Abstract
Morphine given by Patient Controlled Analgesia (PCA) is widely used in hospital settings to manage severe pain during acute painful episodes. Wide variations in prescription patterns occur and some patients are often self-administering sub- or low- therapeutic doses. In this preliminary study, a descriptive design with repeated measures was used to examine the effects of different PCA morphine regimens on the intensity, location and quality of pain as well as on the perceived amount of relief and side effects in patients with sickle cell disease (N=13; mean age 13.7 years; eight males; five females). The preliminary data showed that a regimen with a high background infusion rate and low intermittent push dose (Regimen B) may provide better response to PCA morphine. The difference in trends between the worst and least pain intensity ratings were narrower in this regimen, suggesting that pain peaks and troughs were not occurring as in a regimen with an around the clock nurse administered dosing schedule (Regimen C). The amount of morphine that was administered per day was not significantly different (p > 0.05) among the three morphine regimens. The combination of a high background infusion rate and low intermittent push dose (as in Regimen B) within the first 24 hours of admission, may provide improved response and possibly shorter recovery from the painful episode than the regimen that would routinely be prescribed with lower background infusion rate and high intermittent push dose (as in regimen A).
Keywords: Patient Controlled Analgesia (PCA) morphine, acute painful episodes, sickle cell disease
Introduction
Sickle cell disease is an inherited disorder that affects one in 500 African Americans (1,2). The acute painful episode is the most common problem in children with sickle cell disease. It may be a manifestation of vaso-occlusion or may be a symptom of another process, such as infection. The painful episode due to vaso-occlusion is the most frequent cause for emergency department visits and hospital admissions (3,4).
Painful episodes that are severe enough to require hospitalization typically last 3 to 7 days, but pain may persist for as long as several weeks (5). Children may experience persistent pain of mild to moderate intensity on a daily basis, or may have intermittent acute exacerbations of more severe pain. In most cases during the acute painful episode, pain intensity increases rapidly, plateaus, and then falls, but the pattern may be unpredictable (6,7). Morphine given by PCA is widely used in hospital settings to manage severe pain during acute painful episodes (8-10). However, there were wide variations in prescription patterns, and patients were often self-administering sub- or low-therapeutic doses, commonly 35% on the average of what were prescribed. The most common PCA morphine regimen consisted of high intermittent push dose and low background infusion rate (8-10). With this PCA morphine regimen, children were most likely falling asleep in between the intermittent push doses and waking up with pain, and leading to a “prn” dosing schedule and thus, not providing optimal relief. Therefore, pain intensity ratings remained at moderate to high levels through the course of hospitalization (8,9) and some patients with sickle cell disease had prolonged hospitalizations associated with persistent pain (10). It is not known if other combinations of PCA settings, such as increasing the background infusion, or having larger amounts administered around the clock (ATC) may provide better response to morphine. Such regimens could achieve a more steady concentration of the drug associated with the “ATC” dosing schedule, rather than a “PRN” dosing schedule with peaks and troughs (11,12). Therefore, the purpose of this preliminary study was to examine the effects of the different PCA regimens on the intensity, location and quality of pain as well as on the perceived amount of relief and side effects.
Methods
A descriptive design with repeated measures was used to examine the effects of the different PCA regimens during hospitalization for acute painful episodes. Patients with sickle cell disease were recruited from the hematology unit of a large children's hospital in the central southern United States. The Sickle Cell Program in this facility follows about 900 patients per year and provides comprehensive services for patients with sickle cell disease.
Inclusion Criteria
All hospitalized patients with sickle cell disease, eight years and older, were eligible. The age of eight years was chosen as the cut-off point because the outcome measures for pain were validated for patients eight years and older. In addition, patients younger than 8 years old were less likely prescribed patient controlled analgesia for pain management during acute painful episodes. Patients were included in the study if: 1) they were English-speaking (data collection instruments available only in English); 2) the primary reason for admission was for management of acute pain as documented by the attending physician in the admission records, and 3) the pain was severe enough to require the administration of intravenous morphine.
Exclusion Criteria
Patients with sickle cell disease were excluded if pain was not related to an acute painful episode (infection, surgery, burns, trauma). Patients were also excluded if they had cognitive and neurological impairments that precluded them from completing the pain tool. They were excluded if they were already receiving morphine at doses above the study entry dose (either an intermittent push dose setting greater than 0.07 mg/dose, a background infusion rate setting greater than 0.03 mg/kg/hr, or a four hour limit setting greater than 0.6 mg/kg (equivalent to 0.15 mg/kg one hour limit setting). The decision to exclude patients was made by the PI in consultation with the hematology team. The study was approved by the Institutional Review Board of the medical center.
Procedures
Patients were randomly assigned to one of three morphine regimens (Regimen A, B, or C). The differences among the regimens were in the combination of settings programmed in the PCA device. The settings differed in: 1) the amount given by background infusion, 2) the amount given by the intermittent push button, and 3) the lockout interval settings programmed in the PCA device (see table 1).
Table 1.
Morphine patient controlled analgesia regimens
| Patient Controlled Analgesia Morphine Regimens |
|||
|---|---|---|---|
| A(N=5) | B(N=5) | C(N=3) | |
| Intermittent Push | 0.07 mg/kg/dose | 0.035 mg/kg/dose | 0.15 mg/kg/dose |
| Background Rate | 0.015 mg/kg/hr | 0.03 mg/kg/hr | 0.00 mg/kg/hr |
| Lock-out Interval | 10 minutes | 10 minutes | 180 minutes [q 3 hrs] |
| 4 hour Maximum [Hourly Maximum] | 0.6 mg/kg [0.15 mg/kg/hr] | 0.6 mg/kg [0.15 mg/kg/hr] | 0.6 mg/kg |
| Example PCA Settings for a 40 kg patient for the different regimens: | |||
| Patient Controlled Analgesia Morphine Regimens |
|||
|---|---|---|---|
| A | B | C | |
| Intermittent Push | 2.8 mg | 1.4 mg | 6 mg |
| Background Rate | 0.6 mg/kg/hr | 1.2 mg | 0.0 mg/kg/hr |
| Lock-out Interval | 10 minutes | 10 minutes | 180 minutes [q 3 hrs] |
| 4 hour Maximum [Hourly Maximum] | 24 mg [6 mg/kg/hr] | 24 mg [6 mg/kg/hr] | |
The 4 hour maximum [converted to hourly equivalent] setting prescribed for each regimen was controlled to minimize the confounding effect of dose on the variation in response. The hourly limit setting was standardized in mg/kg/hour based on the amount that is recommended in the Pediatric Drug Dosage Handbook (13), a medication reference book which is widely used in children's hospitals as part of the formulary across the United States.
Patient Controlled Analgesia Morphine Regimens
Patients in Regimen A were prescribed the regimen of higher intermittent push (HIP) dose (0.07 mg/kg/dose; proportionally two times the intermittent push dose prescribed for regimen B) and lower background infusion (LBI) rate (0.015 mg/kg/hr; proportionately half the amount prescribed for regimen B), The maximum 4-hour limit setting for this regimen was 0.6 mg/kg, equivalent to 0.15 mg/kg/hr x 4 hours (9,10). The recommended therapeutic dose range from the Pediatric Drug Dosage Handbook commonly used by children's hospitals across the country is 0.1 to 0.2 mg/kg/hr (13); thus the hourly dose selected for this study is in the midpoint of the therapeutic dose range.
Patients in regimen B were prescribed the opposite regimen of lower intermittent push (LIP) dose (0.03 mg/kg/dose; proportionally half from Regimen A) and higher background infusion (HBI) rate regimen (0.03 mg/kg/hr; proportionately two times the background rate in Regimen A). The 4-hour maximum limit setting for this regimen was also 0.6 mg/kg [0.15 mg/kg/hr x 4 hours], which was the same as the maximum 4-hour limiting setting for regimen A (13). Thus, the hourly limit setting was standardized for the morphine PCA regimens in the study.
Patients in regimen C [Nurse Controlled Analgesia] were prescribed an around the clock (ATC) dose of 0.15 mg/kg/dose, based on the recommended parameters of 0.1 to 0.2 mg/kg/dose every 3 hours; maximum 15 rug/dose (13). This dose was higher than the dose that could be delivered by PCA in a background infusion rate or in an intermittent push dose, but equivalent to the dose that would be prescribed if PCA was not available. However, for this study, regimen C was delivered using the PCA device with the intermittent push dose rate setting at 0.15 mg/kg/dose and background infusion rate set at 0.0; lockout interval was set at 180 minutes (three hours) to allow the nurse to push the dose every three hours, and simulate a “q 3 hour ATC” regimen, that was typically prescribed for patients without using the PCA device. Thus, no intermittent dose for patient to push and no background infusion rate were prescribed for Regimen C. In general, medications administered ATC achieve stead state levels after 4 to 5 half-Iifes (half-life of morphine is two hours) (14). Therefore, even though this regimen was administered intermittently every three hours ATC, the level of morphine in the blood is expected to reach steady state after two to three doses, a level that would be achieved if patient had a background infusion rate.
All patients were enrolled within 48 hours of initiation of PCA and they were prescribed the study dose for 24 hours. In the event that the patient required additional doses beyond the assigned study settings, a PRN dose was prescribed for all regimens at 0.05 mg/kg/dose (33% of the one hour limit study setting, i.e. 0.05 mg/kg/hr). This amount was based on the rescue dose recommended by the American Pain Society Guideline for the Management of Acute and Chronic Sickle Cell Disease, which is recommended at 25% to 50% of the hourly opioid dose (15). If the patient required three additional doses in any one hour period beyond the study PCA settings before hour 24, the physician could change the PCA settings and the patient would be off study.
Outcome Measures
Children were asked to rate pain using 0 to 10 numerical rating scales (NRS; 0=no pain to 10=a lot of pain) for 1) worst pain, 2) least pain, and 3) amount of pain relief during treatment (0=no relief to 10=complete relief). The 0 to 10 NRS (numeric rating scale) is a well-established valid and reliable tool (16), and was previously used by children with sickle cell disease (8-10). A checklist that consisted of common side effects of morphine (hypotension, bradycardia, respiratory depression, drowsiness, dizziness, tremors, sedation, pruritus, nausea, vomiting, constipation, urinary retention), was used to record side effects.
The patient was also asked to complete the Adolescent Pediatric Pain Tool (APPT) to measure the intensity, location, and quality of pain. Reliability and validity of the APPT is well established (17-20). Previous APPT data from children with sickle cell disease showed that pain location and spatial distribution of pain changed during acute painful episodes even when pain intensity remained the same (8).
The patient's Medication Administration Record was reviewed for the dose, route, and time of all medications that were administered for pain (e.g. nonsteroidal anti-inflammatory drugs [NSAIDs] such as ibuprofen and ketorolac) and for side effects (e.g., diphenhydramine for itching, hydroxyzine and odansetron for emesis). In addition, the following information were also collected from the medical records: 1) demographic information such as age, gender, weight, height, hemoglobin genotype; and 2) health related information such as CBC, pain ratings in the ED, presence of fever, oxygen saturation, other signs and symptoms at time of admission, history of past complications and sickle cell related treatments.
Data Analyses
Scatter plots were used to describe worst and least pain patterns across the five days. Descriptive statistics (frequencies, means, standard deviations) were used to describe worst and least pain, location of pain, and quality of pain, type and amount of medications, and amount of relief.
Results
During the two year study period between August, 2005 and July, 2007, 326 sickle cell related admissions were screened in the hematology unit, with an average of 18.1 ±5.6 admissions each month. These admissions represented 160 patients; 30% had three or more repeat admissions. Among the 160 patients, 68 (42.8%) patients were admitted for reasons other than acute painful episodes, 28 (17.6%) patients had acute painful episode, but did not have PCA morphine at time of screening and recruitment, 14 (8,8%) were excluded for various reasons (such as, developmental delay, communication disorder, behavioral problems, disruptive behavior, cognitive dysfunction, neurological impairments, patient not appropriate for randomization, parent noncompliant or not appropriate for consenting procedures, patient on legal custody and parent/legal guardian not known), 8 (5.0%) were weaned from pain medications and were planned to be discharged within 24 hours, 4 (2.5%) were eligible, but enrolled in a concurrent study, 2 (1.3%) did not have parents available for consent, 16 (10.1%) refused. A total of 15 patients were randomized in the PCA morphine regimens; two of them (1 from regimen C; the other from regimen A) had withdrawn early. The 13 patients who completed the study were randomly assigned to Regimen A (n=5), B (n=5), and C (n=5).
Baseline characteristics of patients in regimen A (n=5), B (n=5), and C (n=3) were not significantly different (N=13; mean age 13.7 years; eight males; five females; table 2).
Table 2.
Demographics
| A(N=5) | B(N=5) | C(N=3) | |
|---|---|---|---|
| Age (years) | 13.6 ±2.1 (11-17) | 13.0 ±2.0 (10-16) | 14.7 ±4.2 (range 10-18) |
| Weight (kg) | 49.5 ± 18.3 (31.0 to 76.0) | 70.5 ± 18.6 (42.0 to 93.6) | 48.1 ±24.0 (26.5.0 to 74.0) |
| Height (ran) | 158.5±11.9 (145.5 to 169.0) | 153.7 ±20.7 (127.0 to 174.0) | 151.) ±13.0 (141.0 to 166.0) |
| Gender | |||
| Male | 4 | 3 | 1 |
| Female | 1 | 2 | 2 |
| Hemoglobin | |||
| SS | 4 | 2 | 3 |
| SC | 1 | 1 | -- |
| SBeta | -- | 1 | -- |
| Pain Onset (Number Days PTA) | 1.8 ±1.5(1 to 4) | 1.7 ±0.6 1 to 2) | 3.4 ±4.6(1 to 9) |
| Lowest, Pain in ER (0 to 10 NRS) | 7.7 ±2.1 (6 to 10) | 7.0 ±3.3(0 to 7) | 5.0 ±1.4 (4 to 6) |
| Highest Pain in ER (0 to 10 NRS) | 9.5 ±1.0 (8 to 10) | 7.3 ±1.2 (6 to 8) | 9.0±1.4(8tolO) |
| Morphine in ER (mg/kg/dose) | 0.08 | 0.09 | 0.10 |
| Length of Hospital Stay (days) | 6.0 2.2 3 to 9 | 5.6 2.4 3 to 9 | 7.0 2.6 7 to 10 |
PTA: Prior to Admission; ER: Emergency Room.
The Hemoglobin phenotype was predominantly HgbSS (n=9). Onset of pain was 1.7 to 3.4 days prior to admission. Lowest mean pain intensity ratings in the Emergency Department (ED) prior to admission ranged from 5.0 to 7.7, with the highest pain intensity ratings from 7.3 to 9.5 on 0 to 10 numerical ratings scales (NRS). Patients received 0.08 to 0.10 mg/kg/dose, which were lower or within the lower range of the recommended morphine dose of 0.1 to 0.2 mg/kg/dose (12). Patients in regimen B had an overall mean shorter length of stay (Table 1); however this was not statistically significant (p > 0.05).
Pain Intensity
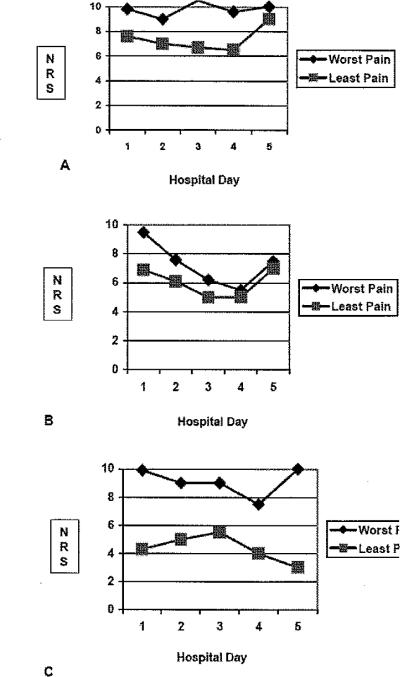
The worst pain intensity ratings in Regimen B decreased significantly during the course of hospitalization (see figure 1). The worst pain intensity rating was 9.5 on day 1 and 5.5 on day 5, p=0.01 for Regimen B. The worst pain intensity ratings for patients on regimen A (9.8 to 9.0, p > 0.05) and regimen C (9.6 to 7.5, p>0.05) did not change significantly during hospitalization (see figure 1). The least pain intensity ratings of patients in regimen C were lower than patients in either regimen A or B (see figure 2). The difference in trends between the worst and least pain intensify ratings across the hospital days was widest in regimen C when compared to either Regimen A or B (see figure 2).
Figure 1.
Pain intensity ratings (A), Number of body areas (B) and number of quality word descriptors (C) Day 1 to Day 5 of hospitalization.
Figure 2.
Comparison of worst and least pain ratings for regimen A, B, C.
Pain Location
The most frequent areas marked for pain by patients in regimen A were the right upper arm (75%), right abdomen (50%), left thigh and knees (50%), right back chest (50%), and lower back (50%). The most frequent areas marked by patients in Regimen B were chest (83%), right knee (50%), and left thigh and knees (50%). Patients in regimen C marked the chest (66.7%), right upper arm (66.7%), right abdomen (66.7%), and lower back (66.7%).
The number of areas marked on the body outline diagram remained constant throughout hospitalization for regimen C (12.3 to 15.5 areas). The number of areas marked decreased for patients in regimen A (10.2 to 1.5) and B (8.8 to 1.5). Worst pain intensity ratings remained at moderate levels (see figure 1A) throughout hospitalization; however, the number of body areas marked with pain decreased for regimen A and B, but not for regimen C (see figure 1B).
Pain Quality
The number of words to describe the quality of pain remained similar throughout hospitalization from 30.2 on day 1 to 11.0 on day 5 (p > 0.05) for patients on regimen A (see figure 1C). The most frequent words were sensory quality (3.7 to 7.5), and fewer affective (1.75 to 2.5), evaluative (2.2 to 2.7), or temporal words (1.7 to 2.5). The number of words to describe the quality of pain decreased from 11.7 to 3.0 for patients on regimen B (see figure 1C). The most frequent words were also sensory quality (3.6 to 6.0) and fewer affective (0.8 to 1.3), evaluative (1.7 to 2.2 ), or temporal words (1.8 to 2.6). For patients in regimen C, the number of words remained similar throughout hospitalization, and slightly increased from 13.0 on day 1 to 19.2 on day 5 (p>0.05). The number of sensory (6.3 to 10.0), affective (2.3 to 3.0), evaluative (2.3 to 4.0), and temporal quality words (2.0 to 3.0) were slightly higher, but not significantly different (p>0.05) from the patients in regimen A and B.
The words that were selected by 75% of patients in regimen A to describe the quality of pain were hurting, sharp, pressure, awful, annoying and continuous. The majority of patients (66.7%) in regimen B selected aching, hurting, like a sharp knife, shooting, dizzy, annoying, and uncomfortable. The majority of patients in regimen C (66.7%) selected similar words. In addition, the majority of patients (66.7%) in regimen C also selected other words such as throbbing, tight, sore, like a pinch, stinging, shocking, and pounding from the sensory words; crying and sickening from the affective words; and never goes away, steady, and always from the temporal words.
Daily Morphine Consumption
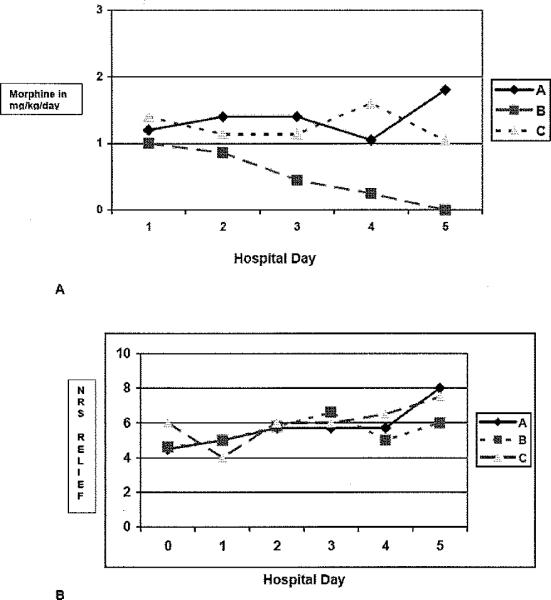
The amount of morphine that was administered per day was not significantly different (p>0.05) among the three regimens (see figure 3 A). Patients in regimen A were administered on the average from 59.4 mg on day 1 to 33.8 mg on day 5. This amount was equivalent to 1.2 mg/kg/day on day 1 to 1.1 mg/kg/day on day 5. The largest amount was administered on day 3 (77.3 mg/day or 1,4 mg/kg/day). Patients in regimen B had consistently decreased in the amount of morphine that was administered during hospitalization from 73.5 mg/day on day 1 to 23.5 mg/day on day 5 (equivalent to 1.1 on day 1 to 0.3 mg/kg/day on day 5). Patients in regimen C were administered on the average 55.7 mg (or 1.1 mg/kg/day) on day I and continued to have a slightly higher amount administered on day 4 (117.9 mg/day or 2.4 mg/kg/day) and day 5 (77.3 mg/day or 1.6 mg/kg/day).
Figure 3.
Amount of morphine in mg/kg/day (A) and perceived amount of relief [0 to 10 NRS] from medications (B).
Other Medications
Ketorolac (administered 15 to 30 mg every 6 hours) was the only other medication that all patients received for pain. A few patients had diphenhydramine (25 to 50 mg) for itching, and odansetron (8 mg prn) or promethazine (12 mg prn) for nausea/vomiting.
Pain Relief
The amount of pain relief did not change significantly (p > 0.05) during hospitalization (figure 3). The mean amount of pain relief (from 0 = no relief to 10 = complete relief) for each day of hospitalization was higher for regimen C (6.0 ± 2.0 to 6.5 ±2.1), when compared with Regimen A (4.6 ±2.5 to 5.7 ± 2.1) or B (4.6 ± 2.7 to 6.6 ± 1.9); however, this was not statistically significant.
Side Effects
Few patients were reporting side effects, mostly drowsiness, dizziness, itching, nausea, and vomiting. Two of five patients from regimen A and three of five patients from regimen B reported drowsiness prior to enrollment. Three patients on regimen A and two patients on regimen B also reported itching on day of enrollment. Two patients had nausea from regimen A and one each from regimen B and C had both nausea and vomiting. No one had low blood pressure, heart rate, or respiratory rate. One patient in regimen B had difficulty stooling, which was reported prior to enrollment
Discussion
This preliminary study evaluated the analgesic response to three different morphine regimens delivered by patient controlled analgesia (PCA). The worst pain intensity ratings in regimen B decreased during the course of hospitalization, while the worst pain intensity ratings in the other two regimens (A and C) did not change. This finding suggests that the regimen with higher background infusion rate (B) may provide a better response to PCA morphine. The difference in trends between the worst and least pain intensity ratings were also narrower in regimen B, suggesting that pain peaks and troughs were not occurring in regimen B, as they were most likely occurring in regimen C, with wide differences in highest pain and lowest pain ratings reported each day.
It is interesting to note that the trend in least pain intensity ratings reported by patients in regimen C was lower across hospitaiization days than reported by patients in either regimen A or B (figure 2). The difference in trends between the worst and least pain intensity ratings across the hospital days was widest in regimen C. This finding suggests that the higher intermittent doses were providing better relief than the amount that were delivered in either regimen A or B; however the every 3 hour interval was most likely not adequate to sustain the low intensity ratings, and may explain the high pain intensity ratings.
Patients in regimen A were reporting the highest worst and least pain intensity ratings and both remained consistently high throughout hospitaiization. This finding is similar to previous reports (9,10), which also reported regimens similar to regimen A and worst and least pain intensity ratings that remained high to moderate throughout hospitaiization. This implies that the routinely prescribed PCA regimen with lower background infusion and high intermittent push doses (similar to the doses prescribed in regimen A for this study) was not providing optimal pain control.
The chest, abdomen, and extremities were the most frequently marked areas for pain in all regimens, which are similar to the pain location marked by patients in previous reports (9,10). The number of areas marked decreased for patients in regimens A and B. It is important to note that pain intensity ratings remained at high to moderate levels throughout hospitaiization (figure 1A), but the number of body areas marked with pain decreased (figure 1B) for regimen A and B. This finding emphasizes the importance of assessing not only pain intensity dimension as routinely done in practice, but also assessing the location and spatial distribution of pain throughout hospitaiization (8), which could be more informative in evaluating response to treatments. This data suggest that regimens A and B were providing better analgesia when compared to regimen C (figure 1B) when the dimension of pain location is used as the outcome criteria.
The most frequently used words selected to describe the quality of pain were predominantly sensory, and less frequently affective, evaluative, or temporal. Patients did not select words that suggest pain with neuropathic component (e.g. shooting, burning, or shock-like). This finding is similar to previous reports of patients with sickle cell disease (8,21).
The amount of morphine prescribed was standardized for all three regimens, which was selected so that the hourly maximum setting is midpoint of the therapeutic dose range (33). We were anticipating that patients in regimen B would consume more morphine since the background infusion rate was higher. However, patients in regimen B showed a consistent decrease in their daily morphine consumption throughout hospitalization (figure 3A). Although not statistically significant, possibly due to small sample size, the length of stay for regimen B is shorter (table 1). This finding is suggesting that a higher background infusion rate (regimen B) than would routinely be prescribed (regimen A) within the first 24 hours of admission for acute painful episode may provide improved response and shorter recovery from the painful episode in patients with sickle cell disease.
An important finding in this study is that on the average, patients in the ED received 0.08 to 0.10 mg/kg/dose, which were lower or within the lower end of the recommended therapeutic dose range of 0.1 to 0.2 mg/kg/dose (13). This finding is similar to previous studies which reported that in children and adolescents with sickle cell disease, the prescribed morphine doses were frequently prescribed and administered in the lower end of the therapeutic dose range (9,10). It would be interesting to determine in future studies whether standardizing the ED protocol to increase the amount prescribed to at least the midpoint of the therapeutic dose range would lead to improved analgesia in the ED and thereby minimize the number of admissions related to acute painful episodes (4,22,23).
The patients in regimen C were reporting a higher relief than patients in regimens A and B. It is possible that the higher intermittent doses administered by the nurse around the clock were providing good relief. These pain relief data are consistent with their reports of least pain intensity ratings.
We did not examine the regimen with high background infusion rate and high intermittent push dose that would be within the therapeutic dose range, a regimen that may be optimal. Future studies need to examine this regimen using larger sample size. There were no differences in side effects reported by patients. The side effects (mostly drowsiness, dizziness, itching, nausea, and vomiting) were present prior to initiation of the doses prescribed in the regimens.
A major limitation in this study is the small sample size. We recommend larger samples in future studies. We screened and recruited patients from the hematology program serving a large population of patients with sickle cell disease. During the two year screening and recruitment period, we were only able to enroll 15 patients (including two early withdrawal patients). We recommend that in future studies, screening and recruitment be done in several sites as the pool of patients with sickle cell disease requiring patient controlled analgesia is relatively low compared to the overall number of all patients with sickle cell disease in a hospital setting.
Several studies including the current study, have documented that the most routinely prescribed regimen (similar to the doses prescribed in Regimen A with high intermittent push with low background infusion rates) was not optimal in providing analgesia. Our current study also showed that patients prescribed a nurse administered around the clock q 3 hour regimen (as prescribed in regimen C) were most likely experiencing pain peaks and troughs, and therefore such regimens should be avoided. We recommend future studies to examine a high background infusion rate with low intermittent push dose (as was prescribed for regimen B) and alternative morphine regimens such as high background infusion with high intermittent push dose, while keeping within the recommended dose range (13). Previous reports have documented that patients with sickle cell disease required higher dosing schedules due to the more intense pain (24), prolonged hospitalizations (10), and altered pharmacokinetics (25). And finally, future studies are warranted that examine alternative PCA opioids such as hydromorphone (26), fentanyl (27), tramadol (28) or a combination of morphine and an adjuvant medication such as ketamine (29) in patients with sickle cell disease who experience persistent severe pain that may be refractory to morphine PCA.
Acknowledgments
Funding from this study was provided by a Mentored Patient-Oriented Research Career Development Award (#5K23NR009192) from the National Institute of Nursing Research at the National Institute of Health.
References
- 1.Platt OS, Brambilia DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, et al. Mortality in sickie cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–44. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 2.Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325(1):11–16. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y, Sha A, Watson M, Mankad V. Comparison of costs to the health sector of comprehensive and episodic health care for sickle cell disease patients. Public Health Reports. 1997;110:80–6. [PMC free article] [PubMed] [Google Scholar]
- 4.Blank FSJ, Li H, Henneman PL, Smithline HA, Santoro JS, Provost D, Maynard AM. A descriptive study of heavy emergency department users at an academic emergency department reveals heavy ED users have better access to care than average users. J Emerg Nurs. 2005;31(2):139–44. doi: 10.1016/j.jen.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Beyer J, Simmons L, Woods GM, Woods PM. A chronology of pain/comfort in children with sickle cell disease. Arch Pediatr Adoiesc Med. 1999;153(9):913–20. doi: 10.1001/archpedi.153.9.913. [DOI] [PubMed] [Google Scholar]
- 6.Balks S. The acute painful episode. Sickle cell pain. Seattle: IASP Press. 1998;11:201–37. [Google Scholar]
- 7.Conner-Warren RL. Pain intensity and home pain management of children with sickle cell disease. Issues Compr Pediatr Nurs. 1996;19(3):183–95. doi: 10.3109/01460869609026860. [DOI] [PubMed] [Google Scholar]
- 8.Jacob E, Miaskowski C, Savedra M, Beyer JE, Treadwell M, Styles L. Changes in intensity, location, and quality of vaso-occlusive pain. Pain. 2003;102(12):187–93. doi: 10.1016/s0304-3959(02)00374-3. [DOI] [PubMed] [Google Scholar]
- 9.Jacob E, Miaskowski C, Savedra M, Beyer JE, Treadwell M, Styles L. Management of vaso-occlusive pain in hospitalized children with sickle cell disease. J Pediatr Hematology/Oncology. 2003;25(4):307–l1. doi: 10.1097/00043426-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Jacob E, Mueller B. Pain and symptom experience in hospitalized children with sickle cell disease who had prolonged hospitalizations. Pain Med. 2007 Feb 01; doi: 10.1111/j.1526-4637.2006.00252.x. [EPub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Sutters KA, Miaskowski C, Holdridge-Zeuner D, Waite S, Paul SM, Savedra MC, Lanier B. A randomized clinical trial of the effectiveness of a scheduled oral analgesic dosing regimen for the management of postoperative pain in children following tonsillectomy. Pain. 2004;110(l2):49–55. doi: 10.1016/j.pain.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Sutters KA, Miaskowski C, Holdridge-Zeuner D, Waite S, Paul SM, Savedra MC, Lanier B. Time-contingent dosing of an opioid analgesic after tonsillectomy does not increase moderate-to-severe side effects in children. Pain Manag Nurs. 2005;6(2):49–57. doi: 10.1016/j.pmn.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Taketomo CK, Hodding JH, Kraus DM. Pediatric dosage handbook [Google Scholar]
- 14.Hudson . Lexi-comp; Ohio: 2007. [Google Scholar]
- 15.Brunton L, Lazo J, Lazo JS. Goodman and Gilman's the Pharmacological basis of therapeutics. 11th ed. McGraw-Hill; New York: 2005. pp. 569–20. [Google Scholar]
- 16.Benjamin LJ, Dampier CD, Jacox AK, Odesina V, Phoenix D, Shapiro B, et al. Guideline for the management of acute and chronic pain in sickle cell disease. Am Pain Society; Glenview, 111: 1999. p. 42. [Google Scholar]
- 17.Burns N, Groves S. The practice of nursing research. WB Saunders; Philadelphia, PA: 2003. p. 292. [Google Scholar]
- 18.Savedra M, Tesler M, Holzemer WL, Wilkie DJ, Ward JA. Testing a tool to assess postoperative pediatric and adolescent pain. In: Tyler DC, Krane EJ, editors. Advances in pain research and therapy: Pediatric pain. Raven Press; New York: 1990. [Google Scholar]
- 19.Savedra MC, Holzemer WL, Tesler MD, Wiliie DJ. Assessment of postoperation pain in children and adolescents using the adolescent pediatric pain tool. Nurs Res. 1993;42(l):5–9. [PubMed] [Google Scholar]
- 20.Savedra MC, Tesler MD, Holzemer WL, Wilkie DJ, Ward JA. Pain location: Validity and reliability of body outline markings by hospitalized children and adolescents. Res Nurs Health. 1989;12:307–14. doi: 10.1002/nur.4770120506. [DOI] [PubMed] [Google Scholar]
- 21.Wilkie DJ, Holzemer WL, Tesler MD, Ward JA, Paul SM, Savedra MC. Measuring pain quality: Validity and reliability of children's and adolescents' pain language. Pain. 1990;41:151–9. doi: 10.1016/0304-3959(90)90019-A. [DOI] [PubMed] [Google Scholar]
- 22.Franck LS, Treadwell M, Jacob E, Vichinsky E. Assessment of sickle cell pain in children and young adults using the adolescent pediatric pain tool. J Pain Sympt Manage. 2002;23:114–20. doi: 10.1016/s0885-3924(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 23.Givens M, Rutherford C, Joshi G, Delaney K. Impact of an emergency department pain management protocol on the pattern of visits by patients with sickle cell disease. J Emerg Med. 2006;32(3):239–43. doi: 10.1016/j.jemermed.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Melzer-Lange MD, Walsh-Kelly CM, Lean G, Hillery CA, Scott JP. Patient-controlled analgesia for sickle cell pain crisis in a pediatric emergency department. Pediatr Emerg Care. 2004;20(l):2–4. doi: 10.1097/01.pec.0000106235.72265.29. [DOI] [PubMed] [Google Scholar]
- 25.Crawford MC, Galton S, Naser B. Postoperative morphine consumption in children with sickle cell disease. Pediatric Anesthesia. 2006;16:152–7. doi: 10.1111/j.1460-9592.2005.01705.x. [DOI] [PubMed] [Google Scholar]
- 26.Dampier CD, Setty BN, Logan J, loli JG, Dean R. Intravenous morphine pharmacokinetics in pediatric patients with sickie cell disease. J Pediatr. 1995;126(3):461–7. doi: 10.1016/s0022-3476(95)70472-8. [DOI] [PubMed] [Google Scholar]
- 27.Perlman KM, Myers-Phariss S, Rhodes JC. A shift from meperidine to hydromorphone improves pain control and decreases admissions for patients in sickle cell crisis. 2004;30(5):439–46. doi: 10.1016/j.jen.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Ruggiero A, Barone G, Liotti L, Chiaretti A, Lazzareschi I, Riccardi R. Safety and efficacy of fentanyl administered by patient controlled analgesia in children with cancer pain. Supportive Care Cancer. 2007;15:569–73. doi: 10.1007/s00520-006-0193-8. [DOI] [PubMed] [Google Scholar]
- 29.Ozalevli M, Unlugenc H, Tuncer U, Gunes Y, Ozcengiz D. Comparison of morphine and tramadol by patient controlled analgesia for postoperative analgesia after tonsillectomy hi children. Pediatric Anesthesia. 2005;15:979–84. doi: 10.1111/j.1460-9592.2005.01591.x. [DOI] [PubMed] [Google Scholar]
- 30.Subramaniam K, Subramaniam B, Stenbrook RA. Ketamine as adjuvant analgesic to opioids: A quantitative and qualitative systematic review. Anesthesia Analgesia. 2004;99:4S2–95. doi: 10.1213/01.ANE.0000118109.12855.07. [DOI] [PubMed] [Google Scholar]