmark). To assess the release kinetics of the colistin following rehydration, the beads were introduced into capped vials and totally covered with 10mL of MilliQ water. Extraction was carried out at 37°C, and the water was exchanged every 24h. The concentration of released colistin sulphate in the extracts was determined by conductometric measurements with a WTW platinated platin electrode LTA 1 at 25°C. Temperature fluctuations were compensated using a WTW TFK 530 temperature electrode in parallel to the conductive electrode. A calibration curve with 40 points (R2=0.999) at concentrations between 0 and 20g/L was recorded to calculate concentrations. All of the measured concentrations were within this range. Placebo beads were used as control, and the conductive values of the placebo beads were subtracted from detected signals before calculating colistin concentrations.
Surgery and antibiotic treatments
All animal studies were performed under University of Rochester Committee for Animal Resources approved protocols. The implant-induced OM surgeries were performed on 6–8 week-old C57Bl/6 female mice as previously described.15 Briefly, mice were anesthetized with ketamine (100mg/kg) and xylazine (10mg/kg), shaved, and the skin was cleansed with 70% ethanol. A small incision was made in the skin on the medial side of the left leg to expose the tibial metaphysis. OM was induced via transcoritcal insertion of a 0.25mm insect pin (Fine Science Tools, Foster City, CA) that was dipped into an overnight culture of A. baumannii or S. aureus (Xen29), which contaminated the pin with ~2.5×105 colony forming units (CFU) as determined by votexing the inoculated pins in PBS and plating out the contents on agar. For prophylactic treatment, mice (n = 24) received either: 1) a control PMMA bead lacking antibiotic, 2) a PMMA bead impregnated with 1.0mg colistin sulphate, or 3) intramuscular (i.m.) injection of 0.2mg (~10mg/kg) of colistinmethate every day for 7 days as previously described in a mouse pneumonia model.21 The 5mm PMMA beads were implanted adjacent to the pin at the time of surgery and were secured with a suture through the skin and muscle. The parenteral colistinmethate was given at the time of surgery and for the next 7 days with no bead. All mice were sacrificed for analysis on day 19.
Radiology
Longitudinal plain film radiographs were obtained using a Faxitron Cabinet x-ray system (Faxitron, Wheeling, IL, USA) as we have previously described.22 Micro-computed tomography (μCT) was performed on tibia after sacrifice at high-resolution (10.5 µm) (VivaCT 40; Scanco Medical AG, Basserdorf, Switzerland) to render 3D images as we have previously described.23
Histologic evaluation of OM
After μCT, the tibial samples were processed for decalcified histology and stained with hematoxylin and eosin (H&E), or Gram-stained as we have described previously.24
Serology
The generation of specific antibodies against A. baumannii proteins during the establishment of chronic OM was determined by western blotting as previously described.15 Briefly, total protein extract was obtained from a 100 ml culture of A. baumannii, strains BAMC 2, BAMC 3 and BAMC 4, using the Complete Bacterial Proteome Extraction Kit (Calbiochem, San Diego, CA). 20 µg of total A. baumannii protein per well was boiled in Laemelli loading buffer and separated in NuPAGE™ 10% Bis-Tris SDS Gels (Invitrogen, Carlsbad, CA) by electrophoresis, and transferred to a PVDF membrane (Millipore, Billerica, MA). The membrane was then cut into single lanes and blocked with PBS, 0.1% Tween 20 (PBST) and 5% non-fat dry milk for 1 hr at room temperature. Afterwards, each lane was incubated with a unique serum (10 µl serum in 5 ml of blocking buffer (PBST + 5% non-fat dry milk)) as the primary antibody, washed 3 times in 0.1% PBST, and then the strips were pooled and incubated with 1.5 µl HRP-conjugated goat anti-mouse IgG antibody (BioRad, Hercules, CA). The strips were then washed 3 times in PBST, 15 minutes each at room temperature. Finally, the strips were reassembled with the molecular weight marker strip and imaged with ECL+ (Amersham) chemiluminescence autoradiograph.
Results
A murine model of implant-associated MDR A. baumannii osteomyelitis
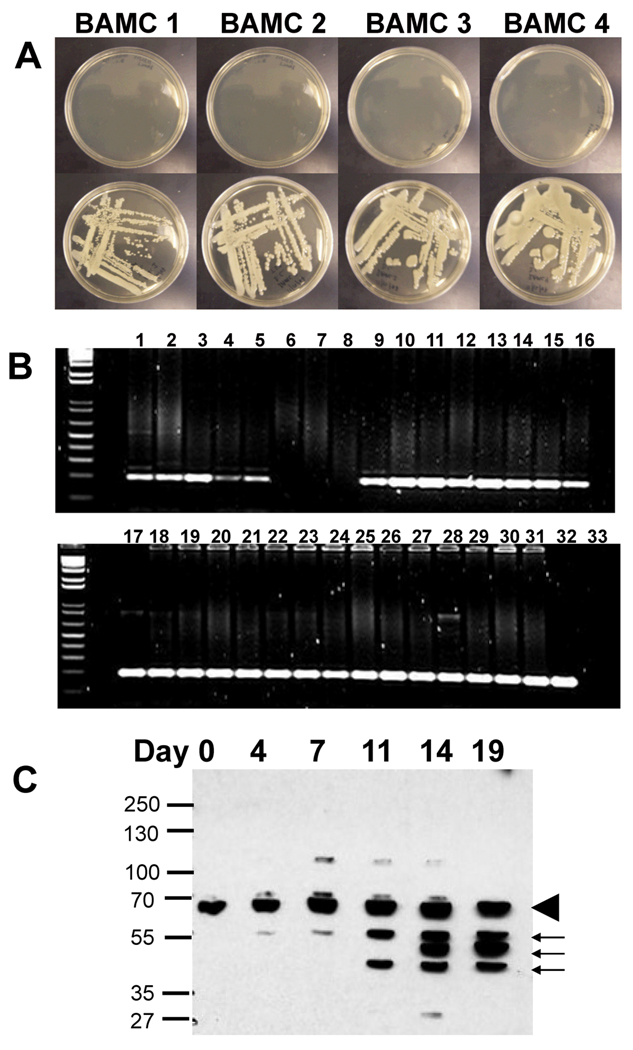
Having recently established a quantitative transtibial model of OM with S. aureus,15 we aimed to utilize this same approach to develop the first mouse model of A. baumannii OM. Thus, we obtained four strains of A. baumannii that were isolated from soldiers wounded in the Middle East and screened them for antibiotic resistance. While these isolates displayed variable resistance to the most commonly used antibiotics in orthopaedic bone cement (gentamicin, tobramycin and vancomycin), they were all sensitive to colistin (Figure 1A). In order to assess the virulence of these strains in our mouse model, stainless steel insect pins were contaminated with an overnight culture of each strain and then surgically implanted into the tibiae of mice. The presence of infection was determined through amplification of the A. baumannii specific parC gene. While all of the mice survived these infections, Figure 1B demonstrates the presence of chronic OM in 100% of the mice challenged with strains BAMC2, BAMC3 and BAMC4. Strain BAMC1 failed to establish infection in all of the mice tested and was therefore excluded from further experiments. Further evidence of this infection was demonstrated by the development of A. baumannii specific antibodies in the sera of challenged mice, which appeared around day 11 and recognized 3 protein antigens 40, 47 and 56KDa that were conserved in all four strains (Figure 1C).
Figure 1. Characterization of MDR A. baumannii strains in the murine model of implant-associated osteomyelitis.
(A) Four MDR A. baumannii clinical isolates were screened for colistin sensitivity by plating on agar medium with (top) and without (bottom) 10µg/ml of colistinmethate. The absence of bacterial growth confirms that all strains were colistin sensitive. (B) The ability of the clinical isolates to establish chronic OM was evaluated in the murine model as described in Materials and Methods. Mice (n=8) were infected with contaminated pins and their tibiae were harvested on day 19 for par C PCR, and the 196bp product was resolved in a 2% agarose gel stained with ethidum bromide. Lanes 1–8 are from strain BAMC1, lanes 9–15 are from strain BAMC2, lanes 16–23 are from strain BAMC3, lanes 24–31 are from strain BAMC4, lane 32 is the pTOPO-parC positive control and lane 33 is the no template negative control. (C) Mice (n=4) were bled on the indicated day following infection, and their sera were used as the primary antibody in western blots of total cell extract of the A. baumannii strains. A representative autoradiograph is shown demonstrating the presence of nonspecific IgG antibodies that were present in all of the sera (arrowhead) and whose titer did not increase over time, and specific IgG antibodies against A. baumannii proteins present in all the strains that initially appeared on day 11 and whose titer increased thereafter in all of the mice tested (arrows).
Implant-associated MDR A. baumannii osteomyelitis is osteoblastic
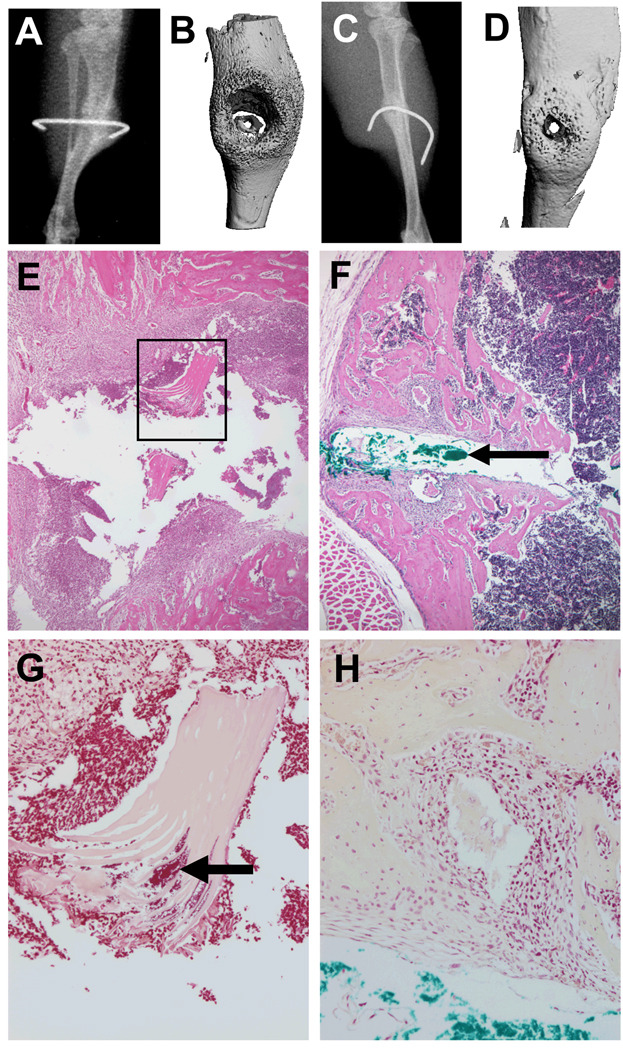
One of the salient features of OM is osteolysis around the implant and the presence of biofilm in the adjacent necrotic bone and soft tissue. Thus, we examined the effects of A. baumannii OM in our model compared to S. aureus and identified several remarkable differences between these bacterial pathogens. Most striking was that in contrast to the osteolytic response to S. aureus, A. baumannii OM induces a robust osteoblastic bone formation response around the infected pin (Figures 2A–D). This dramatic difference in the host bone response to the bacteria was also evident in histology sections of the infected area, which confirmed the large osteolytic lesions in S. aureus OM filled with inflammatory tissue (Figure 2E), contrasted by the new woven bone adjacent to the pin tract (Figure 2F). Examination of Gram stained sections demonstrated another interesting difference between these pathogens in that S. aureus OM is always associated with the presence of biofilm in necrotic bone fragments,25 such as that observed in Figure 2G, while we were unable to identify any biofilm in the necrotic tissue adjacent to the A. baumannii infected pins (Figure 2H). Although this negative finding is not conclusive, it raises the possibility that A. baumannii may persist as an intracellular pathogen in chronic OM.
Figure 2. Differential host bone responses to S. aureus vs. A. baumannii infection.
Representative radiology (A–D) and histology (E–H) of S. aureus (A, B, E & G) and A. baumannii (C, D, F & H) OM in the mouse model are shown. Of note is the remarkable osteolysis that is induced by S. aureus as evidenced in the radiolucent x-ray (A), lytic lesion in the 3D micro-CT image (B) and the inflammatory tissue surrounding the pin tract in H&E stained histology at 20× magnification (E). The necrotic bone fragments in these lesions (boxed region in E) were laden with biofilm that could be readily identified in parallel Gram stained sections at 40× magnification (arrow in G). In contrast, A. baumannii OM was characterized by blastic lesions that were radiodense on x-ray (C), and contained copious amounts of new bone around the infected pin (D & F). This made it difficult to identify the pin tract in histology sections, which had to be confirmed by injecting pathology marking ink (Newcomer Supply Inc., Middleton, WI) into the pin hole before processing (arrow in F). Furthermore, Gram staining failed to identify any foreign material in the necrotic bone fragments adjacent to the pin tract (H).
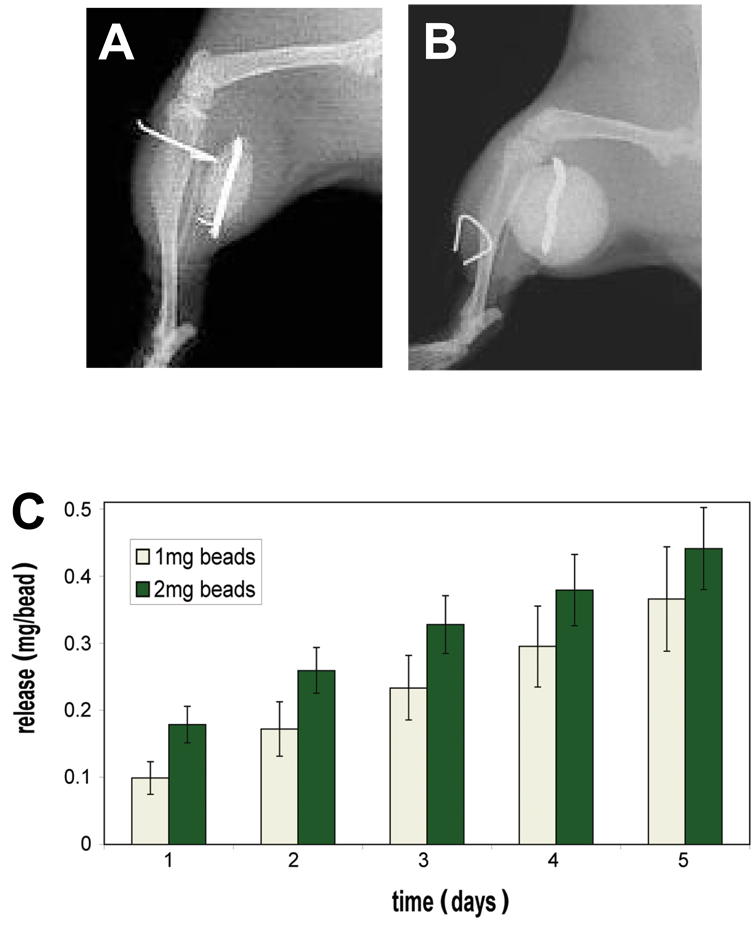
Local prophylactic colistin prevents MDR A. baumannii OM
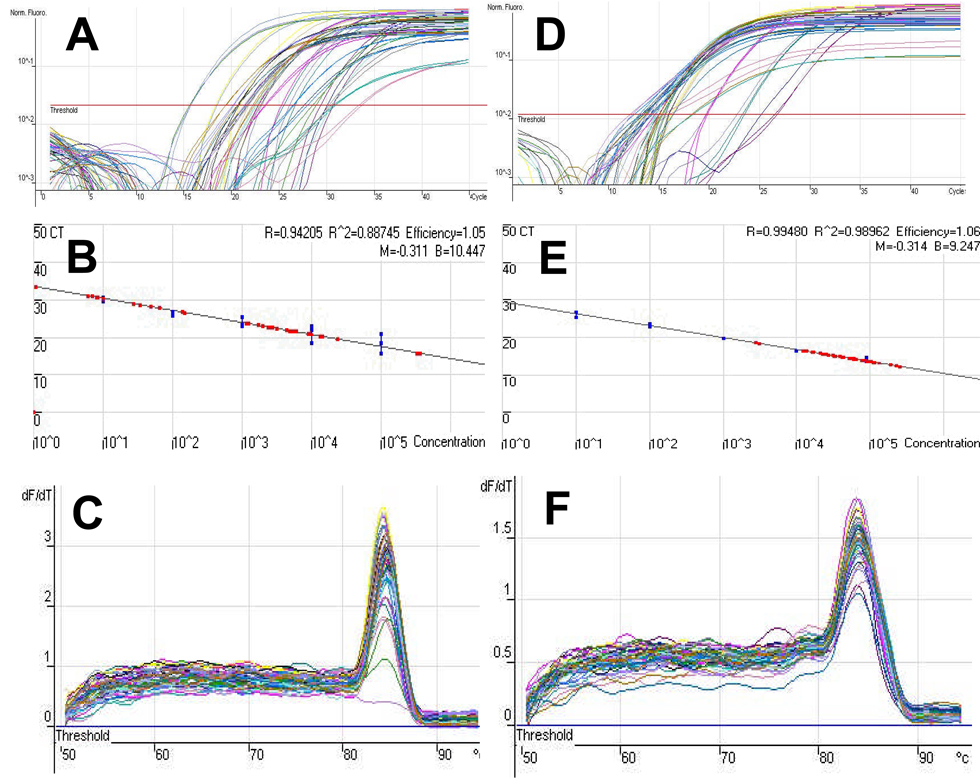
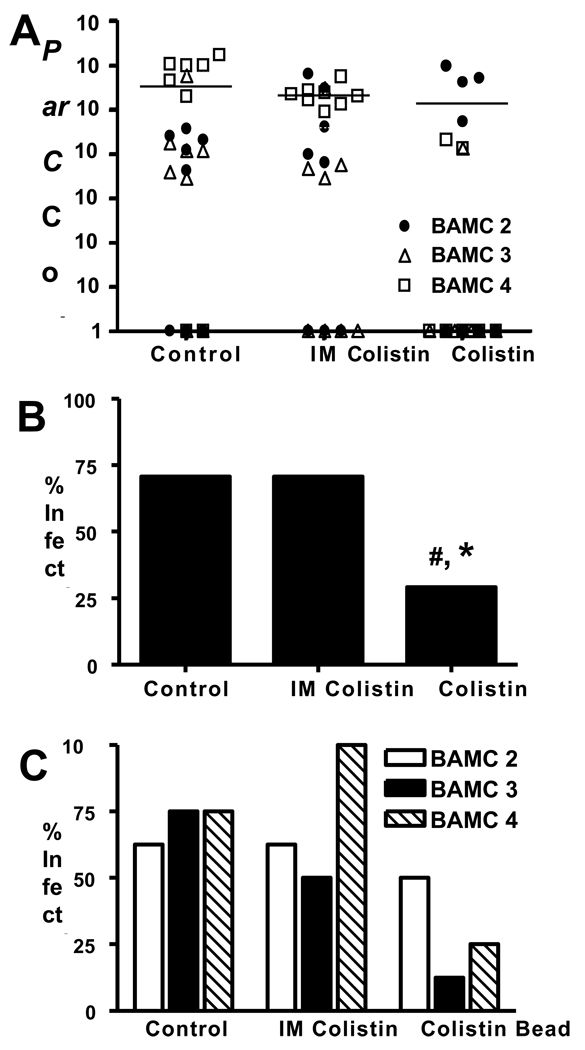
In order to evaluate the efficacy of prophylactic colistin in our model, we first developed a real time quantitative (RTQ) PCR assay to assess the in vivo bacterial load. Figure 3 demonstrates the sensitivity and specificity of this assay, which was able to reproducibly detect as few as 10 parC copies in infected bone. Since we were interested in assessing the difference between parenteral and local colistin, we chose to utilize antibiotic impregnated PMMA beads as the carrier. In pilot studies we found that the 5mm beads could be readily implanted adjacent to the infected pin (Figure 4A), while 1cm beads were too big to be used in this mouse model (Figure 4B). We then evaluated the in vitro release kinetics of the colistin from the 5mm beads to ensure an appropriate biodistribution of the drug over time (Figure 4C). These studies demonstrated that 40–50% of the loaded colistin-sulphate is steadily released into solution from the beads over the first 5 days, which is when the drug would be most effective in killing bacteria initiating the chronic infection. As there were no marked differences between the 1mg and 2mg doses in these studies, we moved forward to in vivo challenge experiments with 1.0mg colistin-sulphate impregnated PMMA beads. BAMC-1 was excluded from this study due to its low virulence as shown in Figure 1B. Following sacrifice on day 19, the bacterial load (Figure 5A), incidence of infection for all strains (Figure 5B), and the incidence of infection for each strain (Figure 5C), was determined by RTQ-PCR. Infection rates were determined to be 75% (18/24), 71% (17/24 and 33% (8/24) for mice receiving a placebo bead, intramuscular colistinmethate injection or colistin-sulphate impregnated PMMA bead, respectively. While the results failed to demonstrate any significant effects of parenteral colistin versus placebo control, local colistin significantly reduced the incidence of chronic OM versus both placebo and parenteral colistin. Moreover, when we analyzed the strains individually, it was clear that most of the infections in the colistin bead group were caused by BAMC-2, suggesting that this strain may have heteroresistance or may be more virulent than the other strains.
Figure 3. Sensitivity and Specificity of RTQ-PCR to quantify bacterial load in A. baumannii OM.
A real time quantitative PCR assay to detect the A. baumannii bacterial load in the infected tibiae was developed by generating standard curves from 10-fold dilutions of pTOPO-parC, which was standardized to mouse DNA (pTOPO-βactin) as we have previously described for S. aureus.15 Syber green RTQ-PCR was performed with parC (A–C) or β-actin (D–F) specific primers. The primary threshold cycle values (CT) data of the dilutions (A & D) were used to generate a standard curve for each template (blue dots in B & E). Then the DNA from the infected tibiae described in Figure 1 was extracted and amplified with the same parC and β-actin specific primers, and extrapolated to the standard curve (red dots in B & E), allowing for an estimate of bacterial load that is presented as parC copies/β-actin copies per sample. Given that contamination can be a problem with real time PCR at >35 cycles, and that we could reproducible quantify 10 copies of parC at 30.1 cycles, we set this value as the upper threshold limit to detect our PCR products. The purity was confirmed using a melt curve, which identified the predicted single peak for the parC (C) and β-actin (F) PCR products respectively.
Figure 4. Colistin impregnated PMMA Beads.
X-rays were taken of mice following implantation of a 5mm (A) and 1cm (B) PMMA bead adjacent to a transcortical pin. The in vitro colistin release kinetics of 5mm PMMA beads impregnated with 1.0 or 2.0mg of colistin-sulfate was determined over the course of 5 days (C). The data are presented as the mean +/− SD (n=5) of the cumulative colistin recovered in solution at the indicated time, such that the values for each day are added to the previous values to give a cumulative total from time 0. No significant differences between the 1.0 and 2.0mg dose were detected by t-test with Bonferroni correction.
Figure 5. Local but not parenteral colistin prevents A. baumannii implant-associated osteomyelitis.
Immediately prior to transtibial implantation of a pin contaminated with the indicated strain of A. baumannii, mice ((n=8) with strains BAMC-2, 3 and 4; 24 total mice per group) received either: i) a sterile PMMA bead (placebo), ii) daily 200µg intramuscular injection of colistinmethate for seven days, or iii) a PMMA bead impregnated with 1mg of colistin-sulphate. Following sacrifice on day 19, par C real-time quantitative PCR was used to measure bacterial load (A), and infection rates (B & C). Of the mice receiving the placebo, 18 out of 24 were infected, in mice receiving intramuscular colistinmethate injection, 17 out of 24 mice were infected and in mice receiving a PMMA bead impregnated with 1mg of colistin-sulphate, 8 out of 24 mice were infected. While no significant effects of IM colistin treatment were observed verses the placebo control, the colistin impregnated bead significantly reduced the incidence of chronic OM compared to placebo (#) and IM colistin (*; p > 0.05 using Fisher’s exact test) (B). However, when the strains were analyzed individually (C) it was clear that BAMC2 was responsible for most of the chronic infections in the mice treated with local colistin.
Discussion
Infections caused by MDR pathogens have long been recognized to be a very serious problem in medicine, requiring vigilance when prescribing antibiotic therapy. Most recently this subject has received tremendous attention, as epidemiology studies seem to indicate that prosthetic infections may be on the rise, 26 27 and methicillin-resistant Staphylococcus aureus (MRSA) has surpassed HIV as the most deadly pathogen in the United States.28 Therefore, the emergence of MDR Acinetobacter OM in orthopaedic trauma patients was initially proposed to be a situation requiring urgent attention.9–11 However, more recent clinical experiences in dealing with this problem suggest that MDR Acinetobacter OM can be managed effectively, but may be the predecessor to more serious super-infections including MRSA.7,29 Of these orthopedic injuries in veterans of Operation Iraqi Freedom and Operation Enduring Freedom (OIF/OEF) we found that Gram-negative pathogens predominate early, and are replaced with staphylococci after treatment, despite nearly universal use of Gram-positive therapy. More specifically, Acinetobacter species were present in 70% of OM cases at presentation, but only 5% of reoccurring OM. In contrast, S. aureus was only present in 13% of initial OM, while 53% of reoccurrences were infected with Staphylococcus, and 31% of the cases were MRSA. Most notable were the type III diaphyseal tibial fractures, of which 13 out of the 35 patients studied had union times of >9 months that appeared to be associated with infection, and 4 that ultimately required limb amputation due to infectious complications. These new findings underscore the importance of effective early treatment of MDR Acinetobacter OM, and warrant investigation of the cause and effect relationship between initial Gram negative infections that evolve into catastrophic MRSA OM. To address these issues, here we investigated the nature of MDR A. baumannii OM versus that of S. aureus, and evaluated the efficacy of local high dose colistin to prevent infection from a contaminated tibial implant in a mouse model.
Although we found MDR Acinetobacter to be highly infectious as expected (Figure 1), we were surprised by several features of A. baumannii OM that are remarkably distinct from S. aureus infection of bone (Figure 2). The first is that A. baumannii induces blastic lesions, in contrast to the osteolytic lesions most commonly associated with OM. Although we have no information on the mechanism by which the bacteria stimulates bone formation, this may occur via induction of anabolic factors (i.e. BMPs, Wnts), and/or the down-regulation of antagonists (i.e. noggin, sost, dkk), in a similar manner as that observed in osteoblastic tumors.30 The other major difference that we found was the absence of biofilm and any histological evidence of colonized necrotic bone in mice infected with A. baumannii, which is always present in chronic S. aureus OM. One possible explanation of these results, which needs to be explored in a focused investigation, is that A. baumannii persists as an obligate intracellular pathogen. In so doing, the bacteria would be immune privileged from humoral immunity (Figure 1C), which should clear extracellular bacteria from the infection site. Additionally, the absence of extracellular bacteria and their pathogen-associated molecular patterns (PAMPs),31 would lead to decreased Toll-like receptor (TLR) activation of innate immunity and osteoclast activation, which causes osteolysis.32–35
Another interesting speculation that is brought by these data and the recent clinical studies on OM cases from OIF/OEF is the possibility that S. aureus infections are opportunistic, and in some cases may depend on an initial colonization by Gram negative bacteria such as Acinetobacter. This scenario further stresses the importance of early eradication of peri-implant infection following orthopaedic surgery, which is most effectively achieved with local drug therapy via antibiotic impregnated bone cement,16–18 and potentially antibiotic coated implants 36,37. Given that most MDR Acinetobacter strains are sentive to colistin,10 and that PMMA beads can be readily impregnated with colistin and steadily release the drug over time (Figure 4), we evaluted this mode of local prophylaxis versus standard parenteral colistinmethane (Figure 5). As predicted, the local treatment, which presumably leads to higher drug concentration levels at the site of infection, was significantly better in preventing MDR Acinetobacter OM. Considering the vast independent clinical experience with antibiotic impregnated PMMA beads and colistin, and the absence of an accepted large animal model of OM that simulates the soft tissue injury associated with war wounds, we find that these results support the evaluation of colistin impregnated immprengnated PMMA beads, or absorbable materials that do not require removal, in a clinical trial to evaluate their efficacy in clearing MDR Acinetobacter OM and reoccuring infections.
Acknowledgments
The authors would like to thank Laura Yanoso for technical assistance with the micro-CT and Krista Scorsone for technical assistance with the histology. This work was supported by research grants from the US Army Medical Research Acquisition Activity (USAMRAA), Orthopaedic Trauma Research Program (OTRP) W81XWH-07-1-0124, and the National Institutes of Health PHS awards AR48681, DE17096, AR52674, AR51469, AR46545, AR54041 and AR53459.
Footnotes
Disclaimer: The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of the Army, Department of Defense or the US government. This work was prepared as part of their official duties and, as such, there is no copyright to be transferred.
References
- 1.Covey DC. Iraq war injuries. Orthopedics. 2006;29(10):884–886. doi: 10.3928/01477447-20061001-08. [DOI] [PubMed] [Google Scholar]
- 2.Owens BD, Kragh JF, Jr, Macaitis J, Svoboda SJ, Wenke JC. Characterization of extremity wounds in Operation Iraqi Freedom and Operation Enduring Freedom. J Orthop Trauma. 2007;21(4):254–257. doi: 10.1097/BOT.0b013e31802f78fb. [DOI] [PubMed] [Google Scholar]
- 3.Owens BD, Kragh JF, Jr, Wenke JC, Macaitis J, Wade CE, Holcomb JB. Combat wounds in operation Iraqi Freedom and operation Enduring Freedom. J Trauma. 2008;64(2):295–299. doi: 10.1097/TA.0b013e318163b875. [DOI] [PubMed] [Google Scholar]
- 4.Covey DC. Combat orthopaedics: a view from the trenches. J Am Acad Orthop Surg. 2006;14(10) Suppl:S10–S17. doi: 10.5435/00124635-200600001-00004. [DOI] [PubMed] [Google Scholar]
- 5.Covey DC, Aaron RK, Born CT, Calhoun JH, Einhorn TA, Hayda RA, Levin LS, Mazurek MT, Murray CK, Powell ET, Schwarz EM, Wenke JC. Orthopaedic war injuries: from combat casualty care to definitive treatment: a current review of clinical advances, basic science, and research opportunities. Instr Course Lect. 2008;57:65–86. [PubMed] [Google Scholar]
- 6.Murray CK, Hsu JR, Solomkin JS, Keeling JJ, Andersen RC, Ficke JR, Calhoun JH. Prevention and management of infections associated with combat-related extremity injuries. J Trauma. 2008;64(3) Suppl:S239–S251. doi: 10.1097/TA.0b013e318163cd14. [DOI] [PubMed] [Google Scholar]
- 7.Yun HC, Branstetter JG, Murray CK. Osteomyelitis in military personnel wounded in Iraq and Afghanistan. J Trauma. 2008;64(2) Suppl:S163–S168. doi: 10.1097/TA.0b013e318160868c. discussion S8. [DOI] [PubMed] [Google Scholar]
- 8.Acinetobacter baumannii infections among patients at military medical facilities treating injured U.S. service. MMWR Morb Mortal Wkly Rep. 2004;53(45):1063–1066. [PubMed] [Google Scholar]
- 9.Murray CK, Hospenthal DR. Treatment of multidrug resistant Acinetobacter. Curr Opin Infect Dis. 2005;18(6):502–506. doi: 10.1097/01.qco.0000185985.64759.41. [DOI] [PubMed] [Google Scholar]
- 10.Davis KA, Moran KA, McAllister CK, Gray PJ. Multidrug-resistant Acinetobacter extremity infections in soldiers. Emerg Infect Dis. 2005;11(8):1218–1224. doi: 10.3201/1108.050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbo A, Navon-Venezia S, Hammer-Muntz O, Krichali T, Siegman-Igra Y, Carmeli Y. Multidrug-resistant Acinetobacter baumannii. Emerg Infect Dis. 2005;11(1):22–29. doi: 10.3201/eid1101.040001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 13.Scott P, Deye G, Srinivasan A, Murray C, Moran K, Hulten E, Fishbain J, Craft D, Riddell S, Lindler L, Mancuso J, Milstrey E, Bautista CT, Patel J, Ewell A, Hamilton T, Gaddy C, Tenney M, Christopher G, Petersen K, Endy T, Petruccelli B. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis. 2007;44(12):1577–1584. doi: 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- 14.Hawley JS, Murray CK, Jorgensen JH. Colistin heteroresistance in acinetobacter and its association with previous colistin therapy. Antimicrob Agents Chemother. 2008;52(1):351–352. doi: 10.1128/AAC.00766-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li D, Gromov K, Soballe K, Puzas JE, O'Keefe RJ, Awad H, Drissi H, Schwarz EM. Quantitative mouse model of implant-associated osteomyelitis and the kinetics of microbial growth, osteolysis, and humoral immunity. J Orthop Res. 2008;26(1):96–105. doi: 10.1002/jor.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marks KE, Nelson CL, Jr, Schwartz J. Antibiotic-impregnated acrylic bone cement. Surg Forum. 1974;25(0):493–494. [PubMed] [Google Scholar]
- 17.Wininger DA, Fass RJ. Antibiotic-impregnated cement and beads for orthopedic infections. Antimicrob Agents Chemother. 1996;40(12):2675–2679. doi: 10.1128/aac.40.12.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop. 2008;79(3):335–341. doi: 10.1080/17453670710015229. [DOI] [PubMed] [Google Scholar]
- 19.Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis. 2005;40(9):1333–1341. doi: 10.1086/429323. [DOI] [PubMed] [Google Scholar]
- 20.Hamouda A, Amyes SG. Development of highly ciprofloxacin-resistant laboratory mutants of Acinetobacter baumannii lacking topoisomerase IV gene mutations. J Antimicrob Chemother. 2006;57(1):155–156. doi: 10.1093/jac/dki397. [DOI] [PubMed] [Google Scholar]
- 21.Montero A, Ariza J, Corbella X, Domenech A, Cabellos C, Ayats J, Tubau F, Borraz C, Gudiol F. Antibiotic combinations for serious infections caused by carbapenem-resistant Acinetobacter baumannii in a mouse pneumonia model. J Antimicrob Chemother. 2004;54(6):1085–1091. doi: 10.1093/jac/dkh485. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Schwarz EM, Young DA, Puzas JE, Rosier RN, O'Keefe RJ. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest. 2002;109(11):1405–1415. doi: 10.1172/JCI15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koefoed M, Ito H, Gromov K, Reynolds DG, Awad HA, Rubery PT, Ulrich-Vinther M, Soballe K, Guldberg RE, Lin AS, O'Keefe RJ, Zhang X, Schwarz EM. Biological Effects of rAAV-caAlk2 Coating on Structural Allograft healing. Mol Ther. 2005;12(2):212–218. doi: 10.1016/j.ymthe.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 24.Ito H, Koefoed M, Tiyapatanaputi P, Gromov K, Goater JJ, Carmouche J, Zhang X, Rubery PT, Rabinowitz J, Samulski RJ, Nakamura T, Soballe K, O'Keefe RJ, Boyce BF, Schwarz EM. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11(3):291–297. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brady RA, Leid JG, Calhoun JH, Costerton JW, Shirtliff ME. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol Med Microbiol. 2008;52(1):13–22. doi: 10.1111/j.1574-695X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- 26.Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection Burden for Hip and Knee Arthroplasty in the United States. J Arthroplasty. 2008 doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466(7):1710–1715. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. Jama. 2007;298(15):1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 29.Johnson EN, Burns TC, Hayda RA, Hospenthal DR, Murray CK. Infectious complications of open type III tibial fractures among combat casualties. Clin Infect Dis. 2007;45(4):409–415. doi: 10.1086/520029. [DOI] [PubMed] [Google Scholar]
- 30.Virk MS, Lieberman JR. Tumor metastasis to bone. Arthritis Res Ther. 2007;9 Suppl 1:S5. doi: 10.1186/ar2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 32.Bi Y, Collier TO, Goldberg VM, Anderson JM, Greenfield EM. Adherent endotoxin mediates biological responses of titanium particles without stimulating their phagocytosis. J Orthop Res. 2002;20(4):696–703. doi: 10.1016/S0736-0266(01)00176-0. [DOI] [PubMed] [Google Scholar]
- 33.Bi Y, Seabold JM, Kaar SG, Ragab AA, Goldberg VM, Anderson JM, Greenfield EM. Adherent endotoxin on orthopedic wear particles stimulates cytokine production and osteoclast differentiation. J Bone Miner Res. 2001;16(11):2082–2091. doi: 10.1359/jbmr.2001.16.11.2082. [DOI] [PubMed] [Google Scholar]
- 34.Bi Y, Van De Motter RR, Ragab AA, Goldberg VM, Anderson JM, Greenfield EM. Titanium particles stimulate bone resorption by inducing differentiation of murine osteoclasts. J Bone Joint Surg Am. 2001;83-A(4):501–508. doi: 10.2106/00004623-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Nalepka JL, Lee MJ, Kraay MJ, Marcus RE, Goldberg VM, Chen X, Greenfield EM. Lipopolysaccharide found in aseptic loosening of patients with inflammatory arthritis. Clin Orthop Relat Res. 2006;451:229–235. doi: 10.1097/01.blo.0000224050.94248.38. [DOI] [PubMed] [Google Scholar]
- 36.Antoci V, Jr, King SB, Jose B, Parvizi J, Zeiger AR, Wickstrom E, Freeman TA, Composto RJ, Ducheyne P, Shapiro IM, Hickok NJ, Adams CS. Vancomycin covalently bonded to titanium alloy prevents bacterial colonization. J Orthop Res. 2007 doi: 10.1002/jor.20348. [DOI] [PubMed] [Google Scholar]
- 37.Parvizi J, Antoci V, Jr, Hickok NJ, Shapiro IM. Selfprotective smart orthopedic implants. Expert Rev Med Devices. 2007;4(1):55–64. doi: 10.1586/17434440.4.1.55. [DOI] [PubMed] [Google Scholar]