Abstract
Newborn screening (NBS) for cystic fibrosis (CF) is increasingly being implemented and is soon likely to be in use throughout the United States, because early detection permits access to specialized medical care and improves outcomes. The diagnosis of CF is not always straightforward, however. The sweat chloride test remains the gold standard for CF diagnosis but does not always give a clear answer. Genotype analysis also does not always provide clarity; more than 1500 mutations have been identified in the CF transmembrane conductance regulator (CFTR) gene, not all of which result in CF. Harmful mutations in the gene can present as a spectrum of pathology ranging from sinusitis in adulthood to severe lung, pancreatic, or liver disease in infancy. Thus, CF identified postnatally must remain a clinical diagnosis. To provide guidance for the diagnosis of both infants with positive NBS results and older patients presenting with an indistinct clinical picture, the Cystic Fibrosis Foundation convened a meeting of experts in the field of CF diagnosis. Their recommendations, presented herein, involve a combination of clinical presentation, laboratory testing, and genetics to confirm a diagnosis of CF.
Cystic fibrosis (CF) is the most common life-threatening autosomal recessive disease in the United States, occurring in approximately 1 in 3500 newborns.1–3 Treatment advances over the past several decades have raised the median predicted survival age in the United States from the mid-teens in the 1970s to more than 36 years old today;4 optimal outcomes depend on timely and accurate diagnosis, however.5–8 Although the vast majority of persons with CF are diagnosed through classic signs and symptoms of the disease (Table I) and corroborative laboratory results, the diagnosis is not as clear-cut in approximately 5% to 10% of individuals with CF.4,9–11 To facilitate the diagnostic process and thereby improve access to vital medical services, in 1996 the Cystic Fibrosis Foundation convened a panel of experts to develop criteria for the diagnosis of CF. The panel’s consensus was that the diagnosis of CF should be based on the presence of 1 or more characteristic clinical features, a history of CF in a sibling, or a positive newborn screening (NBS) test, plus laboratory evidence of an abnormality in the CF transmembrane conductance regulator (CFTR) gene or protein.12 Acceptable evidence of a CFTR abnormality included biological evidence of channel dysfunction (ie, abnormal sweat chloride concentration or nasal potential difference) or identification of a CF disease-causing mutation in each copy of the CFTR gene (ie, on each chromosome). Nevertheless, some patients remain difficult to classify due to the presence of only limited clinical features of CF and inconclusive diagnostic test results.
Table I.
Phenotypic features consistent with a diagnosis of CF
|
Modified from Rosenstein B, Cutting G. The diagnosis of cystic fibrosis: a consensus statement. Cystic Fibrosis Foundation Consensus Panel. J Pediatr 1998;132:589–95. Used with permission.
The significant advances in the diagnosis and treatment of CF over the past decade have increased our understanding of the disease, making this an opportune time to reexamine the criteria for a diagnosis of CF. For example, the age of onset of symptoms is increasingly recognized as being highly variable, ranging from prenatal evidence of echogenic bowel to onset of symptoms in late adolescence or adulthood that nevertheless can cause major morbidity and premature mortality. Our knowledge of the scope and complexity of CFTR gene mutations also has expanded greatly. In 1996, approximately 500 mutations had been identified, with typical commercial panels screening for only 30 of them. Today, more than 1500 mutations have been identified (http://www.genet.sickkids.on.ca/cftr), and comprehensive analysis of the CFTR gene, including sequence determination of the exons and intron splice sites, as well as detection of gross deletions and duplications, is readily available. Extensive genetic studies have produced both greater awareness of the spectrum of mutations in specific population groups13 and increased understanding of genotype–phenotype relationships,14,15 illuminating distinctions between CFTR mutations with limited or no functional effects and those known or predicted to cause CF disease. For the purposes of this article, here “CF mutation” refers only to a CF disease-causing mutated allele, although it is recognized that mutations in the CFTR gene can result in various pathologies, ranging from chronic sinusitis16 to extensive hepatobiliary17 and lung disease.15 Our increased understanding of the wide range of phenotypes in individuals diagnosed with CF is helping to establish a breakpoint for the diagnosis of CF. In addition to the progress in these areas, important advances in defining reference and abnormal ranges of sweat chloride concentrations more clearly also may help improve the accuracy of CF diagnosis.
One of the greatest changes over the past decade has been the way in which individuals with CF come to recognition. In 1996, most people in the United States who presented for diagnostic testing did so based on clinical features or a positive family history; at the time, NBS for CF was routinely operational in only 2 states. Today, CF NBS is in various stages of implementation in 40 states and is likely to be implemented in all states by 2010. Such widespread NBS is rapidly changing the diagnostic paradigm. In contrast to individuals who are diagnosed due to clinical features suggestive of CF, infants referred for diagnostic testing after a positive screen, though they may be underweight,18 often have no clear clinical manifestations of the disease. NBS for CF depends instead on the initial identification of high values of immunoreactive trypsinogen (IRT) in the blood of the newborn (Figure). Because normal IRT reference values vary slightly, the individual NBS program in the state in which the newborn is being tested sets the specific cutoff value that defines an elevated IRT. After an abnormal IRT value is identified, most NBS programs perform DNA testing to identify known CFTR gene mutations (IRT/DNA strategy), while other programs repeat the IRT measurement in a second blood sample obtained from the infant at age approximately 2 weeks (IRT/IRT strategy).19 Both strategies have been reported to provide approximately 90% to 95% sensitivity,20,21 and both identify newborns at risk for a wide spectrum of disease severity.22,23
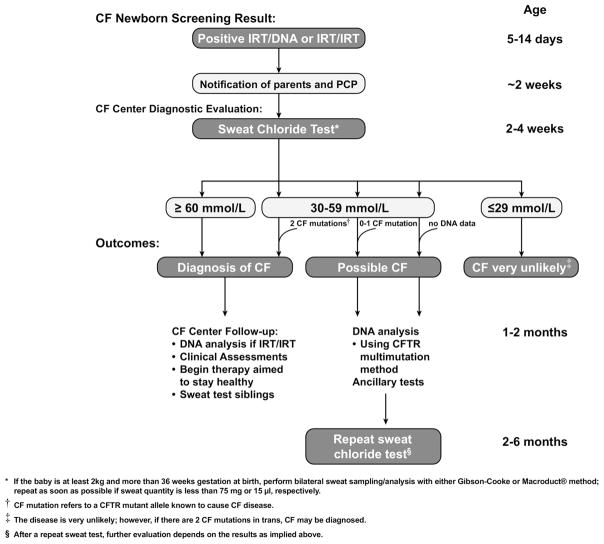
Figure.
The CF diagnostic process for screened newborns.
CF NBS is a screen, not a diagnostic test, and thus identifies only newborns at risk for CF. A positive screening result, indicating persistent hypertrypsinogenemia, must be followed by referral for direct diagnostic testing (ie, sweat chloride test) to confirm a diagnosis of CF. With sufficient experience, sweat testing can be performed adequately in infants, but interpreting the results can be problematic. Some infants have been particularly difficult to classify, such as those with 2 CF mutations and a sweat chloride value <40 mmol/L and those with only 1 CF mutation and a slightly elevated sweat chloride value. Although such infants represent only a small fraction of patients, they may be at risk for developing complications of CF and thus should be identified and followed.
The opportunity provided by NBS to diagnose individuals before symptoms appear and the ability to apply recently acquired knowledge of CF genotype and phenotype relationships to the diagnostic process clearly demonstrate the need for an improved algorithm for diagnosis. Toward this end, in 2007 the Cystic Fibrosis Foundation convened another diagnosis consensus committee of experts, including some members of the panel from 1996 together with representatives from the United States, Canada, Europe, and Australia. In addition to addressing the needs of the clinician faced with the infant with a positive screen, the meeting also provided an opportunity to apply the newly acquired tools to older patients with diagnostic uncertainty. This article presents consensus recommendations for a diagnosis of CF developed by the committee after reviewing recent data, including a diagnostic algorithm formulated by an international group of experts following a European consensus conference.10 In addition, it is intended to present guidance to physicians who are faced with disorders that are related to the partial loss of CFTR function but do not clearly meet the diagnostic criteria for CF. In the end, the diagnosis of CF must be based on good clinical judgment and, in rare cases, may become apparent only over time.
METHODS
Sweat Chloride Test
The measurement of sweat electrolyte concentrations has been the mainstay of diagnosing CF since a standardized procedure, known as the Gibson-Cooke method, was established in 1959.24 Subsequent analysis of isolated single sweat ducts identified chloride as the principle electrolyte affected in CF.25 The discovery of CFTR confirmed the role of electrolyte transport in the etiology of CF and gave a molecular rationale to the sweat test for diagnosing CF. Although the ability to test for CFTR gene mutations gives a new dimension to diagnosing CF, the sweat chloride test remains the standard procedure to confirm a CF diagnosis.
Test Methodology
Appropriate performance of the sweat test is crucial for the accurate diagnosis of CF. Therefore, the Cystic Fibrosis Foundation requires that sweat testing conducted at accredited CF care centers adheres to the standards recommended by a Cystic Fibrosis Foundation committee comprising CF center directors.26 The sweat test involves transdermal administration of pilocarpine by iontophoresis to stimulate sweat gland secretion, followed by collection and quantitation of sweat onto gauze or filter paper or into a Macroduct coil (Wescor Inc, Logan, Utah) and analysis of chloride concentration, as described by the Clinical Laboratory Standards Institute.27 Laboratories accredited by the College of American Pathologists also must follow the procedures and protocols outlined in the College’s Laboratory Accreditation Program Inspection Checklist.28 Because of the additional technical challenges involved in obtaining sweat from newborns, CF NBS algorithms, under local public health department regulations, often recommend that NBS-positive newborns undergo sweat testing only at a Cystic Fibrosis Foundation– certified laboratory.
Details on performing the sweat test can be found in the aforementioned documents. The Cystic Fibrosis Foundation’s diagnosis consensus committee highlighted some important aspects of sweat testing:
To increase the likelihood of collecting an adequate sweat specimen, it is recommended that sweat chloride testing in asymptomatic newborns with a positive CF NBS first be performed when the infant is at least 2 weeks of age and weighs >2 kg.29 In symptomatic newborns (eg, those with meconium ileus), sweat chloride can be evaluated as early as 48 hours after birth if adequate sweat can be collected,26,27 although the likelihood of inconclusive results may be greater at this age.29 A sufficient sweat volume is defined as at least 75 mg of sweat obtained by the Gibson-Cooke method or at least 15 μL obtained by the Macroduct coil collection method.26
Laboratories performing sweat testing should maintain an “insufficient sweat volume” collection rate of <5% for infants over age 3 months. Some data suggest that infants under age 3 months may be at greater risk for insufficient sweat volume collection,30 but this issue requires further investigation with different sweat collection methods before a standard recommendation can be established. Factors that influence sweat volume include age, sex, body weight, race, condition of the skin, and collection system used.29
Sweat chloride is the only analyte on which a diagnosis of CF should be based.
The sensitivity of chloride detection should be validated by the laboratory. The analytical method should be able to accurately detect sweat chloride at the lower end of the normal range (10 mmol/L) without the addition of extraneous chloride to artificially “boost” the sensitivity.
Sweat conductivity or osmolality should not be used for diagnosing CF at the present time. Although determining sweat conductivity by the Nanoduct device (Wescor Inc) may prove to be useful someday,31,32 so far it has produced a high rate of false-negative results.33 Currently, insufficient data are available on which to base a recommendation for its use in diagnosing CF.
Bilateral testing is suggested as a useful means of ensuring that at least 1 adequate sweat sample is obtained (although inadequate samples from 2 sites should never be pooled for analysis). The analysis of 2 separate samples from bilateral collection also could increase the reliability of the result, although it does not provide a substitute for a second sweat test, which is required sometimes.34
It is important that the laboratory performs the sweat collection and analysis on the same day and reports the results and their interpretation to clinicians and parents expeditiously. Standard procedure should be to perform the analysis within a few hours after collection. Although sweat collected in gauze, once reweighed and secured in a vial with a tightly fitting lid, has been shown to be stable for up to 72 hours at 4°C,35 storage time and conditions must be validated for stability by each laboratory individually. Validation of storage is especially important when the Macroduct system is used, because the stability of sweat collected in Macroduct coils has not yet been established.
Test Interpretation
Since the introduction of the original standardized sweat test methodology, universal definitions of normal (≤39 mmol/L), intermediate (40 to 59 mmol/L), and abnormal (≥ 60 mmol/L) sweat chloride values have been applied to all patients regardless of age (see below). This classification of sweat chloride ranges was initially affirmed through an examination of 7200 sweat tests performed between 1959 and 1966.36 Like most published reports of sweat chloride values, the technical aspects of the sweat test methodologies would not meet currently accepted guidelines, and the studies lacked truly healthy controls, making it difficult to derive a valid reference interval.37 But despite these limitations, the traditionally accepted sweat chloride reference range has generally proven satisfactory. In the 2005 Cystic Fibrosis Foundation Patient Registry, only 3.5% of patients with a diagnosis of CF had a sweat chloride value <60 mmol/L, and only 1.2% had a value <40 mmol/L.4
Although such traditionally accepted sweat chloride ranges appear to be adequate for diagnosing CF in children presenting with pancreatic exocrine insufficiency and suppurative lung disease, an increasing number of children are being identified as being at risk for CF in other ways. As NBS for CF becomes more widespread, it is anticipated that up to 90% of infants with CF, who frequently have no apparent symptoms of the disease, will be detected by age 6 weeks,38 creating an urgent need for an accurate reference range for sweat chloride in this age group.
Sweat Chloride Values in Infancy
Studies of sweat chloride testing in infants have demonstrated that the age at which testing is done is an important consideration when interpreting the sweat chloride value. Most infants identified by NBS will undergo sweat testing after 2 weeks of age. Earlier testing could lead to misleading results, because sweat chloride concentrations in healthy newborns gradually decrease over the first weeks of life.30 A study in 103 infants without CF found a mean sweat chloride value (±1 standard deviation) of 23.3 ± 5.7 mmol/L at age 3 to 7 days, decreasing to 17.6 ± 5.6 mmol/L by age 8 to 14 days and then to 13.1 ± 7.4 mmol/L after age 6 weeks.29 This gradual early decline in sweat chloride values suggests that sweat test results are less likely to be difficult to interpret after age 2 weeks. In a small number of individuals, sweat chloride values remain inconclusive for months or even years. Although extensive longitudinal data on sweat chloride testing in individuals age 2 weeks and older are not available, 1 small study compared sweat chloride values in 43 F508del (also known as ΔF508) heterozygous infants at age 6 weeks and again at age 6 to 12 months and found both increases and decreases in sweat chloride values between the 2 time points.39 Indeed, 2 infants with sweat chloride values of 40 to 50 mmol/L at age 6 weeks exhibited values in the clearly diagnostic range (≥60 mmol/L) when tested again at age 12 months. This indicates that repeat sweat testing is sometimes a necessary component of accurate diagnosis.
Reference values for sweat chloride in the first 3 months of life have largely been determined from a detailed study of 725 infants identified as being at risk through NBS or based on clinical presentation who carried 0, 1, or 2 copies of the common CFTR gene mutation F508del.40 All of the infants underwent a standardized sweat chloride test following methodology that meets current published guidelines. The infants without a CF phenotype and without F508del (n = 184) had a mean sweat chloride value of 10.6 ± 5.6 mmol/L; notably, only 1 of these infants had a sweat chloride value >30 mmol/L. Those infants who did not have a CF phenotype but were heterozygous for F508del (n = 128) had a mean sweat chloride value of 14.9 ± 8.4 mmol/L, or 1 SD above the mean for those infants who did not carry F508del; 9 of these infants had a sweat chloride value >30 mmol/L. Although no systematic follow-up of these infants was conducted to determine whether any could be diagnosed with CF, no cases of CF emerged from this cohort in the subsequent 10 years. Thus, it can be concluded that sweat chloride values >30 mmol/L can occur in healthy individuals who are heterozygous for F508del. All of the F508del homozygous infants had sweat chloride concentrations >60 mmol/L. The findings from this study have been supported by similar findings in studies from Australia41 and Massachusetts.30
Although sweat chloride values are generally ≥60 mmol/L in infants with CF, lower values also can occur.1,19,30,40,42–45 In a 4-year cohort of infants detected through the aforementioned Massachusetts NBS program who had clinician-diagnosed CF, 9 of 110 (8.2%) had a sweat chloride concentration of 30 to 59 mmol/L. and 3 of 110 (2.7%) had a concentration <30 mmol/L.11 The findings from this small but significant population lend further support to our recommendation (Figure) that a sweat chloride value ≥ 30 mmol/L in infants <age 6 months should be considered abnormal and trigger further patient evaluation.29,32,40–45
Recommended Sweat Chloride Reference Values in Infancy
Based on the available data on sweat chloride test results in healthy and CF-affected infants, the consensus committee recommends the following sweat chloride reference ranges for infants up to age 6 months: ≤29 mmol/L, CF unlikely; 30 to 59 mmol/L, intermediate; ≥60 mmol/L, indicative of CF (Figure). As more data emerge from NBS programs, the upper limit of the normal reference range may have to be lowered. Individuals with intermediate results should undergo repeat sweat chloride testing and then be referred to a CF center with expertise in diagnosing CF in infancy. Further evaluation should include an early detailed clinical assessment, more extensive CFTR gene mutation analysis, and repeat sweat chloride testing and follow-up at 6- to 12-month intervals until the diagnosis is clear.
Sweat Chloride Values Beyond Infancy
In addition to the burgeoning group of infants identified as at risk for CF through NBS, increasing recognition of the great variations in symptomatology of the disease is increasing the numbers of older children, adolescents, and adults in whom the diagnosis is being considered, including many with an indistinct CF phenotype. Clear sweat chloride reference intervals are required, but studies of normal sweat chloride values beyond infancy using current standardized testing procedures remain limited. One study in unaffected adults age 18 to 39 years found moderately elevated sweat chloride levels (average, 31 mmol/L; range, 14 to 48 mmol/L).46 A more rigorous study of sweat chloride values in 282 carefully screened healthy individuals age 5 to 68 years was recently completed in Australia.47 This study demonstrated that although the median sweat chloride value in each of the 7 age-based cohorts of volunteers was well below the value accepted as diagnostic for CF (≥60 mmol/L), the upper limit of the 95% confidence interval was in the intermediate range (40 to 59 mmol/L) for those over age 15 years and just under 60 mmol/L for those age 20 to 68 years, none of whom carried the F508del mutation. Three healthy subjects age 15 years and older had a sweat chloride value >60 mmol/L. These findings suggest that sweat chloride analysis alone may not be used to diagnose CF.
Although it is apparent that sweat chloride values ≥40 mmol/L can occur in individuals without CF, intermediate sweat chloride values (40 to 59 mmol/L) as well as, rarely, sweat chloride values <40 mmol/L also can occur in individuals with CF.48–51 In a Canadian study, sweat chloride values <60 mmol/L were observed in 5 of 24 patients (21%) with pancreatic-sufficient CF.52 Individuals diagnosed with CF as adults also have lower sweat chloride values;53 a study of the Cystic Fibrosis Foundation Registry found that 13.85% of individuals diagnosed with CF as adults had a value <60 mmol/L.48 Increasing recognition of the wide range of CF phenotypic variability54,55 should lead to increasing diagnosis of CF in individuals with intermediate sweat chloride values. These data add support to our recommendation that sweat chloride values ≥40 mmol/L in individuals over age 6 months should be considered beyond the normal range and merit further evaluation, to include repeat sweat chloride testing and DNA analysis for CFTR mutations as described later.
Recommended Sweat Chloride Reference Values Beyond Infancy
Based on the available data on sweat chloride test results beyond infancy, the consensus committee recommends the following sweat chloride reference ranges for individuals over age 6 months: ≤39 mmol/L, CF unlikely; 40 to 59 mmol/L, intermediate; ≥60 mmol/L, indicative of CF. Individuals with intermediate results should undergo repeat sweat chloride testing and further evaluation, including detailed clinical assessment and more extensive CFTR gene mutation analysis. Clinical follow-up should occur at 6-to 12-month intervals, and repeat sweat chloride testing should be performed periodically, particularly if a change in symptoms occurs, until the diagnosis is clear.
Role of DNA Analysis in CF Diagnosis
For the vast majority of persons with CF, the sweat chloride test remains the best diagnostic indicator. For those individuals with sweat chloride values in the intermediate range, DNA analysis can help establish the diagnosis.3 The analysis and interpretation of CF genotype information requires the use of appropriate testing techniques to identify CFTR mutations, standardized criteria for defining a CF-causing mutation, and an understanding of the contribution of the genetic background to the phenotypic variability of CF. It should be noted that 2 or more CFTR mutations detected in genomic DNA may be located in trans on 2 separate chromosomes or in cis on the same chromosome. The latter situation is not generally associated with disease. This distinction is not made in most commercial laboratories, however, and throughout the rest of the article, we assume that the trans arrangement applies.
Despite the potential usefulness of the information, acquiring a CF genotype can be difficult. Although currently available mutation screening panels can identify 90% of CFTR mutations, 9.7% of genotyped individuals in the Cystic Fibrosis Foundation Patient Registry have at least 1 un-identified mutation.4 Even commercially available “sequencing” tests provide information only about the coding region of the gene and the immediately adjacent intron sequences; large deletions or insertions and many RNA processing or transcriptional mutations are not readily identified. Identification of CF mutations is more challenging in some populations; for example, the nature, distribution, and frequency of CF-causing mutations in populations with Hispanic, African, or Asian origins differ markedly from those identified in Caucasians. Thus, for instance, the screening panel of CFTR mutations recommended by the American College of Medical Genetics (ACMG), which was developed for population or prenatal screening of Caucasians, detects only 68.5% of CF-causing mutations in the Hispanic population.56
Even if the genotype is identified, the consequences of the vast majority of CFTR mutations remain unknown. A mutation is simply a change from the accepted normal sequence of the gene and its control elements. To be considered a cause of CF, the mutation must:
Cause a change in amino acid sequence that severely affects CFTR synthesis or function; or
Introduce a premature termination signal; or
Alter invariant nucleotides of intron splice sites; or
Cause a novel amino acid sequence that does not occur in the normal CFTR genes from at least 100 carriers of CF mutations from the patient’s ethnic group.
Of the 1547 mutations currently listed in the CF Mutation Database (http://www.genet.sickkids.on.ca/cftr/app), 225 are designated as sequence variants with no resulting clinical effect. Of the remaining 1322 potential CF-causing mutations, only 23 (Table II) have been demonstrated by direct or empirical evidence to cause sufficient loss of CFTR function to confer CF disease and thus can be recommended as conclusive genetic evidence for diagnostic purposes. These mutations account for the defects in both CFTR genes in 85% of the CF population; the severe loss of CFTR function in these individuals usually results in pancreatic insufficiency (PI) and pulmonary complications. Many of the remaining 15% of individuals with CF have mutations with unknown effects on CFTR function. The Cystic Fibrosis Foundation is considering the feasibility of characterizing these CFTR mutations to determine the molecular basis for their effects on cell function. The knowledge gained should help scientists determine the usefulness of new targeted therapies that may potentiate channel performance or correct protein trafficking in individuals carrying these mutations, as well as aid diagnosis. As our understanding of the effects of different CFTR mutations develops, the list of mutations that provide acceptable diagnostic evidence will need to be expanded. In the meantime, extensive genetic analysis to identify large deletions or other obviously destructive mutations may be useful in resolving the diagnosis in individual cases.
Table II.
Recommended panel of CF-causing mutations
| Missense, deletion, stop mutations | Splicing, frameshift mutations | |||
|---|---|---|---|---|
| G85E | I507del | R560T | 621+1G>T | 2789+5G>A |
| R117H | F508del | R1162X | 711+1G>T | 3120+1G>A |
| R334W | G542X | W1282X | 1717−1G>A | 3659delC |
| R347P | G551D | N1303K | 1898+1G>A | 3849+10kbC>T |
| A455E | R553X | 2184delA | ||
Revised from the mutation panel for population screening for CF developed by the ACMG.77
Additional or alternative mutations present at significant frequencies in an ethnic population served by an NBS program may be added.
Because the effects of many mutations remain obscure, and because some allow pancreatic sufficiency (PS) due to a slight degree of residual chloride channel function, some individuals with these mutations can remain undiagnosed until adulthood. It may be difficult to distinguish these individuals from those with disease in single organs (eg, congenital absence of the vas deferens, idiopathic pancreatitis, various sinopulmonary disorders), who carry a higher frequency of CFTR gene mutations than the general population.16,57,58 An example of the complexity of mutation analysis is found in the evolving picture of individuals who are compound heterozygotes for a CF-causing mutation and the R117H mutation in the CFTR gene. The likelihood of CF in this group is driven by the length of a polythymidine tract in intron 8 of the R117H allele. The presence of a 5T tract in the R117H background is usually associated with CF, whereas R117H(7T) is more often associated with isolated male infertility or pancreatitis.59 But individuals from both groups may display sweat chloride values in the normal, intermediate, or diagnostic range,60 and some individuals with R117H(7T) can present with CF lung disease. Thus, R117H(7T) is a mutation that when present in trans with a CF-causing mutation, can cause a variable phenotype, ranging from normal to CF. Although the risk of poor outcomes should be weighed against the psychosocial risks of assigning a CF diagnosis,61,62 infants with a known CF-causing mutation (Table II) and R117H(7T) are at sufficiently high risk for lung disease to merit clinical monitoring in a CF care center.60,63
Some individuals with mutations in both copies of the CFTR gene who have partial phenotypes, designated CFTR-related disorders, eventually may receive a diagnosis of CF based on current diagnostic criteria. In others, a very mild or single system phenotype may allow definitive exclusion of the diagnosis.52,55 In a few patients, the diagnosis of CF cannot be confirmed or excluded. In effect, as knowledge of the range of phenotypes associated with CFTR gene mutations has expanded, the demarcation line between patients with CF disease and those with disorders associated with CFTR mutations has blurred. Thus, in this clinical setting, CF cannot be diagnosed simply by the presence of 2 CFTR mutations; these 2 mutations must cause significant loss of function to result in a CF clinical phenotype.
Regardless of the types of mutations found, with the possible exception of male infertility, genotype analysis cannot be used to predict prognosis in individual patients with CF.64,65 Although there is a strong relationship between some mutations and pancreatic function (PI or PS),66 the correlation is not absolute, and the relationship between genotype and pulmonary disease is weak. Even individuals carrying identical Fdel508 mutations on both alleles can exhibit a wide range of pulmonary function and severity of hepatobiliary disease.67 Work is underway to identify various modifier genes that also may play a role in the disease process,68 but the interaction between multiple modifier genes is likely to be complex, and at present, no diagnostic inferences can be drawn as of yet. Consequently, the consensus committee strongly recommends that caregivers avoid making prognostic predictions based on genotype information in any individual with CF.
Role of Ancillary Tests in CF Diagnosis
Ancillary tests may help establish a diagnosis of CF either by revealing a phenotype, such as PI, or by identifying an ion channel abnormality. Information regarding pancreatic exocrine function is valuable for both diagnostic and treatment purposes. Assessment of pancreatic function actually may be needed several times over an individual’s lifetime, because despite the presence of PS in at least 25% of all individuals with CF at the time of identification by NBS, most develop PI over time.18,22,69 A wide range of tests for assessing pancreatic function are available, but all have at least 1 shortcoming for routine clinical testing (eg, low specificity or sensitivity, complexity, high cost). When performed correctly, 72-hour stool collection is very useful for determining pancreatic function and evaluating response to enzyme therapy; however, this test is not used routinely, because of technical and logistical complexities. Alternative screening tests measure the fecal concentration of endogenous pancreatic enzymes. Because fecal trypsin and chymotrypsin tests may be inaccurate due to intraluminal degradation and cross-reactivity with ingested enzymes, the highly specific monoclonal test for fecal elastase, which is resistant to degradation, is preferred. Because of its ease of use, this test is recommended for evaluating pancreatic function at diagnosis and for monitoring individuals with PS.70 This test also has some significant limitations, however; although reference values have been determined for healthy preterm and full-term infants,71 the test has not been extensively studied in infants with CF. Currently, a value of <100 μg/g in individuals over age 2 to 3 years is considered indicative of PI. Higher values of fecal elastase (100 to 200 μg/g) are considered indicative of loss of pancreatic function, although not necessarily of sufficient severity to confer PI and warrant the need for pancreatic enzyme supplementation. Because reference values for fecal elastase measured by the polyclonal antibody test have not been established, and because the antibody displays some cross-reactivity with ingested enzymes, this test is considered less reliable. In patients with CF who are at least 7 to 8 years old, serum trypsinogen values also may be used to assess pancreatic function.72,73 Pancreatic stimulation tests are not indicated for routine assessment of pancreatic function in individuals with CF. Even though pancreatic lipase and co-lipase can be accurately measured to assess pancreatic status, pancreatic electrolyte (principally Cl− and HCO3−) values have not been validated, and reference values for the diagnosis of CF have not been sufficiently established.
Additional ancillary tests are currently in use by clinicians to clarify the diagnostic status of individuals with less CF-specific gastrointestinal or pulmonary symptomatology. The nasal potential difference (NPD) test, which has been used in CF research for decades, has recently been introduced to clinical practice to aid diagnosis;74 it may be particularly helpful in individuals with inconclusive sweat chloride values.75 CF is indicated by the presence of a high potential difference during baseline measurements plus a very low voltage response to zero-chloride perfusate and isoproterenol. An NPD test showing a significant response to zero-chloride perfusate containing isoproterenol may be useful in ruling out a diagnosis of CF. But the quantitative aspects of NPD results that are clearly indicative of CF are not defined consistently across all testing centers. Moreover, some overlap likely occurs between CF and non-CF values for both the basal PD and response to zero-chloride and isoproterenol, analogous to the overlap in sweat chloride values. The NPD test’s predictive capability improves somewhat when analyses of sodium and chloride channel abnormalities are combined. Nevertheless, to date only 12 US centers have been validated by the Cystic Fibrosis Foundation for reproducible, accurate NPD testing using standardized procedures, and only 1 center has sufficient expertise with infant NPD to make this test a useful adjunct to NBS.76 Properly conducted NPD testing at a research center can provide valuable information for diagnosis when clinical evidence is not clear-cut; however, access to the test is limited. Because there are no clear reference values, validation studies, or standardized technical protocols for NPD testing for diagnostic purposes, the test should be used only to provide contributory evidence in a diagnostic evaluation.
Intestinal ion channel measurements, such as Ussing chamber measurements of CFTR function from rectal biopsies, have no clearly established reference values and should be used for research purposes only at present.
Diagnosis of CF: Consensus Statement
Individuals with suspected CF are identified for diagnostic evaluation from different pathways, including prenatal screening or NBS. Diagnosis then may be made through various approaches, depending on age, genotype, and phenotype. Until the advent of widespread NBS for CF, suspicion for CF arose only from the appearance of symptoms or a family history of the disease. But eventually, NBS for CF will be universal throughout the United States, and most individuals will enter the diagnostic algorithm because of a positive NBS.
The primary test for confirming the diagnosis of CF is the sweat chloride test, performed according to the guidelines described earlier. Any individual presenting with signs or symptoms of CF (Table I) should undergo sweat chloride testing, regardless of the NBS results. To increase the likelihood of a successful test in infants, a bilateral sweat chloride test should be performed on individuals who weigh at least 2 kg, are more than 36 weeks gestation at birth, and are at least 2 weeks of age. This section presents diagnostic process recommendations for newborns with positive CF NBS, followed by recommendations for individuals presenting through other means.
Recommended CF Diagnostic Process for Screened Newborns (Figure)
In infants with a positive NBS, a diagnosis of CF can be confirmed if the sweat chloride value is ≥60 mmol/L. These patients are likely to experience life-threatening CF lung disease at varying ages. CFTR mutation assessment is recommended to help confirm the diagnosis and reduce the risk of laboratory error if genotype was not identified during NBS. The panel of 23 mutations recommended by the ACMG in their revised list for population screening77 represents mutations known to cause CF (Table II) and is reasonable for use in most states at present. In the absence of 2 CF-causing mutations, sweat chloride testing should be repeated to ensure a correct diagnosis.
A diagnosis of CF is very unlikely in infants with a sweat chloride value ≤29 mmol/L. However, on rare occasions, infants with 2 identified CF-causing mutations can have a normal sweat chloride value;11,51,63 these infants have CF and should be followed in a CF care center. Analysis of maternal DNA is recommended to determine whether the 2 mutations are present in cis or in trans and to help clarify the diagnosis.
-
Infants with a positive CF NBS result and sweat chloride values in the intermediate range (30 to 59 mmol/L) should undergo at least a basic CFTR gene mutation assessment (Table II) at the initial visit if it is not included in the NBS:
In the presence of 2 CF-causing mutations, a diagnosis of CF can be made. Maternal DNA should be analyzed if necessary to determine whether the 2 mutations are present in cis or in trans.
-
Infants with intermediate sweat chloride values and no or 1 CF-causing mutation cannot be diagnosed definitively with CF. This is an evolving recommendation that will become clearer as experience with NBS accumulates nationwide. Meanwhile, these infants are at increased risk for CF, as demonstrated by the abnormal NBS and increased sweat chloride value, and should be followed. Because clinical manifestations of CF can appear in the first few weeks of life,78,79 a clinical assessment should be performed at a CF care center by age 2 months, and the sweat chloride test should be repeated at age 2 to 6 months; the earlier time might allow resolution of the diagnosis for many families. The diagnosis of CF is then made if it is supported by a preponderance of evidence from a variety of assessments, as described later. To prevent possible cross-infection, the infant should not be assessed in a clinic attended by individuals known to have CF. Assessments may include the following:
Clinical assessment, including anthropometrics, growth, and state of the lungs
Personal medical history
Family history of CF and related phenotypes
Extended genetic testing, if required
Pancreatic function tests
Oropharyngeal culture for CF-associated pathogens, especially Pseudomonas aeruginosa
Chest radiograph
Liver function tests.
Infant pulmonary function testing, chest computed tomography (CT), bronchoalveolar lavage, and NPD measurements are not recommended for diagnosis in this age group. Clinical findings suggestive of CF, such as fecal elastase values indicative of pancreatic damage (<200 μg/g) and a positive respiratory tract culture for CF-associated pathogens, especially P aeruginosa, should be considered indicative of CF. The presence of Staphylococcus aureus or Hemophilus influenzae is not a sufficient indication for diagnosis in the absence of other features of CF. The sweat chloride test should be repeated at age 2 to 6 months. Beyond age 6 months, only sweat chloride values ≥40 mmol/L should be considered beyond the normal range. The committee selected age 6 months as the time for transition to acceptance of a higher range of normal sweat chloride values by consensus. This choice was necessarily somewhat subjective, because very little data are available on the normal values for this age group; data from NBS programs are usually focused on the first few months of life.
An increase in the sweat chloride value to ≥60 mmol/L confirms a diagnosis of CF.
A sweat chloride value ≤39 mmol/L after age 6 months generally is not consistent with a diagnosis of CF, although CF can occur in this group in rare cases.50,51,60,63
If the sweat chloride value remains in an intermediate range (ie, 40 to 59 mmol/L for individuals age 6 months and older), the child should continue to be monitored every 6 to 12 months with clinical evaluations, repeat sweat testing, and use of ancillary tests as appropriate. In the presence of 1 CF-causing mutation and clinical findings suggestive of CFTR dysfunction (eg, bronchiectasis, pancreatitis), a diagnosis of a CFTR-related disorder can be made. Over time, further evaluation may confirm a diagnosis of CF in some of these individuals.
Families of infants diagnosed with CF should receive appropriate education at the first diagnostic visit, and genetic counseling should be provided. Sweat chloride testing should be arranged for all first-degree siblings and for any half-siblings with signs or symptoms of CF or who have 2 parents known to be carriers. In addition, genetic analysis should be provided for these family members if the diagnosed infant demonstrates a sweat chloride value ≤59 mmol/L.
Recommended General Process for Diagnosing CF
In individuals presenting with symptoms of CF (Table I) or a positive family history, the following diagnostic process is recommended:
A diagnosis of CF can be made if the sweat chloride value is ≥60 mmol/L. A second, confirmatory sweat chloride test is recommended unless mutation analysis identifies the presence of 2 CF-causing mutations (Table II). These patients, who may present at any age, are likely to develop CF lung disease.
A sweat chloride value ≤39 mmol/L in individuals over age 6 months is not consistent with a diagnosis of CF. CF is unlikely in this group. However, 2 identified CF-causing mutations can occur in this group; these individuals have CF and should be followed in a CF care center.
-
Individuals with sweat chloride values in the intermediate range (30 to 59 mmol/L for infants under age 6 months; 40 to 59 mmol/L for older individuals) should undergo extensive CFTR mutation analysis (ie, expanded panel of CFTR mutations, evaluation for deletions, or gene sequencing):
In the presence of 2 CF-causing mutations, a diagnosis of CF can be made.
-
Individuals with no or 1 CF-causing mutation and clinical findings suggestive of CFTR dysfunction (ie, obstructive azoospermia, bronchiectasis, or acute, recurrent, or chronic pancreatitis) may be diagnosed with a CFTR-related disorder, depending on their clinical picture or family history, and are at risk for CF. Sweat chloride testing should be repeated in infants by age 2 to 6 months and immediately in older individuals. If sweat chloride values remain in the intermediate range on repeat testing, then further assessment should be performed at a CF care center that can provide basic and ancillary testing to clarify the diagnosis, including:
Clinical assessment
Expanded genetic testing
Exocrine pancreatic function tests
Respiratory tract culture for CF-associated pathogens, especially P aeruginosa.
Depending on clinical presentation, assessment also may include ancillary tests, such as:
Genital evaluation in males (ie, genital examination, rectal ultrasound, semen analysis)
Pancreatic imaging
High-resolution chest CT
Bronchoalveolar lavage, including microbiology assessment
Pulmonary function testing (not routinely recommended in infants at this time)
NPD testing
Exclusionary testing for ciliary dyskinesia and immune deficiency.
Significant clinical signs or symptoms of CF, laboratory indication of PI, or a positive culture for a CF-associated pathogen (especially P aeruginosa), should be considered strongly suggestive of CF. Individuals who have sweat chloride values in the intermediate range and exhibit no significant signs of CF should be monitored periodically for the appearance of symptoms until the diagnosis can be ruled in or out.
CONCLUSION
The diagnostic procedures recommended herein recognize the wide possible range of disease severity and permit some leeway in the diagnosis of an individual while still creating a threshold for a diagnosis of CF. As was the case in 1996, the recommendations are based on the current state of the knowledge and should be considered a “work in progress,” leaving room for improvement resulting from increased insight into CF manifestations, genetics, and pathobiology. Nevertheless, it is hoped that the consensus of opinion presented herein will provide increased guidance for establishing or excluding a diagnosis of CF, thereby permitting timely access to vital medical services and allowing the best possible outcomes for individuals with the disease.
Acknowledgments
Supported by funds from the Cystic Fibrosis Foundation.
The authors thank Cynthia Adams, Associate Director of Medical Meetings, and Andrea Waterman, Coordinator of Medical Meetings for the Cystic Fibrosis Foundation.
- ACMG
American College of Medical Genetics
- CF
Cystic fibrosis
- CFTR
Cystic fibrosis transmembrane conductance regulator
- CT
Computed tomography
- IRT
Immunoreactive trypsinogen
- NBS
Newborn screening
- NPD
Nasal potential difference
- PCP
Primary care provider
- PI
Pancreatic insufficiency
- PS
Pancreatic sufficiency
Footnotes
No reprints are available from the authors.
Changes in CF Descriptive Terminology
Attempts to classify individuals with CF based on sweat chloride values are not as useful as was envisioned originally.80 CF lung disease, the main cause of morbidity and mortality, has been identified in every group. Furthermore, patients diagnosed with CF as newborns who then receive the recommended specialized care may have a delay in pulmonary involvement for decades. Thus, such classification schemes as “atypical” or “typical,” “mild” or “severe,” and “classical” or “nonclassical” are not recommended. The authors recognize that some of these terms are embedded in the literature and that NBS programs will continue to use “classical” and “atypical,” but as time passes the clinical distinctions will not be sharp enough to sustain such terminology. Although once considered an unambiguous disease entity resulting in death in early childhood, CF is now known to cause a wide spectrum of disease, and determining an individual’s prognosis is not possible using currently available tools. In fact, individuals who initially display few deleterious health effects can develop severe disease in 1 or more organ systems. Therefore, careful monitoring and timely treatment are crucial for all affected individuals.
Other conference participants included Leslie Hazle, RN, Cystic Fibrosis Foundation, Bethesda, MD; Michael Knowles, MD, University of North Carolina, Chapel Hill, NC; Bruce Marshall, MD, Cystic Fibrosis Foundation, Bethesda, MD; Mark Montgomery, MD, Alberta Children’s Hospital, Calgary, Alberta, Canada; Clement Ren, MD, University of Rochester, Rochester, NY; and Robert Wilmott, MD, Saint Louis University School of Medicine, St. Louis, MO.
AUTHOR DISCLOSURES
Philip M. Farrell, MD, PhD serves the Cystic Fibrosis Foundation as national facilitator for implementation of newborn screening and receives compensation for his efforts. Terry B. White, PhD who compiled the first draft of the manuscript, is an employee of the Cystic Fibrosis Foundation. Garry R. Cutting, MD holds patents on 2 of the CFTR mutations in the ACMG Panel suggested in this article for use in DNA analysis (Table II), as well as patents on CFTR mutations not included in the ACMG Panel. The following authors have no financial arrangement or affiliation with a corporate organization or a manufacturer of a product discussed in this supplement: Beryl J. Rosenstein, MD, Frank J. Accurso, MD, Carlo Castellani, MD, Peter R. Durie, MD, FRCP, Vicky A. LeGrys, DrA, CLS, John Massie, MBBS, FRACP, PhD, Richard B. Parad, MD, MPH, Michael J. Rock, MD, and Preston W. Campbell, III, MD.
References
- 1.Sontag MK, Hammond KB, Zielenski J, Wagener JS, Accurso FJ. Two-tiered immunoreactive trypsinogen (IRT/IRT)-based newborn screening for cystic fibrosis in Colorado: screening efficacy and diagnostic outcomes. J Pediatr. 2005;147(Suppl):S83–8. doi: 10.1016/j.jpeds.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Parad RB, Comeau AM. Newborn screening for cystic fibrosis. Pediatr Ann. 2003;32:528–35. doi: 10.3928/0090-4481-20030801-10. [DOI] [PubMed] [Google Scholar]
- 3.Comeau AM, Parad RB, Dorkin HL, Dovey M, Gerstle R, Haver K, et al. Population-based newborn screening for genetic disorders when multiple mutation DNA testing is incorporated: a cystic fibrosis newborn screening model demonstrating increased sensitivity but more carrier detections. Pediatrics. 2004;113:1573–81. doi: 10.1542/peds.113.6.1573. [DOI] [PubMed] [Google Scholar]
- 4.Cystic Fibrosis Foundation Patient Registry. Annual Data Report to the Center Directors. Bethesda, MD: Cystic Fibrosis Foundation; 2005. [Google Scholar]
- 5.Farrell PM, Lai HC, Li Z, Kosorok MR, Laxova A, Green CG, et al. Evidence on improved outcomes with early diagnosis of cystic fibrosis through neonatal screening: enough is enough! J Pediatr. 2005;147(Suppl):S30–6. doi: 10.1016/j.jpeds.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Dankert-Roelse JE, Mérelle ME. Review of outcomes of neonatal screening for cystic fibrosis versus non-screening in Europe. J Pediatr. 2005;147(Suppl):S15–20. doi: 10.1016/j.jpeds.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Rosenfeld M. Overview of published evidence on outcomes with early diagnosis from large US observational studies. J Pediatr. 2005;147(Suppl):S11–4. doi: 10.1016/j.jpeds.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Accurso FJ, Sontag MK, Wagener JS. Complications associated with symptomatic diagnosis in infants with cystic fibrosis. J Pediatr. 2005;147(Suppl):S37–41. doi: 10.1016/j.jpeds.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 9.Palomaki GE, Haddow JE, Bradley LA, FitzSimmons SC. Updated assessment of cystic fibrosis mutation frequencies in non-Hispanic Caucasians. Genet Med. 2002;4:90–4. doi: 10.1097/00125817-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 10.De Boeck K, Wilschanski M, Castellani C, Taylor C, Cuppens H, Dodge J, et al. Cystic fibrosis: terminology and diagnostic algorithms. Thorax. 2006;61:627–35. doi: 10.1136/thx.2005.043539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parad RB, Comeau AM. Diagnostic dilemmas resulting from the immunoreactive trypsinogen/DNA cystic fibrosis newborn screening algorithm. J Pediatr. 2005;147(Suppl):S78–82. doi: 10.1016/j.jpeds.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Rosenstein B, Cutting G. The diagnosis of cystic fibrosis: a consensus statement. Cystic Fibrosis Foundation Consensus Panel. J Pediatr. 1998;132:589–95. doi: 10.1016/s0022-3476(98)70344-0. [DOI] [PubMed] [Google Scholar]
- 13.Alper OM, Wong LJ, Young S, Pearl M, Graham S, Sherwin J, et al. Identification of novel and rare mutations in California Hispanic and African-American cystic fibrosis patients. Hum Mutat. 2004;24:353. doi: 10.1002/humu.9281. corr 2005;25:223. [DOI] [PubMed] [Google Scholar]
- 14.Groman JD, Karczeski B, Sheridan M, Robinson TE, Fallin MD, Cutting GR. Phenotypic and genetic characterization of patients with features of ”nonclassic” forms of cystic fibrosis. J Pediatr. 2005;146:675–80. doi: 10.1016/j.jpeds.2004.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mickle JE, Cutting GR. Genotype–phenotype relationships in cystic fibrosis. Med Clin North Am. 2000;84:597–607. doi: 10.1016/s0025-7125(05)70243-1. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Moylan B, Leopold DA, Kim J, Rubenstein RC, Togias A, et al. Mutation in the gene responsible for cystic fibrosis and predisposition to chronic rhinosinusitis in the general population. JAMA. 2000;284:1814–9. doi: 10.1001/jama.284.14.1814. [DOI] [PubMed] [Google Scholar]
- 17.Feranchak AP. Hepatobiliary complications of cystic fibrosis. Curr Gastroenterol Rep. 2004;6:231–9. doi: 10.1007/s11894-004-0013-6. [DOI] [PubMed] [Google Scholar]
- 18.Cipolli M, Castellani C, Wilcken B, Massie J, McKay K, Gruca M, et al. Pancreatic phenotype in cystic fibrosis patients identified by mutation screening. Arch Dis Child. 2007;92:842–6. doi: 10.1136/adc.2006.107581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comeau AM, Accurso FJ, White TB, Campbell PW, III, Hoffman G, Parad RB, et al. Guidelines for implementation of cystic fibrosis newborn screening programs: Cystic Fibrosis Foundation workshop report. Pediatrics. 2007;119:495–518. doi: 10.1542/peds.2006-1993. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Newborn screening for cystic fibrosis: evaluation of benefits and risks and recommendations for state newborn screening programs. Morb Mortal Recomm Rep. 2004;53(RR13):1–36. [PubMed] [Google Scholar]
- 21.Wilcken B, Wiley V, Sherry G, Bayliss U. Neonatal screening for cystic fibrosis: a comparison of two strategies for case detection in 1.2 million babies. J Pediatr. 1995;127:965–70. doi: 10.1016/s0022-3476(95)70040-4. [DOI] [PubMed] [Google Scholar]
- 22.Waters DL, Dorney SF, Gaskin KJ, Gruca MA, O’Halloran M, Wilcken B. Pancreatic function in infants identified as having cystic fibrosis in a neonatal screening program. N Engl J Med. 1990;322:303–8. doi: 10.1056/NEJM199002013220505. [DOI] [PubMed] [Google Scholar]
- 23.Farrell PM, Kosorok MR, Laxova A, Shen G, Koscik RE, Bruns WT, et al. Nutritional benefits of neonatal screening for cystic fibrosis. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. N Engl J Med. 1997;337:963–9. doi: 10.1056/NEJM199710023371403. [DOI] [PubMed] [Google Scholar]
- 24.Gibson LE, Cooke RE. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics. 1959;23:545–9. [PubMed] [Google Scholar]
- 25.Quinton PM. Chloride impermeability in cystic fibrosis. Nature. 1983;301:421–2. doi: 10.1038/301421a0. [DOI] [PubMed] [Google Scholar]
- 26.Legrys VA, Yankaskas JR, Quittell LM, Marshall BC, Mogayzel PJ., Jr Diagnostic sweat testing: the Cystic Fibrosis Foundation guidelines. J Pediatr. 2007;151:85–9. doi: 10.1016/j.jpeds.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Clinical Laboratory Standards Institute (formerly National Committee for Clinical Laboratory Standards) Approved guideline. National Committee for Clinical Laboratory Standards; 2000. Sweat testing: sample collection and quantitative analysis. Document C34-A2. [Google Scholar]
- 28.College of American Pathologists. [Accessed November 6, 2007];Chemistry checklist, laboratory accreditation program. Available from: http://www.cap.org/apps/docs/laboratory_accreditation/checklists/chemistry_and_toxicology_april2006.pdf.
- 29.Eng W, LeGrys VA, Schechter MS, Laughon MM, Barker PM. Sweat-testing in preterm and full-term infants less than 6 weeks of age. Pediatr Pulmonol. 2005;40:64–7. doi: 10.1002/ppul.20235. [DOI] [PubMed] [Google Scholar]
- 30.Parad RB, Comeau AM, Dorkin HL, Dovey M, Gerstle R, Martin T, et al. Sweat testing newborn infants detected by cystic fibrosis newborn screening. J Pediatr. 2005;147(Suppl):S69–72. doi: 10.1016/j.jpeds.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 31.Funk M, LeGrys VA. Testing diagnostic tests: why size matters. J Pediatr. 2005;146:159–62. doi: 10.1016/j.jpeds.2004.10.054. [DOI] [PubMed] [Google Scholar]
- 32.Barben J, Ammann RA, Metlagel A, Schoeni MH. Conductivity determined by a new sweat analyzer compared to chloride concentrations for the diagnosis of cystic fibrosis. J Pediatr. 2005;146:183–8. doi: 10.1016/j.jpeds.2004.08.085. [DOI] [PubMed] [Google Scholar]
- 33.Losty HC, Wheatly H, Doull I. The evaluation of a novel conductometric device for the diagnosis of cystic fibrosis. Ann Clin Biochem. 2006;43:375–81. doi: 10.1258/000456306778520025. [DOI] [PubMed] [Google Scholar]
- 34.LeGrys VA. Assessing quality assurance for sweat chloride testing. Clin Lab Sci. 1992;5:354–7. [PubMed] [Google Scholar]
- 35.LeGrys VA. Stability of chloride in sweat testing. Clin Lab Sci. 1993;6:156–7. [Google Scholar]
- 36.Shwachman H, Mahmoodian A. Pilocarpine iontophoresis sweat testing: results of seven years’ experience. In: Rossi E, Stoll E, editors. Modern Problems in Pediatrics. Basel: Karger; 1967. pp. 158–82. [PubMed] [Google Scholar]
- 37.Mishra A, Greaves R, Massie J. The limitations of sweat electrolyte reference intervals for the diagnosis of cystic fibrosis: a systematic review. Clin Biochem Rev. 2007;28:60–76. [PMC free article] [PubMed] [Google Scholar]
- 38.Massie RJ, Olsen M, Glazner J, Robertson CF, Francis I. Newborn screening for cystic fibrosis in Victoria: 10 years’ experience (1989–1998) Med J Aust. 2000;172:584–7. doi: 10.5694/j.1326-5377.2000.tb124123.x. [DOI] [PubMed] [Google Scholar]
- 39.Massie RJH, Gaskin KJ, Van Asperen PP, Bayliss U, Wilcken B. The reliability of sweat testing at six weeks following newborn screening for cystic fibrosis in NSW, 1995–1996 [abstract] Am J Resp Crit Care Med. 1998;157:126. [Google Scholar]
- 40.Farrell PM, Koscik RE. Sweat chloride concentrations in infants homozygous or heterozygous for ΔF508 cystic fibrosis. Pediatrics. 1996;97:524–8. [PubMed] [Google Scholar]
- 41.Massie J, Gaskin K, Van Asperen P, Wilcken B. Sweat testing following newborn screening for cystic fibrosis. Pediatr Pulmonol. 2000;29:452–6. doi: 10.1002/(sici)1099-0496(200006)29:6<452::aid-ppul7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 42.Padoan RAB, Seia M, Corbetta C. Negative sweat test in hypertrypsinaemic infants with cystic fibrosis carrying rare CFTR mutations. Eur J Pediatr. 2002;161:212–5. doi: 10.1007/s00431-001-0910-8. [DOI] [PubMed] [Google Scholar]
- 43.Massie J, Clements B. Diagnosis of cystic fibrosis after newborn screening: the Australasian experience. Twenty years and five million babies later: a consensus statement from the Australasian Paediatric Respiratory Group. Pediatr Pulmonol. 2005;39:440–6. doi: 10.1002/ppul.20191. [DOI] [PubMed] [Google Scholar]
- 44.Taccetti G, Festini F, Braccini G, Campana S, deMartino M. Sweat testing in newborns positive to neonatal screening for cystic fibrosis. Arch Dis Child Fetal Neonatal Ed. 2004;89:F463–4. doi: 10.1136/adc.2003.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rock MJ, Hoffman G, Laessig RH, Kopish GJ, Litsheim TJ, Farrell PM. Newborn screening for cystic fibrosis in Wisconsin: nine years experience with routine trypsinogen/DNA testing. J Pediatr. 2005;147(Suppl):S73–7. doi: 10.1016/j.jpeds.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Hall SK, Stableforth DE, Green A. Sweat sodium and chloride concentrations: essential criteria for the diagnosis of cystic fibrosis in adults. Ann Clin Biochem. 1990;27 (Pt 4):318–20. doi: 10.1177/000456329002700406. [DOI] [PubMed] [Google Scholar]
- 47.Mishra A, Greaves R, Massie J. Sweat electrolytes: Establishing a reference range in adolescents and adults [abstract] Aust J Med Sci. 2006;27:171. [Google Scholar]
- 48.Rock M, Makholm L, Lai H, Cheng Y. Distribution of sweat chloride values in patients with cystic fibrosis [abstract] Pediatr Pulmonol. 2003;25(Suppl):337. [Google Scholar]
- 49.Highsmith WE, Burch LH, Zhou Z, Olsen JC, Boat TE, Spock A, et al. A novel mutation in the cystic fibrosis gene in patients with pulmonary disease but normal sweat chloride concentrations. N Engl J Med. 1994;331:974–80. doi: 10.1056/NEJM199410133311503. [DOI] [PubMed] [Google Scholar]
- 50.Stewart B, Zabner J, Shuber A, Welsh MJ, McCray PB. Normal sweat chloride values do not exclude the diagnosis of cystic fibrosis. Am J Respir Crit Care Med. 1995;151:899–903. doi: 10.1164/ajrccm/151.3_Pt_1.899. [DOI] [PubMed] [Google Scholar]
- 51.Lebecque P, Leal T, De Boeck C, Jaspers M, Cuppens H, Cassiman JJ. Mutations of the cystic fibrosis gene and intermediate sweat chloride levels in children. Am J Respir Crit Care Med. 2002;165:757–61. doi: 10.1164/ajrccm.165.6.2104073. [DOI] [PubMed] [Google Scholar]
- 52.Wilschanski M, Dupuis A, Ellis L, Jarvi K, Zielenski J, Tullis E, et al. Mutations in the cystic fibrosis transmembrane regulator gene and in vivo transepithelial potentials. Am J Respir Crit Care Med. 2006;174:787–94. doi: 10.1164/rccm.200509-1377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gilljam M, Ellis L, Corey M, Zielenski J, Durie P, Tullis DE. Clinical manifestations of cystic fibrosis among patients with diagnosis in adulthood. Chest. 2004;126:1215–24. doi: 10.1378/chest.126.4.1215. [DOI] [PubMed] [Google Scholar]
- 54.Nick JA, Rodman DM. Manifestations of cystic fibrosis diagnosed in adulthood. Curr Opin Pulm Med. 2005;11:513–8. doi: 10.1097/01.mcp.0000183052.56728.76. [DOI] [PubMed] [Google Scholar]
- 55.Bishop MD, Freedman SD, Zielenski J, Ahmed N, Dupuis A, Martin S, et al. The cystic fibrosis transmembrane conductance regulator gene and ion channel function in patients with idiopathic pancreatitis. Hum Genet. 2005;118:372–81. doi: 10.1007/s00439-005-0059-z. [DOI] [PubMed] [Google Scholar]
- 56.Wong LJ, Wang J, Zhang YH, Hsu E, Heim RA, Bowman CM, et al. Improved detection of CFTR mutations in Southern California Hispanic CF patients. Hum Mutat. 2001;18:296–307. doi: 10.1002/humu.1191. [DOI] [PubMed] [Google Scholar]
- 57.Pignatti PF, Bombieri C, Marigo C, Benetazzo M, Luisetti M. Increased incidence of cystic fibrosis gene mutations in adults with disseminated bronchiectasis. Hum Mol Genet. 1995;4:635–9. doi: 10.1093/hmg/4.4.635. [DOI] [PubMed] [Google Scholar]
- 58.Miller PW, Hamosh A, Macek M, Jr, Greenberger PA, MacLean J, Walden SM, et al. Cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations in allergic bronchopulmonary aspergillosis. Am J Hum Genet. 1996;59:45–51. [PMC free article] [PubMed] [Google Scholar]
- 59.Kiesewetter S, Macek M, Jr, Davis C, Curristin SM, Chu CS, Graham C, et al. A mutation in CFTR produces different phenotypes depending on chromosomal background. Nat Genet. 1993;5:274–8. doi: 10.1038/ng1193-274. [DOI] [PubMed] [Google Scholar]
- 60.Massie RJ, Poplawski N, Wilcken B, Goldblatt J, Byrnes C, Robertson C. Intron-8 polythymidine sequence in Australasian individuals with CF mutations R117H and R117C. Eur Respir J. 2001;17:1195–200. doi: 10.1183/09031936.01.00057001. [DOI] [PubMed] [Google Scholar]
- 61.Scotet V, Audrezet M-P, Roussey M, Rault G, Dirou-Prigent, Journel H, et al. Immunoreactive trypsin/DNA newborn screening for cystic fibrosis: should the R117H variant be included in CFTR mutation panels? Pediatrics. 2006;118:e1523–9. doi: 10.1542/peds.2005-3161. [DOI] [PubMed] [Google Scholar]
- 62.Chmiel JF, Drumm ML, Konstan MW, Ferkol TW, Kercsmar CM. Pitfalls in the use of genotype analysis as the sole diagnostic criterion for cystic fibrosis. Pediatrics. 1999;103:823–6. doi: 10.1542/peds.103.4.823. [DOI] [PubMed] [Google Scholar]
- 63.O’Sullivan BP, Zwerdling RG, Dorkin HL, Comeau AM, Parad R. Early pulmonary manifestation of cystic fibrosis in children with the deltaF508/R117H-7T genotype. Pediatrics. 2006;118:1260–5. doi: 10.1542/peds.2006-0399. [DOI] [PubMed] [Google Scholar]
- 64.Sokol RZ. Infertility in men with cystic fibrosis. Curr Opin Pulm Med. 2001;7:421–6. doi: 10.1097/00063198-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 65.Dörk T, Dworniczak B, Aulehla-Scholz C, Wieczorek D, Böhm I, Mayerova A, et al. Distinct spectrum of CFTR gene mutations in congenital absence of vas deferens. Hum Genet. 1997;100:365–77. doi: 10.1007/s004390050518. [DOI] [PubMed] [Google Scholar]
- 66.Durie PR. Pathophysiology of the pancreas in cystic fibrosis. Neth J Med. 1992;41:97–100. [PubMed] [Google Scholar]
- 67.Kerem E, Reisman J, Corey M, Canny GJ, Levison H. Prediction of mortality in patients with cystic fibrosis. N Engl J Med. 1992;326:1187–91. doi: 10.1056/NEJM199204303261804. [DOI] [PubMed] [Google Scholar]
- 68.Drumm ML, Konstan MW, Schluchter MD, Handler A, Pace R, Zou F, et al. Genetic modifiers of lung disease in cystic fibrosis. N Engl J Med. 2005;353:1443–53. doi: 10.1056/NEJMoa051469. [DOI] [PubMed] [Google Scholar]
- 69.Bronstein MN, Sokol RJ, Abman SH, Chatfield BA, Hammond KB, Hambridge KM, et al. Pancreatic insufficiency, growth, and nutrition in infants identified by newborn screening as having cystic fibrosis. J Pediatr. 1992;120:533–40. doi: 10.1016/s0022-3476(05)82478-3. [DOI] [PubMed] [Google Scholar]
- 70.Daftary A, Acton J, Heubi J, Amin R. Fecal elastase-1: utility in pancreatic function in cystic fibrosis. J Cyst Fibros. 2006;5:71–6. doi: 10.1016/j.jcf.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 71.Kori M, Maayan-Metzger A, Shamir R, Sirota L, Dinari G. Faecal elastase 1 levels in premature and full-term infants. Arch Dis Child Fetal Neonatal Ed. 2003;88:F106–8. doi: 10.1136/fn.88.2.F106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Durie PR, Forstner GG, Gaskin KJ, Moore DJ, Cleghorn GJ, Wong SS, et al. Age-related alterations of immunoreactive pancreatic cationic trypsinogen in sera from cystic fibrosis patients with and without pancreatic insufficiency. Pediatr Res. 1986;20:209–13. doi: 10.1203/00006450-198603000-00002. [DOI] [PubMed] [Google Scholar]
- 73.Sontag MK, Corey M, Hokanson JE, Marshall JA, Sommer SS, Zerbe GO, et al. Genetic and physiologic correlates of longitudinal immunoreactive trypsinogen decline in infants with cystic fibrosis identified through newborn screening. J Pediatr. 2006;149:650–7. doi: 10.1016/j.jpeds.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 74.Knowles MR, Paradiso AM, Boucher RC. In vivo nasal potential difference: techniques and protocols for assessing efficacy of gene transfer in cystic fibrosis. Hum Gene Ther. 1995;6:445–55. doi: 10.1089/hum.1995.6.4-445. [DOI] [PubMed] [Google Scholar]
- 75.Wilson DC, Ellis L, Zielenski J, Corey M, Ip WF, Tsui LC, et al. Uncertainty in the diagnosis of cystic fibrosis: possible role of in vivo nasal potential difference measurements. J Pediatr. 1998;132:596–9. doi: 10.1016/s0022-3476(98)70345-2. [DOI] [PubMed] [Google Scholar]
- 76.Standaert TA, Boitano L, Emerson J, Milgram LJ, Konstan MW, Hunter J, et al. Standardized procedure for measurement of nasal potential difference: an outcome measure in multicenter cystic fibrosis clinical trials. Pediatr Pulmonol. 2004;37:385–92. doi: 10.1002/ppul.10448. [DOI] [PubMed] [Google Scholar]
- 77.Watson MS, Cutting GR, Desnick RJ, Driscoll DA, Klinger K, Mennuti M, et al. Cystic fibrosis population carrier screening: 2004 revision of the American College of Medical Genetics mutation panel. Genet Med. 2004;6:387–91. doi: 10.1097/01.GIM.0000139506.11694.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sims EJ, Clark A, McCormick J, Mehta G, Connett G, Mehta A. Cystic fibrosis diagnosed after 2 months of age leads to worse outcomes and requires more therapy. Pediatrics. 2007;119:19–28. doi: 10.1542/peds.2006-1498. [DOI] [PubMed] [Google Scholar]
- 79.Farrell PM. The meaning of “early” diagnosis in a new era of cystic fibrosis care. Pediatrics. 2007;119:156–7. doi: 10.1542/peds.2006-3074. [DOI] [PubMed] [Google Scholar]
- 80.Kerem E, Corey M, Kerem BS, Rommens J, Markiewicz D, Levison H, et al. The relation between genotype and phenotype in cystic fibrosis: analysis of the most common mutation (delta F508) N Engl J Med. 1990;323:1517–22. doi: 10.1056/NEJM199011293232203. [DOI] [PubMed] [Google Scholar]