Abstract
Nucleosome positioning plays a major role in controlling the accessibility of DNA to transcription factors and other nuclear processes. Nucleosome positions after assembly are at least partially determined by the relative affinity of DNA sequences for the histone octamer. Nucleosomes can be moved, however, by a class of ATP dependent chromatin remodeling complexes. We recently showed that the human SWI/SNF remodeling complex moves nucleosomes in a sequence specific manner, away from nucleosome positioning sequences (NPSes). Here, we compare the repositioning specificity of five remodelers of diverse biological functions (hSWI/SNF, the SNF2h ATPase and the hACF, CHRAC and WICH complexes than each contain SNF2h) on 5S rDNA, MMTV and 601 NPS polynucleosomal templates. We find that all five remodelers act similarly to reduce nucleosome occupancy over the strongest NPSes, an effect that could directly contribute to the function of WICH in activating 5S rDNA transcription. While some differences were observed between complexes, all five remodelers were found to result in surprisingly similar nucleosome distributions. This suggests that remodeling complexes may share a conserved repositioning specificity, and that their divergent biological functions may largely arise from other properties conferred by complex-specific subunits.
INTRODUCTION
Nucleosomes impose a significant barrier to transcription by blocking access of activators and basal factors to their sites on DNA, as well as by inhibiting the elongation of engaged polymerase molecules. Each nucleosome covers ∼146 bp of DNA, while linker DNA between nucleosomes ranges from ∼20 to 60 bp. Since linker DNA is far more accessible than nucleosome bound DNA, the precise location of nucleosomes on DNA is expected to functionally control transcription factor binding. Strikingly, recent studies have indicated that ∼80% of all yeast nucleosomes adopt specific positions, and that these positions are partially controlled by ∼146 bp ‘nucleosome positioning sequence’ (NPS) motifs encoded in the primary DNA sequence. Other genome-scale or genome-wide studies have shown that positioned nucleosomes are also common in a variety of organisms from C. elegans to humans (1,2). This gene-specific arrangement of nucleosomes is likely to directly impact transcription, since functional transcription factor binding sites (consensus sites that are evolutionarily conserved and/or known to be bound in vivo) were much more frequently found in linker regions than in DNA covered by nucleosomes, and since start sites of active genes were frequently devoid of nucleosomes. These studies indicate that nucleosome positioning will be involved in transcriptional regulation much more often than was initially suspected, and emphasize the need for a deeper understanding of how nucleosome positions are functionally controlled.
One mechanism all eukaryotic cells use to regulate the repressive effects of chromatin is the action of ATP-dependent chromatin remodeling complexes, which use the energy of ATP hydrolysis to modify chromatin structure. Several subfamilies exist, many of which are conserved from yeast to mammals, including SWI/SNF, ISWI, CHD/NuRD, Ino80, Swr1, Rad54, CSB and DDM1 (3–5). Each subfamily tends to have distinct biological functions. For instance, human SWI/SNF is an important transcriptional coactivator and corepressor, while Rad54 is involved in DNA damage repair. The most diverse group of remodeling complexes belongs to the ISWI subfamily (6). Human cells have two ISWI-class ATPases: SNF2L (which is part of a single complex, hNURF) and SNF2h (which is part of many complexes, including human ACF, CHRAC, RSF, NoRC and WICH). Mouse SNF2h is essential for development and also for individual cell survival (7), and SNF2h complexes are known or expected (based on studies of homologous complexes in drosophila) to be involved in a wide range of biological processes, including chromatin assembly, linker histone incorporation, nucleosome spacing, transcriptional silencing, transcriptional activation, DNA replication, recombination and replication of heterochromatin [e.g. (8–11) and for review see (6)]. The different non-ATPase subunits of the various SNF2h complexes underlie their divergent biological functions. For instance the human and/or fly ACF and CHRAC complexes (which each contain an ACF1 subunit) have been shown to be important for normal chromatin structure, heterochromatic gene silencing, transcriptional repression through the nuclear receptor corepressor NCoR, and repression of TCF factor-bound promoters in the absence of Wingless/Wnt signaling (12–14). In contrast, the WICH complex (composed of WSTF and SNF2h) plays important roles in promoting rDNA transcription by RNA Polymerase I, 5S rDNA transcription by RNA Pol III, DNA replication, formation of active chromatin after replication, and DNA damage repair (15–19).
The ability to hydrolyze ATP is essential for the biological functions of all remodeling complexes. Furthermore, all tested complexes have the ability to reposition nucleosomes on DNA, in an ATP-hydrolysis-dependent manner. This suggests that nucleosome repositioning may be an important general aspect of remodeling complex function. Of course, the effects of nucleosome repositioning would depend greatly on the specific sequences occupied by nucleosomes both before and after remodeling. Several recent studies have started to examine whether remodeling complexes reposition nucleosomes in a sequence specific manner. Initial studies, by us and others, used linear mononucleosomes (3). While some hints of specificity were seen (20–26), all of these studies were complicated by the fact that remodeling complexes are sensitive to the presence of DNA ends. For instance, SWI/SNF subfamily members all displayed a strong tendency to move nucleosomes to DNA ends, while some ISWI subfamily members moved nucleosomes towards DNA ends and others moved them away from ends.
In order to examine underlying repositioning specificity in the absence of end effects, our recent studies examined the effects of human SWI/SNF on circular mononucleosomal and polynucleosomal templates (27,28). In both systems we found that complete remodeling of several tested templates, to a hSWI/SNF-preferred dynamic equilibrium state, resulted in dramatic reduction of nucleosome occupancy over sequences that were strongly preferred for nucleosome assembly (NPSes). At the same time, nucleosome occupancy often rose at other positions. These and other observations indicated that the chromatin remodeling effects of hSWI/SNF were influenced by DNA sequence, and that this sequence-directed remodeling specificity might be an essential component of hSWI/SNF regulatory function. For instance, the movement of nucleosomes away from genetically-encoded NPSes covering transcription factor binding sites might function to automatically flip promoter chromatin from a default ‘off’ state to a remodeled ‘on’ state.
The observation that hSWI/SNF moves nucleosomes in a sequence specific manner suggested that other remodeling complexes may also ‘read’ DNA sequence, and, furthermore, that potential differences in remodeling specificity might contribute to the divergent functions of remodeling complexes, both between and within remodeler subfamilies. Here, we compare the nucleosome repositioning specificities of hSWI/SNF, the human SNF2h chromatin remodeling ATPase, and three complexes which contain SNF2h, together with additional complex-specific subunits (hACF, CHRAC and WICH). We examine remodeling effects on three polynucleosomal templates, bearing either the 5S rDNA promoter [transactivated by WICH (19)], the MMTV promoter [transactivated by hSWI/SNF, for review see (29)] or the strong, affinity-selected 601 NPS sequence (30). Our results show that all five remodelers greatly reduce nucleosome occupancy over a few strong NPSes, but have a more modest effect on nucleosome occupancy at other positions. They show some differences between remodelers, but also reveal a remarkable similarity in nucleosome repositioning specificity between these divergent complexes.
MATERIALS AND METHODS
Assembly of circular polynucleosomal arrays
The 5S template is the pXP10 plasmid, containing a 241 bp region of the Xenopus borealis somatic 5S rDNA gene (31). The 601 template is the p601 plasmid, containing the strong, artificial 601 NPS (30). The p601/PstI@75 template used for remodeler activity tests was constructed as described previously (32). The MMTV template is the pMMTV-LTR-Luc plasmid, which contains the full length MMTV LTR (33,34). Arrays were assembled from 20 µg of each plasmid by salt dialysis with a 1.5 : 1 weight ratio of human HeLa cell core histones to DNA (28). Complete assembly was monitored by relaxation of assembled chromatin with Topoisomerase I, purification of the DNA, and agarose gel electrophoresis in the presence or absence of chloroquine. Note that each of these NPSes is known to promote nucleosome positioning both in vivo and after nucleosome assembly by salt dialysis in vitro (29–31,35–38). Thus, assembly-favored nucleosome positions observed in these studies are likely to reflect functional positioning.
Remodeling, MNase digestion and isolation of mononucleosome fragments
hSWI/SNF was purified by immunoaffinity chromatography of a FLAG-tagged Ini1 subunit from HeLa Ini1 cells (39). ISWI complexes were purified by M2 affinity chromatography from Sf9 cells (24,32). 90 µl reactions were prepared with 400 ng of polynucleosomes (in 6 µl) and 2.4 µg hSWI/SNF, 6.72 µg SNF2h, 1.44 µg hACF, 0.96 µg CHRAC or 1.92 µg WICH (each in 48 µl of BC100, with BC100 alone as a control), in a final reaction buffer containing an additional 6.67 mM KCl, as well as 2 mM ATP/MgCl2, 0.4 mg/ml BSA, 1 mM MgCl2 and 0.1 units/µl wheat germ topoisomerase I (Promega). The ratios of remodelers to nucleosomes (assuming one nucleosome per 200 bp) were: hSWI/SNF; 0.53, SNF2h; 19, hACF; 1.7, CHRAC; 1.0 and WICH; 2.3. Note that, we do not think the remodelers (at the concentrations used here to give complete remodeling) influence remodeling results passively [e.g. by binding to a favored site and acting as a steric barrier to nucleosome movement by a second remodeling complex—as discussed also in (28)]. This is indicated, for instance, by our prior observation that restriction enzyme accessibility changes representative of sequence-specific repositioning were observed at hSWI/SNF : nucleosome ratios ranging from ∼3 : 1 to 0.1 : 1 (40,43). This is also consistent with the observations that remodelers can track rapidly along DNA in the presence of ATP, such that they will not remain in any one place long enough to present a steric barrier (44). The reactions were incubated at 30°C for 2 h. Remodeling was stopped by addition of ADP to a final concentration of 13 mM. For MNase digestion, each reaction was diluted 4.5-fold and adjusted to 0.4 mg/ml BSA, 0.7 mM MgCl2, 64 mM KCl and 3 mM CaCl2 (final concentrations). Reactions were digested at 30°C for 5 min with 0.4 units/µl MNase for unremodeled, hSWI/SNF, hACF and CHRAC, 0.8 units/µl for SNF2h, and 1.2 units/µl for WICH. The reactions were stopped by adding SDS to 0.2% and EDTA to 15 mM (final concentrations). The digested DNA fragments were purified by phenol extraction, ethanol precipitation in the presence of 40 µg glycogen carrier, and separation by 4% PAGE. Fragments of ∼146 bp in size, corresponding to mononucleosomes, were excised from the gel and eluted in TE at 4°C with continuous shaking overnight.
Restriction enzyme digestion and Southern blotting
For each restriction enzyme reaction, a volume of eluted mononucleosome-sized fragments corresponding to 13.3 ng of input polynucleosomes (prior to MNase digestion) was incubated overnight in a volume of 20 µl under ideal supplier-specified conditions and using 5–20 U of each enzyme. The restriction enzymes used for mapping on 5S were EcoRI, ApaI, EcoRV, ScaI, DdeI, XbaI and HindIII. The enzymes for 601 were EcoRI, XbaI, PmlI, BsiWI, MfeI, NotI, PstI and HindIII. The enzymes for MMTV were BsmI, AlwNI, HaeIII, Hpy188III, SacI, ApoI, HphI and BsrBI. All enzymes were unique within the probe region being mapped. Reactions were separated by PAGE and Southern blotted as described previously (28). The MMTV probe, new to this study, was PCR amplified from pMMTV-LTR-Luc using the primers GGGGAAAGATTTTCCATACCA and ATGGCGAACAGACACAAACA to generate a 375 bp fragment containing NucB.
Analysis of mononucleosome positions and percent occupancy
Analysis of nucleosome positions and occupancy was done essentially as described previously (28). Briefly, Southern blot fragments resulting from each restriction enzyme digest were quantitated for size and for percent cutting [normalized to account for the other cut fragment by multiplying percentage cutting by 146/(fragment length)]. The data was used to determine nucleosome position and abundance respectively. To calculate percent mononucleosome occupancy, raw percent cutting numbers were multiplied by the fraction of MNase digested nucleosomal products that were mononucleosomes (measured by Southern blot of the unseparated, MNase-digested products), and then by the total number of nucleosomes in the probe region. Note that the percentage mononucleosome measurement is most accurate when MNase has fully digested linker DNA but not overdigested nucleosomal DNA. To correct for MNase underdigestion that was evident in some WICH reactions, we digested WICH-remodeled and control 601 chromatin with a broad range of MNase concentrations, separated fragments by PAGE and stained them with ethidium bromide. We then plotted the ratio of [internucleosomal fragment intensity (between mono- and dinucleosomes, representing underdigestion)] over [subnucleosomal fragment intensity (below mononucleosomes, representing overdigestion)] versus observed percentage mononucleosomes. This resulted in a curve that fit to a logarithmic equation, which could be used to calculate actual percentage mononucleosomes given an observed internucleosomal/subnucleosomal ratio and an apparent percentage mononucleosome value. The number of nucleosomes in each probe region was determined as described previously, by measuring the fraction of probe-region DNA left after MNase and/or by measuring restriction enzyme accessibility at sites of peak nucleosome occupancy (28). In the second method, nucleosome number was determined by dividing the percentage of uncut DNA by the raw percentage mononucleosome coverage calculated by our MNase/restriction enzyme mapping results at that same site. Note that this estimate of nucleosome number assumes that the ∼10–20% of non-mononucleosomal species (dinucleosomes, etc.) have distributions that are similar to those of mononucleosomes. This is expected to be a reasonable assumption, based on our prior studies which mapped mononucleosomes, altosomes and dinucleosomes before and after hSWI/SNF remodeling (28).
Error estimates and statistical analysis
Our prior studies showed that the error in percentage occupancy assignments were modest, and that overall nucleosome distribution patterns were highly reproducible in repeat experiments (for both unremodeled and hSWI/SNF-remodeled conditions) (28). Nonetheless, we performed repeat experiments for more than half of all condition and template combinations tested here. We found that the characteristic pattern of bands resulting from a specific remodeling condition on a specific template was always evident in repeated experiments, even in cases where blotting problems prevented the complete analysis of all nucleosome positions on one or the other blot. To determine background values for Figure 7, we used only repeat experiments for which the blots allowed all positions to be assigned. This gave an estimate of average absolute difference per position of 0.76%, which corresponds to background summed absolute difference of 25%, and to an estimated standard deviation per position of 0.54%. The significance of differences between ‘summed absolute difference’ values was computed by converting these values to mean values (dividing by the number of nucleosome positions) and using the estimated standard deviation per position in a t-test. Where multiple comparisons were made (e.g. for all 10 pairwise combinations of conditions within a table row), we adjusted for this using the Bonferroni method. This highly conservative method was chosen, in part, for simplicity of presentation (to allow a single value for significant difference to be applied to each comparison within a row), and also in recognition of the fact that the background standard deviation per position, applied to all conditions, was estimated using complete positioning data from of only a few conditions.
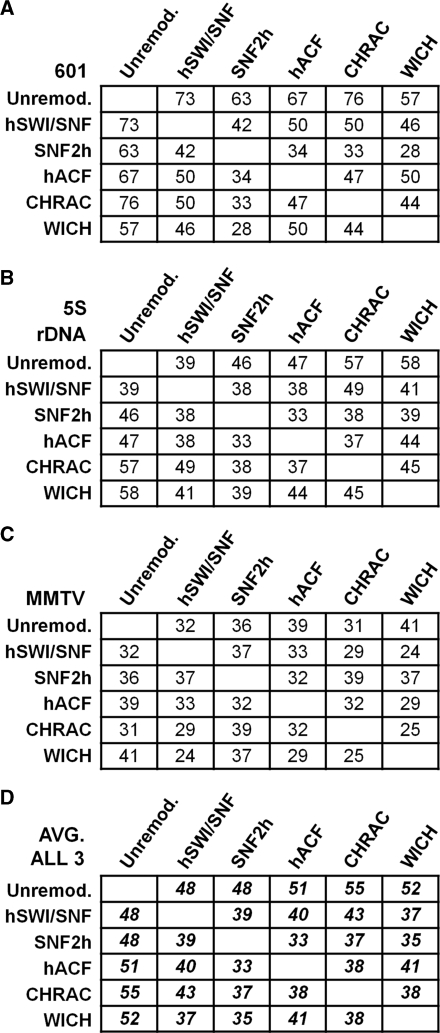
Figure 7.
Summed absolute differences in nucleosome occupancy reveal similarities and differences between remodelers. For each indicated pair of conditions (by column and row), the absolute difference in normalized percentage occupancy at each nucleosome position was calculated and summed, for all data from the 601 (A) 5S (B) or MMTV (C) templates. The average value for all three templates is shown in (D). The background summed absolute difference, for repeats of the same condition and template, is 25%. Differences between any given cell and this background value, or between any two cells in any given column or row, are significant (P < 0.05) if they are >13% (for A through C) or 7.2% (for D).
Remodeler activity tests
Standard remodeling reactions (as described above), using p601/Pst@75 polynucleosomes, were stopped with apyrase before remodeler addition (‘0 time’) or at 15 min, 30 min, 1 h or 2 h. Reactions were then digested with PstI, phenol extracted to purify the DNA, then digested with ScaI. Fragments were separated by electrophoresis through 0.8% agarose, stained with ethidium bromide, and quantitated to calculate percent accessibility. To confirm remodeler activity after preincubation, remodeling reactions were set up as described above using 601 polynucleosomes (at half the normal concentration). After 2 h, p601/Pst@75 polynucleosomes were added (to bring the reaction to the normal template concentration) and remodeled for an additional 30 min, before stopping the reaction and analyzing PstI accessibility.
RESULTS
To map nucleosome positions after chromatin assembly and after chromatin remodeling, we have developed a novel ‘MNase footprint/Restriction Enzyme’ mapping assay (28). Briefly, closed circular plasmid templates are assembled into chromatin by salt dialysis from human histones, followed by incubation with ATP and with or without each remodeling protein or complex. Nucleosome positions are then measured by MNase digestion, purification of ∼146 bp mononucleosome-protected fragments, digestion with locally unique restriction enzymes and Southern blotting with a probe surrounding the promoter or NPS sequence of interest (Figure 1A). The intensity and position of bands resulting from digestion with each enzyme, together with novel analysis methods we have developed for polynucleosomal mapping, allows the accurate assignment of nucleosome positions and the quantitation of nucleosome occupancy (i.e. the fraction of templates bearing a nucleosome in each position) (28). The human remodeling complexes and ATPases compared in this study were: hSWI/SNF, the SNF2h ATPase, hACF (SNF2h + hACF1), CHRAC (SNF2h + hACF1 + CHRAC15 + CHRAC17) and WICH (SNF2h + WSTF) (24,32,39). As will be described further in Figure 4, each remodeling complex was added at a concentration sufficient to give complete remodeling, such that additional remodeler or reaction time resulted in no further changes to the chromatin. Thus, nucleosome positions after remodeling reflect the intrinsic preference of each remodeler for nucleosome placement.
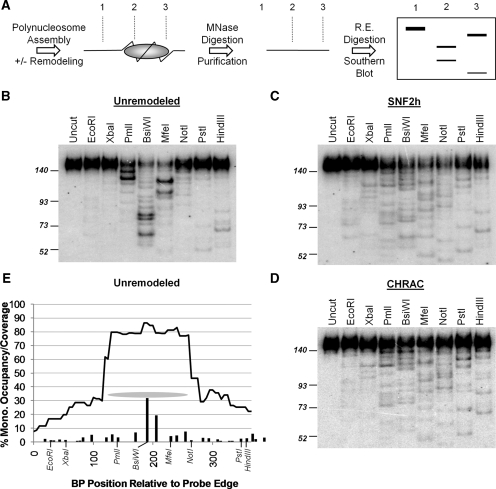
Figure 1.
MNase footprint restriction mapping of nucleosome positions. (A) Outline of method (see text for details). (B–D): representative Southern blots, used to map mononucleosomes over the 601 NPS region, under the indicated conditions. The positions of molecular weight markers (in bp) are shown on the left. (E) Quantitation of the blot shown in (B). Bars: ‘percentage mononucleosome occupancy’ (e.g. the percent of templates with a nucleosome centered over each location). For simplicity of presentation, bars correspond to nucleosome occupancy over 5 bp regions (e.g. the bar at position 50 covers 47.5–52.5 bp). In almost all cases each bar corresponds to a single nucleosome position. Curved line: ‘percentage mononucleosome coverage’ considering all mapped positions (e.g. the fraction of templates on which nucleosomes cover any given site). Grey oval: the area covered by the major position centered over the BsiWI site. The positions of the restriction sites used are shown in italics.
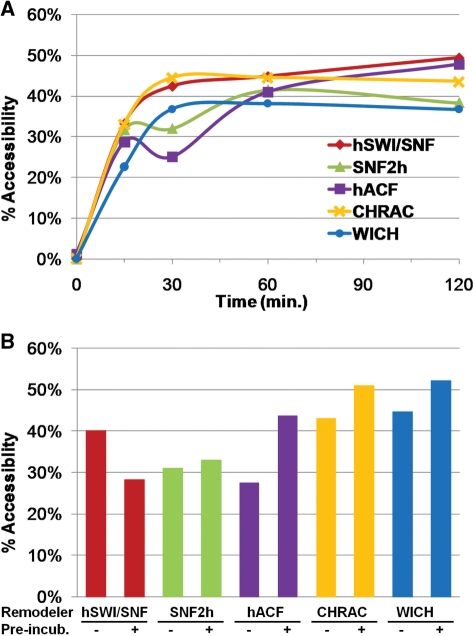
Figure 4.
Remodeling conditions used for mapping result in a fully remodeled state. (A) Percent accessibility at a PstI site inserted at the center of the 601 NPS (75 bp from the edge) was measured after 0, 15, 30, 60 and 120 min of remodeling. (B) Preincubation test: 200 ng of p601 polynucleosomes were preincubated with remodelers for 2 h, then spiked with 200 ng of p601/Pst@75 polynucleosomes for 30 min (‘+Pre-incub.’). ‘−Pre-incub.’: remodelers and both templates were added together and incubated for 30 min.
Similarities and differences in nucleosome distributions upon remodeling of the strong 601 NPS
In our prior studies, we found that human SWI/SNF repositioned nucleosomes away from the 601 NPS, one of the strongest known positioning sequences, on a polynucleosomal plasmid template (28,30). We have now compared the repositioning specificities of SNF2h, hACF, CHRAC, WICH and hSWI/SNF on this same template. Examples of the raw data are shown in Figure 1B (unremodeled), 1C (SNF2h remodeled) and 1D (CHRAC remodeled). Nucleosome intensities were assigned by first measuring the percentage of counts in each lane corresponding to a given restriction fragment. A single band identifies two possible nucleosome orientations (with the long restriction fragment pointed either leftwards or rightwards). Incorporating the results from adjacent restriction enzyme sites, however, allows a single nucleosome position to be assigned (e.g. the strongest two bands in the PmlI lane of Figure 1B correspond to rightward-pointing nucleosome positions, since bands of the correct position and intensity exist in the BsiWI and MfeI lanes, and not in the XbaI and EcoRI lanes). The percent of templates bearing a nucleosome at each individual position, ‘percent occupancy’, was determined by multiplying percentage cutting by the percentage of all MNase digestion products that are 146 bp (mononucleosomes) and then by the average total number of nucleosomes present in the probe region (for details see ‘Materials and methods’ section). The bar graph in Figure 1E shows percent occupancy for each position, with the bar located at the center of each nucleosome. Each ∼146 bp nucleosome covers DNA extending 73 bp to the left and right of this center position (e.g. the oval in Figure 1E indicates the ‘coverage’ of the strongest unremodeled position, which corresponds to the strongest restriction fragments in the PmlI, BsiWI and MfeI lanes in Figure 1B). Summing the percentage occupancies of all 146 bp nucleosomes overlapping each position on the template results in a ‘nucleosome coverage’ curve, where low percentage coverage indicates accessible regions free of nucleosomes and high percentage coverage indicates nucleosome occluded regions (curve in Figure 1E).
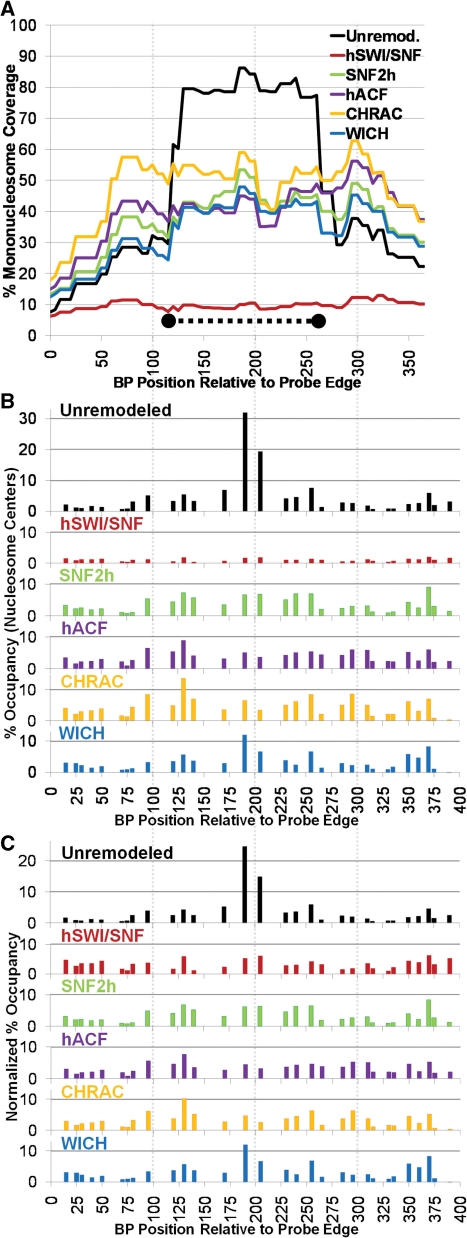
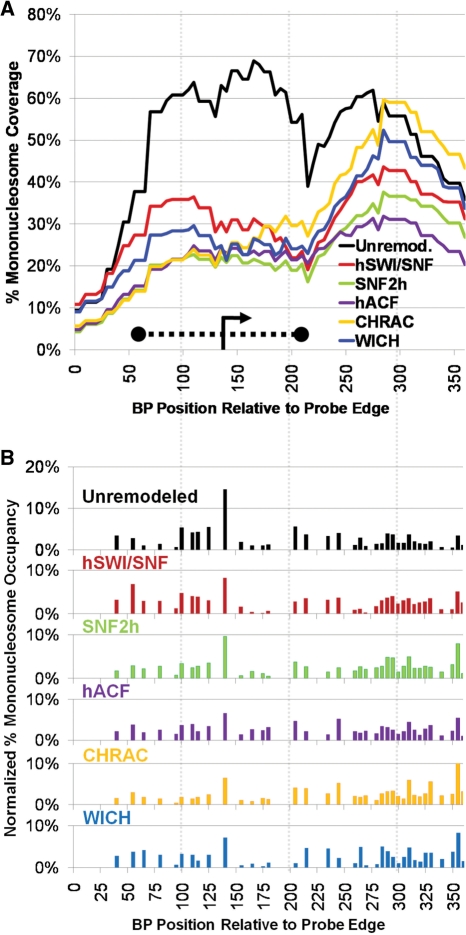
The complete results for the 601 template (unremodeled and remodeled by SNF2h, hACF, CHRAC, WICH or hSWI/SNF) are shown in Figure 2 (with coverage curves shown in 2A and percentage occupancy graphs shown in 2B). Nucleosome assembly results in very high coverage over the strong 601 NPS sequence (Figure 2A black line, from ∼BP 120–266), with low coverage over upstream and downstream sequences. This occupancy peak corresponds to two very strong individual nucleosome positions at BP 190 and 205 (Figure 2B black bars). Every tested remodeling complex greatly reduced coverage over this region (Figure 2A), and occupancy at these positions (Figure 2B). To either side of this NPS sequence, however, coverage and occupancy profiles after remodeling (by all remodelers except for hSWI/SNF) were somewhat similar to those seen before remodeling.
Figure 2.
All remodelers cause a similar redistribution of mononucleosomes around the 601 NPS. (A) Percentage mononucleosome coverage over the 601 polynucleosome probe region for each remodeler tested. The position of the 601 NPS, from prior studies, is shown as a thick dotted line. (B) Percentage mononucleosome occupancy. (C) Normalized percentage occupancy: to better compare the distribution of mononucleosomes between remodelers, the data in (B) was adjusted so that the total percent occupancy for all positions equaled 100%.
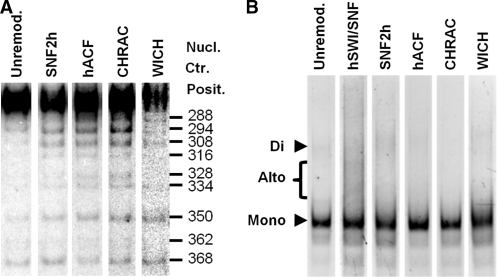
hSWI/SNF converts up to 40% of the well-separated ‘mononucleosomes’ on polynucleosomal templates into altered dinucleosomes (altosomes) (28,40). Prior studies have indicated that ISWI subfamily remodeling complexes are unlikely to form altosomes, since these complexes do not form altered dimers of mononucleosomes and do not reduce nucleosome-constrained supercoiling (3). This is confirmed in the results shown in Figure 3B, where the formation of significant ∼200 bp internucleosomal MNase footprint products (a hallmark of altosomes) is only observed after hSWI/SNF treatment. Note that MNase digestion of the unremodeled and SNF2h-complex remodeled templates did give a small amount of ∼292 bp ‘dinucleosomal’ products. These products result from situations where two normal nucleosomes are immediately adjacent to each other, such that MNase cannot cut between them. Because these products (as well as higher multimers: trinucleosomes, etc.) represent <20% of all unremodeled or SNF2h complex-remodeled products, we did not map them in these studies. However, their presence in the probed region does reduce the fraction of all nucleosomal species that are mononucleosomes (reducing calculated mononucleosome percentage occupancy). For instance, as indicated by our prior studies, the presence of dinucleosomes at low percentage occupancy is why the mononucleosome percentage coverage over the 601 NPS is <100% (28) (Figure 2A).
Figure 3.
Differences between remodeled positions and products on p601 (A) Alignment of PstI digestion lanes from Southern blots for p601 chromatin that was unremodeled or remodeled by SNF2h, hACF, CHRAC or WICH. Note differences in band intensities, especially for positions 288, 294, 308, 328 and 334. (B) Ethidium bromide stain of MNase digestion products from unremodeled or remodeled p601 polynucleosomes. Note the absence of internucleosomal ‘altosome’ bands for all conditions except SWI/SNF remodeled.
Previously, we found that hSWI/SNF created altosomes over the 601 NPS probe region and also moved histone octamers out of the probe region (28). These effects result in a much lower average number of well-separated mononucleosomes in the probe region after hSWI/SNF remodeling, greatly reducing calculated percentage occupancy and percentage coverage (red lines in Figure 2A and B). Altosome formation appears to result when one nucleosome collides with another before hSWI/SNF can finish moving it from a disfavored sequence to a preferred sequence (28). Given this, the intrinsic sequence preference for nucleosome repositioning would be best represented by ignoring altosomes (and other structures) and focusing only on the relative pattern of repositioned normal mononucleosomes. Accordingly, in order to get a better sense of the relative repositioning preferences of hSWI/SNF and the SNF2h complexes, we normalized the percentage mononucleosome occupancy data for each condition such that the occupancy at all positions added to 100%. This presentation makes it much easier to see that the relative distribution of mononucleosomes after remodeling by hSWI/SNF and each of the SNF2h complexes is remarkably similar across the whole probe region (Figure 2C). The similarity in mononucleosome profiles can also be seen in the raw data (e.g. compare Figure 1C: SNF2h with Figure 1D: CHRAC). Surprisingly, with the exception of the two major NPS positions, the pattern was also quite similar to that seen in the unremodeled state. For instance, ignoring the two strongest bands in the PmlI, BsiWI and MfeI lanes of Figure 1B, the overall pattern of bands is similar for unremodeled, SNF2h remodeled and CHRAC remodeled conditions (compare Figure 1B to Figure 1C and D).
Even though remodeled profiles were generally quite similar, differences between complexes were also evident. For instance, CHRAC (and, to a lesser extent, hACF) results in higher normalized mononucleosome occupancy at center positions between 95 and 150 and between 275 and 315 than all other complexes (Figure 2C), corresponding to two peaks of coverage after CHRAC or hACF remodeling, to the left and to the right of the 601 NPS (Figure 2A). This difference in banding patterns can be readily seen when lanes for the same restriction enzyme digestion are compared across complexes (e.g. Figure 3A, note the increasing strength of bands for positions 288, 294 and 308 in SNF2h, hACF and CHRAC lanes, and the relative weakness of these bands in the unremodeled and WICH lanes). In addition to these differences in pattern, there were also differences in the number of nucleosomes present in the probe region. The calculated number of nucleosomes (in all forms) on the unremodeled template was 1.38. This number was decreased slightly by SNF2h, to 1.28, and further decreased by WICH, to 0.89, consistent with removal of histone octamers from the now-disfavored 601 NPS and distribution to other regions of the plasmid template, outside of the probe region. By contrast, hACF and CHRAC remodeling actually increased the number of mononucleosomes in the probe region, to 1.47 and 1.65, respectively. These effects can be seen in Figure 2A, where all of the complexes result in a similar decrease in coverage over the 601 NPS, but hACF and CHRAC result in the strongest increases in coverage at leftward and rightward flanking regions.
Reaction conditions result in a fully remodeled state
In our earlier studies we used a restriction enzyme-accessibility assay to establish hSWI/SNF and ATP concentrations that gave complete remodeling after <1 h. Reactions were then carried out for 2 h under these conditions. This ensured that mapped nucleosome positions represented hSWI/SNF-preferred positions at a dynamic equilibrium state, where movement away from any position was countered by an equal degree of movement back to that position (27,28). To confirm complete remodeling for SNF2h and each SNF2h-containing complex, we performed a similar restriction enzyme accessibility assay, using a variant of the p601 polynucleosomal template (p601/Pst@75) that is identical to p601 except for the presence of a PstI site at position 235 (in the middle of the 601 NPS) (32). Consistent with the mapping results, PstI accessibility is very low at time zero, indicating that nucleosomes block the PstI site on >95% of templates (Figure 4A). Also consistent with the mapping results, remodeling by SNF2h, hACF, CHRAC or WICH greatly increased PstI accessibility, with a plateau reached between 30 and 60 min. This indicates that the nucleosome repositioning reaction is complete well before the standard 2 h incubation time. Complete remodeling was also confirmed using a mixed-template gel shift assay (Supplementary Figure 1A).
One possibility that the above experiment could not rule out was that additional remodeling effects were not seen because remodeling had ceased before the 2 h incubation was complete (either because the complex had stopped functioning or because ongoing ATP hydrolysis had removed all free ATP). We thought this was unlikely, based on studies by us and others that measured hSWI/SNF and SNF2h complex stability and ATP hydrolysis rates (24,32,39,41,42). Nonetheless, we wished to directly confirm that remodeling was still occurring after 2 h of incubation. To do this, remodelers were preincubated with the normal p601 polynucleosomal template (lacking the PstI site) for 2 h. p601/Pst@75 polynucleosomes were then added and the reactions continued for 30 min before assaying for PstI accessibility. This was compared to control conditions in which remodelers were added together with both templates for 30 min (‘-’preincubation). The results in Figure 4B show that the increase in PstI accessibility due to SNF2h, hACF, CHRAC or WICH remodeling (from <5% for the unremodeled template) was essentially the same with or without 2 h preincubation. Similar results were seen when the remodeler was preincubated for 2 h without template (Supplementary Figure 1B). These results confirm that all tested remodeling complexes remain active after 2 h of incubation/remodeling, and indicate that the nucleosome positions observed represent a fully remodeled, complex-and-sequence-specific dynamic equilibrium state. Accordingly, the positions we observe after remodeling do not represent intermediate states, which might be influenced by starting nucleosome positions or by the rate at which the remodeler can move nucleosomes.
Diverse complexes show similar effects when remodeling the 5S rDNA promoter
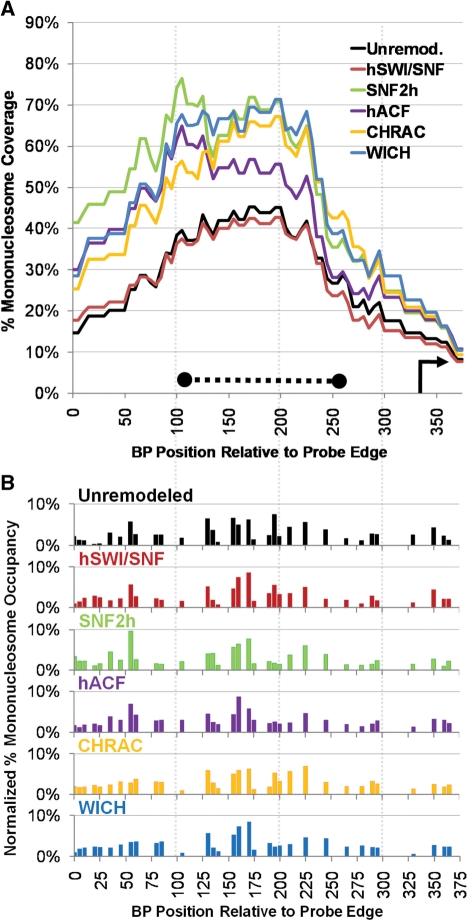
The NPS surrounding the 5S rDNA gene transcription start site, from a variety of organisms, is widely used as a canonical nucleosome positioning element [e.g. (31,45,46) and for review see (35)]. The 5S NPS was also of particular interest here because of recent studies showing that both WICH components (WSTF and SNF2h) are associated with human 5S genes and that shRNA knock down of WSTF decreases 5S transcript levels (19). Here, as was also seen in prior studies by us and others, we found that the 5S NPS is characterized by a cluster of overlapping individual nucleosome positions (Figure 5B, black bars for nucleosomes with centers between 100 and 140 bp) that give rise to a broad peak of nucleosome coverage over the transcription start site located at position 141 [Figure 5A, black lines (27,28,46)]. This is likely to be because the same DNA properties that result in a single high affinity 146 bp sequence (e.g. the highest bar in Figure 5A at 140 bp) can also result in almost equally high affinity 146 bp sequences shifted by 10, 20 or even 30 bp, so long as inhibitory sequences are not introduced in those new positions. Here, as in our prior studies, we found that hSWI/SNF greatly reduced the mononucleosome coverage over the 5S NPS sequence, but had a lesser effect on a weaker downstream peak of coverage [(28) and compare Figure 5A red and black lines]. This was associated with a great decrease in the strongest 5S NPS position centered at 140 bp, and a more modest decrease in the cluster of NPS-associated positions between 100 and 135 (Figure 5B, red and black lines show normalized nucleosome occupancy, as per Figure 2C, to better highlight similarities and differences in overall mononucleosome patterns). Interestingly, SNF2h, hACF, CHRAC and WICH all showed similar overall results, decreasing coverage in the same region and nucleosome occupancy at the same positions. In addition, aside from this cluster of positions at or near the 5S NPS, the distribution of positions was similar between unremodeled and all remodeled states. Together with the p601 results, these data indicate that each of these divergent remodeling complexes has a similar propensity to move nucleosomes away from positions favored by strong NPS sequences. Nonetheless, as was also seen on the p601 template, distinct localized differences in nucleosome distributions could also be seen for the each complex. For instance, between 200 and 250 bp, WICH moves nucleosomes from the high/low/low/high pattern seen upon assembly to a low/high/high/low pattern, while hACF does not.
Figure 5.
Diverse complexes show similar effects when remodeling the 5S rDNA promoter. (A) percentage mononucleosome coverage over the 5S rDNA polynucleosome probe region. The transcription start site is indicated by an arrow, and the region of peak nucleosome occupancy expected from prior in vitro and in vivo studies is indicated by a thick dotted line. (B) Normalized percentage mononucleosome occupancy.
All complexes show similar, modest effects on MMTV nucleosome distribution
The MMTV promoter is a well established model for the roles of chromatin changes during gene activation by hormone-bound glucocorticoid receptor (GR) (29). In vivo and in vitro the promoter is assembled into an array of six positioned nucleosomes. When GR is activated to bind the promoter, one of these nucleosomes, NucB, becomes disrupted, resulting in much greater restriction enzyme accessibility. The human SWI/SNF complex is known to be recruited to MMTV by GR, and is important for gene activation as well as increased restriction enzyme accessibility at sites within NucB. Here, we wished to test whether hSWI/SNF would disrupt nucleosomes positioned over the NucB sequences (similar to its effect at the 5S and 601 NPSes). We also wished to test whether each of the SNF2h complexes exhibited similar or different specificities. The results of these assays are shown in Figure 6A (coverage) and B (normalized percentage occupancy). The peak of nucleosome coverage observed in the unremodeled state (black line in Figure 6A) is consistent with prior studies that mapped the nucleosome positions on an in vitro reconstituted MMTV construct at low resolution (37). Mononucleosome coverage in this position arises from about a dozen separate positions with centers between 120 and 250 bp (black bars in Figure 6B). This is consistent with high resolution in vivo mapping studies of MMTV, which indicated that nucleosome coverage over the NucB was due to contributions of thirteen distinct positions of differing strengths (38). Interestingly, we found that complete remodeling by hACF, CHRAC, WICH, SNF2h or hSWI/SNF all resulted in a similarly localized peak of nucleosome coverage (Figure 6A) and in normalized percentage mononucleosome occupancy distributions that were quite similar to the unremodeled state (Figure 6B). While some differences in normalized percent occupancy can be seen between complexes (such as the high relative percentage occupancies of positions between 35 and 65 bp after SNF2h and hACF remodeling), the distribution preferred after complete remodeling by these divergent complexes is overall quite similar to the distribution after assembly. This is in contrast to the results from the 601 and 5S sequences, where at least one strong NPS position is dramatically reduced. Furthermore, remodeling by SNF2h, hACF, CHRAC and WICH all increased nucleosome coverage in the NucB region, and also increased the total average number of nucleosomes in the probe region (Figure 6A and data not shown). hSWI/SNF also increased the number of nucleosomes in the probe region, although this does not result in a higher mononucleosome percentage coverage curve, because hSWI/SNF converts a portion of well-separated mononucleosomes into altosomes and other structures. These results indicate that not all functional positioning sequences (capable of creating a peak of nucleosome occupancy in vivo and after assembly in vitro) will be disfavored after remodeling.
Figure 6.
Similarities between remodeled and unremodeled distributions in the MMTV NucB region. (A) Percentage mononucleosome coverage (arrow and dotted line: as per Figure 5). (B) Normalized percentage mononucleosome occupancy.
Combined results highlight similarities and differences between complexes and templates
It can be difficult to judge the overall degree of similarity or difference between nucleosome distributions (unremodeled or remodeled by each complex) by simply looking at the percentage occupancy bar graphs. To better make this comparison, we summed the absolute differences between normalized percent occupancy at every position for two conditions [e.g. the absolute value of (601_unremodeled_position1 minus 601_hACF_position1), etc.]. First, we determined a background value by comparing normalized percentage occupancy values from repeat experiments done with the same template and under the same remodeling conditions. The average background summed absolute difference was 25% (corresponding to a 0.76% error in normalized percentage occupancy for each mapped position). Accordingly, a summed absolute difference that is close to 25% indicates a high degree of similarity between two remodeled conditions. Conversely, the maximum summed absolute difference is 200% (corresponding to a situation where two distributions have no positions in common).
The first row in tables A, B and C in Figure 7 show the summed absolute differences between the unremodeled state and the remodeled state on each template. An increase in summed absolute difference of >13% (from 25% to >38%) indicates that the remodeled state is significantly different from the unremodeled state (P < 0.05). Thus, on the 601 and 5S templates, every remodeler creates a nucleosome pattern that is significantly different from the unremodeled pattern (Figure 7A and B, top row). This is consistent with the graphs in Figures 2 and 5, and reflects the great decrease in occupancy observed at the strongest 5S and 601 NPS sites. By contrast, SWI/SNF, SNF2h and CHRAC remodeling of the MMTV template resulted in remodeled states that were not significantly different from unremodeled, with ACF and WICH remodeled states just barely reaching the significance threshold. This confirms the observation that nucleosome positions in the MMTV Nucleosome B probe region are relatively unchanged by any of the tested remodeling complexes. Note that, in the ‘unremodeled’ rows, the summed absolute differences for each remodeled state compared to unremodeled tended to be similar (with the only significant differences being between the SWI/SNF:Unremodeled and CHRAC:Unremodeled and between the SWI/SNF:Unremodeled and WICH:Unremodeled comparisons on the 5S template, Figure 7B). This indicates that each of these disparate remodelers introduce a similar degree of change in nucleosome distributions on each template. This is also evident in Figure 7D, where results from all three templates were combined to provide an overall measure of remodeling effects.
To examine similarities and differences between remodelers, we compared summed absolute differences for each remodeled state on each template and across all three templates (Figure 7, lower rows). Comparing the first row in each table to subsequent rows, it is evident that the pattern for any given remodeler generally differs more from the unremodeled state than from other remodeled states. This is significant or nearly significant for most pairs in Figure 7A, B and D (where differences between entries are significant if >13% for 7A and B or 7.2% for 7D). More generally, the observation that remodeler vs. unremodeled comparisons result in higher summed absolute differences than remodeler vs. remodeler comparisons, across all three templates, is highly significant (P < 0.001). This confirms the general observation that remodeled patterns, especially for 5S and 601, are closer to each other than to unremodeled.
Interestingly, looking at the SNF2h rows, the SNF2h pattern was more similar to the patterns for the SNF2h-containing complexes (between 33 and 37% summed absolute differences for hACF, CHRAC and WICH in Figure 7D) than to the pattern for hSWI/SNF (39%). While these differences were not individually significant, the general observation that SNF2h or SNF2h-containing complexes give patterns closer to SNF2h than to hSWI/SNF was highly significant (P < 0.001). This indicates that sharing the same SNF2h ATPase tends to impart a similar overall specificity to these complexes. Conversely, looking at the ACF, CHRAC and WICH rows, the pattern for any one of these remodelers is almost always closer to the pattern for SNF2h than to the pattern for the other SNF2h-containing complexes. This is significant for some individual pairs (e.g. ACF:SNF2h vs. ACF:WICH in Figure 7D), and is also highly significant in general (ACF, CHRAC and WICH patterns are closer to SNF2h than to each other, P < 0.002). This indicates that the distinct subunits of these complexes alter SNF2h remodeling to result in complex-specific patterns. Interestingly, ACF and CHRAC patterns appear to be further from each other than either complex is from SNF2h (P < 0.1). This suggests that the small CHRAC 15 and 17 subunits alter the remodeling pattern of the SNF2h and ACF1 complex. The effect of the CHRAC 15 and 17 subunits is also suggested, in the SNF2h row, by an increase in summed absolute difference going from the SNF2h : hACF comparison to the SNF2h : CHRAC comparison (from 33 to 37%). These results indicate that, even though there is considerable similarity between remodeled states, the distinct ATPases and subunits present in each remodeling complex can alter the overall remodeled patterns of nucleosome positions.
DISCUSSION
Our studies have revealed similarities and differences between nucleosome repositioning effects of five human ATP-dependent remodeling complexes or ATPases (hSWI/SNF, hACF, CHRAC, WICH and SNF2h) and on three chromatinized DNA sequences. The greatest change relative to unremodeled chromatin was seen at the major NPS-favored positions on the Xenopus 5S rDNA and the 601 sequences, where all five remodelers caused a marked decrease in nucleosome occupancy. This indicates that the movement of mononucleosomes away from certain strong NPSes, that we observed previously for hSWI/SNF, may be a common property of remodeling complexes. This may be consistent with a recent study which showed ATP-dependent chromatin assembly and remodeling by the drosophila ACF complex largely erased the effects of yeast DNA sequence on salt dialysis-assembled nucleosome positions (47). Surprisingly, while we observed localized differences in nucleosome abundance after remodeling by each complex, the overall remodeled patterns were similar. This indicates that differences in ATPases (BRG1 and hBRM in hSWI/SNF and SNF2h in the other complexes) as well as differences in subunit composition (for hACF, CHRAC and WICH) do not confer markedly distinct sequence specificities for repositioning to each complex. This argues against the possibility that differences in the sequence specificity could be a primary determinant of distinct remodeling complex functions. Instead, our data suggest that the divergent functions of hSWI/SNF, hACF, CHRAC and WICH may mostly be determined by differences in their recruitment (e.g. different subunits allow the complexes to be recruited to the binding sites of divergent transcription factors), from other differences in protein–protein interactions (e.g. distinct complex subunits may interact with other chromatin modifying factors such as histone acetyltransferases or demethylases), or even from distinct enzymatic activities of subunits [such as the histone H2A.X tyrosine kinase activity that was recently discovered for WSTF (18)]. For hSWI/SNF, divergent functions might also arise from its ability to cause other remodeling effects, such as altosome formation.
On the other hand, despite considerable similarities in overall remodeled nucleosome distributions, clear differences can also be seen between remodelers. For instance, CHRAC and hACF increase nucleosome density over the 601 probe region, while WICH decreases it. In addition, we observe significantly greater summed absolute differences amongst ACF, CHRAC and WICH (the CHRAC:hACF, CHRAC:WICH or hACF:WICH comparisons) than for comparisons of each of these three complexes with SNF2h (Figure 7). These and other differences in mononucleosome repositioning specificity could potentially contribute to complex-specific remodeling effects that may be important for the transcriptional regulation of select promoter sequences.
In our prior studies of hSWI/SNF remodeling, the ability of hSWI/SNF to convert mononucleosomes to altered and normal dinucleosomes, resulted in a decrease in mononucleosome levels, that was the most striking effect at almost all positions. Normalizing percentage mononucleosome occupancy values, however, allowed us to eliminate these other remodeling effects, and focus on similarities or differences in mononucleosome distribution patterns. This revealed that (with the exception of the strong 5S and 601 NPS positions) the distribution of mononucleosomes after hSWI/SNF remodeling, and after remodeling by any of the SNF2h complexes, was very similar to mononucleosome distribution on each unremodeled template. Furthermore, in both this and previous studies, we found that remodeler-preferred positions were almost never entirely new, but were at least weakly occupied after chromatin assembly. This indicates that, while remodelers avoid putting nucleosomes on certain high affinity sites, they do not generally ignore the underlying energetics of DNA:histone wrapping, and thus have site preferences for repositioning that reflect the site preferences for nucleosome assembly (ostensibly low energy binding sites for the histone octamer). This is consistent with a study done on long linear templates where mononucleosome positions resulting from remodeling by yeast CHD, Isw2 or Isw1a complexes bore similarities to those preferred by thermal motion [after heat treatment of the template (25)]. Given these observations, remodeler recruitment to a promoter or any other region of chromatin would not be expected to completely re-arrange nucleosome positions that had been established over low energy binding sites during DNA replication or by thermal motion. Instead, at all but a few ‘disruptable’ sites, remodelers might function by increasing the fluidity of chromatin (shuffling nucleosomes between favorable positions) without greatly changing the overall distribution of these nucleosomes. This idea is supported by one recent in vitro study showing that hACF could increase the accessibility of a nucleosome-occluded site despite the absence of any apparent aggregate effect on that nucleosome’s position (48).
What is special about the 601 and 5S NPSes that causes remodeling complexes to reposition nucleosomes away from them so effectively? One possibility is that they are favored remodeling targets that either efficiently recruit the remodeler or facilitate rapid repositioning. This would increase the rate of nucleosome movement away from them, resulting in low occupancy at a remodeler-promoted dynamic equilibrium. This is argued against by a recent study showing that the rate of ACF or yeast RSC remodeling was similar on linear templates with a broad range of affinities for the histone octamer [including 601 and 5S sequences (26)]. Another possibility is that they are sites at which the remodeler has a very low probability of disengaging from and depositing a nucleosome that it is moving. One final possibility builds upon models for repositioning mechanism which argue that remodelers engage a nucleosome at two points—near the DNA entry exit point as well as ∼50 bp further into the nucleosome [for a recent review see (44)]. ATP hydrolysis then promotes movement of one contact site relative to the other, forming a loop of DNA whose propagation around the histone octamer results in repositioning. In this model, if a sequence blocked remodeler binding near the entry/exit site, it would prevent the remodeler from translocating the nucleosome towards or over that sequence (but would not prevent movement of a nucleosome already covering that sequence). This is consistent, perhaps, with the observation that SNF2h, hACF or CHRAC remodeling of the 601 template decreases occupancy at the two most favored 601 NPS locations (centered at 190 and 205 bp in Figure 2C), and increases occupancy at two clusters of sites with center positions from 95 to 140 and from 285 to 310. These clusters are ∼50–80 bp away from the 601 NPS sites, about half the length of a nucleosome. Accordingly, the distribution we observe could result if sequences in the center of the 601 NPS (at the pseudodyad of the NPS-positioned nucleosome) prevent the remodeler’s entry/exit binding domain from engaging with DNA. After initial remodeling moves nucleosomes more than 73 bp from these sequences, any attempt to move a nucleosome back over these sequences would be blocked, because SNF2h, hACF or CHRAC could not productively engage these sequences at the entry/exit site. Along these lines it is interesting to note that the sequences at the pseudodyads of the strongest 5S and 601 nucleosome positions share an interesting sequence pattern: they are both extremely GC rich for 11 bp to either side of the pseudodyad (68 and 77%), and these GC rich sequences are flanked, within 10 bp, by AT-rich stretches containing a 3-to-4 bp A or T homopolymer (the only A or T homopolymers longer than 2 bp in the entire NPS).
On the MMTV NucB region we found that remodeling by all tested complexes caused little change in the pattern of nucleosome positions (as indicated by normalized percentage occupancy, Figures 6B and 7C). This indicated that not all valid NPSes (capable of directing a peak of nucleosome occupancy in vivo and in vitro) are disfavored by remodeling. What distinguishes ‘nondisruptable’ NPSes from ‘disruptable’ NPSes is unclear, although it is interesting to note that the MMTV NPS lacks the shared pseudodyad sequence properties of the 601 and 5S NPSes, and is also more distributive (giving a peak of coverage through the combination of many low-occupancy positions, rather than from a few high-occupancy positions). Interestingly, instead of altering the nucleosome distribution profile, remodeling increased the number of nucleosomes in the NucB probe region, indicating that histone octamers from other parts of the plasmid are moved into the NucB region. This might result, perhaps, if surrounding MMTV LTR sequences contain disruptable NPSes, causing remodelers to displace histone octamers that are then distributed over the equally assembly- and remodeler-preferred NucB positions.
The similar repositioning preferences we see between remodelers on polynucleosomes contrasts with the differences often seen on long linear mononucleosome templates (20–26,32) (Supplementary Figure 1A). One reason for this is that remodeling complexes respond differently to DNA ends. For instance, addition of hACF1 to SNF2h, to form the hACF complex, has been shown to increase the length of flanking DNA needed for repositioning (24,32,48). Thus, hACF and CHRAC (which both contain hACF1) move nucleosomes further from DNA ends than SNF2h, an effect that can be seen, in Supplementary Figure 1A, by the presence of strong medium-mobility positions (neither center or end positioned) after SNF2h remodeling, and only strong low-mobility positions (center positioned) after hACF or CHRAC remodeling. It is also possible that intrinsic sequence preferences may be dampened on polynucleosomes, where a remodeler trying to move one nucleosome is constrained by the presence of adjacent nucleosomes. This idea is supported by our recent studies comparing hSWI/SNF remodeling on 5S rDNA and c-myc promoter sequences assembled into 359 bp mononucleosomal minicircles versus assembled into plasmid chromatin (27,28). On the minicircles we found dramatic lessening of occupancy over all sites strongly favored by assembly, with a dramatic increase at other sites. On polynucleosomes, the decrease at assembly favored sites was somewhat less extreme, and sharp increases at other sites were only occasionally seen. This may mean that remodelers, in the absence of any other constraints (DNA ends or adjacent nucleosomes) may vary greatly in their sequence preferences for repositioning, but that much of this variability is functionally masked in the context of polynucleosomal cellular chromatin.
Our results indicate that the regulatory effects of chromatin remodeling complexes will depend greatly on the promoter sequences they are targeted to. On 5S rDNA, by reducing nucleosome occupancy over the major 5S NPS sequences (effectively ‘disrupting’ the positioned nucleosome), each tested complex greatly increases accessibility of the 5S transcriptional start site at position 141. In this way, the intrinsic sequence properties of the 5S NPS may allow recruitment of any SWI/SNF- or ISWI-class remodeling complex to effectively activate 5S gene transcription. This could be directly relevant to 5S rDNA gene regulation, given the importance of human WICH in 5S transcription (19). More generally, this might constitute a common regulatory mechanism: in which gene promoters evolve to include ‘disruptable’ NPSes, allowing remodeling complex recruitment to directly activate transcription. By contrast, remodeling of the MMTV Nucleosome B NPS resulted in an increase in mononucleosome coverage with little change in distribution. How does this relate to the importance of human SWI/SNF in transcriptional activation of MMTV by GR, as well as for increased restriction enzyme accessibility to sites within NucB (29)? The answer may lie in the fact that the MMTV NucB sequences include several transcription factor binding sites, including GR and NF1 sites. Accordingly, even though hSWI/SNF does not (by itself) result in quantitative movement of histones away from MMTV NucB, it may impart greater mobility to the NucB histone octamer, allowing a window of opportunity for GR and NF1 to bind to their sites. The presence of these bound factors might then create a boundary that prevents hSWI/SNF from moving a nucleosome back, resulting in the establishment of a nucleosome-free region. GR, NF1 an hSWI/SNF might also, together, destabilize nucleosome B, allowing the histones to be removed in trans by chaperones. Interestingly, one recent study found that ySWI/SNF moved histone H2A/H2B dimers from MMTV NucB to acceptor DNA, but did not have this effect on flanking nucleosomes or on a mouse rDNA NPS sequence (49). This suggests that, as we see for nucleosome movement, nucleosome disassembly by the combined action of remodeling complexes and acceptor DNA/proteins may also be regulated by DNA sequence.
How nucleosome positions are functionally controlled in vivo remains a mystery, although recent studies make it clear that the contribution of DNA sequence to default positions is just one of many factors (47). Our results suggest that remodeling complexes can promote regulatory changes in nucleosome positioning in two possible ways. Sometimes they may work solo, with functional effects established by remodeler specificity and DNA sequence alone (e.g. the disruption of the 5S NPS nucleosome). At other promoters, such as MMTV, however, repositioning specificity alone will not be sufficient to generate a regulatory effect, and the remodeler will have to either work through another effect (such as histone removal) or work in conjunction with other factors to establish an active chromatin state.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
American Cancer Society (RSG-04-188 to G.R.S.). Funding for open access charge: MCRC institutional funds.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Dr Robert Kingston for p601 constructs and for SNF2h proteins and complexes generated by Dr He when she was in his laboratory, and Dr Trevor Archer for the pMMTV-LTR-Luc plasmid.
REFERENCES
- 1.Jiang C, Pugh BF. Nucleosome positioning and gene regulation: advances through genomics. Nat. Rev. Genet. 2009;10:161–172. doi: 10.1038/nrg2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnitzler GR. Control of nucleosome positions by DNA sequence and remodeling machines. Cell Biochem. Biophys. 2008;51:67–80. doi: 10.1007/s12013-008-9015-6. [DOI] [PubMed] [Google Scholar]
- 3.Ramachandran A, Schnitzler G. Regulating transcription one nucleosome at a time: nature and function of chromatin remodeling complex products. Recent Res. Dev. Mol. Cell Biol. 2004;5:149–170. [Google Scholar]
- 4.Saha A, Wittmeyer J, Cairns BR. Chromatin remodelling: the industrial revolution of DNA around histones. Nat. Rev. Mol. Cell Biol. 2006;7:437–447. doi: 10.1038/nrm1945. [DOI] [PubMed] [Google Scholar]
- 5.de la Serna IL, Ohkawa Y, Imbalzano AN. Chromatin remodelling in mammalian differentiation: lessons from ATP-dependent remodellers. Nat. Rev. Genet. 2006;7:461–473. doi: 10.1038/nrg1882. [DOI] [PubMed] [Google Scholar]
- 6.Dirscherl SS, Krebs JE. Functional diversity of ISWI complexes. Biochem. Cell Biol. 2004;82:482–489. doi: 10.1139/o04-044. [DOI] [PubMed] [Google Scholar]
- 7.Stopka T, Skoultchi AI. The ISWI ATPase Snf2h is required for early mouse development. Proc. Natl Acad. Sci. USA. 2003;100:14097–14102. doi: 10.1073/pnas.2336105100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burgio G, La Rocca G, Sala A, Arancio W, Di Gesu D, Collesano M, Sperling AS, Armstrong JA, van Heeringen SJ, Logie C, et al. Genetic identification of a network of factors that functionally interact with the nucleosome remodeling ATPase ISWI. PLoS Genet. 2008;4:e1000089. doi: 10.1371/journal.pgen.1000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corona DF, Siriaco G, Armstrong JA, Snarskaya N, McClymont SA, Scott MP, Tamkun JW. ISWI regulates higher-order chromatin structure and histone H1 assembly in vivo. PLoS Biol. 2007;5:e232. doi: 10.1371/journal.pbio.0050232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Langst G, Grummt I. NoRC-dependent nucleosome positioning silences rRNA genes. EMBO J. 2006;25:5735–5741. doi: 10.1038/sj.emboj.7601454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou J, Chau CM, Deng Z, Shiekhattar R, Spindler MP, Schepers A, Lieberman PM. Cell cycle regulation of chromatin at an origin of DNA replication. EMBO J. 2005;24:1406–1417. doi: 10.1038/sj.emboj.7600609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fyodorov DV, Blower MD, Karpen GH, Kadonaga JT. Acf1 confers unique activities to ACF/CHRAC and promotes the formation rather than disruption of chromatin in vivo. Genes Dev. 2004;18:170–183. doi: 10.1101/gad.1139604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu YI, Chang MV, Li HE, Barolo S, Chang JL, Blauwkamp TA, Cadigan KM. The chromatin remodelers ISWI and ACF1 directly repress Wingless transcriptional targets. Dev. Biol. 2008;323:41–52. doi: 10.1016/j.ydbio.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ewing AK, Attner M, Chakravarti D. Novel regulatory role for human Acf1 in transcriptional repression of vitamin D3 receptor-regulated genes. Mol. Endocrinol. 2007;21:1791–1806. doi: 10.1210/me.2007-0095. [DOI] [PubMed] [Google Scholar]
- 15.Poot RA, Bozhenok L, van den Berg DL, Steffensen S, Ferreira F, Grimaldi M, Gilbert N, Ferreira J, Varga-Weisz PD. The Williams syndrome transcription factor interacts with PCNA to target chromatin remodelling by ISWI to replication foci. Nat. Cell Biol. 2004;6:1236–1244. doi: 10.1038/ncb1196. [DOI] [PubMed] [Google Scholar]
- 16.Percipalle P, Fomproix N, Cavellan E, Voit R, Reimer G, Kruger T, Thyberg J, Scheer U, Grummt I, Ostlund Farrants AK. The chromatin remodelling complex WSTF-SNF2h interacts with nuclear myosin 1 and has a role in RNA polymerase I transcription. EMBO Rep. 2006;7:525–530. doi: 10.1038/sj.embor.7400657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimura K, Kitagawa H, Fujiki R, Tanabe M, Takezawa S, Takada I, Yamaoka I, Yonezawa M, Kondo T, Furutani Y, et al. Distinct function of 2 chromatin remodeling complexes that share a common subunit, Williams syndrome transcription factor (WSTF) Proc. Natl Acad. Sci. USA. 2009;106:9280–9285. doi: 10.1073/pnas.0901184106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Xiao A, Li H, Shechter D, Ahn SH, Fabrizio LA, Erdjument-Bromage H, Ishibe-Murakami S, Wang B, Tempst P, Hofmann K, et al. WSTF regulates the H2A.X DNA damage response via a novel tyrosine kinase activity. Nature. 2009;457:57–62. doi: 10.1038/nature07668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavellan E, Asp P, Percipalle P, Ostlund Farrants AK. The WSTF-SNF2h chromatin remodelling complex interacts with several nuclear proteins in transcription. J. Biol. Chem. 2006;281:16264–16271. doi: 10.1074/jbc.M600233200. [DOI] [PubMed] [Google Scholar]
- 20.Ramachandran A, Omar M, Cheslock P, Schnitzler GR. Linker histone H1 modulates nucleosome remodeling by human SWI/SNF. J. Biol. Chem. 2003;278:48590–48601. doi: 10.1074/jbc.M309033200. [DOI] [PubMed] [Google Scholar]
- 21.Gutierrez J, Paredes R, Cruzat F, Hill DA, van Wijnen AJ, Lian JB, Stein GS, Stein JL, Imbalzano AN, Montecino M. Chromatin remodeling by SWI/SNF results in nucleosome mobilization to preferential positions in the rat osteocalcin gene promoter. J. Biol. Chem. 2007;282:9445–9457. doi: 10.1074/jbc.M609847200. [DOI] [PubMed] [Google Scholar]
- 22.Rippe K, Schrader A, Riede P, Strohner R, Lehmann E, Langst G. DNA sequence- and conformation-directed positioning of nucleosomes by chromatin-remodeling complexes. Proc. Natl Acad. Sci. USA. 2007;104:15635–15640. doi: 10.1073/pnas.0702430104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flaus A, Owen-Hughes T. Dynamic properties of nucleosomes during thermal and ATP-driven mobilization. Mol. Cell Biol. 2003;23:7767–7779. doi: 10.1128/MCB.23.21.7767-7779.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He X, Fan H, Garlick JD, Kingston RE. Diverse regulation of SNF2h chromatin remodeling by noncatalytic subunits. Biochemistry. 2008;47:7025–7033. doi: 10.1021/bi702304p. [DOI] [PubMed] [Google Scholar]
- 25.Stockdale C, Flaus A, Ferreira H, Owen-Hughes T. Analysis of nucleosome repositioning by yeast ISWI and CHD1 chromatin remodeling complexes. J. Biol. Chem. 2006;281:16279–16288. doi: 10.1074/jbc.M600682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Partensky PD, Narlikar GJ. Chromatin remodelers act globally, sequence positions nucleosomes locally. J. Mol. Biol. 2009;391:12–25. doi: 10.1016/j.jmb.2009.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sims HI, Lane JM, Ulyanova NP, Schnitzler GR. Human SWI/SNF drives sequence-directed repositioning of nucleosomes on C-myc promoter DNA minicircles. Biochemistry. 2007;46:11377–11388. doi: 10.1021/bi7008823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sims HI, Baughman CB, Schnitzler GR. Human SWI/SNF directs sequence-specific chromatin changes on promoter polynucleosomes. Nucleic Acids Res. 2008;36:6118–6131. doi: 10.1093/nar/gkn623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Kinyamu HK, Archer TK. Changes in attitude, changes in latitude: nuclear receptors remodeling chromatin to regulate transcription. Mol. Endocrinol. 2006;20:1–13. doi: 10.1210/me.2005-0192. [DOI] [PubMed] [Google Scholar]
- 30.Lowary PT, Widom J. New DNA sequence rules for high affinity binding to histone octamer and sequence-directed nucleosome positioning. J. Mol. Biol. 1998;276:19–42. doi: 10.1006/jmbi.1997.1494. [DOI] [PubMed] [Google Scholar]
- 31.Hayes JJ, Wolffe AP. Histones H2A/H2B inhibit the interaction of transcription factor IIIA with the Xenopus borealis somatic 5S RNA gene in a nucleosome. Proc. Natl Acad. Sci. USA. 1992;89:1229–1233. doi: 10.1073/pnas.89.4.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He X, Fan HY, Narlikar GJ, Kingston RE. Human ACF1 alters the remodeling strategy of SNF2h. J. Biol. Chem. 2006;281:28636–28647. doi: 10.1074/jbc.M603008200. [DOI] [PubMed] [Google Scholar]
- 33.Lefebvre P, Berard DS, Cordingley MG, Hager GL. Two regions of the mouse mammary tumor virus long terminal repeat regulate the activity of its promoter in mammary cell lines. Mol. Cell Biol. 1991;11:2529–2537. doi: 10.1128/mcb.11.5.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fryer CJ, Kinyamu HK, Rogatsky I, Garabedian MJ, Archer TK. Selective activation of the glucocorticoid receptor by steroid antagonists in human breast cancer and osteosarcoma cells. J. Biol. Chem. 2000;275:17771–17777. doi: 10.1074/jbc.M908729199. [DOI] [PubMed] [Google Scholar]
- 35.Owen-Hughes T, Workman JL. Experimental Analysis of Chromatin Function in Transcription Control. Crit. Rev. Eukaryot. Gene Expr. 1994;4:403–441. [PubMed] [Google Scholar]
- 36.Lomvardas S, Thanos D. Modifying gene expression programs by altering core promoter chromatin architecture. Cell. 2002;110:261–271. doi: 10.1016/s0092-8674(02)00822-x. [DOI] [PubMed] [Google Scholar]
- 37.Fletcher TM, Ryu BW, Baumann CT, Warren BS, Fragoso G, John S, Hager GL. Structure and dynamic properties of a glucocorticoid receptor-induced chromatin transition. Mol. Cell Biol. 2000;20:6466–6475. doi: 10.1128/mcb.20.17.6466-6475.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fragoso G, John S, Roberts MS, Hager GL. Nucleosome positioning on the MMTV LTR results from the frequency-biased occupancy of multiple frames. Genes Dev. 1995;9:1933–1947. doi: 10.1101/gad.9.15.1933. [DOI] [PubMed] [Google Scholar]
- 39.Schnitzler G, Sif S, Kingston RE. Human SWI/SNF interconverts a nucleosome between its base state and a stable remodeled state. Cell. 1998;94:17–27. doi: 10.1016/s0092-8674(00)81217-9. [DOI] [PubMed] [Google Scholar]
- 40.Ulyanova NP, Schnitzler GR. Human SWI/SNF generates abundant, structurally altered dinucleosomes on polynucleosomal templates. Mol. Cell Biol. 2005;25:11156–11170. doi: 10.1128/MCB.25.24.11156-11170.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phelan ML, Sif S, Narlikar GJ, Kingston RE. Reconstitution of a core chromatin remodeling complex from SWI/SNF subunits. Mol. Cell. 1999;3:247–253. doi: 10.1016/s1097-2765(00)80315-9. [DOI] [PubMed] [Google Scholar]
- 42.Narlikar GJ, Phelan ML, Kingston RE. Generation and interconversion of multiple distinct nucleosomal states as a mechanism for catalyzing chromatin fluidity. Mol. Cell. 2001;8:1219–1230. doi: 10.1016/s1097-2765(01)00412-9. [DOI] [PubMed] [Google Scholar]
- 43.Schnitzler GR, Cheung CL, Hafner JH, Saurin AJ, Kingston RE, Lieber CM. Direct imaging of human SWI/SNF-remodeled mono- and polynucleosomes by atomic force microscopy employing carbon nanotube tips. Mol. Cell Biol. 2001;21:8504–8511. doi: 10.1128/MCB.21.24.8504-8511.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cairns BR. Chromatin remodeling: insights and intrigue from single-molecule studies. Nat. Struct. Mol. Biol. 2007;14:989–996. doi: 10.1038/nsmb1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simpson RT, Thoma F, Brubaker JM. Chromatin reconstituted from tandemly repeated cloned DNA fragments and core histones: a model system for study of higher order structure. Cell. 1985;42:799–808. doi: 10.1016/0092-8674(85)90276-4. [DOI] [PubMed] [Google Scholar]
- 46.Ura K, Hayes JJ, Wolffe AP. A positive role for nucleosome mobility in the transcriptional activity of chromatin templates: restriction by linker histones. EMBO J. 1995;14:3752–3765. doi: 10.1002/j.1460-2075.1995.tb00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Moqtaderi Z, Rattner BP, Euskirchen G, Snyder M, Kadonaga JT, Liu XS, Struhl K. Intrinsic histone-DNA interactions are not the major determinant of nucleosome positions in vivo. Nat. Struct. Mol. Biol. 2009;16:847–852. doi: 10.1038/nsmb.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang JG, Madrid TS, Sevastopoulos E, Narlikar GJ. The chromatin-remodeling enzyme ACF is an ATP-dependent DNA length sensor that regulates nucleosome spacing. Nat. Struct. Mol. Biol. 2006;13:1078–1083. doi: 10.1038/nsmb1170. [DOI] [PubMed] [Google Scholar]
- 49.Vicent GP, Nacht AS, Smith CL, Peterson CL, Dimitrov S, Beato M. DNA Instructed Displacement of Histones H2A and H2B at an Inducible Promoter. Mol. Cell. 2004;16:439–452. doi: 10.1016/j.molcel.2004.10.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.