Abstract
Bacillus subtilis and most Gram positive bacteria possess four SMC like proteins: SMC, SbcC, RecN and the product of the yhaN gene, termed SbcE. SbcE is most similar to SbcC but contains a unique central domain. We show that SbcE plays a role during transformation in competent cells and in DNA double-strand break (DSB) repair. The phenotypes were strongly exacerbated by the additional deletion of recN or of sbcC, suggesting that all three proteins act upstream of RecA and provide distinct avenues for presynapsis. SbcE accumulated at the cell poles in competent cells, and localized as a discrete focus on the nucleoids in 10% of growing cells. This number moderately increased after treatment with DNA damaging agents and in the absence of RecN or of SbcC. Damage-induced foci of SbcE arose early after induction of DNA damage and rarely colocalized with the replication machinery. Our work shows that SMC-like proteins in B. subtilis play roles at different subcellular sites during DNA repair. SbcC operates at breaks occurring at the replication machinery, whereas RecN and SbcE function mainly, but not exclusively, at DSBs arising elsewhere on the chromosome. In agreement with this idea, we found that RecN-YFP damage-induced assemblies also arise in the absence of ongoing replication.
INTRODUCTION
Proteins belonging to the structural maintenance of chromosomes (SMC) family perform key functions in various chromosome dynamics in almost all organisms. SMC proteins are central components of chromosome condensation and segregation complexes, and are thus essential for either mitosis or for DNA break repair (1). SMC proteins are characteristically composed of conserved N- and C-terminal domains, and two central long stretches of heptad repeat regions predicted to form coiled coils. The heptad repeat regions fold back upon themselves, forming a long coil that can be as long as 25 nm. N- and C-terminal regions form a single head domain having an ATP cassette fold and ATPase activity (2–6). The SMC protein family can be divided into two major groups: true SMC proteins (i.e. SMC 1 to SMC 6 in eukaryotes and bacterial SMC/MukB) and SMC-like proteins (i.e. eukaryotic Rad50, or bacterial SbcC and RecN) (1,7). SMCs contain a conserved hinge domain between the two coiled coil sequences, and SMC-like proteins various other domains/sequence motifs. The monomeric proteins consist of a globular head domain, a long-coiled coil and the hinge domains/motifs. The latter forms a specific dimer with another SMC protein (e.g. SMC 1 with SMC 3, or homodimers for bacterial SMC) (8,9). Instead of a hinge domain, Rad50 contains a CXXC motif that can form a Zn-bridge with another Rad50 monomer (3). Like Rad50, bacterial SbcC forms a complex with an exonuclease (SbcD, or Mre11 for Rad50) and forms a dimer (10,11). Bacterial RecN has much shorter coils than most other SMC like proteins and has a 50 aa domain between the heptad repeat sequences, which does not bear any similarity to the hinge domain. RecN forms higher multimers in solution (12,13), but their architecture is still unclear. Similarly, it is also uncertain if RecN has a specific complex partner. For all other SMC-like proteins, this has been shown to be the case the case. SMC 1/3 form the cohesin complex together with at least two additional proteins and SMC 2/4 are the central components of condensin (14). Bacterial SMC forms a complex with ScpA and ScpB (MukB in Escherichia coli with MukE and MukF) (15,16). ScpA belongs to the family of kleisins, like Scc1, which interacts with the SMC 1/3 cohesin complex from yeast. Scc1 further interacts with additional proteins such as Scc3. Similarly, a member of kleisins (Ycs4 in yeast) is also present in condensin (1). SMC head domains can dimerize through sandwiching of two ATP molecules (17). This way, SMC proteins can form a ring around DNA, in which DNA is trapped. In cohesin, Scc1 tightly bridges SMC head domains (18). Cohesin rings are opened during the metaphase to anaphase transition by specific cleavage of the Scc1 subunit (19), allowing commencement of pole-ward chromosome movement. Condensin is bound to chromosomes throughout the cell cycle but mediates chromosome compaction at the onset of prophase (1). Bacterial SMC is essential for chromosome segregation and compaction (20) and usually forms two subcellular assemblies, one in each cell half (15,21). Both, condensin and bacterial SMC complex appear to affect chromosome compaction through the generation of writhe in DNA, resulting in increased negative supercoiling (22,23). However, the precise mode of action of condensin and bacterial SMC is still unclear. The condensin complex has also been involved in DNA repair (24). Conversely, SMC 5/6, Rad50, SbcC and RecN are exclusively involved in repair of DNA damage and of double-strand breaks (DSBs) (10,25–28). The Rad50/Mre11 complex is involved in homologous recombination, probably by generating ssDNA overhangs at the break site, and apparently also in non-homologous end joining (NHEJ), where broken DNA ends are tethered together by a dedicated ligase system (3).
In response to DSBs or to the generation of inter strand cross links in Bacillus subtilis, DNA repair centres (RCs) are formed. RecN, an ssDNA binding protein, is the first protein to visibly accumulate at break sites, sequentially followed by RecO, RecA, RecF and RecU (13,29,30). RCs are formed during or after generation of ssDNA overhangs at DSBs, mediated through RecJ/RecQ or AddAB endonucleases/helicases, and appear to be organized by RecN (31). RecO aids in loading of RecA onto ssDNA, enabling the formation of RecA/ssDNA nucleofilaments, which appear to extend across the nucleoids in search of the homologous non-broken region on the sister chromosome. This leads to the formation of crossovers, the hallmark of homologous recombination. At a late stage during DSB repair, RecU is recruited to RCs, where it resolves crossovers in conjunction with the RuvAB complex (30), while RecF may down-regulate formation of RecA/ssDNA nucleofilaments. Through this pathway, a DSB is repaired according to the information on the non-broken sister chromosome. SbcC protein has been shown to accumulate at the centrally located replication machinery (RM) upon induction of DNA damage (25) and also when expressed from an ectopic location in non-stressed cells (32). On the contrary, RCs are also formed away from the RM (29), indicating that different pathways may exist for DSB repair occurring at the replication forks, or elsewhere on the chromosome.
We have analysed the function of SbcE, a fourth and novel member of the SMC protein family in B. subtilis. SbcE is most similar to SbcC, yet highly diverged from other SMC-like proteins, and has a novel type of central domain. We show that SbcE is involved in transformation of competent cells, where a subset of cells take up DNA from the environment and incorporate it into the chromosome, if sufficient homology exists between incoming DNA and a region on the chromosome. We also show that SbcE is involved in DSB repair, apparently in a pathway that parallels that of RecN, and predominantly at DSBs that occur away from the replication forks.
MATERIALS AND METHODS
Growth conditions and media
Escherichia coli XL1-Blue (Stratagene) and B. subtilis were grown in Luria Bertani (LB) rich medium supplemented with 50 μg/ml ampicillin for E. coli and appropriate antibiotic for B. subtilis. For microscopy, cells were grown in S750 medium.
Clonings and constructs
All strains used in this study are listed in Table 1. The C-terminal fusion of SbcE with YFP was created by PCR amplifying the C-terminal 500 bps of the yhaN/SbcE gene and cloning the fragment into ApaI and EcoRI sites of pSG1164 using primers 5′-GCAGGGCCCGCACAGCTTCAAGGCGG-3′ and 3′-TTAGAATTCACCCCCTGACACCAAATGGATGATTTGGC-5′. B. subtilis PY79 wild type cells were transformed with the resulting plasmid (selected for cm), which integrated in the original locus on the Bacillus chromosome, to accrue SbcE-Yfp protein being the only form of SbcE protein in the cells. Expression of YhaO/SbcF downstream was driven by addition of 0.1% xylose in growth medium. SbcE-YFP competent cells was transformed with ΔcomK chromosomal DNA to create MK23. SbcE-YFP chromosomal DNA was transformed into DnaX-CFP to obtain MK29. Similarly SbcF was cloned using primers 5′-GGCGGGCCCTCGTTCATGTGACAAACG-3′ and 5′-GGCGAATTCGAATCCCCCTGTATCAAGCACCTTAAGCTG-3′ into pSG1164 which integrates in the original locus resulting in SbcF-Cfp (MK34). MK02 and MK35 were constructed by transforming pcm::tet into ΔyhaN (cm) and MK34, respectively and selecting for tetracycline resistance. MK35 chromosomal DNA was transformed into ΔyhaN (cm) competent cells and selected on appropriate antibiotic places to generate MK36. Chromosomal DNA of ΔrecN was transformed appropriately to achieve different strains. It was transformed into HO cutsite, HO endonuclease I-SceI endonuclease and HO endonuclease HO cutsite to obtain MK13, MK15, MK20 and MK07, respectively. Similarly, chromosomal DNA of ΔrecN (tet) was prepared and transformed into ΔsbcE, MK12, I-SceI endonuclease I-SceI cutsite, ΔsbcE HO endonuclease, ΔsbcE I-SceI endonuclease and in I-SceI cut site and were selected for appropriate antibiotic resistance to obtain MK04, MK11, MK08, MK09, MK10 and MK18. MK03 was created by transforming pcm::Nm in ΔrecN (cm) strain and was used to transform MK17 competent cells, to achieve MK16. MK01chromosmal DNA was transformed into HO endonuclease HO cut site, HO cutsite, HO endonuclease and I-SceI endonuclease; and resultant strains MK05, MK12, MK14 and MK19 was selected according to appropriate antibiotic resistance. MK02 chromosmal DNA was transformed into I-SceI cut site to obtain MK17. Chromosomal DNA of HO cutsite and HO endonuclease was transformed into ΔrecA to create MK21 and MK22, respectively. MK26, MK25 and MK24 were created by transforming RecA-Gfp chromosomal DNA into ΔrecN, ΔsbcE and MK04 competent cells. MK30 and MK32 were generated by transforming chromosomal DNA from sbcE-yfp into dnaBts competent cells or into dnaAts competent cells, respectively, and selecting for the antibiotic resistance. Further on, MK30 and MK32 competent cells were transformed with ΔrecN chromosomal DNA to obtain MK31 and MK33 strains.
Table 1.
Strains used in the present study
| ST41 | ΔsbcE, cm | Present work |
| MK02 | ΔsbcE, tet | Present work |
| BG277 | ΔrecN, cm | (45) |
| DK35 | ΔrecN, tet | (13) |
| MK03 | ΔrecN, kan | Present work |
| MK04 | ΔrecN ΔsbcE, cm, tet | Present work |
| AKR07 | HO endonuclease, HO cut site, spec kan | Present work |
| LAS210 | I-SceI endonuclease, I-SceI cut site, cm spec | (44) |
| MK05 | ΔsbcE, HO endonuclease, HO cut site, cm spec kan | Present work |
| MK06 | ΔsbcE, I-SceI endonuclease, I-SceI cut site, tet cm spec | Present work |
| MK07 | ΔrecN, HO endonuclease HO cut site cm spec kan | Present work |
| MK08 | ΔrecN, I-SceI endonuclease I-SceI cut site tet cm spec | Present work |
| MK09 | ΔsbcE, ΔrecN, HO endonuclease cm spec kan | Present work |
| MK10 | ΔsbcE, ΔrecN, I-SceI endonuclease tet cm spec | Present work |
| MK11 | ΔsbcE, ΔrecN, HO cutsite cm tet kan | Present work |
| MK12 | ΔsbcE, HO cut site cm kan | Present work |
| MK13 | ΔrecN, HO cut site cm kan | Present work |
| MK14 | ΔsbcE, HO endonuclease cm spec | Present work |
| MK15 | ΔrecN, HO endonuclease cm spec | Present work |
| MK16 | ΔsbcE, ΔrecN, I-SceI cut site tet kan cm | Present work |
| MK17 | ΔsbcE, I-SceI cut site cm tet | Present work |
| MK18 | ΔrecN, I-SceI cut site cm tet | Present work |
| MK19 | ΔsbcE, I-SceI endonuclease cm spec | Present work |
| MK20 | ΔrecN, I-SceI endonuclease cm spec | Present work |
| MK21 | ΔrecA, HO cut site cm tet kan | Present work |
| MK22 | ΔrecA, HO endonuclease cm tet kan | Present work |
| MK23 | sbcE-yfp, ΔcomK, cm kan | Present work |
| MK24 | recA-gfp, ΔsbcE, ΔrecN, cm tet spec | Present work |
| MK25 | recA-gfp, ΔsbcE, cm spec | Present work |
| MK26 | recA-gfp, ΔrecN, cm spec | Present work |
| ST39 | sbcE-yfp, cm | Present work |
| MK28 | sbcE-yfp, tet | Present work |
| DK40 | recA-yfp, spec | (37) |
| MK29 | sbcE-yfp, DnaX-Cfp cm spec | Present work |
| MK30 | sbcE-yfp, DnaBts cm | Present work |
| MK31 | sbcE-yfp, ΔrecN, DnaBts cm tet | Present work |
| MK32 | sbcE-yfp, DnaAts cm | Present work |
| MK33 | sbcE-yfp, ΔrecN, DnaAts cm | Present work |
| MK34 | sbcF-cfp, cm | Present work |
| MK35 | sbcF-cfp, tet | Present work |
| MK36 | sbcF-cfp, sbcE-yfp, cm tet | Present work |
| MK37 | recN-cfp, sbcE-yfp, cm tet | Present work |
| PG747 | ΔrecA | (45) |
DNA double-strand break repair assays
DNA double-stranded break (DSB) assays were performed similarly for both HO endonuclease/HO cut site (close to the origin region) and I-SceI endonuclease/I-SceI cut site (close to the terminus region) strains. Endonuclease expression is controlled by xylose addition, and was induced with 0.5% xylose during the exponential growth phase (OD600 0.6–0.8 nm). The induced cells were further incubated for 30 min at 37°C. Then the cultures were serially diluted in LB medium. One-hundred microlitres of 10−5 and 10−6 dilutions were plated on LB plates and incubated at 37°C overnight. Induced and non-induced cultures of wt versus mutants CFU units counts on the incubated LB plates was further plotted on a graph and compared.
Western blot
Competent cells were made by inoculating overnight cultures into SpC media and were grown at 37°C till they reached a stable and unchanging OD measured at 600 nm. This indicates that the cells are at stationary phase. Then the cultures were transferred to SpII media and grown for 90 min at 37°C. When the cells were grown to competence, samples were taken at different time points. These cells were precipitated and resuspended in lysis buffer containing 1 mg/ml lyzozyme and was incubated on ice for 30 min. The lysate was resuspended in loading buffer, boiled at 95°C for 5 min and then run on 8% PAGE. The proteins were transferred to the nitrocellulose membrane, which was further probed with Rabbit Anti-GFP primary antibody and Anti-Rabbit secondary antibody coupled to HRP. Signals emitted after ECL reactions were captured and detected on X-ray films.
Image acquisition
Fluorescence microscopy was performed on an Olympus AX70 microscope. The respective competent cells were mounted on agarose gel coated slides. Images were acquired with a digital charge-coupled device camera (Princeton Instruments MicroMax or CoolSnap ES) driven by Metamorph 5.0 program (Universal Imaging Corp., USA). DNA was stained with 4′,6-diamidino-2-phenylindole (DAPI; final concentration, 0.2 ng/ml), and membranes were stained with FM-4-64 (final concentration, 1 nM). Filters used were: DAPI—ex360–370, dc400, em420–460, CFP: ex D436/20, dc 455DCLP, em D480/40, YFP: ex HQ500/20, dc Q515LP, em HQ535/30, GFP—ex460–495, dc505, em510–550, FM4-64 ex480-550, dc570, em590.
RESULTS
SbcE (YhaN) is a novel type of SMC protein
The product of the yhaN gene is predicted to be 106 kDa large and to contain two central long heptad repeat regions (Supplementary Figure S1A). N- and C-terminal regions bear low but significant similarity to head domains of SMC proteins; sequence similarity is highest (15–18% identity) to bacterial SbcC proteins and to RecN, while it is slightly lower to BsSMC, or SMC5/6 and Rad50 from yeast (13–15%). The Walker A and B motifs and the signature (‘C’) motif are deviant from that of canonical SMC (especially the Walker A box), but recognizable and somewhat more similar to SbcC than to SMC or to RecN (Supplementary Figure S1A). heptad repeat regions are predicted to be ∼300 aa long, their length being similar to that of coiled coils in SbcC, and much longer than those of RecN. Heptad repeat regions of SbcE are separated by a central domain, which lacks similarity to any other protein domain in the database. However, the central domain is conserved in all homologs of SbcE/YhaN, which are found in many Gram positive bacteria. Within the central domain, several residues are highly conserved (Supplementary Figure S1B), especially a proline (shown in bold), and the first half of the domain contains a high amount of hydrophobic amino acids. In fact, when overproduced in E. coli cells, the central domain is completely insoluble (data not shown). Thus, the yhaN product is clearly a member of the SMC protein family, and, due to its highest similarity to SbcC, we term it SbcE. However, SbcE lacks the CxxC motif as in SbcC and in Rad50 proteins, and due to its unique central domain and deviated sequence, it is a novel class of SMC like proteins. SbcE sequences are well conserved in the genomes of Gram positive bacteria. Thus, B. subtilis and many, if not all, Gram positive bacteria contain at least four different SMC-like proteins, SMC, SbcC, SbcE and RecN. The yhaN gene lies upstream of yhaM, encoding for a presumed exoribonuclease (33), and downstream of yhaO, which is highly similar to sbcD. SbcC forms a complex with SbcD (10), and according to yeast 2 hybrid data, SbcE also interacts with the yhaO gene product (34), which we term SbcF. We will term the yhaN gene ‘sbcE’ from hereon.
The deletion of sbcE (yhaN) results in sensitivity to DSBs, to various DNA damages and to a mild reduction in transformation efficiency
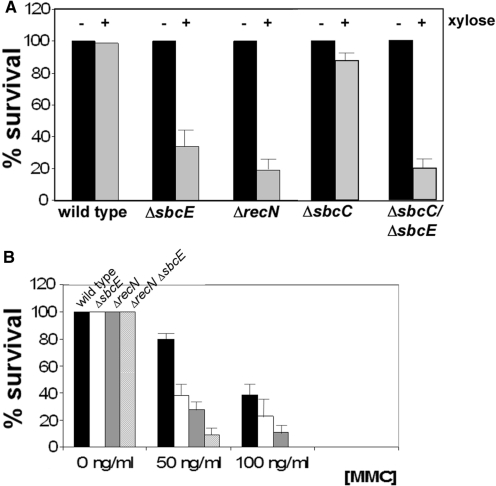
To shed light on the function of the fourth SMC protein in B. subtilis, we generated a strain carrying a truncated sbcE gene by integrating a plasmid containing the central part of the sbcE gene, resulting in SbcE protein that lacks its last one-third of the residues. Addition of xylose ensured transcription of the downstream yhaM gene. The sbcE deletion did not result in any noticeable growth defect or in a detectable difference in nucleoid morphology (data not shown). To investigate if SbcE is involved in DSB repair, we used two systems to generate DSBs during exponential growth, based on the induction of HO or I-SceI endonucleases. These were integrated in strains carrying the corresponding recognition sites (‘cut sites’) close to the origin region of replication or close to the terminus, respectively. Induction of HO endonuclease led to decrease in the number of viable cells, which was marginally larger for sbcE mutant cells than for wild type cells (data not shown). However, induction of I-SceI markedly reduced the number of surviving sbcE mutant cells: only a third of the cells survived the induction of a specific DSB, compared with >95% of wild type cells (Figure 1A). These data show that SbcE plays a role in the repair of DSBs. We also tested the sensitivity of SbcE mutant cells towards Mitomycin C (MMC) and found that only 40% of the cells survived a 30 min treatment with 50 ng/ml MMC, while 80% of wild type cells survived the same condition (Figure 1B). About 40% of wild type cells survived treatment with 100 ng/ml of MMC (200 ng/ml is toxic), whereas only 30% of sbcE mutant cells were able to withstand this treatment. We also found that sbcE mutant cells are more sensitive to the DNA-alkylating agent MMS than wild type cells (data not shown). Taken together, it is clear that the loss of SbcE activity results in a marked sensitivity to DSBs and to DNA-modifying agents.
Figure 1.
(A) Survival of cells without (−) and with (+) induction of I-SceI endonuclease for 30 min. (B) Survival of cells after 30 min of addition of different concentrations of MMC. Data are collected from at least three independent experiments run in duplicated.
We also investigated the effect of the loss of SbcE on transformation efficiency. For transformation with chromosomal DNA, we found that the number of transformants in the absence of SbcE drops ∼20-fold compared to wild type cells (Table 2), showing that SbcE also plays an important role during competence. Transformation with chromosomal DNA is strictly dependent on the RecA protein, while transformation with self-replicating plasmid DNA does not require RecA, but follows a different route involving RecO and RecU proteins (35). We found that plasmid transformation was not affected in the absence of sbcE (data not shown), showing that SbcE does not play a role in this process.
Table 2.
Transformation efficiency
| Strain | Transformation efficiencya |
|---|---|
| PY 79 | 100% (1.13 × 107) |
| ΔsbcE | 5% (5.63 × 105) |
| ΔsbcE ΔrecN | 1.2% (1.34 ×105) |
aThe number of transformants obtained per 0.1 µg chromosomal DNA. The number of transformants varied ∼5% between the repeated experiments.
Deletion of sbcE and recN or of sbcC and sbcE results in synthetic repair and transformation defects
Considering the similarity between SbcE and RecN sequences, we wondered if the proteins provide redundant functions, or if they act at different aspects in DNA repair and in competence. We therefore constructed a recN sbcE double mutant strain and tested its ability to survive treatment of MMC. Strikingly, the number of surviving recN sbcE double mutant cells was even more reduced compared to that of recN mutant cells, which are more severely affected in survival of MMC treatment than sbcE mutant cells (Figure 1B). Whereas 25% of recN mutant cells withstood treatment with 50 ng/ml of MMC, <10% of the double mutant cells survived this insult. Double mutant cells did not grow on plates containing 100 ng/ml of MMC and did not survive 30 min treatment with 100 ng/ml MMC (Figure 1B), which is also true for recA mutant cells, which show the strongest repair defect of any known recombination protein (36). However, MMC sensitivity of double mutant cells was less severe than that of recA mutant cells, because the latter do not survive treatment with 50 ng/ml of MMC (29), while a fraction of recN sbcE does survive this treatment.
We also used the HO or I-SceI cut systems to test for DSBs sensitivity of the double mutant. It was not possible to combine both recN and sbcE deletions in a strain with both endonuclease and cut site, in any of the two systems. It was possible to move either cut site or endonuclease constructs into recN sbcE double mutant cells, but not both. It was also impossible to combine a recA deletion with a cut site and an endonuclease gene. Therefore, a low amount of DSBs generated by even non-induced HO or I-SceI endonucleases is lethal for recA deleted cells or for recN sbcE double mutant cells, showing that the loss of both, SbcE and RecN, causes a strong synthetic defect in DSBR.
We also analysed the genetic interaction between SbcE and SbcC. An sbcC mutant strain was only very mildly sensitive to the induction of DSBs through I-SceI (Figure 1A), but the double sbcC and sbcE mutant stain was more sensitive than each of the two single mutant strains (Figure 1A). Similarly, double mutant cells were more sensitive to the addition of MMC than the single mutants (data not shown), revealing that SbcC and SbcE act at different aspects of DSBR. SbcC/sbcE double mutant cells were less sensitive to DSBs than recN/sbcE double mutant cells (Figure 1A) (and also to the addition of MMC, data not shown), revealing the different degrees of importance for resistance against DNA damaging agents of the tree SMC-like proteins.
We also tested the effect of the double deletion on transformation efficiency. RecN mutant cells show ∼5-fold less transformants than wild type cells (37) and are thus less impaired than sbcE mutant cells. Similar to the epistatic effect on DNA repair, the lack of SbcE and RecN strongly exacerbated the effect of the single gene deletions. The transformation efficiency of double mutant cells was ∼100-fold reduced compared with wild type cells (Table 2). Thus, absence of SbcE and RecN is as severe as absence of DrpA/Smf. DrpA/Smf plays a significant role in loading RecA onto the incoming ssDNA (38,39), while the deletion of recA results in a drop in transformation efficiency by 1000-fold or more, dependent on the B. subtilis strain background.
Deletion of sbcE and of recN strongly reduces the formation of RecA threads during transformation
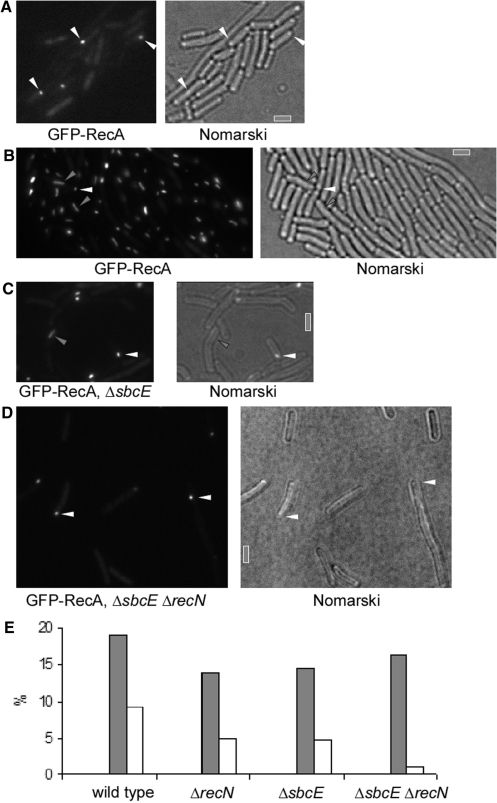
RecA has been shown to form filamentous structures termed ‘threads’ upon addition of DNA to competent cells. Threads emanate from the polar DNA uptake machinery and extend through the cells. They are thought to be key intermediate structures for strand invasion and crossovers during homologous recombination. We wished to investigate if SbcE and RecN have an effect on RecA localization and on RecA thread formation. We visualized GFP-RecA in wild type and mutant backgrounds. Wild type cells grown to competence contained polar GFP-RecA foci in 19% of the cells (Figure 2A and E), and mutant strains showed between 14 and 16.5% of polar foci (Figure 2E). The fact that the number of polar GFP-RecA foci [which depend on the presence of a polar DNA uptake machinery (37)] was similar in recN and in sbcE mutant cells compared with wild type cells suggests that there is no effect of the gene deletions on the DNA uptake complex. However, in the absence of SbcE or RecN, clear GFP-RecA threads were only seen in 5% of the cells after addition of chromosomal DNA (Figure 2C and E), compared to 9% in wild type cells (Figure 2B and E). Strikingly, in recN sbcE double mutant cells, formation of RecA threads was highly impaired; only 1% of the double mutant cells contained clear threads in response to DNA uptake (Figure 2D and E). These experiments strongly suggest that SbcE and RecN act upstream of RecA during transformation, and show that a reduction in the number of RecA threads leads to a reduction in transformation efficiency.
Figure 2.
Localization of GFP-RecA in wild type and mutant cells. (A) GFP-RecA foci at a single cell pole in cells grown to competence (indicted by white triangles), (B) GFP-RecA threads (indicated by grey triangles) and polar foci (white triangles) in wild type cells 30 min after addition of chromosomal DNA, (C) same as (B) in SbcE mutant cells, (D) same as in (B) in SbcE recN double mutant cells. Grey bars 2 µm. (E) Graph depicting number of GFP-RecA foci at the cell pole (grey bars) or of GFP-RecA threads 30 min after addition of chromosomal DNA to cells grown to competence (>600 cells analysed for each experiment). Names of strains are indicated.
SbcE localizes to the cell pole in competent cells and forms DNA damage-induced foci, which are exacerbated in the absence of RecN protein or of SbcC
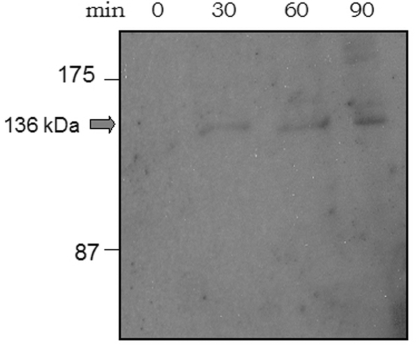
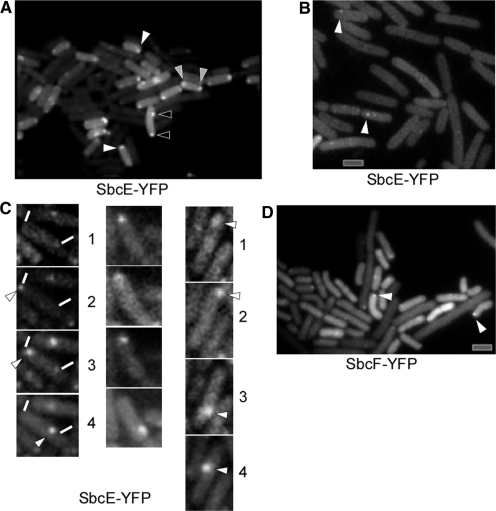
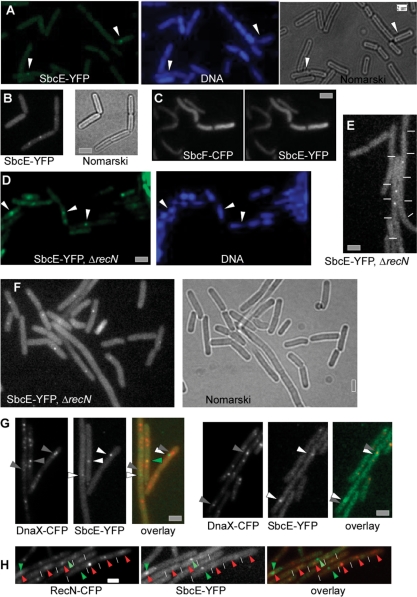
We created a C-terminal fusion of SbcE with YFP, which was integrated at the original locus in such a way that the SbcE-YFP fusion was driven by the original promoter. It is the only form of SbcE expressed in the cell. The fusion is fully functional, based on the observation that the cells have a transformation efficiency and sensitivity towards MMC similar to that of wild type cells (data not shown). Western blot analysis showed that only full length SbcE-YFP is expressed (Figure 3), and no cleavage product is formed (no YFP band is visible on Western blots, data not shown). Expression of SbcE-YFP during exponential growth is extremely low, compared with other YFP fusions generated in our laboratory (e.g. SMC-YFP or RecN-YFP, few hundreds of which are present in exponentially growing cells (20,21), suggesting that only few molecules are present during exponential growth. In agreement with an important function during competence, the level of SbcE-YFP increased considerably in cells grown to competence, whereas SbcE-YFP was barely visible in exponentially growing cells. A maximum of 20–25% of all cells grown to competence induce the master transcription factor ComK and thus become competent (40). These competent cells express a machinery that mediates the transport of DNA across the cell envelope, which assembles at one or both cell poles. Consistent with a role in transformation, SbcE-YFP accumulated at a single cell pole in 10% of the cells grown to competence (Figure 4A), and 3% contained two polar SbcE-YFP foci. Five percent contained a polar SbcE-YFP focus and a second focus at a random position within the cell, and in 5% of cells grown to competence SbcE-YFP foci were present at random positions within the cells (Figure 4A). Time lapse microscopy revealed that SbcE foci are not static like RecA foci, but are quite dynamic (Figure 4C). Within 1 min intervals, SbcE-YFP foci moved from one cell pole to the other, or to a position close to the other pole and moved back. The faint SbcE-YFP signal precluded longer time lapse captures, but the short movies we were able to monitor suggest that like RecN, SbcE also moves between the cell poles, with longer intervals at the poles and short movement across the cell. Foci could also split up into two foci and reunite (data not shown). The fact that a majority of competent cells showed polar SbcE-YFP foci supports the findings that SbcE-YFP assemblies stay at a pole for an extended period and rapidly move to the other pole, such that intermediate localization is only seen in few cells. Transcription of SbcE is not regulated by the major competence transcription factor, ComK, but by ComA, which in turn is necessary for ComK induction (41). SbcE-YFP showed foci only in 6% of comK mutant cells grown to competence and, importantly, never at a cell pole (Figure 4B), showing that its recruitment to the cell poles is mediated or influenced by a component of the DNA uptake machinery, in contrast to RecN, which also assembles at the poles in comK mutant cells grown to competence.
Figure 3.
Western blot showing accumulation of SbcE-YFP in cells grown to competence. 0 min = time point of cessation of growth in competence medium, other time points later than the onset of competence.
Figure 4.
Localization of SbcE-YFP in cells grown to competence. (A) SbcE-YFP foci at single cell pole (indicated by white arrowheads), at both cell poles (grey arrowheads), or at a single cell pole and an additional site between the poles (black arrowheads). (B) SbcE foci within comK mutant cells (indicated by white arrowheads). (C) Localization of SbcE-YFP in time lapse experiments, numbers indicate time in minutes. White lines indicate ends of cell, white arrowheads moving foci. (D) SbcF-YFP accumulates at the cell poles of cells grown to competence (indicated by white arrowheads). Grey bars 2 µm.
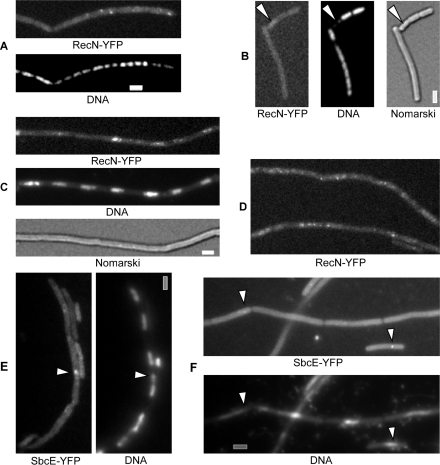
RecN is the first protein that visibly assembles at defined DSBs. RecN accumulates in so called RCs between 15 and 30 min in ∼70% of cells treated with MMC or induced for HO cuts, while only 0.05% of exponentially growing cells show RecN-YFP foci (13). During exponential growth, 9% of all cells showed a single SbcE-YFP focus, usually on the nucleoids (Figure 5A, 250 cells analysed), whereas 20 min after addition of MMC, 14% of the cells contained a single focus (Figure 5B). Sixty minutes after addition of MMC, 16% of all cells contained SbcE-YFP foci (15% contained a single focus and 1% two foci, data not shown, 320 cells analysed). These observations show that SbcE assemblies exist in a considerable number of exponentially growing cells, probably in cells affected by DNA damage or collapsed replication forks. Different from RecN, SbcE assemblies do not dramatically increase after induction of additional DNA damage. Because the sensitivity of cells lacking SbcE and RecN is much higher than that of single mutant cells, we investigated the localization of SbcE in recN mutant cells. Interestingly, 36% of recN mutant cells showed SbcE-YFP foci 30 min after MMC treatment (Figure 5D) (380 cells analysed) (and 37% 60 min after addition of MMC, data not shown), showing that the number of cells containing SbcE-YFP foci strongly increases in the absence of RecN protein.
Figure 5.
Localization of SbcE-YFP in exponentially growing cells or after addition of DNA damage. (A) SbcE-YFP foci (indicated by white arrowheads) in exponentially growing cells, or (B) 20 min after addition of MMC. (C) Exponentially growing cells expressing SbcE-YFP and SbcF-CFP. (D) SbcE-YFP (indicated by white arrowheads) in recN mutant cells 30 min after addition of MMC. (E) SbcE-YFP foci in sbcC mutant cells during exponential growth (ends of cells are indicated by white lines), or (F) 30 min after addition of MMC, (G) SbcE-YFP foci in DnaX-CFP expressing cells 30 min after addition of MMC, co-localization indicated by green arrowheads, non-overlapping localization indicated by grey (DnaX) and white (SbcE) arrowheads. (H) Cells expressing both RecN-CFP and SbcE-YFP. Ends of cells are indicated by white lines. SbcE-YFP foci are indicated with green arrowheads, and are green in the overlay, while RecN-CFP foci are indicated by red arrowheads. Grey and white bars 2 µm.
In the absence of RecA protein, RecN was found to form foci in 35% of all exponentially growing cells (13), suggesting that DSBs accumulate in RecA deficient cells. To find out if the number of growing cells containing SbcE-YFP foci also increases in recA mutant cells, we moved the YFP fusion into a recA null background. Fifteen percent of these mutant cells showed discrete SbcE-YFP foci (350 cells analysed, data not shown), which is a moderate but statistically significant increase over the number wild type cells having foci (9%). Thus, SbcE also accumulates in the absence of RecA, but to a lesser degree than RecN, in agreement with the idea that SbcE represents a rather constitutive system, while RecN a strongly inducible system.
We also wished to analyse if the absence of SbcC has an effect on the formation of SbcE-YFP foci. We therefore moved the SbcE-YFP fusion into a sbcC null background. Interestingly, 14% of exponentially growing mutant cells contained an SbcE-YFP focus on the nucleoid (with 380 cells analysed) (Figure 5E), which is a significant increase over the number in wild type cells (chi square test significance 0.01). Moreover, 30 min after addition of MMC, 26% of the cells showed SbcE-YFP foci in the absence of SbcC (Figure 5F), and 28% of the cells 60 min after addition of MMC (data not shown), which is also a significant increase over MMC-induced foci in wild type cells. Thus, in cells lacking SbcC, more cells contain SbcE-YFP foci during exponential growth as well as after induction of DNA damage.
SbcE foci frequently form at sites on the nucleoids away from the replication forks and rarely colocalize with RecN assemblies
We addressed the question whether upon induction of DNA damage, SbcE accumulates at the RM, as was shown for SbcC (25), or whether it also accumulates at sites on the nucleoids away from the replication forks, as was shown for RecN (29). We visualized SbcE-YFP in cells also expressing DnaX-CFP, a component of the RM. In exponentially growing cells upon addition of MMC, SbcE-YFP and DnaX-CFP colocalized in five cells, while both signals were separate from each other in 26 cells (Figure 5G). Therefore, SbcE appears to be recruited to presumably collapsed replication forks in few cases, but predominantly accumulates at DNA damage occurring elsewhere on the chromosomes. Interestingly, SbcE has been found to interact with DnaG in the yeast 2H system (34), suggesting that SbcE may be recruited to replication forks by DNA primase.
The effect that a double sbcE recN deletion increases the severity of sensitivity to DNA damage compared with both single gene deletions and the fact that both proteins form assemblies on the nucleoids (SbcE also in the absence of induced DNA damage, and RecN only after induction of DNA damage) prompted us to investigate the simultaneous localization of both proteins. We therefore generated a strain carrying an SbcE-YFP and a RecN-CFP fusion (the latter is only barely visible) and monitored fluorescence 30 min after addition of MMC. Interestingly, 70% of the cells contained RecN-CFP foci but no SbcE-YFP foci, while 15% contained SbcE-YFP foci but no RecN-CFP foci (Figure 5H). Only 4% of the cells contained both, RecN and SbcE foci, and in 75% of these, the signals were not coincident, while in 25%, they colocalized (Figure 5H). Eleven percent of the cells did not show any signal. These data show that generally, cells either have a RecN assembly after induction of DNA damage (that is most of the cells), or an SbcE assembly, while few cells have both assemblies. These data support the idea that RecN and SbcE induce two different pathways for an assembly of a DNA RC.
RCs are also induced in the absence of ongoing replication
To test if the induction of DNA RCs depends on ongoing replication, we moved the RecN-YFP fusion into strains containing thermosensitive (ts) alleles of the DnaA initiator protein, or the DnaB helicase loader protein. After a shift from 30 to 42°C, cells growth arrested within 1 h, indicating that replication was blocked at the higher temperature. Under this condition (that is after 1–2 h after shift up), RecN-YFP foci were detectable in ∼15% of the cells (Figure 6A), probably due to collapsed replication forks that have led to DSBs, and that are attempted to be rescued by homologous recombination. A block in replication, however, does not necessarily result in a DSB, because many cells showing clear block in replication did not show any RecN-YFP foci (Figure 4B). In contrast, when MMC was added 1 h after a shift to 42°C and incubated further for 1 h, RecN-YFP foci appeared in 80% of the cells (Figure 6C and D), showing that RCs can form independent of ongoing replication.
Figure 6.
Localization of RecN-YFP or SbcE-YFP in dnaBts cells. (A–B) RecN-YFP foci in cells shifted to 42°C (non-permissive temperature) for 2 h, white arrowheads in (B) indicate cell with obvious segregation defect lacking any RecN-YFP foci. (C–D) RecN-YFP foci in cells shifted to 42°C for 60 min, after addition of MMC for further 60 min. (E) SbcE-YFP foci (indicated by white arrowheads) in cells shifted to 42°C for 2 h. (F) SbcE-YFP foci (indicated by white arrowheads) in cells shifted to 42°C for 60 min, after addition of MMC for further 60 min. Grey bars 2 µm.
SbcE-YFP foci were present in 11% of DnaBts cells shifted to 42°C for 1 h (Figure 6E). After addition of MMC, 20% of the cells showed clear SbcE-YFP foci (Figure 6F), showing that to a moderate extent, SbcE-YFP foci are also inducible in the absence of ongoing replication. These data reinforce the idea that RecN initiates RCs at sites of DSBs occurring at any position on the nucleoid, which are primed for repair by homologous recombination and that SbcE present a rather constitutive system that operates in the absence of artificially induced DSBs. Because of the low RecN-CFP and SbcE-YFP signal intensity, and the low number of SbcE-YFP foci, we were not able to determine if SbcE is also recruited to some RCs initiated by RecN.
SbcF forms foci at a single cell pole in competent cells
Like sbcC, sbcE is located next to a gene encoding for a predicted nuclease, yhaO. The yhaO gene product bears 24.5% identity/29.1% similarity to the sbcD gene product, and 18% identity/25.6% similarity to mre11 (S. cerevaciae). Due to this degree of similarity, we will term YhaO as SbcF from hereon. We attempted to coprecipitate SbcE and SbcF in B. subtilis cells, but were unsuccessful, most likely due to the low abundance of SbcE. When expressed in E. coli cells, SbcE and SbcF were completely insoluble, even when special E. coli expression strains and conditions were employed. When SbcE and SbcF were simultaneously expressed from two compatible plasmids, both proteins remained in the nun-soluble fraction, and could not be successfully refolded. Therefore, we are unable to provide biochemical evidence for a complex of SbcE and SbcF, which however has been supported to exist in Y2H experiments (34).
Several additional lines of observations support the idea that SbcE and SbcF form a functional complex. The SbcF-CFP fusion showed fluorescence throughout the cells, because a large fraction (∼80%) of SbcF molecules were degraded, and CFP remained present in the cells, as seen by Western blotting (data not shown). This is intriguing, because SbcD-GFP is also degraded except for the GFP tag (25), the only two cases we have so far observed in our laboratory. Possibly, the nucleases SbcD and SbcF are prone to proteolysis to keep their amount low. Thus, soluble GFP remains within the cells, which precludes visualization of the low amount of full length SbcF-GFP. In any event, cells expressing SbcF-GFP grow normally, like cells expressing SbcE-YFP. In contrast to this, cells expressing both SbcE-YFP and SbcF-CFP no longer showed any SbcE-YFP foci, be it during growth or after addition of MMC (Figure 5C). Additionally, these cells grew more slowly than wild type cells, suggesting that interfering with complex formation causes a problem for the cells. These data suggest that dual tagging of SbcE with YFP and SbcF with CFP interferes with complex formation and leads to a loss of proper localization of SbcF.
Interestingly, SbcF also accumulated at a single cell pole in 14% of the cells grown to competence (Figure 4D), similarly to SbcE. Probably, SbcF-CFP accumulates to a higher extend at the cell pole than presumably at DSBs in association with SbcE or becomes more stable in competent cells, making it more clearly visible against the background of free CFP. These data support the idea that SbcE and SbcF form a complex, as deduced from the finding that SbcE and SbcF interact in the yeast two hybrid system (34).
DISCUSSION
In this work, we characterize a unique novel member of the SMC protein family, we term SbcE, from B. subtilis. SbcE has an ATPase head-domain/extended coiled coil/central domain arrangement typical of SMC proteins. SbcE is highly diverged from SbcC, and even more so from other SMC proteins, in its sequence, and contains a central domain that is not found in any other SMC-like protein. It is therefore a distinct member of SMC proteins, and is present in all genomes from Gram positive bacteria analysed. The name is appropriate because SbcE plays a role in DNA repair, like SbcC, and interacts with a nuclease, termed SbcF, analogous to SbcC, which interacts with the nuclease SbcD (10). We show that the loss of SbcE renders the cells sensitive to the induction of specific DSBs through an endonuclease, as well as towards MMC. Thus, B. subtilis contains four SMC like proteins, SMC, SbcC, RecN and SbcE, all of which are involved in DNA repair, either directly, or indirectly [the ScpA subunit of the SMC complex has been shown to be important for repair of MMC induced damage (42)]. It is quite striking to see how many proteins in the Bacillus cell (or in fact Gram positive bacteria) are involved in the repair of breaks and modifications in its chromosome. Clearly, the importance of DNA repair must justify such provision of genetic resources. However, it has become clear that proteins involved in DNA repair are also recruited to act during transformation with environmental DNA. Like RecN and RecA, SbcE gains novel functional specificity in cells grown to competence, as it is recruited to the polar DNA uptake machinery, where RecA, RecN, DprA/Smf and SsbB/YwpH are also present (the latter two being specific for competence) (37–39,43). Recruitment of SbcE to the cell pole was dependent on ComK, the master regulator of competence, which is required for the induction of the polar DNA uptake machinery (43). Interestingly, SbcE moved between the cell poles in an irregular manner, similar to RecN (37), indicating that this dynamic localization is common for the B. subtilis SMC like proteins, in contrast to RecA protein that remains at the pole containing the DNA uptake machinery (37). Clearly, SbcE plays an important role in transformation, because efficiency drops 20-fold in the absence of the protein. In agreement with its function during competence, the amount of SbcE, which is very low in exponentially growing cells, increases in cells grown to competence. Again, it is intriguing how many proteins are implicated in transformation, because loss of any of the above listed proteins reduces transformation efficiency between 5- and 1000-fold.
The analysis of competent cells has revealed that SbcE acts upstream of RecA, the central player in homologous recombination, and thus the most important player in transformation with chromosomal DNA. After addition of DNA to cells grown to competence, RecA forms filamentous structures called threads that are thought to be the active forms of RecA searching for homology on the chromosome (37). In the absence of SbcE or of RecN, the number of RecA threads is slightly reduced, but in the absence of both proteins, thread formation is strongly reduced concomitant with an ∼100-fold reduction in transformation efficiency. The visible effect on RecA activity strongly suggests that RecN and SbcE play a role in the loading of RecA to ssDNA, or in the protection of incoming ssDNA from nuclease attack. Indeed, RecN is an ssDNA binding protein (12,37), and SbcE could have a similar activity. Further work is required to shed light onto the detailed mode of action of SbcE in competent cells.
Our work provides further insight into the question of why 3 SMC-like systems are employed for DNA repair by B. subtilis. Firstly, RecN, SbcC and SbcE act at different sites of DSBs or DNA modifications. SbcC exclusively assembles at the RM in response to DNA damage (25), while RecN is the first protein detectable to assemble at breaks occurring away from the replication forks (13,29). Several further proteins are sequentially recruited to the so-called RCs, including RecA, which sets up crossovers between the break site and the homologous region on the sister chromosome. RCs disassemble after ∼3 h, at a time when cell growth resumes (29). SbcE takes a somewhat intermediate position between these two systems, because it assembles at the RM in some cells, while in most cells, SbcE accumulations are clearly located at different sites on the nucleoid, away from the replication forks. Yet different from SbcC and RecN, SbcE accumulations were present in a considerable number of exponentially growing cells (∼10%). It is not unreasonable to assume that DNA damage and/or collapsing of replication forks is generally taking place in this fraction of growing cells, and that SbcE is employed to deal with these normally occurring situations. In response to externally added DNA damage, the number of cells showing SbcE accumulations moderately increased (∼2-fold), while after induction of DSBs, 40 or 70% of the cells contain SbcC or RecN accumulations, respectively (13,25). Thus, SbcE appears to be a rather constitutive system that deals with a low number of DSBs or DNA damages occurring during growth, while SbcC and RecN systems are turned on in response to a dramatic increase in DNA damage. However, in addition, SbcE appears to act as a backup system for RecN and for SbcC, because up to 36% of recN mutant cells showed SbcE accumulations in response to induced DSBs, or 28% of sbcC mutant cells. In sbcC mutant cells, the number of SbcE-YFP foci was also increased during exponential growth (from 9 to 14%), indicating that SbcE can partially compensate for the loss of RecN or of SbcC. Dual visualization of SbcE and of RecN showed that cells generally induce (or contain) an SbcE accumulation or a RecN accumulation is response to DNA damage, but rarely contain assemblies of both proteins, supporting the idea that both factors can induce a distinct avenue to DNA repair via homologous recombination, and that cells use either one of these, and rarely both. Interestingly, we found that the induction of RecN assemblies in response to DNA damage also occurs in cells, in which ongoing replication was suppressed, showing that RCs also form in the absence of ongoing replication. Again for SbcE, we found that a moderate increase in the number of assemblies can occur in the absence of replication, suggesting that DNA damage occurring at any position on the chromosome can induce RecN and SbcE assembly, and at least in case of RecN, the establishment of RCs. In contrast to this, the accumulation of RecA at the RM in response to DNA damage is dependent on ongoing replication (44).
In agreement with the idea that SbcE is involved in a presynaptic stage, the moderate increase in SbcE accumulations was observed early after induction of DNA damage, i.e. within 20–30 min, and thus, in a similar time frame as RecN, and before RecO, RecA, RecU or RecF assemble at RCs (13). Our genetic data support the idea that two avenues exist towards loading of RecA, namely one involving RecN, and a further one involving SbcE, because loss of both RecN and SbcE resulted in an exacerbation of the single gene losses. Therefore, RecN and SbcE act at an early step in DSB repair, they are not epistatic, and SbcE somewhat complements for the loss of RecN during induction of DSBs. Similarly, the deletion of both, sbcC and sbcE also increases the severity of the single mutations [note that the loss of sbcC shows hardly any phenotype (25)]. Because SbcC plays a role at the replication forks, these data suggest that SbcE can also be employed during problems arising during replication. It will be important to determine, which further proteins RecN and SbcE interact with at a biochemical and at a genetic level. Clearly, SbcE is a novel member of the SMC protein family, and extends the spectrum of SMC proteins involved in DNA repair.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
This work was funded in part by the Deutsche Forschungsgemeinschaft.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Lyle Simons and Graham Walker (MIT) for the kind gift of the I-SceI system, and Luise Simon for technical assistance.
REFERENCES
- 1.Hirano T. At the heart of the chromosome: SMC proteins in action. Nat. Rev. Mol. Cell Biol. 2006;7:311–322. doi: 10.1038/nrm1909. [DOI] [PubMed] [Google Scholar]
- 2.Koshland D, Strunnikov A. Mitotic chromosome condensation. Annu. Rev. Cell Dev. Biol. 1996;12:305–333. doi: 10.1146/annurev.cellbio.12.1.305. [DOI] [PubMed] [Google Scholar]
- 3.Hopfner KP, Putnam CD, Tainer JA. DNA double-strand break repair from head to tail. Curr. Opin. Struct. Biol. 2002;12:115–122. doi: 10.1016/s0959-440x(02)00297-x. [DOI] [PubMed] [Google Scholar]
- 4.Lowe J, Cordell SC, van den Ent F. Crystal structure of the SMC head domain: an ABC ATPase with 900 residues antiparallel coiled-coil inserted. J. Mol. Biol. 2001;306:25–35. doi: 10.1006/jmbi.2000.4379. [DOI] [PubMed] [Google Scholar]
- 5.Weitzer S, Lehane C, Uhlmann F. A model for ATP hydrolysis-dependent binding of cohesin to DNA. Curr. Biol. 2003;13:1930–1940. doi: 10.1016/j.cub.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 6.Hirano T. SMC protein complexes and higher- order chromosome dynamics. Curr. Opin. Cell Biol. 1998;10:317–322. doi: 10.1016/s0955-0674(98)80006-9. [DOI] [PubMed] [Google Scholar]
- 7.Graumann PL, Knust T. Dynamics of the bacterial SMC complex and SMC-like proteins involved in DNA repair. Chromosome Res. 2009;17:265–275. doi: 10.1007/s10577-008-9014-x. [DOI] [PubMed] [Google Scholar]
- 8.Hirano T, Mitchison TJ. A heterodimeric coiled-coil protein required for mitotic chromosome condensation in vitro. Cell. 1994;79:449–458. doi: 10.1016/0092-8674(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 9.Hirano M, Hirano T. ATP-dependent aggregation of single-stranded DNA by a bacterial SMC homodimer. EMBO J. 1998;17:7139–7148. doi: 10.1093/emboj/17.23.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connelly JC, Kirkham LA, Leach DR. The SbcCD nuclease of Escherichia coli is a structural maintenance of chromosomes (SMC) family protein that cleaves hairpin DNA. Proc. Natl Acad. Sci. USA. 1998;95:7969–7974. doi: 10.1073/pnas.95.14.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dolganov GM, Maser RS, Novikov A, Tosto L, Chong S, Bressan DA, Petrini JH. Human Rad50 is physically associated with human Mre11: identification of a conserved multiprotein complex implicated in recombinational DNA repair. Mol. Cell Biol. 1996;16:4832–4841. doi: 10.1128/mcb.16.9.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez H, Cardenas PP, Yoshimura SH, Takeyasu K, Alonso JC. Dynamic structures of Bacillus subtilis RecN DNA complexes. Nucleic Acids Res. 2008;36:110–120. doi: 10.1093/nar/gkm759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidane D, Sanchez H, Alonso JC, Graumann PL. Visualization of DNA double-strand break repair in live bacteria reveals dynamic recruitment of Bacillus subtilis RecF, RecO and RecN proteins to distinct sites on the nucleoids. Mol. Microbiol. 2004;52:1627–1639. doi: 10.1111/j.1365-2958.2004.04102.x. [DOI] [PubMed] [Google Scholar]
- 14.Nasmyth K, Haering CH. The structure and function of SMC and kleisin complexes. Annu. Rev. Biochem. 2005;74:595–648. doi: 10.1146/annurev.biochem.74.082803.133219. [DOI] [PubMed] [Google Scholar]
- 15.Mascarenhas J, Soppa J, Strunnikov AV, Graumann PL. Cell cycle-dependent localization of two novel prokaryotic chromosome segregation and condensation proteins in Bacillus subtilis that interact with SMC protein. EMBO J. 2002;21:3108–3118. doi: 10.1093/emboj/cdf314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamazoe M, Onogi T, Sunako Y, Niki H, Yamanaka K, Ichimura T, Hiraga S. Complex formation of MukB, MukE and MukF proteins involved in chromosome partitioning in Escherichia coli. EMBO J. 1999;18:5873–5884. doi: 10.1093/emboj/18.21.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lammens A, Schele A, Hopfner KP. Structural biochemistry of ATP-driven dimerization and DNA-stimulated activation of SMC ATPases. Curr. Biol. 2004;14:1778–1782. doi: 10.1016/j.cub.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 18.Gruber S, Haering CH, Nasmyth K. Chromosomal cohesin forms a ring. Cell. 2003;112:765–777. doi: 10.1016/s0092-8674(03)00162-4. [DOI] [PubMed] [Google Scholar]
- 19.Uhlmann F, Lottspeich F, Nasmyth K. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature. 1999;400:37–42. doi: 10.1038/21831. [DOI] [PubMed] [Google Scholar]
- 20.Britton RA, Lin DC, Grossman AD. Characterization of a prokaryotic SMC protein involved in chromosome partitioning. Genes Dev. 1998;12:1254–1259. doi: 10.1101/gad.12.9.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindow JC, Kuwano M, Moriya S, Grossman AD. Subcellular localization of the Bacillus subtilis structural maintenance of chromosomes (SMC) protein. Mol. Microbiol. 2002;46:997–1009. doi: 10.1046/j.1365-2958.2002.03235.x. [DOI] [PubMed] [Google Scholar]
- 22.Sawitzke JA, Austin S. Suppression of chromosome segregation defects of Escherichia coli muk mutants by mutations in topoisomerase I. Proc. Natl Acad. Sci. USA. 2000;97:1671–1676. doi: 10.1073/pnas.030528397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura K, Rybenkov VV, Crisona NJ, Hirano T, Cozzarelli NR. 13S condensin actively reconfigures DNA by introducing global positive writhe: implications for chromosome condensation. Cell. 1999;98:239–248. doi: 10.1016/s0092-8674(00)81018-1. [DOI] [PubMed] [Google Scholar]
- 24.Schar P, Fasi M, Jessberger R. SMC1 coordinates DNA double-strand break repair pathways. Nucleic Acids Res. 2004;32:3921–3929. doi: 10.1093/nar/gkh716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mascarenhas J, Sanchez H, Tadesse S, Kidane D, Krishnamurthy M, Alonso JC, Graumann PL. Bacillus subtilis SbcC protein plays an important role in DNA inter-strand cross-link repair. BMC Mol. Biol. 2006;7:20. doi: 10.1186/1471-2199-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kupiec M, Simchen G. Cloning and mapping of the RAD50 gene of Saccharomyces cerevisiae. Mol. Gen. Genet. 1984;193:525–531. doi: 10.1007/BF00382094. [DOI] [PubMed] [Google Scholar]
- 27.Picksley SM, Attfield PV, Lloyd RG. Repair of DNA double-strand breaks in Escherichia coli K12 requires a functional recN product. Mol. Gen. Genet. 1984;195:267–274. doi: 10.1007/BF00332758. [DOI] [PubMed] [Google Scholar]
- 28.Sergeant J, Taylor E, Palecek J, Fousteri M, Andrews EA, Sweeney S, Shinagawa H, Watts FZ, Lehmann AR. Composition and architecture of the Schizosaccharomyces pombe Rad18 (Smc5-6) complex. Mol. Cell Biol. 2005;25:172–184. doi: 10.1128/MCB.25.1.172-184.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kidane D, Graumann PL. Dynamic formation of RecA filaments at DNA double strand break repair centers in live cells. J. Cell Biol. 2005;170:357–366. doi: 10.1083/jcb.200412090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez H, Kidane D, Reed P, Curtis FA, Cozar MC, Graumann PL, Sharples GJ, Alonso JC. The RuvAB branch migration translocase and RecU Holliday junction resolvase are required for double-stranded DNA break repair in Bacillus subtilis. Genetics. 2005;171:873–883. doi: 10.1534/genetics.105.045906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez H, Kidane D, Castillo Cozar M, Graumann PL, Alonso JC. Recruitment of Bacillus subtilis RecN to DNA double-strand breaks in the absence of DNA end processing. J. Bacteriol. 2006;188:353–360. doi: 10.1128/JB.188.2.353-360.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meile JC, Wu LJ, Ehrlich SD, Errington J, Noirot P. Systematic localisation of proteins fused to the green fluorescent protein in Bacillus subtilis: identification of new proteins at the DNA replication factory. Proteomics. 2006;6:2135–2146. doi: 10.1002/pmic.200500512. [DOI] [PubMed] [Google Scholar]
- 33.Oussenko IA, Abe T, Ujiie H, Muto A, Bechhofer DH. Participation of 3′-to-5′ exoribonucleases in the turnover of Bacillus subtilis mRNA. J. Bacteriol. 2005;187:2758–2767. doi: 10.1128/JB.187.8.2758-2767.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noirot-Gros MF, Dervyn E, Wu LJ, Mervelet P, Errington J, Ehrlich SD, Noirot P. An expanded view of bacterial DNA replication. Proc. Natl Acad. Sci. USA. 2002;99:8342–8347. doi: 10.1073/pnas.122040799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kidane D, Carrasco B, Manfredi C, Rothmaier K, Ayora S, Tadesse S, Alonso JC, Graumann PL. Evidence for different pathways during horizontal gene transfer in competent Bacillus subtilis cells. PLoS Genet. 2009;5:e1000630. doi: 10.1371/journal.pgen.1000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez S, Ayora S, Alonso JC. Bacillus subtilis homologous recombination: genes and products. Res. Microbiol. 2000;151:481–486. doi: 10.1016/s0923-2508(00)00165-0. [DOI] [PubMed] [Google Scholar]
- 37.Kidane D, Graumann PL. Intracellular protein and DNA dynamics in competent Bacillus subtilis cells. Cell. 2005;122:73–84. doi: 10.1016/j.cell.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 38.Mortier-Barriere I, Velten M, Dupaigne P, Mirouze N, Pietrement O, McGovern S, Fichant G, Martin B, Noirot P, Le Cam E, et al. A key presynaptic role in transformation for a widespread bacterial protein: DprA conveys incoming ssDNA to RecA. Cell. 2007;130:824–836. doi: 10.1016/j.cell.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 39.Tadesse S, Graumann PL. DprA/Smf protein localizes at the DNA uptake machinery in competent Bacillus subtilis cells. BMC Microbiol. 2007;7:105. doi: 10.1186/1471-2180-7-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubnau D. DNA uptake in bacteria. Annu. Rev. Microbiol. 1999;53:217–244. doi: 10.1146/annurev.micro.53.1.217. [DOI] [PubMed] [Google Scholar]
- 41.Comella N, Grossman AD. Conservation of genes and processes controlled by the quorum response in bacteria: characterization of genes controlled by the quorum-sensing transcription factor ComA in Bacillus subtilis. Mol. Microbiol. 2005;57:1159–1174. doi: 10.1111/j.1365-2958.2005.04749.x. [DOI] [PubMed] [Google Scholar]
- 42.Dervyn E, Noirot-Gros MF, Mervelet P, McGovern S, Ehrlich SD, Polard P, Noirot P. The bacterial condensin/cohesin-like protein complex acts in DNA repair and regulation of gene expression. Mol. Microbiol. 2004;51:1629–1640. doi: 10.1111/j.1365-2958.2003.03951.x. [DOI] [PubMed] [Google Scholar]
- 43.Hahn J, Maier B, Haijema BJ, Sheetz M, Dubnau D. Transformation proteins and DNA uptake localize to the cell poles in Bacillus subtilis. Cell. 2005;122:59–71. doi: 10.1016/j.cell.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simmons LA, Grossman AD, Walker GC. Replication is required for the RecA localization response to DNA damage in Bacillus subtilis. Proc. Natl Acad. Sci. USA. 2007;104:1360–1365. doi: 10.1073/pnas.0607123104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alonso JC, Stiege AC, Luder G. Genetic recombination in Bacillus subtilis 168: effect of recN, recF, recH and addAB mutations on DNA repair and recombination. Mol. Gen. Genet. 1993;239:129–136. doi: 10.1007/BF00281611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.