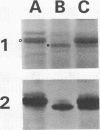
Abstract
The major outer membrane protein (OmpP2) of nontypeable Haemophilus influenzae (NTHI) has been shown to vary markedly with respect to both size and the presence of specific surface-exposed epitopes among strains of this unencapsulated pathogen. In contrast, the OmpP2 proteins of H. influenzae type b (Hib) strains are well conserved at the level of primary protein structure and have in common several surface-exposed antigenic determinants that have not been detected in NTHI strains. The availability of an isogenic, avirulent Hib ompP2 mutant made it possible to investigate whether an NTHI OmpP2 protein could function properly in the Hib outer membrane. A plasmid shuttle vector (pGJB103) was used to clone the ompP2 gene from NTHI TN106 into a recombination-deficient H. influenzae strain in which expression of the NTHI OmpP2 protein was detected by means of an NTHI TN106 OmpP2-specific monoclonal antibody. The amino acid sequence of this NTHI OmpP2 protein, as deduced from the nucleotide sequence of the NTHI TN106 ompP2 gene, was determined to be 83% identical to that of the Hib OmpP2 protein. Transformation of this cloned NTHI ompP2 gene into the Hib ompP2 mutant yielded a Hib transformant strain that expressed the NTHI OmpP2 protein. Expression of this NTHI OmpP2 protein allowed the Hib ompP2 mutant, which normally grows poorly in vitro, to grow in a manner indistinguishable from that of the wild-type Hib strain. More importantly, the introduction of this functional NTHI ompP2 gene into the avirulent Hib ompP2 mutant restored the virulence of this strain to wild-type levels. These results indicate that an NTHI OmpP2 protein can be expressed and function properly in the Hib outer membrane.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barenkamp S. J., Munson R. S., Jr, Granoff D. M. Outer membrane protein and biotype analysis of pathogenic nontypable Haemophilus influenzae. Infect Immun. 1982 May;36(2):535–540. doi: 10.1128/iai.36.2.535-540.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk S. L., Holtsclaw S. A., Wiener S. L., Smith J. K. Nontypeable Haemophilus influenzae in the elderly. Arch Intern Med. 1982 Mar;142(3):537–539. [PubMed] [Google Scholar]
- Bluestone C. D., Stephenson J. S., Martin L. M. Ten-year review of otitis media pathogens. Pediatr Infect Dis J. 1992 Aug;11(8 Suppl):S7–11. doi: 10.1097/00006454-199208001-00002. [DOI] [PubMed] [Google Scholar]
- Burns J. L., Mendelman P. M., Levy J., Stull T. L., Smith A. L. A permeability barrier as a mechanism of chloramphenicol resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1985 Jan;27(1):46–54. doi: 10.1128/aac.27.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. L., Smith A. L. A major outer-membrane protein functions as a porin in Haemophilus influenzae. J Gen Microbiol. 1987 May;133(5):1273–1277. doi: 10.1099/00221287-133-5-1273. [DOI] [PubMed] [Google Scholar]
- Cope L. D., Pelzel S. E., Latimer J. L., Hansen E. J. Characterization of a mutant of Haemophilus influenzae type b lacking the P2 major outer membrane protein. Infect Immun. 1990 Oct;58(10):3312–3318. doi: 10.1128/iai.58.10.3312-3318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope L. D., Yogev R., Mertsola J., Latimer J. L., Hanson M. S., McCracken G. H., Jr, Hansen E. J. Molecular cloning of a gene involved in lipooligosaccharide biosynthesis and virulence expression by Haemophilus influenzae type B. Mol Microbiol. 1991 May;5(5):1113–1124. doi: 10.1111/j.1365-2958.1991.tb01884.x. [DOI] [PubMed] [Google Scholar]
- Deininger P. L. Random subcloning of sonicated DNA: application to shotgun DNA sequence analysis. Anal Biochem. 1983 Feb 15;129(1):216–223. doi: 10.1016/0003-2697(83)90072-6. [DOI] [PubMed] [Google Scholar]
- Eskola J., Peltola H., Käyhty H., Takala A. K., Mäkelä P. H. Finnish efficacy trials with Haemophilus influenzae type b vaccines. J Infect Dis. 1992 Jun;165 (Suppl 1):S137–S138. doi: 10.1093/infdis/165-supplement_1-s137. [DOI] [PubMed] [Google Scholar]
- Forbes K. J., Bruce K. D., Ball A., Pennington T. H. Variation in length and sequence of porin (ompP2) alleles of non-capsulate Haemophilus influenzae. Mol Microbiol. 1992 Aug;6(15):2107–2112. doi: 10.1111/j.1365-2958.1992.tb01384.x. [DOI] [PubMed] [Google Scholar]
- Groeneveld K., van Alphen L., Voorter C., Eijk P. P., Jansen H. M., Zanen H. C. Antigenic drift of Haemophilus influenzae in patients with chronic obstructive pulmonary disease. Infect Immun. 1989 Oct;57(10):3038–3044. doi: 10.1128/iai.57.10.3038-3044.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., Patrick C. C., Hermanstorfer L., McCracken G. H., Jr, Hansen E. J. Conservation of epitopes in the oligosaccharide portion of the lipooligosaccharide of Haemophilus influenzae type b. Infect Immun. 1987 Mar;55(3):513–520. doi: 10.1128/iai.55.3.513-520.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase E. M., Campagnari A. A., Sarwar J., Shero M., Wirth M., Cumming C. U., Murphy T. F. Strain-specific and immunodominant surface epitopes of the P2 porin protein of nontypeable Haemophilus influenzae. Infect Immun. 1991 Apr;59(4):1278–1284. doi: 10.1128/iai.59.4.1278-1284.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel J., Dugourd D., Martin D., Proulx C., Chong P., Brodeur B. R. Localization of conserved B-cell epitopes among encapsulated and non-encapsulated Haemophilus influenzae P2 porin proteins using synthetic peptides. J Gen Microbiol. 1992 Jan;138(1):161–168. doi: 10.1099/00221287-138-1-161. [DOI] [PubMed] [Google Scholar]
- Hansen E. J., Gonzales F. R., Chamberlain N. R., Norgard M. V., Miller E. E., Cope L. D., Pelzel S. E., Gaddy B., Clausell A. Cloning of the gene encoding the major outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1988 Oct;56(10):2709–2716. doi: 10.1128/iai.56.10.2709-2716.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Hasemann C., Clausell A., Capra J. D., Orth K., Moomaw C. R., Slaughter C. A., Latimer J. L., Miller E. E. Primary structure of the porin protein of Haemophilus influenzae type b determined by nucleotide sequence analysis. Infect Immun. 1989 Apr;57(4):1100–1107. doi: 10.1128/iai.57.4.1100-1107.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Pelzel S. E., Orth K., Moomaw C. R., Radolf J. D., Slaughter C. A. Structural and antigenic conservation of the P2 porin protein among strains of Haemophilus influenzae type b. Infect Immun. 1989 Nov;57(11):3270–3275. doi: 10.1128/iai.57.11.3270-3275.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Juni E. Simple genetic transformation assay for rapid diagnosis of Moraxella osloensis. Appl Microbiol. 1974 Jan;27(1):16–24. doi: 10.1128/am.27.1.16-24.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Gulig P. A., McCracken G. H., Jr, Loftus T. A., Hansen E. J. A minor high-molecular-weight outer membrane protein of Haemophilus influenzae type b is a protective antigen. Infect Immun. 1985 Jan;47(1):253–259. doi: 10.1128/iai.47.1.253-259.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980 Dec;30(3):709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D., Hamel J., Brodeur B. R., Musser J. M. Antigenic relationships among the porin proteins of encapsulated Haemophilus influenzae clones. J Clin Microbiol. 1990 Aug;28(8):1720–1724. doi: 10.1128/jcm.28.8.1720-1724.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Moxon E. R., Glode M. P., Sutton A., Robbins J. B. The infant rat as a model of bacterial meningitis. J Infect Dis. 1977 Aug;136 (Suppl):S186–S190. doi: 10.1093/infdis/136.supplement.s186. [DOI] [PubMed] [Google Scholar]
- Moxon E. R., Wilson R. The role of Haemophilus influenzae in the pathogenesis of pneumonia. Rev Infect Dis. 1991 May-Jun;13 (Suppl 6):S518–S527. doi: 10.1093/clinids/13.supplement_6.s518. [DOI] [PubMed] [Google Scholar]
- Munson R. S., Jr, Shenep J. L., Barenkamp S. J., Granoff D. M. Purification and comparison of outer membrane protein P2 from Haemophilus influenzae type b isolates. J Clin Invest. 1983 Aug;72(2):677–684. doi: 10.1172/JCI111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson R., Jr, Bailey C., Grass S. Diversity of the outer membrane protein P2 gene from major clones of Haemophilus influenzae type b. Mol Microbiol. 1989 Dec;3(12):1797–1803. doi: 10.1111/j.1365-2958.1989.tb00165.x. [DOI] [PubMed] [Google Scholar]
- Murphy T. F., Bartos L. C. Human bactericidal antibody response to outer membrane protein P2 of nontypeable Haemophilus influenzae. Infect Immun. 1988 Oct;56(10):2673–2679. doi: 10.1128/iai.56.10.2673-2679.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musher D. M., Kubitschek K. R., Crennan J., Baughn R. E. Pneumonia and acute febrile tracheobronchitis due to haemophilus influenzae. Ann Intern Med. 1983 Oct;99(4):444–450. doi: 10.7326/0003-4819-99-4-444. [DOI] [PubMed] [Google Scholar]
- Musser J. M., Barenkamp S. J., Granoff D. M., Selander R. K. Genetic relationships of serologically nontypable and serotype b strains of Haemophilus influenzae. Infect Immun. 1986 Apr;52(1):183–191. doi: 10.1128/iai.52.1.183-191.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradise J. L. Otitis media in infants and children. Pediatrics. 1980 May;65(5):917–943. [PubMed] [Google Scholar]
- Patrick C. C., Kimura A., Jackson M. A., Hermanstorfer L., Hood A., McCracken G. H., Jr, Hansen E. J. Antigenic characterization of the oligosaccharide portion of the lipooligosaccharide of nontypable Haemophilus influenzae. Infect Immun. 1987 Dec;55(12):2902–2911. doi: 10.1128/iai.55.12.2902-2911.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras O., Caugant D. A., Gray B., Lagergård T., Levin B. R., Svanborg-Edén C. Difference in structure between type b and nontypable Haemophilus influenzae populations. Infect Immun. 1986 Jul;53(1):79–89. doi: 10.1128/iai.53.1.79-89.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikkema D. J., Murphy T. F. Molecular analysis of the P2 porin protein of nontypeable Haemophilus influenzae. Infect Immun. 1992 Dec;60(12):5204–5211. doi: 10.1128/iai.60.12.5204-5211.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. L., Smith D. H., Averill D. R., Jr, Marino J., Moxon E. R. Production of Haemophilus influenzae b meningitis in infant rats by intraperitoneal inoculation. Infect Immun. 1973 Aug;8(2):278–290. doi: 10.1128/iai.8.2.278-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikumar R., Chin A. C., Vachon V., Richardson C. D., Ratcliffe M. J., Saarinen L., Käyhty H., Mäkelä P. H., Coulton J. W. Monoclonal antibodies specific to porin of Haemophilus influenzae type b: localization of their cognate epitopes and tests of their biological activities. Mol Microbiol. 1992 Mar;6(5):665–676. doi: 10.1111/j.1365-2958.1992.tb01514.x. [DOI] [PubMed] [Google Scholar]
- Srikumar R., Dahan D., Gras M. F., Ratcliffe M. J., van Alphen L., Coulton J. W. Antigenic sites on porin of Haemophilus influenzae type b: mapping with synthetic peptides and evaluation of structure predictions. J Bacteriol. 1992 Jun;174(12):4007–4016. doi: 10.1128/jb.174.12.4007-4016.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H., Walter R. B. Effect of glycerol on plasmid transfer in genetically competent Haemophilus influenzae. Mol Gen Genet. 1986 May;203(2):296–299. doi: 10.1007/BF00333969. [DOI] [PubMed] [Google Scholar]
- Tomb J. F., Barcak G. J., Chandler M. S., Redfield R. J., Smith H. O. Transposon mutagenesis, characterization, and cloning of transformation genes of Haemophilus influenzae Rd. J Bacteriol. 1989 Jul;171(7):3796–3802. doi: 10.1128/jb.171.7.3796-3802.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon V., Laprade R., Coulton J. W. Properties of the porin of Haemophilus influenzae type b in planar lipid bilayer membranes. Biochim Biophys Acta. 1986 Sep 25;861(1):74–82. doi: 10.1016/0005-2736(86)90373-1. [DOI] [PubMed] [Google Scholar]
- Vachon V., Lyew D. J., Coulton J. W. Transmembrane permeability channels across the outer membrane of Haemophilus influenzae type b. J Bacteriol. 1985 Jun;162(3):918–924. doi: 10.1128/jb.162.3.918-924.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Musher D. M., Septimus E. J., McGowan J. E., Jr, Quinones F. J., Wiss K., Vance P. H., Trier P. A. Haemophilus influenzae infections in adults: characterization of strains by serotypes, biotypes, and beta-lactamase production. J Infect Dis. 1981 Aug;144(2):101–106. doi: 10.1093/infdis/144.2.101. [DOI] [PubMed] [Google Scholar]
- Zhang H., Scholl R., Browse J., Somerville C. Double stranded DNA sequencing as a choice for DNA sequencing. Nucleic Acids Res. 1988 Feb 11;16(3):1220–1220. doi: 10.1093/nar/16.3.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alphen L., Eijk P., Geelen-van den Broek L., Dankert J. Immunochemical characterization of variable epitopes of outer membrane protein P2 of nontypeable Haemophilus influenzae. Infect Immun. 1991 Jan;59(1):247–252. doi: 10.1128/iai.59.1.247-252.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]