Abstract
Orai1 was reported to function as a calcium channel subunit that facilitates store operated calcium entry (SOCE) in T cells and is necessary for formation of the immune synapse. We reasoned that SOCE via Orai1 might regulate PMNs activation during recruitment to inflamed endothelium. Orai1 function was assessed by real-time imaging of calcium transients as PMNs were stimulated to roll, arrest, and migrate on E-selectin and ICAM-1 in shear flow. Calcium entry was significantly reduced when Orai1 function was impaired by heterozygous knockout in a mouse model or by siRNA knockdown in HL-60 cells. Reduced Orai-1 expression correlated with the delayed onset of arrest and reduced ability to transition to a polarized migratory phenotype. Inhibition of SOCE by treatment with 2-APB, or blocking phospholipase C (PLC) mediated calcium store release with U73122, abrogated formyl peptide induced calcium elevation, and delayed subsequent cell arrest and polarization. These results suggest that calcium entry via Orai1 is the predominant SOCE that cooperates with cytoplasmic calcium store release in coordinating integrin-dependent PMN arrest and migration in the acute response to inflammation.
Introduction
In leukocytes, calcium signaling is triggered by the activity of phospholipase C (PLC), which opens IP3-gated channels embedded in the membranes of internal calcium stores. During G-protein coupled receptor (GPCR)–stimulated calcium release, endoplasmic reticulum stores are depleted, resulting in store-operated Ca2+ entry (SOCE) through plasma membrane channels. Store release and SOCE are an interconnected system that cooperates to raise cytoplasmic calcium concentration. Normal T-cell function is dependent on SOCE mediated by low conductance and highly ion-specific Ca2+–release-activated calcium (CRAC) channels. Recently, the sensor of calcium store depletion, STIM1,1,2 and an essential transmembrane component of the CRAC channel, Orai1, have been identified in T cells, B cells, and mast cells.3–6 Orai1 is one of several 4-transmembrane CRAC channels that regulates influx of calcium from outside of the cell. Because Orai1-mediated calcium signaling was demonstrated to be essential to the function of lymphocytes, we hypothesized that Orai1 is also a component of signaling in innate immunity and particularly in the acute inflammatory response of neutrophils (PMNs) under shear stress of flowing blood. Stimulation of PMNs with formyl peptide activates SOCE that has properties consistent with calcium entry mediated by CRAC. Indeed, Orai1 appears to cooperate with transient receptor potential channels (TRPCs) to mediate calcium entry in PMNs.7 Human neutrophils contain TRPC1, 3, 4, and 6, whereas only the latter has been implicated as the SOCE channel-regulating calcium influx in PMNs activated via soluble E-selectin and GPCR.8–10 Using newly available tools to alter Orai1 expression in PMNs, we examined the signaling role of calcium entry in cell arrest and shape polarization.
The majority of formyl peptide-induced calcium up-regulation originates from extracellular calcium,11 possibly entering through channels formed from Orai1 subunits. For example, production of bactericidal reactive oxygen species and GPCR-induced production of superoxide are partially dependent on extracellular calcium as it is sensitive to SOCE blockade by La3+.12,13 Much of this calcium entry and signaling occurs in the context of a coordinated process of PMN recruitment, which involves capture by inflamed vascular endothelium, rolling and signaling via selectin and chemokine receptor engagement, arrest supported by integrin bond formation, and subsequent migration toward the site of tissue insult. Several lines of evidence support a role for SOCE in PMN recruitment. A rise in calcium causes membrane up-regulation and an upshift in affinity of the β2-integrin Mac-1,14 which is known to participate in both arrest and migration of PMNs.15,16 Although chelation of calcium appears to exert a small effect on PMN migration,17,18 calcium release can trigger cation depolarization and restructuring of the actin cytoskeleton, which may be involved in fine-tuning and directing PMN migration and phagocytosis.19,20 Furthermore, calcium transients are associated with cytoskeletal coordination of phagocytosis and spreading of adherent PMNs.21–23 Migration of PMNs involves intracellular integrin trafficking and uropod detachment, both of which are calcium dependent.24,25 Thus, there is evidence that localized calcium entry and cytoplasmic flux modulate integrin-binding activity and cytoskeletal functions that underlie PMN recruitment, but questions remain regarding the mechanism by which transients in cytosolic release can signal sequential events, such as adhesion and motility.
PMN recruitment occurs under conditions of fluid shear stress, which drives initial cell capture and transient rolling adhesion. Tensile forces exerted on selectin and integrin bonds also profoundly influence signaling events necessary for contact-mediated guidance of leukocyte migration.26–29 Unanswered questions regarding how calcium signaling regulates recruitment under shear flow remain; for instance, it is not known how the dynamics of calcium entry correlate with the onset of arrest in a rolling PMN. Specifically, are integrin activation and PMN arrest triggered during calcium entry via SOCE or is calcium store release preeminent? One reason such questions remain is that extracellular calcium ions are required for cell capture via selectin bond formation.30 Therefore, studying subsequent rolling and arrest in the absence of calcium signaling is technically difficult. Altering Orai1 expression in human PMNs through RNAi or gene deletion in a knockout mouse model makes it possible to study its role in recruitment under shear stress. For these studies, we have used a custom microfluidic channel that enables precise delivery of agonists and Ca2+ cation during neutrophil-endothelial recruitment and real-time ratiometric calcium imaging.31 This approach was applied to analyze the dynamics in intracellular calcium concentration during the multistep process of PMN rolling, arrest, and shape polarization.
Methods
Reagents and antibodies
Human and mouse ICAM-1-Fc and E-selectin-Fc were purchased from R&D Systems. Protein A/G was purchased from Pierce Chemical. 2-Aminoethoxydiphenyl borate (2-APB) was purchased from EMD Biosciences, resuspended in H2O free dimethyl sulfoxide (DMSO) at a concentration of 100mM, and stored at −80°C under dry N2. PLC inhibitor U73122 was purchased from EMD Biosciences and resuspended to 1mM in dry DMSO, and stored at −80°C under dry N2. Thapsigargin was purchased from Invitrogen and resuspended to 1mM in dry DMSO the same day as the experiment. The potassium channel inhibitors TRAM-34 and PAP-1 were a gift from the laboratory of Heike Wulff. Anti-CD18 antibody 327C was obtained as a generous gift from ICOS, now an entity of Eli Lilly. Polyclonal rabbit anti–human Orai1 was purchased from Alomone Labs. Polyclonal anti–mouse Orai1 was purchased from Abcam. Goat anti–rabbit-Alexa 488 polyclonal secondary antibody and normal goat serum were purchased from Thermo-Fisher Scientific.
Neutrophil isolation
PMNs were isolated from whole human blood as previously described.32 In brief, whole blood was obtained from consenting donors and layered over PMN separation media purchased from Thermo-Fisher Scientific. After centrifugation, PMNs were extracted from the appropriate density layer and washed with N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid-buffered salt solution. Mice heterozygous for Orai1 deletion were a generous gift from the laboratory of Dr Anjana Rao (Harvard Medical School).33 Mice were genotyped from tail clippings by polymerase chain reaction (PCR), and PMNs were isolated from the bone marrow of littermate Orai1−/+ and wild-type (WT) ICR strain mice as previously described.33,34 This study was approved by the Institutional Review Board of University of California–Davis (approval identification number 200911635-7).
Cell culture
L cells stably expressing E-selectin or coexpressing E-selectin and ICAM-1 were derived as previously described.32 Both cell types support PMN arrest after GPCR stimulation through Mac-1, although the presence of ICAM-1 facilitates LFA-1 and Mac-1–dependent arrest even in absence of GPCR signaling.31 ICAM-1 expression was necessary to observe arrest in PMNs stimulated with thapsigargin and extracellular calcium in the absence of formyl Met-Leu-Phe (fMLP) stimulation. L cells expressing only E-selectin were used in experiments where PMNs were stimulated with fMLP to avoid premature LFA-1–dependent arrest of PMNs. L cells were grown in RPMI media supplemented with selection agents (geneticin and mycophenolic acid) until confluence, then serially passaged up to 10 times to maintain cell numbers. Two days before the experiment, L cells were transferred from culture flasks to glass coverslips and grown again to confluence. HL-60 cells were maintained in Advanced RPMI containing 10% United States Department of Agriculture–certified low endotoxin fetal bovine serum by serial dilution (JRS Scientific).
siRNA
HL-60 cells were transfected with negative control siRNA (AllStars; QIAGEN) labeled with Alexa 488, with sterile water (a mock transfection), or with Orai1-specific siRNA (QIAGEN) by electroporation using the MXCell electroporator (Bio-Rad) according to the manufacturer's instructions. HL-60 cells were given fresh media 24 hours before electroporation to maximize survival. Cells were pelleted from media, resususpended in high resistance electroporation buffer (Bio-Rad) in the presence of 200nM siRNA, and electroporated with an exponential waveform at 250 V, 1000 ohms, 500 μF. Immediately after electroporation, cells were transferred to 37°C media containing 1.3% DMSO and differentiated for 2 days under the influence of siRNA. Transfection efficiency was optimized by measuring control siRNA delivery by flow cytometry.
Cytometry
Expression of Orai1, in HL-60 cells as well as in human and mouse PMNs, was measured by flow cytometry. Cells were fixed with 4% paraformaldehyde for 5 minutes at 0°C, washed with phosphate-buffered saline (PBS), permeablized with 0.1% Triton X-100 for 2 minutes, and resuspended in PBS plus 10% normal goat serum. Cells were incubated with primary antibodies in 10% normal goat serum for 1 hour at 0°C, washed once in PBS, and then labeled with secondary goat anti–rabbit Alexa 488 for 30 minutes at 0°C in 10% goat serum. Samples were then read by a FACScan laser cytometer and quantified for median fluorescence intensity at constant amplifier settings.
Expression of high-affinity β2-integrin was measured by incubating PMNs with 5 μg/mL the Dylight-488–conjugated monoclonal antibody 327C, which recognizes an extended conformation of the β2-subunit.25,35 PMNs or differentiated HL-60 cells were stimulated for 5 minutes with 0.1μM fMLP in the presence of the reporter antibody and relevant inhibitors and then immediately placed on ice for 30 minutes. After stimulation and labeling, cells were analyzed by flow cytometry for median fluorescence intensity after washing with PBS.
Shear/calcium assay
PMNs (human, mouse, or differentiated HL-60) were suspended at a concentration of 106/mL in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid-buffered salt solution and labeled with fura-2 AM for 30 minutes at 37°C. Cells were then washed and resuspended in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid-buffered salt solution. Labeled cells were perfused into a microfluidic flow chamber and imaged as previously described.29,31 Briefly, cells were drawn into the microfluidic chamber at a calculated shear stress of 2 dyne/cm2 and sequentially imaged over time with alternating excitation by 340nM and 380nM light generated by a mercury arc lamp attached to a filter wheel with 0.1 second switch time. Images were acquired with an Orca-ER camera (Hamamatsu) coupled to a Nikon 1200 microscope running SimplePCI 5.3 software. Image sequences were analyzed for the ratio between emission at 340nM and 380nM using custom macros written for Image Pro Plus 5.1. During analysis, the average intensity of each cell was identified in a confined area of interest around each cell for both the 340nM and 380nM exposure. This method of cell identification and local overlap accounted for moderate motion of rolling PMNs during image acquisition. The shape of each cell was measured by applying an intensity threshold to the image under 380nM illumination for every 10th frame. Cells with a length-to-width ratio greater than 1.5 (ie, 50% deformation from a circular shape) were considered polarized. Rolling versus arrested PMNs were determined by tracking the centroid of each cell image over 10-second increments by an automated macro in Image Pro Plus. PMNs that moved less than 4 μm in 10 seconds (approximately half a cell diameter) were considered arrested rather than rolling. In certain experiments, PMNs were fixed with 2% paraformaldehyde at various time points during the experiment and then incubated with 5 mg/mL 327C conjugated to Dylight-488 for 1 hour at 4°C. An optical slice within 1 μm of the surface of contact between the PMNs and substrate was captured using confocal microscopy.
TIRF microscopy of calcium
Human and mouse PMNs were loaded with 1μM fluo-5f for 30 minutes at 37°C, washed, and perfused over E-selectin, and ICAM-1 coated glass coverslips in a microfluidic flow chamber at a calculated shear stress of 2 dyne/cm2. Human or mouse Fc-recombinant proteins were derivatized to protein-A/G coated coverslips to study each species as previously described.29 Arresting PMNs were excited with a 488-nm laser and imaged via total internal reflection (TIRF) microscopy at 1 frame per second to observe changes in intracellular calcium in a focal section of the PMNs approximately 100 nm from the surface of the coverslip.
Quantitative PCR
Cellular expression of Orai1 mRNA was determined by quantitative real-time PCR. To determine the extent of Orai1 expression, RNA was extracted from 106 HL-60 cells using an RNeasy mini kit (QIAGEN). To confirm siRNA-dependent knockdown of mRNA expression, RNA was also extracted 1 day after transfection of HL-60 cells with Orai1-specific siRNA, negative control siRNA, or an equal volume of sterile water (mock transfection). Quantitative PCR was carried out in the presence of primers for ribosomal protein L (RPL, a control gene) or Orai1 primers using a prevalidated TaqMan gene expression assay (Applied Biosystems) in conjunction with a Mastercycler PCR system according to the manufacturer's instructions.
Statistical analysis
Differences between single pairs of conditions were analyzed for significance by 2-tailed t test. For figures containing exclusively paired observations, P values were obtained by 2-tailed paired t test. Unpaired multiple comparisons were conducted by 1-way analysis of variance with Tukey post-test in GraphPad Prism 5. All error bars represent mean plus or minus SEM based on several independent experiments indicated in the figure legends.
Results
Suppression of Orai1 expression in mouse and human PMNs
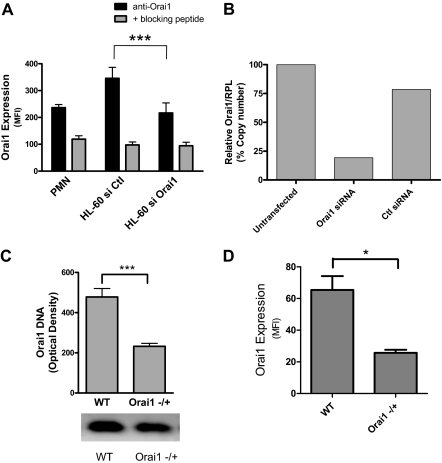
Orai1 expression has previously been confirmed in PMNs and HL-60 cells by real-time quantitative PCR and in HL-60 cells by Western blot.7 We endeavored to knock down Orai1 expression in human PMNs using siRNA on myeloid-differentiated HL-60 cells and in mice heterozygous for Orai1 gene deletion (Orai1−/+). Expression of Orai1 on human PMNs and differentiated HL-60 was detected with a polyclonal primary antibody and quantified by a labeled secondary antibody using fluorescence flow cytometry (Figure 1A). Membrane expression of Orai1 was significantly greater than the background fluorescence as determined by competitive binding of primary antibody in the presence of excess Orai1 decoy peptide. HL-60 were electroporated in the presence of 200nM gene-specific siRNA, which reduced surface expression of Orai1 by approximately 50% from that detected in cells treated with an equal concentration of negative control siRNA. Knockdown of Orai1 mRNA relative to a control protein (RPL) was confirmed by quantitative PCR, which registered a 77% decrease compared with the control siRNA transfectants (Figure 1B). This ratio of protein inhibition relative to mRNA knockdown is consistent with previously reported data for Orai1 RNAi and may stem from slow degradation and turnover of surface proteins in differentiating HL-60 cells.7 Taken together, these data indicate that PMNs and HL-60 cells express Orai1, which can be reduced by siRNA knockdown in HL-60 cells.
Figure 1.
Neutrophils express membrane Orai1. Isolated mouse and human PMNs, or 2-day differentiated HL-60 were assessed for RNA, DNA, or protein expression. (A) Protein expression levels were measured from the average mean fluorescence intensity (MFI) of paired flow cytometry samples of HL-60 cells transfected with negative control or Orai1-specific siRNA (n = 10) and PMNs (n = 4). Background fluorescence resulting from nonspecific antibody binding was measured by analyzing cells treated with a blocking decoy peptide against which the Orai1 antibody was originally raised. (B) Relative expression levels of Orai1 mRNA compared with RPL mRNA were measured for siRNA-transfected, control siRNA-transfected, or mock-transfected HL-60 cells by quantitative PCR. Data expressed as Orail mRNA copy number relative to RPL after PCR amplification. (C) Relative genomic DNA expression of Orai1 in WT versus Orai1−/+ mice measured by electrophoresis of amplified DNA from mouse tissues. Bars represent average densitometry measurements from 18 Orai1−/+ and 6 littermate WT mice ± SE. (D) Orai1 protein expression measured from the average MFI of paired cytometric measurements in WT and Orai1+/− mouse PMNs (n = 3). *P < .05; ***P < .001.
Orai1-deficient mice were recently developed that exhibit many of the defects associated with natural mutations in humans that render Orai1 dysfunctional. Because mice homozygous for the Orai1 deletion often die in utero, are prone to premature death, and exhibit severe skin defects, we used Orai1−/+ PMN as a model of Orai1 deficiency relative to PMNs from WT Orai1+/+ littermates. Heterozygous Orai1−/+ mice were derived in a B6 strain background by homologous recombination, then backcrossed for 6 generations with outbred ICR mice to improve the survivability of knockouts.3,5 Orai1−/+ mice were confirmed to have 50% of the gene expression of WT based on electrophoresis of amplified DNA (Figure 1C). This correlated with an approximate 50% decrease in Orai1 protein expression as measured by flow cytometry (Figure 1D). This deficiency was previously shown to result in defective regulation of calcium entry in T cells and B cells compared with WT counterparts.5
Ca2+ dynamics during neutrophil arrest and shape polarization
Bacterial peptides, such as fMLP, produce an elevation of intracellular calcium through the combination of GPCR-signaled PLC-dependent store release and SOCE. Using human and murine PMNs, we examined whether SOCE mediated by Orai1 was responsible for the transition from rolling to arrest and subsequent shape polarization during fMLP stimulation under shear stress. PMNs were infused under a fluid shear stress of 2 dyne/cm2 on a monolayer of L cells stably expressing E-selectin, which supports CD11b/CD18-dependent arrest and migration.36 Activation of rolling PMNs with fMLP resulted in an immediate increase in intracellular calcium and transition to arrest and subsequent shape polarization (as defined by PMNs length/width ratio > 1.5; Figure 2A). PMNs from Orai1−/+ mice had 47% reduced peak calcium up-regulation compared with WT littermates, consistent with Orai1 functioning as a primary store-operated channel (Figure 2B). Orai1−/+ PMNs exhibited a significant defect in transition from rolling to arrest compared with Orai1+/+ during the first minute after fMLP infusion (25% vs 65% at 30 seconds, P < .05), suggesting that SOCE contributes to adhesion function via integrin activation. The rate of polarization of Orai1−/+ PMNs was substantially reduced compared with Orai1+/+ PMNs, resulting in up to 50% fewer polarized neutrophils after 60 seconds of stimulation (58% vs 18%, P < .05). These data demonstrate that Orai1 is a key regulator of PMN SOCE and that loss of half of the gene expression has a profound effect on calcium signaling and the efficiency of PMNs transition from arrest to a migratory phenotype.
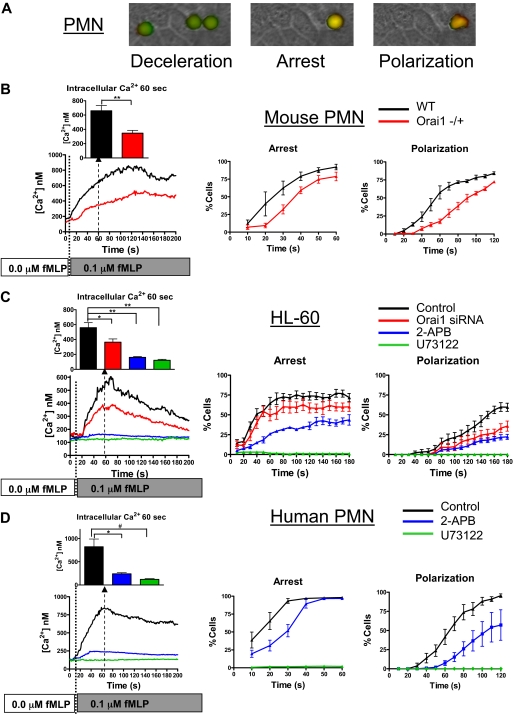
Figure 2.
Calcium dynamics via Orai1 supports GPCR-mediated PMN recruitment. PMNs isolated from murine Orai1+/+ (WT), or Orai1−/+ littermates, 2-day differentiated HL-60 cells, or freshly isolated human PMNs were loaded with fura-2 AM, perfused at a shear stress of 2 dyne/cm2 into a microfluidic flow chamber consisting of an L-cell monolayer substrate expressing E-selectin, and then 0.1μM fMLP was perfused just after time 0 and ratio-imaged by fluorescence microscopy. (A) Representative images of a human PMN decelerating, arresting, and undergoing shape polarization show fura-2 emission, with the intensity of red representing 340 nm excitation (calcium bound) and green representing 380nM excitation (calcium unbound). The average calcium concentration is shown in 1-second increments, and the percentage of cells arrested is shown in 10-second increments. (B) Percentage of arrested and polarized in WT and Orai1−/+ PMNs is based on analysis of more than 150 cells from 4 paired experiments. WT cells had significantly higher arrest and polarization than Orai1−/+ at time points beyond 30 seconds. **P < .01. (C) HL-60 were transfected with negative control, or Orai1-specific siRNA as indicated, were differentiated for 2 days with 1.3% DMSO and were imaged in the flow channels responding to 0.1μM fMLP as in panel A (n = 10 separate experiments). Negative control transfectants were treated with 2-APB or U73122 to completely block SOCE or store release. For Ca2+ quantified at 60 seconds. *P < .05. **P < .01. (D) Human PMNs were observed in absence of inhibition (n = 6), in the presence of 100μM 2-APB (n = 4), or in the presence of 1μM U73122 (n = 4) as indicated. Cells treated with 2-APB had significantly lower arrest fraction at 30 seconds (P = .003) and significantly lower polarization between 50 seconds and 100 seconds (P values range from .007 to .045 compared with the controls). *P < .05; #P < .05 (1-tailed t test, P = .059 by 2-tailed t test).
Stimulation of HL-60 cells with fMLP caused a sequence of increased intracellular calcium, cell arrest, and polarization similar to that seen in freshly isolated PMNs (Figure 2C). The kinetics of arrest and polarization were somewhat slower than freshly isolated PMNs, which was attributed to the incomplete maturity of 2-day differentiated HL-60. Knockdown of Orai1 with siRNA in HL-60 reduced the extent of the rise of intracellular calcium by 44%, which correlated with a significant drop in the fraction of cells arresting and polarized in response to the stimulus over time (individual HL-60 fura-2 traces, supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Orai1 knockdown PMNs had significantly higher rolling fractions at 130 seconds, 160 seconds, and 170 seconds (P = .04, .03, and .02, respectively) and lower polarization fractions at 150 seconds, 160 seconds, and 170 seconds (P = .03, .005, and .04, respectively) than control transfected cells. Similar to the Orai1−/+ mouse PMNs, RNA knockdown of Orai1 induced an approximate 20% decrease in arrest compared with control and an effective 30-second delay in polarization in response to fMLP.
To determine the relative contributions of SOCE and PLC-dependent cytosolic stores in facilitating the transition from arrest to a migratory phenotype, we examined the effects of the pharmacologic inhibitors 2-APB and U73122 on HL-60 and human PMNs freshly isolated from whole blood. Pretreatment with 2-APB effectively inhibited calcium entry inhibiting its up-regulation more than 80% in both HL-60 and isolated PMNs (Figure 2C-D). This result was equivalent to PMN treatment with 10μM LaCl3, which effectively blocks all channel-mediated calcium entry (supplemental Figure 2). Similar to the defect observed in mouse Orai1−/+, 2-APB treatment delayed the onset and reduced the extent of PMN arrest and shape polarization. Because activation of PLC underlies both release of cytoplasmic calcium stores as well as resulting SOCE, PMNs were preincubated with the PLC inhibitor U73122. This treatment completely abrogated the elevation in intracellular calcium and the transition to arrest and shape polarization, implying that calcium entry via SOCE and kinase signaling downstream from PLC is required for activation of these responses.
SOCE induces arrest of rolling PMNs through Orai1
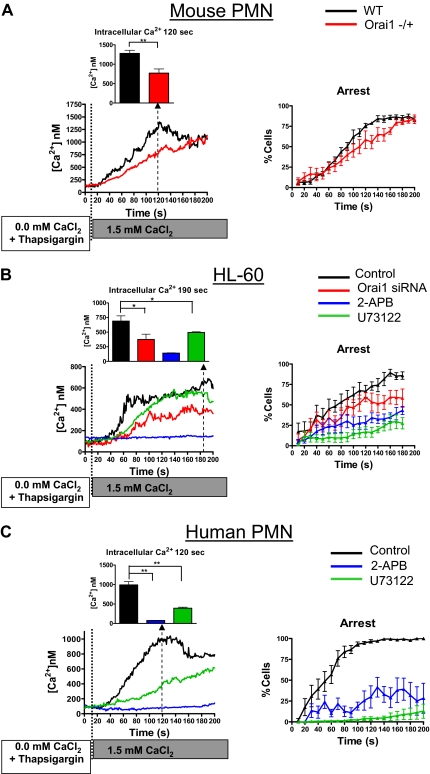
To isolate the function of SOCE in activating the transition from rolling to arrest, PMNs were labeled with fura-2, and then calcium stores were depleted by incubation with 1 μM thapsigargin in Ca2+ free media. PMNs were then perfused over an E-selectin/ICAM-1–coated surface within a microfluidic flow chamber and exposed to a bolus containing 1.5mM CaCl2, which served as an agonist to initiate rolling on E-selectin and Mac-1– and LFA-1–dependent arrest on ICAM-1.29 After infusion of CaCl2, tethered and rolling mouse PMNs, human PMNs, or HL-60 cells registered a progressive elevation of intracellular calcium (Figure 3). As with infusion of the fMLP stimulus, CaCl2 exposure activated β2-integrin–dependent arrest in store-depleted PMNs within a minute. Under these conditions, WT PMNs had approximately double the rate of intracellular calcium rise as Orai1−/+ PMNs and a significantly more rapid onset of arrest (Figure 3A). Thus, the rate of calcium entry is related to Orai1 expression and appears to determine the extent of SOCE-mediated activation of arrest. We confirmed the effects of Orai1 knockdown in HL-60 cells on calcium influx and transition to arrest after thapsigargin treatment (Figure 3B). As with Orai1−/+ PMNs, Orai1 knockdown in HL-60 cells significantly reduced peak calcium concentration compared with control transfected cells (689nM vs 375nM, P = .03) and had a significant effect on the arrest fraction after 2 minutes of rolling (75% vs 50%, P = .03). This functional response was dependent on plasma membrane calcium channel influx because pretreatment with 2-APB virtually eliminated calcium entry and significantly diminished arrest to a similar extent in differentiated HL-60 and freshly isolated human PMNs (Figure 3B-C). Inhibition of PLC with U73122 reduced the rise of intracellular calcium induced by store depletion but to a lesser extent than blocking SOCE with 2-APB. PLC inhibition was more effective than 2-APB in abrogating PMN arrest, indicating that the lipid intermediates created by PLC were downstream of the Ca2+ rise induced by SOCE and Orai1.35,37 These data confirm that, in calcium-depleted PMNs, the influx of extracellular calcium is sufficient to trigger PMN arrest and that SOCE is significantly modulated by Orai1 in both human and mouse.
Figure 3.
Calcium influx through SOCE and Orai1 facilitates neutrophil arrest. (A) PMNs isolated from murine Orai1−/+, WT littermates (ie, Orai1+/+), 2-day differentiated HL-60 cells, or freshly isolated human PMNs were loaded with fura-2, incubated with 1 μM thapsigargin as indicated, and then perfused over a monolayer of L cells expressing E-selectin and ICAM-1 in the presence of 0.1mM ethyleneglycoltetraacetic acid. At time 0, 1.5mM CaCl2 was injected into the inlet reservoir triggering selectin-dependent rolling and calcium influx through store-operated calcium channels. (A) Panels indicate intracellular calcium concentration and arrested fraction from WT or Orai1−/+ PMNs as indicated. (B) HL-60 cells were transfected with scrambled control or specific Orai1 siRNA as indicated (both n = 10). Orai1 siRNA-transfected cells had significantly higher rolling fractions at 150 seconds, 160 seconds, and 170 seconds (P = .03, .01, and .03, respectively) than control transfected cells. (C) Human PMNs were analyzed in the presence of vehicle control (n = 6), 100μM 2-APB (n = 4), or 1μM U73122 (n = 4) as a function of time. Cells treated with 2-APB had significantly lower arrest fraction than control at 60 seconds (P = .01). All statistical comparisons in this figure are by 2-tailed t test: *P < .05; **P < .01.
Synchrony in calcium rise, arrest, and polarization is regulated by Orai1
PMN recruitment is a multistep process in which receptor-ligand–mediated signaling via selectins and GPCR superpose to activate β2-integrin–dependent arrest and subsequent migration.38 These signaling events are highly synchronized among a population of PMNs with a variation of no more than 10 seconds in the timing of changes in intracellular calcium with respect to arrest and shape polarization (Figure 4A-B). We performed high resolution temporal analysis of the kinetics of intracellular calcium in more than 200 individual PMNs and display the dynamics of cell capture and deceleration to arrest on a substrate of recombinant E-selectin-Ig and ICAM-1-Ig under shear flow. In comparing the kinetics of fMLP stimulation with direct activation via SOCE in thapsigargin-depleted PMNs, we observed arrest with a t1/2 of 20 seconds for fMLP, compared with 50 seconds for thapsigargin. Intracellular calcium reached a maximum at 60 seconds in fMLP-activated PMNs, compared with 120 seconds in thapsigargin-treated PMNs. In addition, thapsigargin-induced SOCE caused PMN polarization that was comparable, albeit slower, than that caused by fMLP. In both cases, deceleration to arrest was contemporaneous with the calcium rise, which subsequently rose to a plateau, heralding an increase in fraction of PMNs adopting a polarized shape.
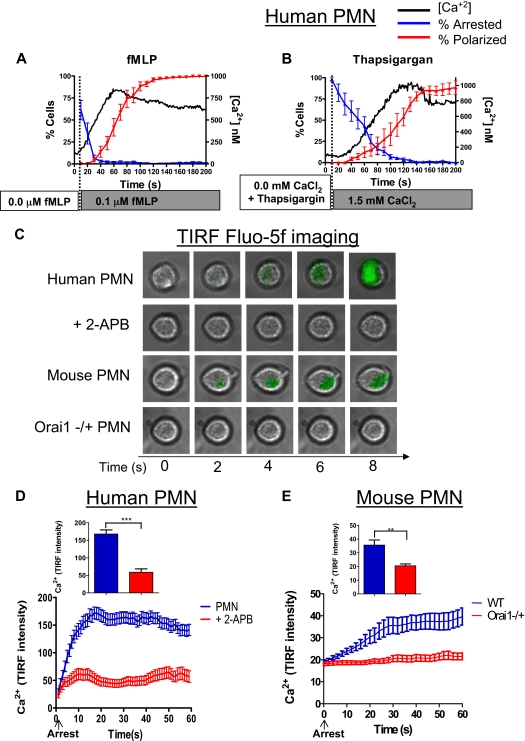
Figure 4.
Intracellular calcium rises in synchrony with PMN arrest and polarization. (A) Data for human PMNs responding to fMLP stimulation are compiled from more than 200 individual observations onto 1 plot to compare the dynamics of calcium, arrest, and polarization. Arrest is represented here as the fraction of cells remaining rolling (ie, the inverse of arrest). (B) Data for human PMNs treated with thapsigargin and stimulated by the addition of 1.5mM CaCl2 are also compiled onto 1 plot to compare the dynamics of calcium arrest and polarization. Arrest is represented as in panel A. (C) Human and mouse PMNs were loaded with fluo-5f, perfused over a glass coverslip coated with recombinant E-selectin-Fc and ICAM-1-Fc, and imaged by TIRF microscopy. Representative images from 3 separate experiments are indicated. (D) Calcium (fluo-5F) intensity over 60 seconds, untreated PMNs had significantly higher relative fluo-5f signal than 2-APB treated cells at 15 seconds and higher time points (P < .01). (E) Calcium (fluo-5F) signal in mouse WT versus Orai1−/+ PMNs over 60 seconds. **P < .01. ***P < .001. Error bars represent the SE for each measured time point.
We examined in more detail the dynamics in Ca2+ rise within the plane of adhesive contact just after PMNs rolled to arrest, relying on E-selectin–mediated activation of β2-integrin–dependent recruitment.32,39 Human and mouse PMNs were labeled with fluo-5f, a fluorescent Ca2+ indicator with a fast on rate, and perfused across a glass slide coated with E-selectin-Ig and ICAM-1-Ig. The intensity of fluo-5f emission was imaged by TIRF microscopy, which limits photon excitation of the fluo-5f reporter to within approximately 100 nm of the substrate at sub-second resolution (Figure 4C). Because these cells were not stimulated with fMLP or thapsigargin, this ramp in intracellular calcium was initiated at the membrane during rolling and signaling on E-selectin under shear stress. Calcium flux increased rapidly, reaching a peak within 10 seconds from the baseline value after arrest (Figure 4D; supplemental Video 1). SOCE was the source of this calcium flux because treatment with 2-APB suppressed the TIRF signal by 70% in human PMNs (supplemental Video 2). The rise in calcium in WT mouse PMNs was significantly slower than human requiring more than 30 seconds to reach maximum (Figure 4E). In Orai1−/+ PMNs, the calcium signal remained at the background activated in response to rolling on E-selectin, and no sharp increase on arrest was observed as in the WT controls. Taken together, these data implicate Orai1 as a predominant SOCE in mediating calcium entry and activation of β2-integrin–dependent PMN arrest within the plane of adhesive contact on E-selectin and ICAM-1 under shear stress.
Discussion
Neutrophil recruitment to a vascular site of inflammation is a serial molecular event that is initiated by selectin and chemokine bond formation that superpose to activate a transition from cell rolling to integrin-dependent arrest and migration. Deletion or disruption of any single receptor-ligand interaction in the multistep process results in defective emigration across inflamed endothelium to the site of injury or infection.38 Uninterrupted PMN recruitment is a robust and synchronous process that by nature's design is faithfully reproduced a million-fold in healthy people during the innate immune response to inflammatory stimuli. Calcium signaling is an integral part of the integrin and chemokine signaling pathways that govern this recruitment process.40–42 Recently, a point mutation in the Orai1 gene was shown to be responsible for the genetic defect in SOCE in T cells and B cells of SCID patients. These patients with defects in Orai1 have severe immunodeficiencies manifest as defects in hair, skin, and sweat gland morphogenesis.3,43 Similar phenotypes are found in Orai1-deficient mice, which exhibit disruption of SOCE and impaired intracellular signaling of cell proliferation, cytokine production, integrin activation, and immune cell recruitment to sites of inflammation.5,44 Orai1 mRNA is reported to be broadly expressed in mammalian tissues, which may account for defects in adaptive immune responses against infection and pathogen invasion. Although this is the first report of Orai1 function in PMN recruitment in an in vitro model of inflammation, ongoing studies indicate that recruitment to skin inflamed with tumor necrosis factor-α in an air pouch model is also defective (E.P. and S.I.S, unpublished data, 2009).
We report that the dynamics of intracellular calcium entry facilitated by Orai1 are precisely synchronized with adhesive activation events and function to accelerate PMN recruitment. Neutrophils possess 4 TRPC channels along with Orai1 for facilitating SOCE,7,10 and the following observations support a predominant role for Orai1 in PMNs store-operated function: (1) Knocking down Orai1 with siRNA or deletion of Orai1 in heterozygous mice decreased the rate of intracellular calcium increase by approximately 50%, in proportion to the reduction in the number of active store-operated channels. (2) Activation via the addition of Ca2+ to PMNs in shear flow revealed that SOCE alone could induce arrest and polarization in calcium-depleted cells treated with thapsigargin. Partial deletion of Orai1 in HL-60 resulted in a proportional reduction in the initial rate of calcium entry. These data are comparable with previous measurements of the effect of thapsigargin followed by calcium infusion on T cells and B cells in Orai1 heterozygous mice.33 (3) Blocking SOCE with 2-APB decreased intracellular calcium influx almost to baseline in response to stimulation with fMLP or thapsigargin, similar to observed impairment of calcium flux in PMNs treated with LaCL3. (4) Finally, we discovered that Orai1 was the predominant SOCE supporting membrane Ca+2 entry within the plane of adhesive contact with the inflammatory substrate.
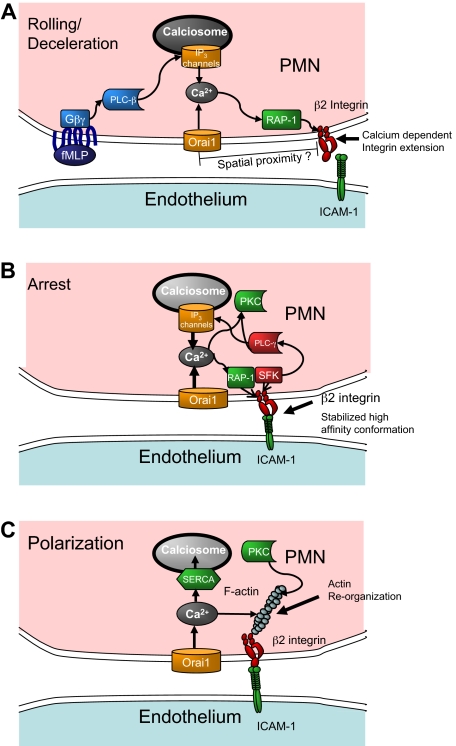
The observation that Orai1 acting through SOCE coordinates and accelerates adhesive and signaling events during inflammatory recruitment raises several questions for future study. Foremost of these questions is this: through what pathway does SOCE influence signaling that leads to leukocyte recruitment? Orai1 could facilitate signaling by the direct local action of calcium entry on activation of β2-integrins via the small GTPase regulator for cell adhesion and polarization type I (Rap-1), as depicted in the model of Figure 5A. In addition, Orai1 appears to cooperate with IP3 channels on calciosomes to refill depleted stores that otherwise would produce less robust calcium release. The finding that store repletion directly activated PMN recruitment by enhancing expression of high-affinity β2-integrin (supplemental Figure 3) and that this effect was blocked by 2-APB addition lends credence to the direct local action model. On the other hand, the reduced rate of the early rise in calcium observed in Orai1-deficient mice PMNs and siRNA treated HL-60 cells after fMLP stimulation suggests that initial store release may have been suppressed along with SOCE by these interventions. Because SOCE is vital for maintenance and refilling of stores, it is possible that suppressing Orai1 inhibits PMN signaling through reduced store volume.45 This question can be directly addressed by measurement of the calcium concentration within the stores, as has previously been done to determine the calcium affinity of STIM1 and STIM2.46 After arrest, we envision that release of intracellular calcium stores in concert with SOCE results in additional active integrin recruitment as depicted in the schematic model of Figure 5B.
Figure 5.
Conceptual model of calcium regulation and integrin engagement during PMN recruitment in shear flow. (A) During rolling, release of calcium stores initiated by GPCR and PLC-β signals IP3-sensitive calciosomes that cooperate with Orai1 SOCE eliciting an upshift in β2-integrin activation via Rap-1 that is sensitive to the rapid and local release of calcium stores resulting in PMN arrest. (B) As the PMN transitions to arrest, integrin engagement under stress stabilizes a high affinity conformation, which triggers activation of PLC-γ. The additional calcium release caused by integrin engagement sustains SOCE-mediated calcium influx and reinforces signaling for strengthening of arrest. (C) Calcium signaling causes rearrangement of the actin cytoskeleton and activation of secondary messengers (PKC) necessary for shape polarization. Electrical depolarization of the membrane and refilling of calcium stores through SERCA pumps slowly diminish intracellular calcium.
A second major question is how cell arrest is activated by calcium entry given the relatively low cytoplasmic calcium concentration detected during capture and rolling on E-selectin in the microfluidic channels. Because decreasing Orai1 expression or 2-APB inhibition of SOCE function correlated with an arrest defect, it is probable that focal regions of elevated calcium during the initial seconds of calcium entry are sufficient to activate integrins at focal sites of adhesion. Supporting this is the observation of β2-integrin colocalization with Orai1 in T cells during immune synapse formation.47 Binding of integrins may in turn enhance store release and SOCE through the action of associated PLC-γ isoforms, leading to the postarrest spike in calcium as depicted in Figure 5B.48,49 Previous studies show that inside-out signaling of integrin engagement is contemporaneous with rapid increases in calcium, which in turn activates the calcium diacylglycerol guanine nucleotide exchange factor I and Rap-1.50–52 TIRF in-plane imaging revealed that the spike in calcium from the low baseline value on PMN rolling on E-selectin correlated with the onset of arrest, supporting the premise that integrin-mediated adhesion precedes and can trigger calcium entry within the region of contact. We speculate that feedback between integrin activation and local PLC-γ–coupled calcium entry is responsible for the observed synchrony between the rapid rise in intracellular calcium after arrest perhaps via outside-in signaling from high affinity LFA-1 (Figure 5B).
A final question is how SOCE is linked to the signaling processes that underlie PMN conversion to a polarized migratory phenotype. The rate of conversion of Orai1−/+ PMN- and RNAi-treated HL-60 cells to a polarized shape was found to be reduced by up to 50% compared with their respective controls. Thus, the SOCE appears to have a larger role in precipitating polarization than for arrest, which is reduced by only 20% to 30% for SOCE interventions. Based on their relative timing, we conjecture that arrest induces an outside-in integrin signaling complex, which includes Src family kinase and PLC-γ, initiating peak concentrations of calcium and other secondary messengers, such as PKC responsible for subsequent polarization (Figure 5C). Calcium entry may fall off after completion of cell polarization resulting from depolarization of the membrane caused by entry of positive Ca2+ ions. Unlike T cells, which sustain SOCE and elevated calcium for hours, PMNs do not show sensitivity to selective inhibitors against the potassium channels Kv1.3 and KCa3.1, which are required to sustain electrical polarization in T cells despite an influx of positive ions (supplemental Figure 4).53,54 Reduced intracellular calcium may set the stage for the fine-tuning of directional guidance as the PMNs migrate over the course of minutes.
Clearly, there are some limitations to the interpretation of our data, as pharmacologic inhibition of PLC may also eliminate lipid intermediate signals generated by PLC, such as diacylglycerol. Therefore, some of the functional impact of U73122 may be attributed to blockade of downstream kinase signaling rather than direct effects on calcium. Because thapsigargin-induced PMN arrest was abrogated by U73122 despite continued calcium entry via SOCE, we speculate that lipid products significantly contribute to upshift in integrin affinity and the role of PLC in mediating cell arrest. In the same vein, although 2-APB has well-characterized interactions with Orai1-mediated SOCE, under some conditions it has been demonstrated to inhibit IP3-gated channels within calcium stores.35 Thus, interpreting 2-APB inhibition data requires caution. At concentrations less than 10μM, 2-APB enhances SOCE possibly by interaction with Orai1. In contrast, at concentrations more than 75μM as used here, 2-APB interacts with STIM1 and effectively inhibits SOCE.37,55 Consistent with this dose dependence, we found that interleukin-8–stimulated intracellular calcium was suppressed by 100μM 2-APB29 but enhanced by 1 to 10μM 2-APB (supplemental Figure 5). Our measurements were consistent with 2-APB inhibiting SOCE: the compound brought thapsigargin-stimulated intracellular calcium to baseline in PMNs but allowed 20% of the increase in calcium stimulated by fMLP in line with previous measurements of fMLP-induced PLC-dependent store release.56
In conclusion, Orai1 expressed on circulating PMNs is a component of a predominant SOCE channel. It cooperates with IP3-gated channels downstream of PLC in activation of β2-integrins and functions to synchronize and hasten the transition from PMN rolling to arrest and shape polarization. Calcium influx appears to be initiated within the plane of adhesive contact and reaches a peak after arrest, perhaps because of membrane shear stresses transmitted from flowing blood to integrins that anchor PMNs to endothelial ICAM-1. We speculate that this local maxima in calcium cooperates with PLC-dependent store release in coordinating actin rearrangement and polarization of the cell, the initial steps in integrin- and chemokine-mediated guidance of PMNs at vascular sites of inflammation.
Acknowledgments
The authors thank Dr Anjana Rao for generously supplying Orai1−/+ mice and for assistance in editing the manuscript, and D. Genetos and K. Passerini for conducting quantitative PCR and advice on siRNA.
This work was supported by the National Institutes of Health (grant AI47294; S.I.S.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: U.Y.S. designed and conducted siRNA transfections, shear flow, cytometry, and TIRF experiments and wrote the first draft of the manuscript; N.D. designed and conducted cytometry and shear flow experiments and assisted in writing the manuscript; E.P., I.Y., and T.T. designed and conducted cytometry and shear flow experiments; and S.I.S. designed experiments, assisted in writing the manuscript, and provided funds for the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Scott I. Simon, Department of Biomedical Engineering, 451 E Health Sciences Dr, Davis, CA 95616; e-mail: sisimon@ucdavis.edu.
References
- 1.Roos J, DiGregorio PJ, Yeromin AV, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169(3):435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang SL, Yu Y, Roos J, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437(7060):902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feske S, Gwack Y, Prakriya M, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441(7090):179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 4.Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281(30):20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 5.Gwack Y, Srikanth S, Oh-Hora M, et al. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol Cell Biol. 2008;28(17):5209–5222. doi: 10.1128/MCB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 7.Bréchard S, Melchior C, Plancon S, Schenten V, Tschirhart EJ. Store-operated Ca(2+) channels formed by TRPC1, TRPC6 and Orai1 and non-store-operated channels formed by TRPC3 are involved in the regulation of NADPH oxidase in HL-60 granulocytes. Cell Calcium. 2008;44(5):492–506. doi: 10.1016/j.ceca.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 8.McMeekin SR, Dransfield I, Rossi AG, Haslett C, Walker TR. E-selectin permits communication between PAF receptors and TRPC channels in human neutrophils. Blood. 2006;107(12):4938–4945. doi: 10.1182/blood-2005-09-3803. [DOI] [PubMed] [Google Scholar]
- 9.Heiner I, Eisfeld J, Halaszovich CR, et al. Expression profile of the transient receptor potential (TRP) family in neutrophil granulocytes: evidence for currents through long TRP channel 2 induced by ADP-ribose and NAD. Biochem J. 2003;371(3):1045–1053. doi: 10.1042/BJ20021975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itagaki K, Kannan KB, Singh BB, Hauser CJ. Cytoskeletal reorganization internalizes multiple transient receptor potential channels and blocks calcium entry into human neutrophils. J Immunol. 2004;172(1):601–607. doi: 10.4049/jimmunol.172.1.601. [DOI] [PubMed] [Google Scholar]
- 11.Krause KH, Campbell KP, Welsh MJ, Lew DP. The calcium signal and neutrophil activation. Clin Biochem. 1990;23(2):159–166. doi: 10.1016/0009-9120(90)80030-m. [DOI] [PubMed] [Google Scholar]
- 12.Granfeldt D, Samuelsson M, Karlsson A. Capacitative Ca2+ influx and activation of the neutrophil respiratory burst: different regulation of plasma membrane- and granule-localized NADPH-oxidase. J Leukoc Biol. 2002;71(4):611–617. [PubMed] [Google Scholar]
- 13.Demaurex N, Monod A, Lew DP, Krause KH. Characterization of receptor-mediated and store-regulated Ca2+ influx in human neutrophils. Biochem J. 1994;297(3):595–601. doi: 10.1042/bj2970595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sengeløv H, Kjeldsen L, Diamond MS, Springer TA, Borregaard N. Subcellular localization and dynamics of Mac-1 (alpha m beta 2) in human neutrophils. J Clin Invest. 1993;92(3):1467–1476. doi: 10.1172/JCI116724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding ZM, Babensee JE, Simon SI, et al. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J Immunol. 1999;163(9):5029–5038. [PubMed] [Google Scholar]
- 16.Solovjov DA, Pluskota E, Plow EF. Distinct roles for the alpha and beta subunits in the functions of integrin alphaMbeta2. J Biol Chem. 2005;280(2):1336–1345. doi: 10.1074/jbc.M406968200. [DOI] [PubMed] [Google Scholar]
- 17.Zigmond SH, Slonczewski JL, Wilde MW, Carson M. Polymorphonuclear leukocyte locomotion is insensitive to lowered cytoplasmic calcium levels. Cell Motil Cytoskeleton. 1988;9(2):184–189. doi: 10.1002/cm.970090210. [DOI] [PubMed] [Google Scholar]
- 18.Jaconi ME, Theler JM, Schlegel W, Appel RD, Wright SD, Lew PD. Multiple elevations of cytosolic-free Ca2+ in human neutrophils: initiation by adherence receptors of the integrin family. J Cell Biol. 1991;112(6):1249–1257. doi: 10.1083/jcb.112.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bengtsson T, Jaconi ME, Gustafson M, et al. Actin dynamics in human neutrophils during adhesion and phagocytosis is controlled by changes in intracellular free calcium. Eur J Cell Biol. 1993;62(1):49–58. [PubMed] [Google Scholar]
- 20.Downey GP, Chan CK, Trudel S, Grinstein S. Actin assembly in electropermeabilized neutrophils: role of intracellular calcium. J Cell Biol. 1990;110(6):1975–1982. doi: 10.1083/jcb.110.6.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dewitt S, Hallett MB. Cytosolic free Ca(2+) changes and calpain activation are required for beta integrin-accelerated phagocytosis by human neutrophils. J Cell Biol. 2002;159(1):181–189. doi: 10.1083/jcb.200206089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kruskal BA, Shak S, Maxfield FR. Spreading of human neutrophils is immediately preceded by a large increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1986;83(9):2919–2923. doi: 10.1073/pnas.83.9.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pettit EJ, Hallett MB. Release of ‘caged’ cytosolic Ca2+ triggers rapid spreading of human neutrophils adherent via integrin engagement. J Cell Sci. 1998;111(15):2209–2215. doi: 10.1242/jcs.111.15.2209. [DOI] [PubMed] [Google Scholar]
- 24.Lawson MA, Maxfield FR. Ca(2+)- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 1995;377(6544):75–79. doi: 10.1038/377075a0. [DOI] [PubMed] [Google Scholar]
- 25.Eddy RJ, Pierini LM, Matsumura F, Maxfield FR. Ca2+-dependent myosin II activation is required for uropod retraction during neutrophil migration. J Cell Sci. 2000;113(7):1287–1298. doi: 10.1242/jcs.113.7.1287. [DOI] [PubMed] [Google Scholar]
- 26.Cinamon G, Grabovsky V, Winter E, et al. Novel chemokine functions in lymphocyte migration through vascular endothelium under shear flow. J Leukoc Biol. 2001;69(6):860–866. [PubMed] [Google Scholar]
- 27.Cinamon G, Shinder V, Shamri R, Alon R. Chemoattractant signals and beta 2 integrin occupancy at apical endothelial contacts combine with shear stress signals to promote transendothelial neutrophil migration. J Immunol. 2004;173(12):7282–7291. doi: 10.4049/jimmunol.173.12.7282. [DOI] [PubMed] [Google Scholar]
- 28.Green CE, Schaff UY, Sarantos MR, Lum AF, Staunton DE, Simon SI. Dynamic shifts in LFA-1 affinity regulate neutrophil rolling, arrest, and transmigration on inflamed endothelium. Blood. 2006;107(5):2101–2111. doi: 10.1182/blood-2005-06-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaff UY, Yamayoshi I, Tse T, Griffin D, Kibathi L, Simon SI. Calcium flux in neutrophils synchronizes beta2 integrin adhesive and signaling events that guide inflammatory recruitment. Ann Biomed Eng. 2008;36(4):632–646. doi: 10.1007/s10439-008-9453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kogan TP, Revelle BM, Tapp S, Scott D, Beck PJ. A single amino acid residue can determine the ligand specificity of E-selectin. J Biol Chem. 1995;270(23):14047–14055. doi: 10.1074/jbc.270.23.14047. [DOI] [PubMed] [Google Scholar]
- 31.Schaff UY, Xing MM, Lin KK, Pan N, Jeon NL, Simon SI. Vascular mimetics based on microfluidics for imaging the leukocyte-endothelial inflammatory response. Lab Chip. 2007;7(4):448–456. doi: 10.1039/b617915k. [DOI] [PubMed] [Google Scholar]
- 32.Simon SI, Hu Y, Vestweber D, Smith CW. Neutrophil tethering on E-selectin activates beta 2 integrin binding to ICAM-1 through a mitogen-activated protein kinase signal transduction pathway. J Immunol. 2000;164(8):4348–4358. doi: 10.4049/jimmunol.164.8.4348. [DOI] [PubMed] [Google Scholar]
- 33.Gwack Y, Feske S, Srikanth S, Hogan PG, Rao A. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium. 2007;42(2):145–156. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Lowell CA, Fumagalli L, Berton G. Deficiency of Src family kinases p59/61hck and p58c-fgr results in defective adhesion-dependent neutrophil functions. J Cell Biol. 1996;133(4):895–910. doi: 10.1083/jcb.133.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16(10):1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Schaff UY, Green CE, et al. Impaired integrin-dependent function in Wiskott-Aldrich syndrome protein-deficient murine and human neutrophils. Immunity. 2006;25(2):285–295. doi: 10.1016/j.immuni.2006.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prakriya M, Lewis RS. Potentiation and inhibition of Ca(2+) release-activated Ca(2+) channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP(3) receptors. J Physiol. 2001;536(1):3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon SI, Green CE. Molecular mechanics and dynamics of leukocyte recruitment during inflammation. Annu Rev Biomed Eng. 2005;7:151–185. doi: 10.1146/annurev.bioeng.7.060804.100423. [DOI] [PubMed] [Google Scholar]
- 39.Zarbock A, Lowell CA, Ley K. Spleen tyrosine kinase Syk is necessary for E-selectin-induced alpha(L)beta(2) integrin-mediated rolling on intercellular adhesion molecule-1. Immunity. 2007;26(6):773–783. doi: 10.1016/j.immuni.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ng-Sikorski J, Andersson R, Patarroyo M, Andersson T. Calcium signaling capacity of the CD11b/CD18 integrin on human neutrophils. Exp Cell Res. 1991;195(2):504–508. doi: 10.1016/0014-4827(91)90402-g. [DOI] [PubMed] [Google Scholar]
- 41.Schorr W, Swandulla D, Zeilhofer HU. Mechanisms of IL-8-induced Ca2+ signaling in human neutrophil granulocytes. Eur J Immunol. 1999;29(3):897–904. doi: 10.1002/(SICI)1521-4141(199903)29:03<897::AID-IMMU897>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 42.Petersen M, Williams JD, Hallett MB. Cross-linking of CD11b or CD18 signals release of localized Ca2+ from intracellular stores in neutrophils. Immunology. 1993;80(1):157–159. [PMC free article] [PubMed] [Google Scholar]
- 43.Feske S. ORAI1 and STIM1 deficiency in human and mice: roles of store-operated Ca2+ entry in the immune system and beyond. Immunol Rev. 2009;231(1):189–209. doi: 10.1111/j.1600-065X.2009.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sjaastad MD, Lewis RS, Nelson WJ. Mechanisms of integrin-mediated calcium signaling in MDCK cells: regulation of adhesion by IP3- and store-independent calcium influx. Mol Biol Cell. 1996;7(7):1025–1041. doi: 10.1091/mbc.7.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smyth JT, Dehaven WI, Bird GS, Putney JW., Jr Ca2+-store-dependent and -independent reversal of Stim1 localization and function. J Cell Sci. 2008;121(6):762–772. doi: 10.1242/jcs.023903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell. 2007;131(7):1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lioudyno MI, Kozak JA, Penna A, et al. Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proc Natl Acad Sci U S A. 2008;105(6):2011–2016. doi: 10.1073/pnas.0706122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hellberg C, Molony L, Zheng L, Andersson T. Ca2+ signalling mechanisms of the beta 2 integrin on neutrophils: involvement of phospholipase C gamma 2 and Ins(1,4,5)P3. Biochem J. 1996;317(Pt 2):403–409. doi: 10.1042/bj3170403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanner SB, Grosmaire LS, Ledbetter JA, Damle NK. Beta 2-integrin LFA-1 signaling through phospholipase C-gamma 1 activation. Proc Natl Acad Sci U S A. 1993;90(15):7099–7103. doi: 10.1073/pnas.90.15.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pasvolsky R, Feigelson SW, Kilic SS, et al. A LAD-III syndrome is associated with defective expression of the Rap-1 activator CalDAG-GEFI in lymphocytes, neutrophils, and platelets. J Exp Med. 2007;204(7):1571–1582. doi: 10.1084/jem.20070058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eierman D, Hellberg C, Sjolander A, Andersson T. Chemotactic factor receptor activation transiently impairs the Ca2+ signaling capacity of beta 2 integrins on human neutrophils. Exp Cell Res. 1994;215(1):90–96. doi: 10.1006/excr.1994.1319. [DOI] [PubMed] [Google Scholar]
- 52.Pettit EJ, Hallett MB. Two distinct Ca2+ storage and release sites in human neutrophils. J Leukoc Biol. 1998;63(2):225–232. doi: 10.1002/jlb.63.2.225. [DOI] [PubMed] [Google Scholar]
- 53.Chandy KG, Wulff H, Beeton C, Pennington M, Gutman GA, Cahalan MD. K+ channels as targets for specific immunomodulation. Trends Pharmacol Sci. 2004;25(5):280–289. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fanger CM, Rauer H, Neben AL, et al. Calcium-activated potassium channels sustain calcium signaling in T lymphocytes: selective blockers and manipulated channel expression levels. J Biol Chem. 2001;276(15):12249–12256. doi: 10.1074/jbc.M011342200. [DOI] [PubMed] [Google Scholar]
- 55.DeHaven WI, Smyth JT, Boyles RR, Bird GS, Putney JW., Jr Complex actions of 2-aminoethyldiphenyl borate on store-operated calcium entry. J Biol Chem. 2008;283(28):19265–19273. doi: 10.1074/jbc.M801535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kelher MR, Ambruso DR, Elzi DJ, et al. Formyl-Met-Leu-Phe induces calcium-dependent tyrosine phosphorylation of Rel-1 in neutrophils. Cell Calcium. 2003;34(6):445–455. doi: 10.1016/s0143-4160(03)00067-8. [DOI] [PubMed] [Google Scholar]