Abstract
Postmortem prefrontal cortices (PFC) (Brodmann’s areas 10 and 46), temporal cortices (Brodmann’s area 22), hippocampi, caudate nuclei, and cerebella of schizophrenia patients and their matched nonpsychiatric subjects were compared for reelin (RELN) mRNA and reelin (RELN) protein content. In all of the brain areas studied, RELN and its mRNA were significantly reduced (≈50%) in patients with schizophrenia; this decrease was similar in patients affected by undifferentiated or paranoid schizophrenia. To exclude possible artifacts caused by postmortem mRNA degradation, we measured the mRNAs in the same PFC extracts from γ-aminobutyric acid (GABA)A receptors α1 and α5 and nicotinic acetylcholine receptor α7 subunits. Whereas the expression of the α7 nicotinic acetylcholine receptor subunit was normal, that of the α1 and α5 receptor subunits of GABAA was increased when schizophrenia was present. RELN mRNA was preferentially expressed in GABAergic interneurons of PFC, temporal cortex, hippocampus, and glutamatergic granule cells of cerebellum. A protein putatively functioning as an intracellular target for the signal-transduction cascade triggered by RELN protein released into the extracellular matrix is termed mouse disabled-1 (DAB1) and is expressed at comparable levels in the neuroplasm of the PFC and hippocampal pyramidal neurons, cerebellar Purkinje neurons of schizophrenia patients, and nonpsychiatric subjects; these three types of neurons do not express RELN protein. In the same samples of temporal cortex, we found a decrease in RELN protein of ≈50% but no changes in DAB1 protein expression. We also observed a large (up to 70%) decrease of GAD67 but only a small decrease of GAD65 protein content. These findings are interpreted within a neurodevelopmental/vulnerability “two-hit” model for the etiology of schizophrenia.
The symptoms of schizophrenia appear to be associated with functional and structural changes in a number of neocortical regions, including heteromodal prefrontal and temporal-association cortices, as well as in the connections and integrative interactions among these regions, corticolimbic areas, and the thalamus (1–7).
Selemon et al. (2), applying stereologic counting methods to prefrontal cortex (PFC; Brodmann’s areas 9 and 46), reported increased neuronal density and reduced cortical volume with schizophrenia. In Brodmann’s area 46, these findings are predominantly the result of a reduction in the interneuronal neuropil in layer II. In agreement with this observation, a cerebral ventricle dilation is present in a significant proportion of patients when schizophrenia is first diagnosed (8).
Uptake and release of γ-aminobutyric acid (GABA) have been reported to be reduced in cortical synaptosomes prepared from brains of schizophrenia patients (5); the relative expression density of the GABA transporter in the axon cartridges of chandelier neurons also has been found to be decreased by 40% in the PFC of schizophrenia patients compared with nonpsychiatric subjects (9). When similar comparisons were extended to the activity of glutamic acid decarboxylase (GAD) and the expression of its mRNA, they were found to be reduced (40–50%) in the PFC of schizophrenia patients, i.e., layers I and II (10). In contrast, GABAA receptor density was found to be increased in the dentate gyrus and corticolimbic structures of patients with schizophrenia (4, 5), reminiscent of a putative denervation supersensitivity.
The “Two-Hit” Model.
Although some genetic abnormalities may contribute to the cause of schizophrenia, their poor penetration suggests that this disorder is not exclusively related to either single- or multiple-gene defects (11–13). The absence of obvious early psychopathology in children who are diagnosed with schizophrenia later in life has provided support for the hypothesis that schizophrenia etiology may involve a “two-hit” process (14).
In this two-hit process, genetic load, adverse embryonic events, and perinatal events may be considered a neurodevelopmental first hit that leads to vulnerability to schizophrenia (15, 16). The most frequently cited embryonic and perinatal factors include viral illness during the second trimester of pregnancy (17), low birth weight, short gestational period, and perinatal brain damage (18).
Hormonal events, such as altered neurosteroid biosynthesis (i.e., dehydroepiandrosterone or allopregnanolone) during puberty acting per se or presumably integrating with the residues of developmentally related changes could act as a second hit, facilitating excitotoxicity or oxygen radical formation due to environmental factors (19–22).
Possible Role of Reelin in the Two-Hit Model.
The name Reelin derives from reeler, referring to the characteristic gaiting behavior of the mouse phenotype that has a null mutation of a gene with about 450 kb (reln) (23, 24) encoding a 3,461-aa protein residue, RELN, that regulates cortical pyramidal neurons, interneuron and Purkinje cell positioning, and/or trophism during brain development (24). RELN contains a cleavage-signal peptide at the N terminus and is devoid of other hydrophobic amino acid residue sequences, indicating that RELN can be secreted (24). This sequence shows 25% identity with F-spondin and is followed by a series of eight epidermal growth factor-like repeats similar to those of the tenascin C and X and the β-subunit of integrins (24). Another factor that plays an important role in guiding the migration of embryonic cortical neurons to their final destinations in the subcortical plate is the mouse disabled-1 (dab1) gene, which encodes for an adaptor protein (DAB1) that is a phosphorylation target for a signaling cascade putatively triggered by the RELN protein interaction with extracellular matrix (ECM) proteins (25, 26). DAB1 expression is deficient in another neurological genetic phenotype, the scrambler mouse, which is neurologically and behaviorally similar to the reeler mouse (25, 26).
During mammalian brain ontogenesis, including that of the human brain, RELN is abundantly synthesized by the Cajal–Retzius cells and other pioneer neurons located in the telencephalic marginal zone and by granule cells of the external granular layer of the cerebellum (24, 27). In wild-type and scrambler mice, RELN is secreted into the ECM, but the reeler mouse neither synthesizes nor secretes typical RELN protein (24, 28). During development, telencephalic migrating neurons and interneurons express DAB1, but they neither express nor secrete RELN (29). In the reeler mouse, the telencephalic neurons—which are misplaced following migration—express approximately 10-fold more DAB1 than their wild-type counterpart (29). Such an increase in the expression of a protein that virtually functions as a receptor is expected to occur when the specific signal for the receptor is missing. It has been suggested that the function of RELN in embryos may ultimately depend on the phosphorylation of DAB1 expressed selectively in migrating telencephalic pyramidal neurons and cerebellar Purkinje neurons (25, 26, 29).
The nature of the molecular mechanisms mediating the interactions between RELN in ECM and DAB1 in the cytosol of neurons is not clear, but one possibility is that the RELN protein secreted into the ECM binds to specific molecules, such as neural cell adhesion molecules and and/or substrate adhesion molecules, which interact with integrins (predominantly α8β1 heterodimers in neurons) located in the membrane of cortical pyramidal neurons and interneurons. Integrins mediate the intraneuronal cascade of events leading to tyrosine kinase activation, which presumably phosphorylates DAB1, thereby allowing this protein to express its adaptor function.
Investigations with rats (28) indicate that, after the postnatal disappearance of Cajal–Retzius cells that provide the major source of RELN protein functioning in embryonic corticogenesis, a second generation of telencephalic RELN is expressed in adult brains by horizontal and bitufted GABAergic interneurons in the cortex. Immunohistochemical studies indicate that in the adult rat brain, RELN is not only stored in the neurons but is also present extracellularly as a diffuse, albeit intense, immunostain appearing in cortical layers I and II and in the hilus of the hippocampus. This may represent a pool of RELN that is located in the neuropil and, in part, in the ECM (28). When we became aware that RELN mRNA expression occurs in the adult human brain (30, 31), we reasoned that if there was a genetic defect in the transcription of RELN with schizophrenia [for instance, related to a RELN/DNA polymorphism (32)], a reflection of such abnormality should persist as a generalized deficit of RELN expressed in adult brain. Based on these inferences, we hypothesized that a defect in RELN expression may not only be an effective factor in inducing the first-hit vulnerability for schizophrenia, but also that if such a deficit persisted, it may play a contributory role to the second hit, which elicits a neuronal dysfunction responsible for the onset of the symptoms of schizophrenia.
The demonstration that subventricular zone neurogenesis in brains of rodents, carnivores, and primates can occur throughout life (33, 34) strongly suggests that RELN-mediated signaling, similar to that operative in neuronal development, may be associated with adulthood neurogenesis (24, 29).
MATERIALS AND METHODS
Tissue Collection and RNA Isolation.
Brain samples from 18 chronic schizophrenia patients and 18 nonpsychiatric subjects were obtained from two different banks (Table 1). Schizophrenia patients included 10 males and 8 females; the control group included 11 males and 7 females. Mean age ± SD for schizophrenia patients was 53.9 ± 21.5 yr and for the nonpsychiatric subjects was 62.2 ± 17.3 yr. The diagnosis of chronic schizophrenia was determined by two psychiatrists by using a combination of prospective and retrospective analyses based on DSM-IV criteria (35). They also classified the schizophrenia patients into undifferentiated or paranoid subtypes (Table 1). All of the patients were receiving neuroleptics at the time of death.
Table 1.
Demography, anamnesis and RELN mRNA in post mortem prefrontal cortices of schizophrenia patients and nonpsychiatric subjects
| Schizophrenia patients | |||||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H |
| 1/N | P | 19 | M/21 | S | 37 | 0.22 | 46 |
| 2/N | P | 17 | M/28 | S | 41 | 0.25 | 46 |
| 3/H | P | 23 | F/29 | C | 22.8 | 0.12 | 10 |
| 4/N | P | 20 | M/30 | C | 33.5 | 0.21 | 46 |
| 5/N | P | 21 | M/36 | S | 35 | 0.34 | 46 |
| 6/N | P | 40 | M/74 | R | 13.5 | 0.22 | 46 |
| 7/N | P | 26 | F/82 | C | 16 | 0.28 | 46 |
| 8/N | P | 44 | F/64 | I | 39 | 0.15 | 46 |
| 9/N | U | 15 | M/25 | S | 20 | 0.33 | 46 |
| 10/H | U | 17 | M/44 | S | 17.8 | 0.14 | 10 |
| 11/H | U | 29 | F/49 | I | 29 | 0.19 | 10 |
| 12/H | U | 25 | F/74 | C | 27.7 | 0.28 | 10 |
| 13/H | U | 22 | F/78 | C | 12.2 | 0.18 | 10 |
| 14/H | U | 34 | F/79 | R | 5.5 | 0.23 | 10 |
| 15/N | U | 33 | F/46 | R | 40 | ⊗ | |
| 16/N | U | 28 | M/72 | C | 15 | ⊗ | |
| 17/N | U | NA | M/82 | C | 22.5 | ⊗ | |
| 18/N | U | NA | M/58 | C | 19.5 | ⊗ | |
| Nonpsychiatric patients | |||||
|---|---|---|---|---|---|
| A | D | E | F | G | H |
| 19/N | M/24 | A | 26 | 0.37 | 46 |
| 20/N | M/26 | S | 14.5 | 0.48 | 46 |
| 21/H | F/49 | C | 23.4 | 0.29 | 10 |
| 22/N | M/66 | C | 19 | 0.31 | 46 |
| 23/N | M/45 | C | 19.5 | 0.58 | 46 |
| 24/N | M/71 | R | 25 | 0.33 | 46 |
| 25/N | F/77 | A | 19 | 0.42 | 46 |
| 26/N | F/47 | A | 20 | 0.28 | 46 |
| 27/H | M/72 | C | 12.4 | 0.27 | 46 |
| 28/N | M/69 | C | 23 | 0.40 | 10 |
| 29/H | F/58 | C | 13.3 | 0.41 | 10 |
| 30/H | F/69 | C | 16.6 | 0.45 | 10 |
| 31/H | F/70 | I | 18.5 | 0.46 | 10 |
| 32/H | M/82 | C | 28.3 | 0.31 | 10 |
| 33/N | F/82 | R | 7.8 | ⊗ | |
| 34/N | M/65 | C | 11 | ⊗ | |
| 35/N | M/86 | R | 13.5 | ⊗ | |
| 36/N | M/61 | A | 12.8 | ⊗ | |
A, Subject no./sample source (N, National Neurological Research Specimen Bank, Veteran’s Administration Medical Center, Los Angeles, CA; H, Harvard Brain Tissue Resource Center, McLean Hospital, Belmont, MA). B, DSM-IV diagnosis (P, paranoid; U, undifferentiated); C, Age of onset, yrs; D, Sex/age, yr; E, Cause of death (S, suicide; C, cardiac failure; I, infection; R, respiratory failure; A, accident); F, Post-mortem interval, hr; G, RELN mRNA in PFC, fmol/μg RNA; H, Brodmann’s area; ⊗, for analysis of temporal cortex, hippocampus, and caudate nucleus only; NA, not available.
Total RNA was extracted as described (36). Extracts with evidence of RNA degradation as evidenced by smearing of 28S and 18S ribosomal RNA on agarose/formaldehyde gel electrophoresis were excluded.
Quantitative Reverse Transcription–PCR (RT-PCR) Analysis.
RELN, and α1 and α5 GABAA receptor subunit and α7 nicotinic acetylcholine receptor (nAChR) subunit mRNA content was measured by RT-PCR analysis as described (36), with internal standards (IS; see below) and the following amplification primers: RELN-I (forward, bp 1261–1284; reverse, bp 1556–1579); RELN-II (forward, bp 9211–9234; reverse, bp 9549–9572; GenBank accession no. HSU79716); GABAA α1-receptor subunit (forward, bp 1193–1216; reverse, bp 1536–1556; GenBank Accession no. 14766); GABAA α5-receptor subunit (forward, bp 1579–1602; reverse, bp 1868–1891; GenBank accession no. A28104); nAChR α7-subunit (forward, bp 471–494; reverse, bp 758–781; GenBank accession no. X70297).
Each IS targeted by the same primers used to amplify the canonic sequence was generated by site-directed mutagenesis to introduce a BanI (RELN-IS-I) or BglII (RELN-IS-II, GABAA α1 and α5, and nAChR α7 subunit) restriction endonuclease site midway between the amplification primers (37) so that the digestion of the IS amplicon would generate two fragments of approximately equal molecular size as follows: RELN-IS-I (amplicon 319, BanI-digested fragments 160 and 159 bp); RELN-IS-II (amplicon 359, BglII-digested fragments 179 and 180 bp); GABAA α1 (amplicon 363, BglII-digested fragments 198 and 165 bp); GABAA α5 (amplicon 313, BglII-digested fragments 167 and 146 bp), and nAchR α7 (amplicon 311, BglII-digested fragments 156–155 bp). Because the intron/exon boundaries of the RELN gene are known (23), we targeted our primers to DNA sequences located on different exons so that the amplicons were free of DNA contamination. Absence of DNA contamination was also ascertained by running the PCR without prior addition of reverse transcriptase. Rat RELN mRNA was assayed by using primers and IS as described (28).
Alternative Splice Variant Analysis.
The extent of alternative splicing of microexon 64 of the RELN coding sequence was determined by RT-PCR analysis of 1 μg of total RNA by using the primer pairs: forward (10406–10492), reverse (10492–10515); GenBank accession no. HSU79716). To assess the ratio of spliced versus unspliced transcripts in the total amplified template, the 32P-labeled RT-PCR amplicon was digested with MaeI restriction endonuclease, which specifically recognizes the unspliced variant. The spliced and unspliced variants were separated by agarose gel electrophoresis, and the radioactivity incorporated in the respective bands was counted.
Western Blot Analysis.
Tissues were homogenized at 4°C in 10 vol of extraction buffer (50 mM Tris/5 mM EDTA/150 mM sodium chloride/2 mM phenylmethylsulfonyl chloride/10 mM N-ethylmaleimide, pH 7.6) and then centrifuged at 20,000 × g for 20 min at 4°C. The proteins present in the supernatants were precipitated with 7 vol of 100% methanol followed by 100 × g centrifugation for 15 min at 4°C.
The extract samples were resolved on acrylamide gel and then blotted for 60 min on enhanced chemiluminescence membranes (Amersham; ref. 38). The membranes were then incubated overnight with primary antibodies monoclonal RELN 142 directed to the N-terminal region of RELN (amino acids 40–189, ref. 39), monoclonal RELN G-10 (39), polyclonal RELN R-58 directed against peptide (amino acids 593–639), polyclonal DAB1-B3 (26), polyclonal GAD65/GAD67 (Chemicon), and specific polyclonal GAD67 (Chemicon) or monoclonal GAD65 (Boehringer Mannheim).
RELN and DAB1 Immunostaining.
Flash-frozen brain tissue samples were dropped into ice-cold fixative (4% paraformaldehyde in 0.1 M PBS, pH 7.4) for 20 hr, embedded in 30% sucrose in PBS at room temperature, and refrozen on dry ice for sectioning. Twenty-micron sections were rinsed with PBS, mounted on uncoated superfrost plus slides (Fisher Scientific), and air-dried for 2 hr. Sections were rinsed several times in Tris-buffered saline (TBS, pH 7.4), microwaved in citrate buffer (pH 6) at 80% (microwave power) for 2 min, followed by 20% for 5 min, and rinsed in TBS. The immunostaining protocol is described in detail by Pesold et al. (28), and the antibody concentrations used were 1:500 (RELN 142) and 1:1000 (DAB1 B3). For double-labeling with the neuronal nuclei-specific marker (NeuN) (40), sections were immunostained for DAB1, blocked with an avidin/biotin blocking kit (Vector Laboratories), and labeled with the NeuN monoclonal antibody (1:200; Chemicon). For each brain section, the number of RELN-positive cells in each layer was counted without knowledge of the specimen diagnosis in six 1-mm wide columns from three or more 20-μm sections. The density of RELN-positive cells in each section was expressed as the number per 1 mm2.
RESULTS
Measurements of RELN mRNA Expression.
RELN mRNA expressed in adult brain of nonpsychiatric subjects was higher in the cerebellum than in the temporal cortex (Brodmann’s area 22), PFC (Brodmann’s areas 10 and 46), hippocampus, or caudate nucleus (Table 2). Although the brain samples were obtained from two different banks (see Table 1 legend), there were no major differences in the content of RELN mRNA in the same brain structures when obtained from both banks. In the nonpsychiatric subjects, the mean value ± SE of RELN mRNA did not differ significantly (F = 0.55; df = 1, 42; P not significant) between Brodmann’s area 10 (388 ± 29 amol/μg RNA, n = 6) and Brodmann’s area 46 (382 ± 38 amol/μg RNA, n = 8; see Table 1 for values), nor did the two PFC samples differ from the temporal cortex (Brodmann’s area 22: 418 ± 63 amol/μg RNA, n = 9; F = 1.8; df = 1, 7; P not significant).
Table 2.
Demography and brain RELN mRNA in schizophrenia patients (SP) and nonpsychiatric subjects (NPS)
| Brain region | Age of onset, years | Age at death, years | Post-mortem interval, hours | RELN, fmol/μg RNA |
|---|---|---|---|---|
| SP | ||||
| PFC | 25 ± 2.4 | 51 ± 6.2 | 25 ± 3.0 | 0.22 ± 0.018* |
| TC | 25 ± 1.8 | 55 ± 6.7 | 22 ± 3.0 | 0.25 ± 0.04† |
| CBL | 25 ± 2.4 | 52 ± 7.6 | 23 ± 3.8 | 1.4 ± 0.24† |
| HIP | 24 ± 3.2 | 55 ± 9.6 | 24 ± 3.8 | 0.15 ± 0.038§ |
| CN | NA | 71 ± 7.0 | 19 ± 2.2 | 0.023 ± 0.0018¶ |
| NPS | ||||
| PFC | 59 ± 4.9 | 20 ± 1.3 | 0.38 ± 0.020 | |
| TC | 61 ± 6.0 | 18 ± 1.7 | 0.42 ± 0.060 | |
| CBL | 61 ± 5.4 | 20 ± 1.5 | 2.3 ± 0.17 | |
| HIP | 69 ± 5.3 | 15 ± 2.0 | 0.30 ± 0.030 | |
| CN | 74 ± 6.2 | 11 ± 1.3 | 0.080 ± 0.019 |
All values are the mean ± SE. Age of death and PMI (Post-mortem interval) do not differ between SP and NPS, with the exception of PMI of CN (caudate nucleus) (P < 0.022). NA, not available; PFC, prefrontal cortex from SP: 1–14; NPS: 19–32. TC = temporal cortex; SP: 3, 4, 7, 9, 10, 11, 12, 13, 14, 15, 16; NPS: 20, 21, 23, 25, 27, 29, 30, 31, 32. CBL = cerebellum from NPS 19, 20, 21, 22, 24, 25, 27, 28, 29, 30, 31, 32, SP: 1, 2, 3, 5, 6, 7, 10, 11, 13, 14; HIP, hippocampus from SP: 1, 7, 9, 15, 16, 17, 18; NPS: 23, 25, 28, 33, 34, 35, 36, CN, caudate nucleus from SP: 15, 16, 17, 18, NPS: 33, 34, 35, 36. ∗, P < 0.00001;
, P < 0.03;
P < 0.006;
, P < 0.007;
, P < 0.05. SP compared with NPS (ANOVA with SP vs. NPS and brain bank as factors. Statistical Package of Social Sciences, Chicago). RELN data were obtained by using primers and RELN–ISI. Similar results were obtained using primers and RELN–ISII.
In brain areas of schizophrenia patients homologous to those of the nonpsychiatric subjects, the RELN mRNA content was significantly decreased (40–50%; see Table 2). The extent of this decrease was similar in the two cohorts of schizophrenia patients analyzed (Table 1). This table also shows that the RELN mRNA content of the PFC dissected from the brains of patients affected by undifferentiated schizophrenia (244 ± 29 amol/μg RNA; n = 6) was not different than that of patients affected by paranoid schizophrenia (222 ± 24; n = 8; P = 0.35), but both group values were lower than those of the nonpsychiatric subjects (384 ± 24; n = 14) (analysis of variance with Helmert contrast; F = 10.6; df = 2, 22; P = 0.001).
Differences in age at the time of death and postmortem intervals in the PFC, temporal cortex, hippocampus, and cerebellum of schizophrenia patients vs. nonpsychiatric subjects were not statistically significant (Table 2). Moreover, differences in age, gender, and postmortem interval, when evaluated with analysis of covariance, could not account for the RELN mRNA differences between schizophrenia patients and nonpsychiatric subjects.
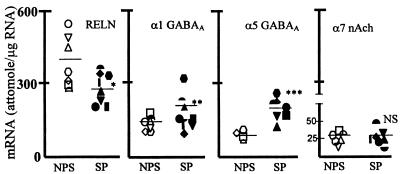
In contrast to the significant decrease in RELN mRNA levels, the content of α1 and α5 GABAA receptor subunit mRNA found in schizophrenia patients was significantly greater than that of the nonpsychiatric subjects (see Fig. 1). No differences were found in α7 nAChr subunit mRNA content in PFC between schizophrenia patients and nonpsychiatric subjects.
Figure 1.
In PFC of schizophrenia patients (SP), the decrease in RELN mRNA is paralleled by an increase of GABAA α1 and α5 and no change in nACh α7-receptor subunit mRNA. ∗, P < 0.01; ∗∗, P < 0.02; ∗∗∗, P < 0.05; NS, not significant; SP vs. nonpsychiatric subjects (NPS); Student’s t test. NPS: = 19, ▿ = 20, □ = 22, ○ = 23, ⋄ = 24, ▵ = 25, ⌓ = 26; SP: ▾ = 1, ▴ = 2, • = 4, = 5, ■ = 6, █ = 7, ♦ = 9, = 15.
In PFC (Brodmann’s area 46) of schizophrenia patients (patients 1, 2, 3, 4, 5, 6, 8, 9, and 15) and nonpsychiatric subjects (subjects 19, 22, 23, 24, 25, and 26), we analyzed the abundance of RELN mRNA splice variants at microexon 64 (see Materials and Methods). The amplicon ratio of spliced/unspliced RELN mRNA was similar in schizophrenia patients (0.14 ± 0.020) and in nonpsychiatric subjects (0.13 ± 0.019) despite the observation that the groups differed in RELN mRNA expression.
RELN, DAB1, and GAD Western Blotting.
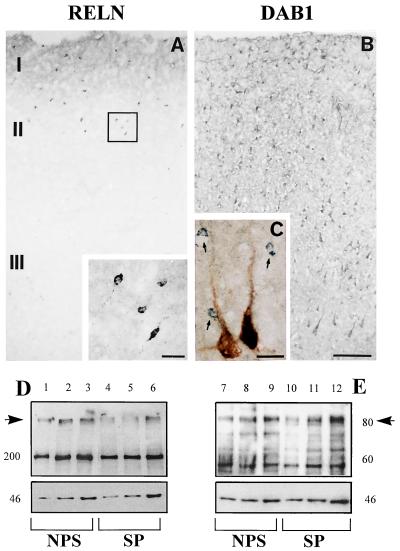
Western immunoblotting of PFC, cerebellum (not shown), and temporal-cortex extracts of nonpsychiatric subjects (Fig. 2) with RELN 142 antibody revealed an immunoreactive band of ≈400 kDa. This band was also present in PFC, temporal-cortex, and cerebellar extracts from schizophrenia patients; however, as shown in Table 3, the band density in the temporal cortex of schizophrenia patients was significantly lower than that observed in nonpsychiatric subjects (Table 3). In addition to the 400-kDa RELN-like immunoreactive band, another immunoreactive band (≈200 kDa) was also detected in every brain extract, and in addition, this band was significantly decreased in the temporal-cortex extracts from brain of schizophrenia patients. Similar results were obtained by using G-10 and R-58 anti-RELN antibodies.
Figure 2.
Photomicrographs of 20-μm sections through human PFC immunolabeled for RELN (A) and DAB1 (B). The Inset in A is a higher magnification of the boxed area showing RELN-immunopositive interneurons. C shows double-labeling with DAB1 (blue-gray) and neuronal nuclei-specific protein [NeuN (40)] antisera, which labels most neurons (brown). Note that small cells (arrows) are immunonegative for the neuronal marker and that not all pyramidal cells are immunopositive for DAB1 (left pyramidal cell not double-labeled). (Bar = 150 μm for A and B and 20 μm for C and for the inset in A.) Western blotting of RELN (D) and DAB1 (E) in representative extracts from the temporal cortex of schizophrenia patients (SP) and nonpsychiatric subjects (NPS). Note that RELN ≈400-kDa immunoreactive bands (indicated by the arrow) have higher intensity in NPS (lanes 1, 2, and 3) than in SP (lanes 4, 5, and 6). In contrast, DAB1 ≈86-kDa bands (indicated by the arrow)are similar in the SP (lanes 10, 11, and 12) and NPS (lanes 7, 8, and 9). In D, 200, 400, and 1,000 μg of protein was resolved on 7.5% acrylamide gel electrophoresis using RELN 142 (1:500) and β-actin (1:5000) antiserum. In E, 25, 50, and 100 μg of protein was resolved on 7.5% acrylamide gel using DAB1 B3 antiserum (1:1000) and β-actin (1:50000) antiserum. Optical densities of the bands on the autoradiogram were quantified by using the Loats Image Analyses System (38).
Table 3.
RELN, DAB1, and GAD in temporal cortex of schizophrenia patients (SP) and nonpsychiatric subjects (NPS)
| Protein | SP | NPS | SA |
|---|---|---|---|
| RELN | 0.70 ± 0.060 | 1.9 ± 0.18 | P = 0.043 |
| DAB1 | 0.31 ± 0.02 | 0.33 ± 0.012 | P = NS |
| GAD 67 | 0.55 ± 0.078 | 2.0 ± 0.25 | P = 0.01 |
| GAD 65 | 0.80 ± 0.070 | 1.1 ± 0.10 | P = NS |
Protein determined from O.D. ratio with β–actin. All values are the mean ± SE of 8 temporal cortices from schizophrenic patients (3, 7, 10, 11, 12, 13, 14, 15) and 8 temporal cortices from nonpsychiatric subjects (20, 21, 25, 27, 28, 29, 30, 31). SA, Statistical Analysis; student’s t test, two-tailed. NS, not significant.
In the same temporal-cortex extracts used to determine RELN-like immunoreactivity, we estimated the relative expression density of GAD67 and GAD65-like immunoreactivity by Western blotting. The ratio of GAD67/β-actin optical densities was significantly decreased—by ≈70%—in the schizophrenia patients, whereas that of the GAD65/β-actin was decreased by ≈30% (Table 3), which was not significant. In addition to the 67-kDa band, the GAD67 polyclonal antibody revealed several other smaller molecular weight bands; therefore, we also conducted similar analyses with another antibody that immunoreacts with each of the two molecular forms of GAD. With this antibody, we could confirm a large decrease of GAD67 with a relatively smaller decrease of the GAD65 immunoreactive band. The difference in postmortem autopsy interval between schizophrenia patients (26 hr ± 8.4) and nonpsychiatric subjects (16 ± 3.9) evaluated by analysis of covariance could not account for the GAD67 differences between the schizophrenia patients and the nonpsychiatric subjects (r2 = 0.2; F = 0.18; P = 0.17).
In the same temporal-cortex extracts used to determine RELN, GAD67, or GAD65 immunoreactivity, we also determined with B3 antibody the expression density of the DAB1-like immunoreactive protein. The optical-density ratio of DAB1 (immunoreactive band of ≈85 kDa) over the optical density of the band of β-actin (Fig. 2) was similar in the temporal cortices of schizophrenia patients and nonpsychiatric subjects (Table 3). An additional band of ≈60 kDa was revealed by the DAB1 antibody.
RELN and DAB1 Immunohistochemistry.
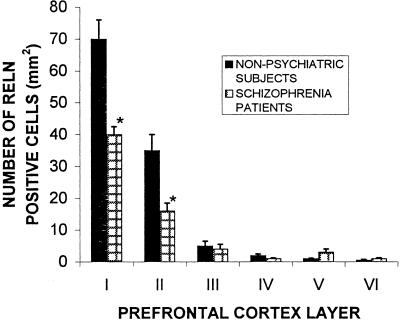
Immunohistochemistry reveals that human PFC expresses numerous RELN-immunoreactive interneurons in addition to a diffuse band of RELN in layer I (Fig. 2). The number of RELN-immunopositive neurons is significantly higher in layers I and II than in the other cortical layers (Fig. 3). In the PFC of schizophrenia patients, the number of RELN-immunopositive neurons in layers I and II is decreased significantly (Fig. 3). The Inset in Fig. 2A shows the morphological characteristics of the small interneurons expressing RELN.
Figure 3.
Mean (±SE) of RELN-positive neurons in PFC layer I through VI of schizophrenia patients (patients 1, 2, 4, 5, 7, 8, 9) and nonpsychiatric subjects (subjects 10, 21, 22, 24, 25, 26). ∗, P < 0.01, Student’s t test.)
Immunohistochemically, DAB1 (Fig. 2) is preferentially expressed in pyramidal neurons, although some small DAB1-positive cells are found throughout the various cortical layers. These small cells not only fail to exhibit RELN immunoreactivity, but also fail to stain for neuronal nuclei-specific protein, a marker that labels the nuclei of most neurons (with the exception of some GABAergic neurons) (40).
Protracted Haloperidol Treatment.
So far, we have been unable to obtain appropriate brain samples from schizophrenia patients that either had never been treated with neuroleptics or had been without such a treatment for at least 6 months before their death. Thus, as an interim strategy, we have treated Sprague–Dawley rats (250–275 g) with haloperidol (1 mg/kg) subcutaneously twice daily for 21 days (41). Such treatment failed to change the RELN mRNA content in the cortex (control 210 ± 15 amol/μg total RNA; haloperidol-treated, 220 ± 25; n = 5) or the cerebellum (control = 490 ± 50; haloperidol-treated, 510 ± 27; n = 5).
DISCUSSION
RELN, RELN mRNA, and DAB1 in Brains of Nonpsychiatric Subjects.
Qualitative evidence that RELN mRNA expression occurs in adult human brains has already been reported (30, 31), but the present data are a quantitative analysis of RELN mRNA and a comparative estimation of RELN protein expression in different regions of the adult human brain.
Because RELN mRNA is a large (>12 kb) molecule (23), it was necessary to investigate whether we measured the full-length RELN mRNA in the adult human brain. Because the results obtained with RT-PCR using two different sets of primers (Table 2) were virtually identical, we conclude that a full-length RELN mRNA is expressed in adult human brain.
A comparative evaluation of RELN content in the brain was carried out by using Western blot densitometry using the RELN 142 antibody (39). In PFC, temporal-cortex, and cerebellar extracts from nonpsychiatric subjects, this antibody reveals a ≈400-kDa immunoreactive band, which presumably represents a full-length RELN protein, and a ≈200-kDa band, which very likely indicates that RELN protein is either processed or degraded into a smaller molecular form. The ≈200-kDa product was found also in extracts from rat granule cells in culture and in the media of these cultures, and in brain of rat and mouse killed immediately before Western blotting (see also ref. 39). The putative RELN specificity of these immunopositive bands is additionally supported by the observation that two other RELN antibodies directed to the N terminus of RELN [i.e., G-10 (39) and R-58 (see Materials and Methods)] also yielded two bands. These bands were absent in the reeler mouse brain and in adult rat and mouse liver and kidney extracts, in which RELN mRNA expression is virtually undetectable.
Preliminary histochemical studies indicate that in the human cortex, a subpopulation of interneurons expresses RELN and GAD67. In the PFC (Brodmann’s area 46) and in the temporal cortex (Brodmann’s area 22), there was a diffuse RELN immunostaining in layers I and II (Fig. 2), which represents RELN located in the neuropil and presumably, in part, also reflects a RELN protein pool located in the ECM. We have never been able to detect RELN or RELN mRNA in cortical pyramidal neurons. Instead, these neurons, similar to those in rodent brains (29), express DAB1 (Fig. 2).
The preferential expression of DAB1 in neocortical pyramidal neurons is in keeping with the hypothesis that RELN is released into the ECM from a selected population of GABAergic neocortical interneurons and may indirectly influence pyramidal-cell function by regulating dendritic sprouting via DAB1 phosphorylation (29).
Decrease of RELN, RELN mRNA, and GAD67 but Not DAB1 in Brains of Schizophrenia Patients.
The extent of the RELN mRNA decrease was similar and statistically significant in all of the brain areas of schizophrenia patients studied, when compared with the corresponding areas of nonpsychiatric subjects (Tables 1 and 2).
Analysis of covariance shows that the decrease of RELN mRNA is unrelated to sex, age of onset of the disease, or postmortem interval before the sample collection. Moreover, the decrease of RELN mRNA cannot be ascribed to a nonspecific postmortem event because in the same brain areas, other mRNAs such as those encoding GABAA α1, α5, and nicotinic α7-receptor subunits (Fig. 1) were either normal or overexpressed, but they were never decreased.
RELN.
mRNA amplicons of RT-PCR reaction from PFC of two nonpsychiatric subjects, 21 and 27, and two schizophrenia patients, 3 and 10, in which RELN mRNA content was significantly decreased (see Table 1) were sequenced and found to be structurally identical, suggesting that the decrease of RELN mRNA expression with schizophrenia does not reflect an abnormality in the amplicon structure with a consequent defect in the efficacy of the RT-PCR assay.
In addition to a decrease of RELN mRNA, the brain content of RELN estimated by Western blotting using three different antibodies (see Materials and Methods) is also significantly decreased in brains of schizophrenia patients (see Table 3). Moreover, in the PFC and temporal cortex of schizophrenia patients, the number of interneurons expressing RELN protein is significantly decreased in layers I and II, which includes the most abundant RELN-positive neurons (Fig. 3).
In temporal-cortex extracts where we found a decrease of RELN but not of DAB1 protein, we also detected a consistently large (≈70%) decrease of GAD67 over a statistically nonsignificant decrease of GAD65 in schizophrenia patients compared with nonpsychiatric subjects. The decrease of GAD67 in schizophrenia patients was not caused by protein degradation attributable to a difference either in the interval between death and autopsy or in age or sex.
This finding, if verified by quantitative analyses of GAD67 and GAD65 mRNA content, will support and extend the seminal study of Akbarian et al. (10), which showed that in schizophrenia, the expression of GABAergic neurons is normal but the amount of regulated GAD67 expressed in GABAergic interneurons is significantly decreased. It would be interesting to verify whether the decrease of GAD67 is selective for the interneurons that express RELN.
Possible Role of RELN in the Two-Hit Model of Schizophrenia.
Overall, the present findings are compatible with a two-hit neurodevelopmental/vulnerability model of schizophrenia. In every brain area studied, schizophrenia patients expressed lower levels of RELN mRNA and RELN protein than nonpsychiatric subjects; this difference is shared by patients with paranoid and undifferentiated forms of schizophrenia. RELN is located primarily in interneurons of the telencephalic areas examined (PFC, temporal cortex, and hippocampus), whereas DAB1 is located in the pyramidal neurons of neocortex, limbic cortex, and hippocampus. It remains to be ascertained whether DAB1 is also located in selected GABAergic neuronal populations. It is remarkable that in various areas of schizophrenia brains, the mRNA encoding for RELN is decreased by ≈40–50%, which is reminiscent of the extent of the RELN mRNA decrease observed in the heterozygous reeler (rl/+) mouse. Using our quantitative assay (see Materials and Methods), we found that there is no detectable mRNA encoding for RELN in the telencephalon of 50-day-old rl/rl mice even after RT-PCR amplification. However, in the telencephalon of rl/+ mice, we found approximately 110 amol of RELN mRNA/μg total RNA, whereas we found approximately 190 amol/μg RNA in the telencephalon of wild-type mice. Thus, there is a similar RELN haplo-insufficiency in the brain of rl/+ mice and in the brain of schizophrenia patients.
From our experience, it appears that RELN mRNA haplo-insufficiency per se cannot generate a phenotype reminiscent of the reeler mouse. The rl/+ mouse expresses a number of neuroanatomical abnormalities similar to the changes associated with schizophrenia, but in the rl/+ mouse, these changes are symptomatically silent (unpublished data). It is probable that these changes may be a residual of neurodevelopmental abnormalities that could play a role in increasing the vulnerability to excitotoxicity elicited by oxygen radicals. Could this vulnerability be further increased by hormonal abnormalities that occur during or after puberty? If this was the case, is RELN haplo-insufficiency a favorable background for the accentuation or summation of various vulnerability factors operative in schizophrenia?
These considerations raise the question of whether we can obtain a model of schizophrenic etiopathology by applying an experimentally induced second hit to rl/+ mice. We know that the rl/+ mouse has a down-regulation of prepulse inhibition, which is reminiscent of the inhibition of auditory sensory gating observed in schizophrenia patients and in many healthy members of schizophrenia families. The possibility that a latent excitotoxicity exists in rl/+ mice has been observed in preliminary findings on a possible restriction-dependent polymorphism detected with a probe consisting of a 3′ stretch of human RELN cDNA (nucleotides 8,500–11,500); this polymorphism has been detected in a number of schizophrenic family members (32).
It is not surprising that a polymorphism exists in the RELN gene that includes approximately 450 kb and 65 exons. The alternative splicing events occurring at the 3′ terminal portion of the RELN gene and at an alternative polyadenylation site at exon 63 (terminal exon 63a) are known to participate in the regulation of RELN protein secretion. The frequency of RELN DNA polymorphism in schizophrenia patients and the location of these events in a stretch of genomic DNA important for the regulation of RELN protein secretion (23, 24) increases the clinical interest of RELN gene abnormalities as putative vulnerability factors in schizophrenia.
Acknowledgments
We thank Dr. A. M. Goffinet, Department of Human Physiology, Facultes Universitaires Notre-Dame de la Pix School of Medicine, Namur, Belgium, for the generous gift of RELN 142 and G-10 antibodies, and Dr. B. W. Howell, Fred Hutchinson Cancer Research Center, Seattle, WA, for the generous gift of the DAB1 B3 polyclonal antibody. We are indebted to Dr. F. E. Bloom (Department of Neuropharmacology, Scripps Research Institute, La Jolla, CA), Dr. T. Curran, (Department of Developmental Neurobiology, St. Jude Children’s Research Hospital, Memphis, TN), and Dr. P. S. Goldman-Rakic, (Section of Neurobiology, Yale University School of Medicine, New Haven, CT) for reading this paper and for their constructive comments, suggestions, and critiques. Brain samples were obtained from the National Neurological Research Specimen Bank, Veterans Administration Medical Center, Los Angeles, CA (which is sponsored by the National Institute of Neurological Disorders and Stroke/National Institute of Mental Health, National Multiple Sclerosis Society, Hereditary Disease Foundation, and Veterans Health Services and Research Administration, Department of Veteran’s Affairs) and from the Harvard Brain Tissue Resource Center, McLean Hospital, Belmont, MA. The research is supported in part by a Theodore and Vada Stanley Foundation research award to J.D.
ABBREVIATIONS
- PFC
postmortem prefrontal cortices
- GABA
γ-aminobutyric acid
- nAChr
nicotinic acetylcholine receptors
- ECM
extracellular martix
- IS
internal standard
- GAD
glutamic acid decarboxylase
References
- 1.Akbarian S, Bunney W E, Potkin S G, Wigal S B, Hagman J O, Sandman C A, Jones E G. Arch Gen Psychiatry. 1993;50:169–177. doi: 10.1001/archpsyc.1993.01820150007001. [DOI] [PubMed] [Google Scholar]
- 2.Selemon L D, Rajkowska G, Goldman-Rakic P S. J Comp Neurol. 1998;392:402–412. [PubMed] [Google Scholar]
- 3.Arnsten A F, Goldman-Rakic P S. Arch Gen Psychiatry. 1998;55:362–368. doi: 10.1001/archpsyc.55.4.362. [DOI] [PubMed] [Google Scholar]
- 4.Benes F M, Davidson J, Bird E D. Arch Gen Psychiatry. 1986;43:31–35. doi: 10.1001/archpsyc.1986.01800010033004. [DOI] [PubMed] [Google Scholar]
- 5.Benes F. Schizophr Bull. 1998;24:219–230. doi: 10.1093/oxfordjournals.schbul.a033322. [DOI] [PubMed] [Google Scholar]
- 6.Kerwin R W, Murray R M. Schizophr Res. 1992;7:1–12. doi: 10.1016/0920-9964(92)90067-f. [DOI] [PubMed] [Google Scholar]
- 7.Andreasen N C, Arndt S, Swayze V, Cizadlo T, Flaum M, O‘Leary D, Ehrhardt J C, Yuh W T C. Science. 1994;266:294–298. doi: 10.1126/science.7939669. [DOI] [PubMed] [Google Scholar]
- 8.Weinberger D R. Schizophr Bull. 1997;23:537–540. doi: 10.1093/schbul/23.3.537. [DOI] [PubMed] [Google Scholar]
- 9.Woo T U, Whitehead R E, Melchitzky D S, Lewis D A. Proc Natl Acad Sci USA. 1998;95:5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akbarian S, Kim J J, Potkin S G, Hagman J O, Tafazzoli A, Bunney W E, Jones E J. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 11.McGuffin P, Owen M J. Cold Spring Harbor Symp Quant Biol. 1996;61:815–822. [PubMed] [Google Scholar]
- 12.Levinson D F, Mahtani M M, Nancarrow D J, Brown D M, Kruglyak L, Kirby A, Hayward N K, Crowe R R, Andreasen N, C, Black, et al. Am J Psychiatry. 1998;155:741–750. doi: 10.1176/ajp.155.6.741. [DOI] [PubMed] [Google Scholar]
- 13.Pritchard D J. Ann Human Genet. 1966;60:105–123. doi: 10.1111/j.1469-1809.1996.tb01182.x. [DOI] [PubMed] [Google Scholar]
- 14.Anderson J E, O’Donnell B F, McCarley R W, Shenton M E. Restorative Neurol Neurosci. 1998;12:1–10. [PubMed] [Google Scholar]
- 15.Bloom F E. Arch Gen Psychiatry. 1993;50:224–227. doi: 10.1001/archpsyc.1993.01820150074008. [DOI] [PubMed] [Google Scholar]
- 16.Rakic P, Caviness V S., Jr Neuron. 1995;14:1101–1104. doi: 10.1016/0896-6273(95)90258-9. [DOI] [PubMed] [Google Scholar]
- 17.Rantakallio P, Jones P, Moring J, Von Wendt L. Int J Epidemiol. 1997;26:837–843. doi: 10.1093/ije/26.4.837. [DOI] [PubMed] [Google Scholar]
- 18.Jones P B, Rantakallio P, Hartikainen A L, Isohanni M, Siplia P. Am J Psychiatry. 1998;155:355–364. doi: 10.1176/ajp.155.3.355. [DOI] [PubMed] [Google Scholar]
- 19.Majewska M D. Ann NY Acad Sci. 1995;774:111–120. doi: 10.1111/j.1749-6632.1995.tb17375.x. [DOI] [PubMed] [Google Scholar]
- 20.Compagnone N A, Mellon S H. Proc Natl Acad Sci USA. 1998;95:4678–4683. doi: 10.1073/pnas.95.8.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olney J W, Farber N B. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 22.Coyle J T. Harvard Rev Psychiatry. 1996;3:241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- 23.Royaux I, Lambert de Rouvroit C, D’Arcangelo G, Demirov D, Goffinet A M. Genomics. 1998;46:240–250. doi: 10.1006/geno.1997.4983. [DOI] [PubMed] [Google Scholar]
- 24.Curran T, D’Arcangelo G. Brain Res Brain Res Rev. 1998;26(2–3):285–294. doi: 10.1016/s0165-0173(97)00035-0. [DOI] [PubMed] [Google Scholar]
- 25.Sheldon M, Rice D S, D’Arcangelo G, Yoneshima H, Nakajima K, Mikoshiba K, Howell B W, Cooper J A, Goldowitz D, Curran T. Nature (London) 1997;389:730–733. doi: 10.1038/39601. [DOI] [PubMed] [Google Scholar]
- 26.Howell B W, Hawkes R, Soriano P, Cooper J A. Nature (London) 1997;389:733–737. doi: 10.1038/39607. [DOI] [PubMed] [Google Scholar]
- 27.Meyer G, Goffinet A M. J Comp Neurol. 1998;397:29–40. [PubMed] [Google Scholar]
- 28.Pesold C, Impagnatiello F, Pisu M G, Uzunov D P, Costa E, Guidotti A, Caruncho H J. Proc Natl Acad Sci USA. 1998;95:3221–3226. doi: 10.1073/pnas.95.6.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rice D S, Sheldon M, D’Arcangelo G, Nakajima K, Goldwitz D, Curran T. Development (Cambridge, UK) 1998;125:3719–3729. doi: 10.1242/dev.125.18.3719. [DOI] [PubMed] [Google Scholar]
- 30.Impagnatiello, F., Costa, E. & Guidotti, A. (1997) Soc. Neurosci. Abs. 655.2 23, 1675.
- 31.deSilva U, D’Arcangelo G, Braden V, Chen J, Miao G G, Curran T, Green E D. Genome Res. 1997;7:157–166. doi: 10.1101/gr.7.2.157. [DOI] [PubMed] [Google Scholar]
- 32.Uzunov, D. P., Impagnatiello, F., Sharma, R., Guidotti, A. & Costa, E. (1998) Soc. Neurosci. Abs. 211.4 24, 525.
- 33.Gould E, Tanapat P, McEwen B S, Flugge G, Fuchs E. Proc Natl Acad Sci USA. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gould E, Cameron H A, McEwen B S. J Comp Neurol. 1994;340:551–565. doi: 10.1002/cne.903400408. [DOI] [PubMed] [Google Scholar]
- 35.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: Am. Psychiatr. Assoc.; 1994. pp. 274–289. [Google Scholar]
- 36.Impagnatiello F, Pesold C, Longone P, Caruncho H, Fritschy J M, Costa E, Guidotti A. Mol Pharmacol. 1996;49:822–831. [PubMed] [Google Scholar]
- 37.Grayson D R, Bovolin P, Santi R M. Methods Neurosci. 1993;12:191–208. [Google Scholar]
- 38.Dwivedi Y, Janicak P G, Pandey G N. Psychopharmacology. 1998;138:47–54. doi: 10.1007/s002130050644. [DOI] [PubMed] [Google Scholar]
- 39.de Bergeyck, V., Naerhuyzen, B., Goffinet, A. M. & Lambert de Rouvroit, C. (1998) J. Neurosci. Methods, in press. [DOI] [PubMed]
- 40.Mullen R J, Buck C R, Smith A M. Development (Cambridge, UK) 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 41.Guidotti A, Gale K, Toffano G, Vargas F M. Life Sci. 1978;23:501–505. doi: 10.1016/0024-3205(78)90161-3. [DOI] [PubMed] [Google Scholar]