Abstract
Objective
To report the first tertiary monosomy in a pregnancy loss to a female t(11;22) carrier.
Methods
The patient was a 34-year-old G10P1 female known to have a balanced translocation t(11;22)(q23;q11.2). She had one female livebirth (a translocation carrier) and eight miscarriages. Five female relatives known to be translocation carriers had a history of breast cancer, three of them premenopausally. The patient herself had a malignant melanoma.
Results
During the 10th pregnancy, ultrasound showed a viable embryo at 6 weeks of gestation, but loss of embryonic heartbeat by 7.5 weeks. Culture of the products of conception at 8 weeks of gestation showed the karyotype: 46,XY,+2,der(11)t(11;22)(q23;q11.2)mat, −22[4]/45,XY,der(11)t(11;22)(q23;q11.2)mat,−22[4], resulting from fertilization of the maternal 3 : 1 segregation product containing only the der(11) by a normal gamete. Subsequently, she became pregnant with a normal 46,XX fetus. FISH analysis indicated that the breakpoints on 11q and 22q in the patient were in the previously described region common to typical recurrent t(11;22). In addition, a nested-PCR-based approach showed that they were located within the same palindromic AT-rich sequence previously described.
Conclusion
This case demonstrates that the tertiary monosomy resulting from the 3 : 1 segregation is compatible with embryonic survival into the first trimester. It is also another example of apparent association of the constitutional translocation t(11;22) and breast cancer.
Keywords: t(11;22), breast cancer, 3 : 1 segregation, tertiary monosomy
INTRODUCTION
The t(11;22)(q23;q11.2) is the only recurring constitutional reciprocal translocation in humans, and has been observed in more than 100 unrelated families of different ethnic and racial backgrounds (Fraccaro et al., 1980; Zackai and Emanuel, 1980; Iselius et al., 1983). Most carriers of this translocation are ascertained because of abnormal offspring with the karyotype 47,XX or XY,+der(22)t(11;22)(q23;q11.2), resulting from 3 : 1 meiosis I segregation (Shaikh et al., 1999). This suggests either that this is a preferential mode of segregation or that this segregation product is the most likely to be viable to the time of amniocentesis and to term.
The clinical picture has been recorded often: these children have craniofacial anomalies, congenital heart defects and mental retardation (Zackai and Emanuel, 1980; Lin et al., 1986). An association between female carriers of the t(11;22) and breast cancer has been previously reported (Lindblom et al., 1994), but this has not been observed in other series (Kurahashi et al., 2000a).
Here, we report the first example of a conception with 45 chromosomes that received only the der(11) by 3 : 1 segregation from a female balanced t(11;22)(q23p;q11.2) carrier. This family is also remarkable for a strong family history of early onset breast cancer segregating with the balanced translocation.
CASE REPORT
The patient was a 34-year-old G10P1 female with a 46,XX,t (11;22)(q23;q11.2) karyotype referred for reproductive planning and breast cancer risk evaluation. A karyotype had been performed after her maternal first cousin was diagnosed with an unbalanced 47,XX,+der(22)t(11;22)(q23;q11.2) translocation associated with severe mental retardation. Subsequent karyotypic analysis of the family demonstrated that her two maternal aunts, mother, maternal female cousin, sister and daughter were all balanced translocation carriers. The patient had a total of eight miscarriages in the first trimester. There was no karyotypic information on any of these miscarriages. Notably, no other female balanced translocation carriers in the family had a history of infertility or multiple miscarriages.
The patient’s family history was remarkable for six cases of breast cancer on the maternal side of the family, three of which were premenopausal. Aside from the patient and her daughter, who are the youngest translocation carriers, each of the other five women carrying the balanced t(11;22) had a history of breast cancer. In addition, a deceased maternal great aunt of unknown carrier status had been diagnosed with breast cancer in her 40 s. The patient’s sister who had breast cancer diagnosed at the age of 40 had comprehensive BRCA1 and BRCA2 testing, and no mutations were identified. The patient herself was diagnosed with malignant melanoma at the age of 30.
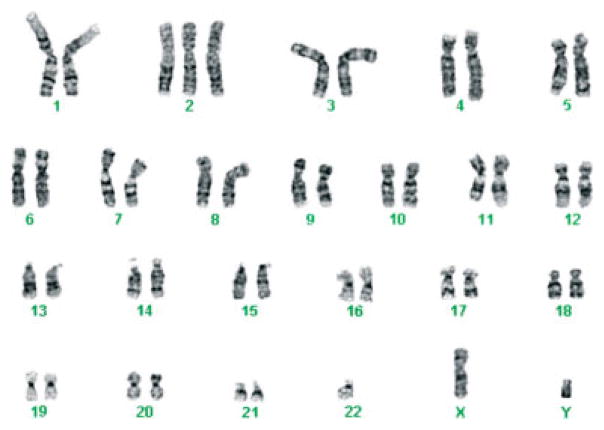
During the 10th pregnancy, ultrasound showed a viable embryo at 6 weeks of gestation, but loss of embryonic heartbeat by 7.5 weeks. Culture of the products of conception after a D&C at 8 weeks of gestation showed the karyotype: 46,XY,+2,der(11)t (11;22)(q23;q11.2)mat,−22[4]/45,XY,der(11)t(11;22) (q23;q11.2)mat,−22[4] (Figure 1). In the next pregnancy, the patient delivered a healthy 46,XX child.
Figure 1.
46,XY,+2,der(11)t(11;22)(q23;q11.2)mat, −22 karyotype of the products of conception from the 10th pregnancy of the patient
FISH and molecular studies were carried out on a blood sample from the carrier mother. FISH using a probe for the ATM gene mapping to 11q22 hybridized to its normal position on both the chromosomes 11, indicating that the translocation breakpoint was distal to the ATM locus. Further FISH analysis was performed using the probes described by Shaikh et al. (1999). BAC 442e11, from the RPCI11 human BAC library (Roswell Park Cancer Institute), was used as a FISH probe. Signal from BAC 442e11 was detected on the normal 11, the der(11) and the der(22) chromosomes, suggesting that BAC 442e11 spans the t(11;22) breakpoint on chromosome 11 (data not shown). These results are the same as have been observed in multiple t(11;22) carriers (Shaikh et al., 1999).
In addition, a nested-PCR-based approach described by Kurahashi et al. (2000a) was used to analyze the breakpoints in this patient and in a normal control individual. With the primers and conditions described by the authors, the junction fragments from the der(11) and from the der(22) could be amplified from the patient who is the carrier of the t(11;22). The translocation-specific PCR products were absent from a normal control individual (results not shown). These findings suggest that the t(11;22) breakpoints are located within the same PATRR (palindromic AT-rich repeat) on chromosome 11 and chromosome 22, as was found in other individuals carrying this translocation (Kurahashi et al., 2000a). Sequence analysis of the PCR products confirmed that the breakpoints were within the same PATRRs on chromosome 11 and 22 as previously reported.
DISCUSSION
The intervals on 22q11 (Funke et al., 1999) and 11q23 (Edelmann et al., 1999) that contain the region of chromosome breakage have been mapped and cloned (Kurahashi et al., 2000a,b; Edelmann et al., 2001). The sites of chromosome breakage on 11q23 and 22q11 occur in AT-rich palindromic sequences. Such sequences are known to be unstable in eukaryotic genomes. The sites of chromosome breakage occur in the center of the palindromes of both parental chromosomes 11q23 and 22q11 (Edelmann et al., 2001; Kurahashi and Emanuel, 2001). Kurahashi et al. (2002b) and Gotter et al. (2004) hypothesized that double-strand breaks at the tip of a putative hairpin, the center of the palindrome, lead to nonhomologous end-joining mechanisms that result in the formation of a stable, nonpalindromic sequence. Kurahashi et al. (2004) have demonstrated that these palindromic sequences form cruciform structures that are inherently unstable.
Table 1 shows the unbalanced segregants that have been reported for carriers of the t(11;22). Almost all reported cases have the karyotype 47,XX/XY,+der(22), which is the result of 3 : 1 meiosis I malsegregation in the parent carrying a balanced t(11;22). This was demonstrated by Shaikh et al. (1999) using short-tandem-repeat polymorphism markers on both chromosome 11 and 22 for all 16 families they investigated. Only rare cases of apparent 2 : 2 segregation have been identified. The single example with a 46,XX,der(22) karyotype, with monosomy for 22q and trisomy for 11q, was found in an early embryonic death resulting in an empty sac (Soler et al., 1993). The other cases produced viable offspring due to subsequent events that rescued the monosomy 22: in one case through a maternal MI nondisjunction of 22 (Dawson et al., 1996), and, in others, most probably by postzygotic nondisjunction involving the der(22) chromosome (Kulharya et al., 2002). Five cases with karyotype 47,t(11;22),+der(22) and phenotype similar to the usual der(22) have been reported (Lockwood et al., 1989; Abeliovich and Carmi, 1990; Lurie and Podelschuk, 1992; Simi et al., 1992; Petkovic et al.,1996). The first four groups postulated that this was the result of a second meiosis or postzygotic nondisjunction following 2 : 2 alternate segregation. However, Lindenbaum (1990) pointed out that a crossover within the chromosome 22 interstitial segment, followed by 3 : 1 segregation, would have the same outcome. Petkovic et al. (1996), using the evidence of polymorphism for inheritance of the paternal chromosome 22 in their case, supported Lindenbaum’s suggestion. Armstrong et al. (2000) also proposed that this rare outcome of 47,t(11;22)(q23;q11),+der(22) can be produced only by 3 : 1 segregation from a complete ring, a configuration that has interstitial chiasmata in both chromosome 11 and 22.
Table 1.
Review of the unbalanced karyotypes resulting from different segregation patterns in the carriers of t(11;22)
| Karyotype | Segregation | Outcome | Reference |
|---|---|---|---|
| 47,XX/XY,+der(22)t(11;22)(q23;q11.2) | 3 : 1 | der(22) syndrome | Zackai and Emanuel (1980); Shaikh et al. (1999) |
| 47,XX,+der(22)t(11;22)(q23;q11.2) | 2 : 2 adjacent-1(pat. de novo) + mat. MI nondisjunction of chr 22 |
der(22) syndrome | Dawson et al. (1996) |
| 47,XX/XY,t(11;22)(q23;q11.2)pat, +der(22) | ? 2 : 2 alternate + MII or postzygotic nondisjunction of der(22) |
der(22) syndrome | Lockwood et al. (1989); Abeliovich and Carmi (1990); Lurie and Podelschuk (1992); Simi et al. (1992) |
| 47,XX,t(11;22)(q23;q11.2)pat,+der(22) | Crossover within chr 22 + 3 : 1 segregation | der(22) syndrome | Petkovic et al. (1996) |
| 46,XX,der(22)t(11;22)(q23;q11.2)pat | 2 : 2 adjacent-1 | Empty sac (one of triplets) | Soler et al. (1993) |
| 46.XY,der(22)t(11;22)(q23;q11.2)mat/46,XY | 2 : 2 or 3 : 1 with multiple postzygotic errors | 26 year old with moderate mental retardation | Kulharya et al. (2002) |
| 45,XY,der(11)t(11;22)(q23;q11.2)mat,−22 | 3 : 1 | Embryonic death at 7 weeks. | This report |
We present here the first reported case of the tertiary monosomy resulting from the same 3 : 1 segregation that leads to live-born offspring with the der(22) syndrome. This specimen, with the 45,XY,der(11)t(11;22)(q23; q11.2),−22 is the result of the fertilization of the reciprocal 3 : 1 segregation product, containing the left over der(11), by a normal haploid gamete. The result is monosomy for 22q proximal to q11.2 and monosomy for 11q distal to q23. The mosaicism for trisomy 2 in this specimen is likely due a postzygotic mitotic error, resulting either in the loss of a chromosome 2 in an originally trisomic conception, or the duplication of one of the parental chromosomes 2. A trisomy arising in culture is also possible. The fact that this pregnancy had a normal ultrasound with a beating heart at 6 weeks of gestation demonstrates that the embryo was viable up to that point, though it died shortly thereafter.
The question of whether 3 : 1 segregation products are especially common in the t(11;22) translocation is in dispute. Drect studies of meiotic segregation have been published from four male carriers. Martin (1984) examined just 13 sperm chromosome preparations from a t(11;22) carrier and found 10 to be unbalanced, of which only 2 were 3 : 1 segregants. Estop et al. (1999) used FISH to examine segregation patterns in 1925 sperm from a t(11;22) carrier and found 73% to be unbalanced, with 40% having 3 : 1 segregation patterns. Van Assche et al. (1999) studied 1012 sperm using FISH and found 34.6% 3 : 1 segregants. In both the latter cases, monosomies greatly outnumbered those with an extra chromosome, raising the possibility that hybridization failure might account for some of these results. Armstrong et al. (2000) examined meiotic chromosomes from a testis biopsy of a t(11;22) carrier, and concluded that regular quadrivalent formation with an increased tendency for crossovers in the interstitial segments was the usual situation, not one favoring 3 : 1 segregation. From their meiosis II preparations, they estimated that only 11.5% of sperm would be the result of 3 : 1 segregation. However, almost all unbalanced progeny from t(11;22) carriers result from maternal segregation, not paternal, and these patterns could be quite different. It is noteworthy that in the few reported cases that are not a result of 3 : 1 segregation, the translocation was paternal in origin in all except one case (Table 1). Karyotyping of early abortuses from the female translocation carriers might be able to provide more information on the segregation patterns.
Whether or not 3 : 1 segregation is unusually frequent in this t(11;22) translocation, the virtual absence of other viable pregnancies with other unbalanced segregation products indicates the lack of viability of unbalanced embryos other than those with tertiary trisomy. Analysis of prenatal diagnoses in known carriers of the common t(11;22) translocation have shown about a 7% probability that the fetus will have tertiary trisomy resulting from 3: 1 segregation (where the gamete contains two normal chromosomes of the tetravalent plus one of the translocation chromosomes) (Daniel et al., 1989). Carrier mothers have more offspring than carrier fathers, pointing to a reduced fertility in the male heterozygote. The risk of miscarriage in the translocation carrier is greater (in the range of 20–30%) than the background population risk of 15% for a recognized pregnancy to miscarry (Stengel-Rutkowski et al., 1988). However, our patient’s very high rate of pregnancy loss (9/11 pregnancies) is much higher than the quoted risk, as well as greater than other carriers in her own family. It is unknown whether this was the result of nonviable products of the translocation, since karyotypic studies were performed only on the last pregnancy.
The other interesting feature of our family is the apparent co-segregation of breast cancer and the t(11;22) translocation. This association has been previously suggested by the study of Lindblom et al. (1994), where the incidence of breast cancer was noted to be significantly higher among carriers of the balanced translocation. This suggested the involvement of genes on 11q and/or 22q in the tumorigenesis of breast cancer. In our patient, FISH eliminated the possibility that the translocation could have disrupted the ATM gene located near the t(11;22) breakpoint on 11q23, and showed that there was no apparent difference between the breakpoints in our family and those that have been described for other cases of t(11;22). Kurahashi et al. (2000a) also demonstrated the same breakpoint in one of their patients who came from a family with breast cancer. Another possibility suggested by Kurahashi et al. (2000a) would be a ‘position effect’ generated by the translocation or that the loss of the der(22) in breast tissue could unmask a mutation of a gene on the normal homologue of 11 or 22. The possible increased risk of breast cancer in the carriers of t(11;22) needs confirmation in an appropriately designed study. Until then, increased cancer surveillance would seem warranted in female carriers, particularly if there is a family history of breast cancer.
Acknowledgments
This work was supported in part by grant from the National Institutes of Health to B.S.E. (CA-39926).
References
- Abeliovich D, Carmi R. The translocation 11q;22q: a novel unbalanced karyotype. Am J Med Genet. 1990;37:288. doi: 10.1002/ajmg.1320370227. [DOI] [PubMed] [Google Scholar]
- Armstrong SJ, Goldman AS, Speed RM, Hulten MA. Meiotic studies of a human male carrier of the common translocation, t(11;22), suggests postzygotic selection rather than preferential 3 : 1 MI segregation as the cause of liveborn offspring with an unbalanced translocation. Am J Hum Genet. 2000;67:601–609. doi: 10.1086/303052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel A, Hook EB, Wulf G. Risks of unbalanced progeny at amniocentesis to carriers of chromosome rearrangements: data from United States and Canadian laboratories. Am J Med Genet. 1989;33:14–53. doi: 10.1002/ajmg.1320330105. [DOI] [PubMed] [Google Scholar]
- Dawson AJ, Mears AJ, Chudley AE, Bech-Hansen T, McDermid H. Der(22)t(11;22) resulting from a paternal de novo translocation, adjacent 1 segregation, and maternal heterodisomy of chromosome 22. J Med Genet. 1996;33:952–956. doi: 10.1136/jmg.33.11.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann L, Spiteri E, Koren K, et al. AT-rich palindromes mediate the constitutional t(11;22) translocation. Am J Hum Genet. 2001;68:1–13. doi: 10.1086/316952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann L, Spiteri E, McCain N, et al. A common breakpoint on 11q23 in carriers of the constitutional t(11;22) translocation. Am J Hum Genet. 1999;65:1608–1616. doi: 10.1086/302689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estop AM, Cieply KM, Munne S, Feingold E. Multicolor fluorescence in situ hybridization analysis of the spermatozoa of a male heterozygous for a reciprocal translocation t(11;22)(q23;q11) Hum Genet. 1999;104:412–417. doi: 10.1007/s004390050977. [DOI] [PubMed] [Google Scholar]
- Fraccaro M, Lindsten J, Ford CE, Iselius L. The 11q;22q translocation: a European collaborative analysis of 43 cases. Hum Genet. 1980;56:21–51. doi: 10.1007/BF00281567. [DOI] [PubMed] [Google Scholar]
- Funke B, Edelmann L, McCain N, et al. Der(22) syndrome and velo-cardio-facial syndrome/DiGeorge syndrome share a 1.5-Mb region of overlap on chromosome 22q11. Am J Hum Genet. 1999;64:747–758. doi: 10.1086/302284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotter AL, Shaikh TH, Budarf ML, Rhodes CH, Emanuel BS. A palindrome-mediated mechanism distinguishes translocations involving LCR-B of chromosome 22q11.2. Hum Mol Genet. 2004;13:103–115. doi: 10.1093/hmg/ddh004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iselius L, Lindsten J, Aurias A, et al. The 11q;22q translocation: a collaborative study of 20 new cases and analysis of 110 families. Hum Genet. 1983;64:343–355. doi: 10.1007/BF00292366. [DOI] [PubMed] [Google Scholar]
- Kulharya AS, Lovell CM, Flannery DB. Unusual mosaic karyotype resulting from adjacent 1 segregation of t(11;22): importance of performing skin fibroblast karyotype in patients with unexplained multiple congenital anomalies. Am J Med Genet. 2002;113:367–370. doi: 10.1002/ajmg.b.10801. [DOI] [PubMed] [Google Scholar]
- Kurahashi H, Emanuel BS. Long AT-rich palindromes and the constitutional t(11;22) breakpoint. Hum Mol Genet. 2001;10:2605–2617. doi: 10.1093/hmg/10.23.2605. [DOI] [PubMed] [Google Scholar]
- Kurahashi H, Inagaki H, Yamada K, et al. Cruciform DNA structure underlies the etiology for palindrome-mediated human chromosomal translocations. J Biol Chem. 2004;279:35 377–35 383. doi: 10.1074/jbc.M400354200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi H, Shaikh TH, Zackai EH, et al. Tightly clustered 11q23 and 22q11 breakpoints permit PCR-based detection of the recurrent constitutional t(11;22) Am J Hum Genet. 2000a;67:763–768. doi: 10.1086/303054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi H, Shaikh TH, Hu P, et al. Regions of genomic instability on 22q11 and 11q23 as the etiology for the recurrent constitutional t(11;22) Hum Mol Genet. 2000b;9:1665–1670. doi: 10.1093/hmg/9.11.1665. [DOI] [PubMed] [Google Scholar]
- Lin AE, Bernar J, Chin AJ, Sparkes RS, Emanuel BS, Zackai EH. Congenital heart disease in supernumerary der(22),t(11;22) syndrome. Clin Genet. 1986;29:269–275. doi: 10.1111/j.1399-0004.1986.tb01254.x. [DOI] [PubMed] [Google Scholar]
- Lindblom A, Sandelin K, Iselius L, et al. Predisposition for breast cancer in carriers of constitutional translocation 11q;22q. Am J Hum Genet. 1994;54:871–876. [PMC free article] [PubMed] [Google Scholar]
- Lindenbaum RH. Unusual segregation of constitutional 11q;22q translocation may be explained by crossover in interchange segment, followed by 3 : 1 segregation at meiosis I. Hum Genet. 1990;85:143. doi: 10.1007/BF00276346. [DOI] [PubMed] [Google Scholar]
- Lockwood DH, Farrier A, Hecht F, Allanson J. Not all chromosome imbalance resulting from the 11q;22q translocation is due to 3 : 1 segregation in first meiosis. Hum Genet. 1989;83:287–288. doi: 10.1007/BF00285174. [DOI] [PubMed] [Google Scholar]
- Lurie IW, Podelschuk LV. 11q;22q translocation: third case of imbalance not due to 3 : 1 nondisjunction in first meiosis. Am J Med Genet. 1992;42:216. doi: 10.1002/ajmg.1320420218. [DOI] [PubMed] [Google Scholar]
- Martin RH. Analysis of human sperm chromosome complements from a male heterozygous for a reciprocal translocation t(11;22)(q23;q11) Clin Genet. 1984;25:357–361. doi: 10.1111/j.1399-0004.1984.tb02004.x. [DOI] [PubMed] [Google Scholar]
- Petkovic I, de Capoa A, Giancotti P, Barisic I. Unusual segregation of t(11;22) resulting from crossing-over followed by 3 : 1 disjunction at meiosis I. Clin Genet. 1996;50:515–519. doi: 10.1111/j.1399-0004.1996.tb02725.x. [DOI] [PubMed] [Google Scholar]
- Shaikh TH, Budarf ML, Celle L, Zackai EH, Emanuel BS. Clustered 11q23 and 22q11 breakpoints and 3 : 1 meiotic malsegregation in multiple unrelated t(11;22) families. Am J Hum Genet. 1999;65:1595–1607. doi: 10.1086/302666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simi P, Ceccarelli M, Barachini A, Floridia G, Zuffardi O. The unbalanced offspring of the male carriers of the 11q;22q translocation: nondisjunction at meiosis II in a balanced spermatocyte. Hum Genet. 1992;88:482–483. doi: 10.1007/BF00215688. [DOI] [PubMed] [Google Scholar]
- Soler A, Carrio A, Perez-Vidal MT, Borrell A, Fortuny A. Unusual segregation for 11q;22q parental translocation in a triplet pregnancy: prenatal diagnosis in chorionic villi and amniotic fluid. Prenat Diagn. 1993;13:137–141. doi: 10.1002/pd.1970130209. [DOI] [PubMed] [Google Scholar]
- Stengel-Rutkowski S, Stene J, Gallano P. Monographie des Annales de Genetique. Expansion Scientifique Francaise; Paris: 1988. Risk estimates in balanced parental reciprocal translocations. [Google Scholar]
- Van Assche E, Staessen C, Vegetti W, et al. Preimplantation genetic diagnosis and sperm analysis by fluorescence in-situ hybridization for the most common reciprocal translocation t(11;22) Mol Hum Reprod. 1999;5:682–690. doi: 10.1093/molehr/5.7.682. [DOI] [PubMed] [Google Scholar]
- Zackai EH, Emanuel BS. Site-specific reciprocal translocation, t(11;22) (q23;q11), in several unrelated families with 3 : 1 meiotic disjunction. Am J Med Genet. 1980;7:507–521. doi: 10.1002/ajmg.1320070412. [DOI] [PubMed] [Google Scholar]