Abstract
Some of the sleep disruption seen in seniors (>65 yrs) may be due to alteration of the circadian pacemaker phase and/or its phase angle with bedtime. The purpose of this study was to determine the effects of 2 h changes in the timing of bedtime (both earlier and later) on the sleep of seniors. Ten healthy seniors (9 F, 1 M, age 70–82 yrs) were each studied individually during three 120 h sessions (each separated by >2 weeks) in a time-isolation laboratory. On nights 1 and 2, bedtime and rise-time occurred at the subjects’ habitual times; on nights 3–5, bedtime was specified by the experiment, but rise-time was at the subjects’ discretion (without knowledge of clock time). Under the control condition, subjects went to bed at their habitual bedtime (HBT), under the earlier bedtime condition at (HBT – 2 h), and under the later bedtime condition at (HBT + 2 h). Sleep was polysomnnographically recorded and rectal temperature continuously monitored. Although total sleep time increased in the earlier compared to the later condition (p < 0.01), sleep efficiency decreased and wake after sleep onset increased (p < 0.01). Subjective ratings of sleep were also worse under the earlier (HBT – 2 h) than under later (HBT+ 2 h) condition (p < 0.05). Performance did not differ between the earlier and later conditions. The larger the phase angle between actual bedtime and circadian temperature minimum (Tmin), the longer the time spent in bed and total sleep time, and the worse the sleep efficiency and subjective sleep ratings. There were no effects related to the phase angle between Tmin and rise-time. The relative benefits of longer vs. more efficient sleep in the elderly require further investigation.
Keywords: Aging, Human, Sleep, Circadian rhythms, Bedtime
INTRODUCTION
Sleep changes as we age. It changes not only in its length, depth, and fragility (Bliwise, 1993; Prinz, 2004), but also in its timing. It is likely that at least some of the sleep disruption experienced by healthy older adults (seniors) is the result of circadian timing issues (Monk, 2005) that are orchestrated principally by the master brain clock, the suprachiasmatic nuclei (Dardente & Cermakian, 2007), although sleep may also be affected by various life events, such as illness or the death of a spouse (Monk et al., 2008). Even in healthy seniors, there are significant age-related circadian phase differences (Czeisler et al., 1992; Monk, 2005). In particular, seniors often choose to go to bed and wake-up several hours earlier than young adults (Buysse et al., 1992; Czeisler et al., 1992) and have higher “morningness” scores (more “morning lark” like) on the Horne-Östberg (Horne & Östberg, 1976) instrument (Czeisler et al., 1992; Monk et al., 1991). Seniors have also been found to have circadian phases (as indicated by temperature and/or melatonin rhythms) that are 1 or 2 h earlier than those of the young, and to have greater phase angles between bedtime and circadian pacemaker phase (Duffy et al., 2002; Monk et al., 1995). When we studied the sleep and morningness scores of healthy older seniors (80 yrs +), we found that those within this age group who had the higher morningness scores (i.e., were morning “lark”-like) were the ones who slept the longest (Monk et al., 1991). Because higher morningness scores are associated with earlier bedtimes, this finding suggests that earlier bedtimes might lead to more sleep being obtained by the elderly.
The fact that seniors are often troubled by extreme levels of early evening sleepiness and wakefulness in the very early morning hours, sometimes warranting a diagnosis of Advanced Sleep Phase Syndrome (Sack et al., 2007), has led researchers to develop treatments based upon the need to change circadian pacemaker phase to a later time (i.e., a phase delay). Thus, the question has arisen as to whether the sleep of elderly people with unwanted early morning awakening could be improved by changing the timing of their circadian pacemaker to more closely follow that of a younger adult (e.g., by delaying pacemaker phase by a couple of hours). The intervention of choice has been evening bright-light therapy (Czeisler et al., 1986). Several authors have shown short-term beneficial effects on sleep of evening bright-light treatment designed to accomplish such a phase delay (Campbell et al., 1995). Unfortunately, though, many older people do not like light therapy, and a recent report has shown that maintenance light therapy for such patients is unlikely to work in the long term (Suhner et al., 2002).
One can instead choose to approach the problem differently. Rather than change the timing of the circadian pacemaker to a later phase, one might instead change the bedtime to an earlier phase. This would move the sleep episode to an earlier phase position relative to the circadian pacemaker; thus, at least in the short term, rectifying the problem of an aberrant phase angle between circadian pacemaker and sleep episode onset without directly intervening to change the timing of the circadian pacemaker. In an earlier publication (Monk et al., 2006), we reported an archival diary-based study of the relation between spontaneous changes in bedtime and the amount of sleep obtained. A total of 128 healthy seniors (63F, 65 M) aged 70–92 yrs each provided a week of sleep diary data yielding a total of 896 individual subject-nights for analysis. Separately for each subject-night, diary data were used to derive measures of time in bed (TIB) and total sleep time (TST). Although there were strong inter-individual and intra-individual differences, the particular timing of bedtime on any individual night had a statistically significant effect (p < 0.001) on both TIB and TST. We observed that earlier bedtimes were associated with longer TIB and more TST. On average, between 7 and 8 min of extra TIB and TST were associated with each 10 min advance in bedtime.
These results prompted us to develop an intervention study in which the bedtimes of seniors were deliberately changed, both earlier and later, by 2 h from the habitual bedtime in a study conducted in a time-isolation laboratory setting. Sleep was measured by polysomnography (PSG) and by post-sleep questionnaire (PSQ); circadian rhythm phase was measured by continuous rectal temperature records, and mood, alertness, and performance were measured using a battery of tests. Three conditions were considered in three separate ~ 120 h laboratory sessions using a within-subjects design that was counter-balanced in the earlier and later conditions: control = habitual bedtime (HBT); earlier = 2 h before HBT; and later = 2 h after HBT. The design also allowed subjects to determine their own rise-time for the last three nights of each condition, thus enabling TIB to become a dependent variable under conditions of temporal isolation, with no knowledge of clock time. This also meant that there were no external time constraints on TST, making TST essentially ad libitum, although there were, of course, biological constraints, some probably related to the advanced age of the subjects. We hypothesized that, in terms of the amount of time spent in bed and the amount of sleep obtained, the earlier condition would be better than the control condition, and the later condition worse than the control condition. We also sought to determine whether sleep efficiency and/or subjective ratings of sleep quality would also show an improvement in the earlier, and worsening in the later, condition relative to the control condition, and to evaluate how these changes related to the phase angle both between the bedtime and circadian temperature minimum and between the rise-time and circadian temperature minimum, using an inexact (masked) measure of the latter.
METHODS
Subjects
Ten healthy paid volunteer subjects, without complaint of sleep problems, took part in the study (9F, 1M, age range 70–82 yrs). All passed a strict medical screening that included a complete medical history, physical examination, and review of current medical records from each subject’s personal physician. Some subjects had stable, non-acute, chronic medical problems, such as well-controlled hypertension or hypothyroidism, but none evidenced unstable medical problems or central nervous system diseases. Medical screening also included a routine laboratory panel (i.e., complete blood count, electrolytes, blood glucose, BUN, creatinine, liver function tests, thyroid function tests, urinalysis, and electrocardiogram). All potential subjects were required to be non-obese (i.e., body mass index (BMI) <27 kg/m2) and non-smokers. None were seeking help for a sleep problem, and none were taking medication known to affect sleep or circadian rhythms. No subject had a current or past history of psychiatric illness, as assessed by the Structured Clinical Interview for DSM-III-R adapted for DSM-IV. In addition, all subjects scored <10 on the Hamilton Rating Scale for Depression (HRSD) and >27 on the Mini-Mental State Examination (MMSE). All subjects were also given the Pittsburgh Sleep Diary (Monk et al., 1994) to complete for 14 nights as well as one screening night of polysomnography in the laboratory with oximetry. All subjects were required habitually to sleep at least 6 h per night (at night). No subject had >20 apneas or hypopneas per hour of sleep or >20 periodic limb movements with arousals per hour of sleep as measured in the laboratory screening night. The protocol complied with the University of Pittsburgh Biomedical Internal Review Board (IRB), as well as the ethical standards of this journal (Portaluppi et al., 2008). Full informed consent was obtained from each participant.
Procedure Overview
A within-subject design was used with each subject attending for three 5-day/night (~ 120 h) sessions, each starting at about 09:00 h. Each session was separated from the others by at least two weeks. During the three laboratory sessions, complete temporal isolation was enforced. At each session, for the first two nights, bed- and rise-time were at the subject’s habitual times from their two-week sleep diary (Monk et al., 1994) completed before the first laboratory study. For the next three nights, bedtime was specified, but rise-time was at the subject’s discretion (whenever you feel you have slept enough and want to get up) without any knowledge of clock time. Under the control condition, which was always the first condition experienced, these three bedtimes (i.e., nights 3, 4, and 5 of the ~ 120 h session) were at the subject’s habitual bedtime (HBT); under the earlier condition, the three bedtimes were 2 h before HBT; and under the later condition, the three bedtimes were 2 h after HBT. The order of earlier and later conditions was counterbalanced.
Each night of sleep was polysomnographically (PSG) recorded and circadian phase estimated from continuous rectal temperature measurement and evening salivary dim light melatonin onset (DLMO) assessments. Upon rising from bed, the subject completed a post-sleep questionnaire (PSQ), which among other things contained self-evaluations of the previous night’s sleep, including ratings of whether enough sleep was obtained, sleep soundness, and how well-rested the subject felt. Assessments were made four times per day of global vigor (alertness), global affect (mood), and performance using our usual battery of tests (Monk et al., 1997).
Facilities and Procedure
The laboratory studies took place in the University of Pittsburgh Neuroscience Clinical and Translational Research Center (N-CTRC) time-isolation laboratories. These laboratories are comprised of two one-bedroom apartments with living and eating areas that are isolated from daylight, protected from noise, and allow the precise monitoring of subjects. Thus, the subject lived in a windowless apartment with no timepieces, no knowledge of clock time, no telephone calls, and no “real time” radio or TV. Only technicians and project staff specially trained to avoid giving time cues were allowed to enter the apartment. Subjects knew that bedtimes might change by up to 2 h, but were not told on which runs, on which nights, or by how much. Thus, potential expectancies concerned with the clock time at which they went to bed and requested to get up were eliminated. The daily procedure allowed subjects to pursue activities, such as hobbies, reading, watching videotapes, etc., during the day as the protocol allowed. Unknown to subjects, breakfast, lunch, and dinner were specified at times related to rise-time (for breakfast) and to bedtime (for lunch and dinner) on that particular day. Thus, breakfast was 1 h after rise-time (whether experimenter-specified or at the subject’s request), lunch was 12 h before bedtime, and dinner was 6 h before bedtime. Snacks were also available ad libitum. Caffeine was restricted to one or two cups of tea or coffee, only in the morning with the breakfast meal, and was forbidden at all other times. Exercise and showers were limited to a 90 min interval prior to lunch. Mood and performance tests were given before breakfast, between breakfast and lunch, between lunch and dinner, and after dinner. As the subject started dinner, the lighting in the apartment was dimmed. Instead of the usual combination of fluorescent tubes and bulbs that gave a light level of about 250–350 lux, dim incandescent bulbs (<20 lux) comprised the only lighting for the 6 h between dinner and bedtime. This was to allow DLMO assessments to be made (see below). At bedtime, lights were extinguished (light levels <3 lux), although infra-red lights and TV cameras still allowed subject monitoring for safety reasons.
Sleep was recorded both by polysomnography (PSG) and by a post-sleep questionnaire (PSQ). PSG studies included one channel of EEG (C3 or C4, referenced to A1 + A2), two channels of electrooculogram (EOG; asymmetric lateral canthus from each eye, referenced to A1 + A2), and one channel of electromyogram (EMG; bipolar submentalis). Review and scoring of sleep records was conducted by trained technicians on-screen with the digitized records divided into 20 sec epochs using modified Rechtshaffen and Kales criteria (Rechtschaffen & Kales, 1968). The PSG measures considered in the present analysis were: time in bed (TIB), defined as the duration between lights-out and rise-time (final awakening as specified by the experimenter or requested by the subject); sleep latency (SL), defined as the time elapsed between lights-out and the first epoch beginning 10 consecutive min of sleep stage 2 or deeper (interrupted by no more than 2 min of wakefulness or Stage 1); wake after sleep onset (WASO), defined as the number of minutes of wakefulness recorded between sleep onset and final awakening; total sleep time (TST), defined as the total number of minutes of objective sleep (NREM stages 1 through 4 and REM) during the night; and sleep efficiency (SE), defined as 100 times TST divided by TIB. The PSQ contained various questions concerning the previous night’s sleep. The present study focuses on three measures from the PSQ—slept as much as needed?, slept soundly?, and well rested?—each response being expressed as a numerical value on a 0–100 mm visual analogue scale (VAS), with higher numbers representing better perceived sleep.
On nights 3–5 of each condition, subjects were free to rise out of bed at the moment of their choice (whenever you feel you have slept enough and want to get up). Upon the subject announcing over the continuously monitored intercom that she or he wanted to get up, the lights were switched on by the technician, who then arranged for their sleep electrodes to be removed and the subject’s “day” to start. The post-sleep questionnaire was completed immediately, and breakfast was given 1 h later. The requested rise-time was recorded and comprised that night’s rise-time for TIB determinations. Subjects were continuously monitored by closed-circuit TV and audio, as well as by personal contact with the monitoring technicians. This ensured the comfort and well-being of the subject, as well as strict protocol compliance, including the avoidance of daytime naps.
Following our standard laboratory procedures (Monk et al., 1995), core body temperature was measured continuously by means of a Yellow Springs Instruments thermistor inserted 10–15 cm into the rectum. The thermistor was connected to a computer via a long cord, allowing freedom of movement throughout the apartment. Missing readings, due to bowel movements and showers, were interpolated. The computer recorded rectal temperature every minute around the clock and alerted technicians to probe slippage. This minimized the loss of data to <5%.
The DLMO was used as a secondary measure of circadian phase. Each evening, commencing 1 h after dinner, a total of eight saliva samples were collected at 30 min intervals to assess melatonin concentration. The last sample was collected 90 min before bedtime. This reduced collection schedule was used to lessen subject burden and study cost, as it was not known whether useful estimates of circadian phase could be obtained. A standardized procedure was enforced, mostly following methods described in Voultsios et al. (1997).
Four times per day, each participant was subjected to a computerized mood and performance assessment battery (Monk et al., 1997). The tests were given in the following order:
Nine visual analogue scales yielding scores of global vigor (alertness) and global affect (mood) (2 min).
Purdue pegboard measuring manual dexterity speed separately in left and right hands, with the two scores being averaged (~5 min).
Serial four-choice task requiring key press to homologous signals on the screen (~10 min).
Stop-signal task, requiring inhibition of a response when a tone sounded (~10 min).
In the afternoon test session, a fifth test was added, the Mackworth Clock Visual Vigilance Task (~20 min). This task required the detection of an infrequent “double jump” in a pointer clicking around a circle.
Data Analysis
The first 48 h (including two nights) of each five-day/night session were considered as baseline and/or adaptation and were not included in the present analysis. This left three comparable 72 h periods in which only the three bedtimes were specified by the experimenter: control (HBT), earlier (HBT − 2 h), and later (HBT + 2 h). Rise-times during this 72 h were always self-selected (see above). Each 72 h period included three nights of both PSG variables (TIB, SL, TST, SE, and WASO) and PSQ variables (i.e., slept as much as needed?, slept soundly?, and well rested?). Both PSG and PSQ variables were averaged across the three nights for each subject-session. Performance and mood variables were taken from the final two full days of each 120 h session (i.e., only after the first shifted night had been slept). Because the day following night 2 occurred before any change in bedtime, and because subjects went home immediately after night 5, alertness, mood, and performance analyses were taken from the days following nights 3 and 4, rather than from the three nights of the sleep measures. For simplicity of presentation, all data were averaged to yield a single value of the given variable for that 72 h period for a given bedtime condition. A repeated-measures ANOVA comparing earlier, control, and later bedtime conditions for the PSG and PSQ sleep variables, and for the performance, mood, and alertness variables, was then applied, and the results reported.
To derive the circadian temperature rhythm endpoints, each subject’s ~4000 min time series, starting at noon on the day before night 3 and covering the three non-baseline nights and intervening days, was considered as the temperature time series for that subject-condition, averaging into 10 min time bins. A separate cosinor analysis was applied to each subject’s temperature time-series for each bedtime condition. A mixed-model, repeated-measures ANOVA was used to fit 12 and 24 h sine and cosine curves to each individual’s temperature time series under each of the three conditions (earlier, control, and later bedtimes). These analyses use an adaptation of SAS Proc Mixed (details of which can be found at http://www.stat.cmu.edu/~hseltman/SASMixed/primer.pdf). Each individual’s fitted curve was then used to estimate the subject’s circadian amplitude (maximum value minus minimum value [Tamp]) and phase (time of the minimum value [Tmin]) for each of the three conditions (earlier, control, and later bedtime). Fitted curves that failed to account for more than 50% of the variance (R2 < 0. 5) were not incorporated into the calculation of the amplitude and phase estimates. Only the eight subjects with good fits for all three conditions were included in the analysis. Mean values of phase (Tmin) and amplitude (Tamp) in the earlier, control, and later bedtime conditions were then reported and statistical significance determined by repeated-measures ANOVA. While it was recognized that our estimate of phase by Tmin was not the true estimate of the circadian pacemaker phase, which would only be obtained using a formal unmasking protocol (see discussion), we felt that using a Tmin value from a sinusoidal fit to three successive cycles allowed a useful, albeit inexact, measure of circadian phase from which phase angles could be calculated.
There were two sets of phase angle analyses, one between bedtime and Tmin (PA-B), the other between Tmin and rise-time (PA-R). Considering only the eight subjects with good estimates of phase (see above), the phase angle between bedtime and Tmin, (PA-B) for each subject-run, was calculated as the number of minutes that elapsed between bedtime and the clock time of Tmin for that subject-run (all Tmin times occurred after bedtime). Similarly, the phase angle between Tmin and rise-time for each subject-run (PA-R) was calculated as the number of minutes that elapsed between Tmin and rise-time for that subject-run (all Tmin times occurred before rise-time). PA-B and PA-R values were then correlated with measures of TIB, TST, and SE from the PSG, and with measures of slept as much as needed?, slept soundly?, and well rested? from the PSQ, using the non-parametric Spearman’s rho statistic. These correlations were calculated using all 24 subject-runs (eight subjects with good circadian phase estimates × three runs) as well as using only using the eight control condition subject-runs.
To derive the melatonin-based circadian phase estimate, the timing of the DLMO was calculated using a simple algorithm on each of the three evenings for a given subject. A threshold value of 3.0 pg/ml was used, following procedures developed by other investigators (Gooneratne et al., 2003). However, as noted below, little useful circadian phase information was obtained from the melatonin analysis.
RESULTS
Effect of Bedtime Condition on Sleep
The effects of the bedtime changes on sleep are summarized in Table 1, which presents the averages of values for the last three nights of each run. Included are the results of both the PSG analyses and the PSQ questionnaire ratings (for which the sample size was reduced to nine in some cases). The PSG results indicated that subjects spent more TIB under the earlier than later bedtime condition, with the control condition intermediate. Also, there was more TST with the earlier bedtime condition. The increase in TST may have been achieved at the cost of a decrease in SE and increase in WASO, although other factors may have also have played a role. There was no effect of condition on SL. The later bedtime condition was found to be significantly superior in measures of slept as much as needed? and slept soundly?, but not in well rested?, from subjects’ ratings of their sleep using the PSQ.
TABLE 1.
Results (mean and standard error of mean) on sleep parameters, averaged across the last three nights of each five-night session (n = 10)
| Variable | Earlier | Control | Later | F | d.f. | p |
|---|---|---|---|---|---|---|
| Time in bed (h) | 8.48 (0.43) | 7.55 (0.28) | 6.48 (0.23) | 17.91 | 2,18 | <.0001 |
| Total sleep time (h) | 6.25 (0.27) | 5.87 (0.16) | 5.43 (0.21) | 6.58 | 2,18 | 0.007 |
| Sleep latency (min) | 29 (9.2) | 27 (7.0) | 14 (3.0) | 2.26 | 2,18 | 0.133 |
| Sleep efficiency (%) | 74.0 (2.8) | 78.3 (3.0) | 83.9 (1.6) | 6.31 | 2,18 | 0.008 |
| Wake after sleep onset (min) | 106 (12.8) | 74 (11.1) | 49 (5.4) | 10.35 | 2,18 | 0.001 |
| PSQ, Slept as much as needed? (0–100) |
80.9 (10.5) | 76.9 (13.3) | 88.3 (5.2) | 4.32 | 2,14 | 0.035 |
| PSQ, Slept soundly? (0–100) | 67.6 (15.9) | 70.5 (19.2) | 88.0 (6.5) | 6.83 | 2,14 | 0.009 |
| PSQ, Well rested? (0–100) | 78.4 (11.7) | 77.5 (11.6) | 86.1 (9.2) | 1.85 | 2,14 | 0.193 |
The F and p values reported from the repeated-measure ANOVA are for the main effect of bedtime condition in a within-subjects comparison. Some PSQ measures were not properly collected, thus reducing the n. Means showing significant differences are bolded.
Effects of Bedtime Condition on Alertness, Mood, and Performance
The results of alertness, mood, and performance analyses are given in Table 2. No significant effect of bedtime condition was detected on daytime alertness, as measured by global vigor, or on mood, as measured by global affect (p > 0.10). For the performance measures, there was a striking similarity between the earlier and later bedtime conditions, although in all but the vigilance test, the control condition, which was always the first condition experienced by subjects, showed worse performance than the other two. This was probably because of practice effects, resulting in a significant repeated-measures ANOVA (in the dexterity, inhibition, and four-choice tasks [p < 0.01 or better]), but with the earlier and later bedtime conditions essentially identical, and better than the control condition.
TABLE 2.
Mood and performance results, means and standard deviations (n = 10)
| Bedtime condition |
Global vigor (0–100 mm) |
Global affect (0–100 mm) |
Vigilance hits (ex 20) |
Dexterity latency (sec) |
Inhibition failures (ex 24) |
Serial four- choice (msec) |
|---|---|---|---|---|---|---|
| Earlier | 78.20 (10.6) | 81.66 (14.2) | 15.05 (3.7) | 52.26 (6.8) | 5.35 (3.1) | 420 (9) |
| Control | 77.61 (9.8) | 84.26 (12.4) | 13.35 (3.9) | 56.44 (6.9) | 7.44 (4.0) | 470 (8) |
| Later | 76.51 (11.5) | 83.79 (12.7) | 14.80 (3.1) | 52.61 (7.4) | 5.53 (3.9) | 420 (11) |
In the headings, “ex” denotes “out of a possible.”
Effect of Bedtime Condition on Circadian Rhythms
Two of the ten subjects failed to have all three time series (earlier, control, later) show reliable 24 + 12 h cosinor fits (R2 > 0.50), and were thus excluded from the circadian temperature rhythm analysis. In the remaining eight subjects, with regard to circadian phase, there was a reliable difference in the timing of the fitted Tmin between the three conditions, being 01:53 h for the earlier, 02:44 h for the control, and 03:57 h for the later bedtime conditions (F(2,14) = 18.41, p < 0.001). Thus, the temperature rhythm shifted, on average, 51 min earlier in the earlier bedtime condition and 73 min later in the later bedtime condition, compared to control bedtime condition, even though the bedtime, and thus the onset of darkness, had been shifted by 120 mins in each direction. This analysis, though, includes the masking effect of the change in bedtime, and was averaged across the three days. There was no consistent difference between the conditions in the fitted amplitude (earlier = 0.91°C, control = 0.83°C, later = 0.87°C; F (2,14) = 1.18, p = 0.33).
Because of problems with sample collection, transportation, and/or storage, melatonin assays were only possible for seven of the ten subjects. As expected, given the limited time frame during which saliva collection was possible (i.e., eight samplings starting 5 h before and ending 1.5 h before actual bedtime), there were many subject-evenings in which no discernable DLMO could be identified. From the data that were available, there appeared to be no clear change in phase detected in DLMO when the three conditions were compared; thus, no conclusion could be reached.
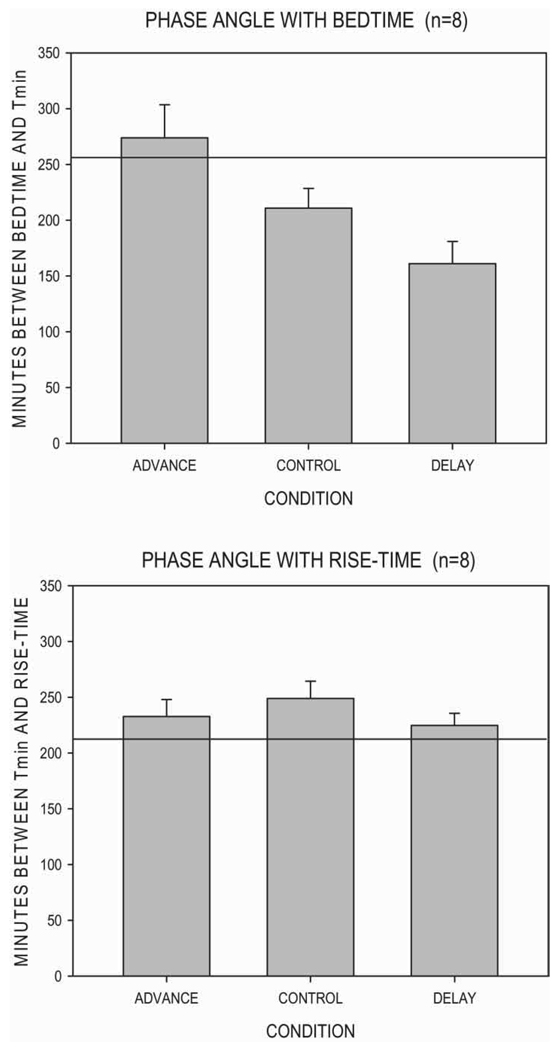
Phase Angle and Bedtime Condition
The phase angle between the actual bedtime and Tmin (PA-B) was calculated for each of the 24 subject-runs. Mean values for the three conditions are plotted in the upper panel of Figure 1. The horizontal line at 258 min represents the mean PA-B value we found in a previous investigation of 22 young male adults living under a normal nychthemeral routine (Monk et al., 1995). Under the control bedtime condition, the phase angle was smaller than that typical of younger adults. Under the earlier bedtime condition, the phase angle was more like that of younger adults, and under the later bedtime condition, the phase angle was even smaller. When all 24 subject-runs were considered, significant positive correlations appeared between PA-B and TIB (rho = 0.833, p < 0.001) and between PA-B and TST (rho = 0.488, p < 0.02). In contrast, significant negative correlations appeared between PA-B and SE (rho = −0.662, p < 0.001) as well as between PA-B and slept soundly? (rho = −0.452, p < 0.05) from the PSQ; the other PSQ measures showed non-significant negative correlations. When only the control condition was considered, the n was substantially reduced to eight. However, a positive correlation was nonetheless detected between PA-B and TIB (rho = 0.833, p < 0.001), though not between PA-B and TST (rho < 0.1, n.s.). In contrast, negative correlations appeared for SE (rho = −0.662, p < 0.001) and (not significantly) for measures of slept as much as needed? (rho = −0.405, p = 0.32) and slept soundly? (rho = −0.524, p = 0.18) from the PSQ. Thus, in general, the more time that elapsed between bedtime and Tmin, the longer the sleep duration (TST) and greater the time spent in bed (TIB), but the lower the sleep efficiency (SE) and the worse the subjective sleep rating of slept soundly?.
FIGURE 1.
Mean (± standard error of the mean) values of PA-B and PA-R. The horizontal line represents PA-B and PA-R values found previously in young male adults (Monk et al., 1995).
In a similar fashion, the phase angle between actual bedtime and Tmin and actual rise-time (PA-R) was calculated for each of the 24 subject-runs. Mean values for the three conditions are plotted in the lower panel of Figure 1. The horizontal line at 214 min represents the mean PA-R value found previously in a study of 22 young male adults living under a normal nychthemeral routine (Monk et al., 1995). Essentially, the mean values of PA-R under the three conditions were very similar, about 230 min, being only slightly longer than that found previously in the young male adults. Similarly, in contrast to the PA-B analyses, there were no significant correlations between PA-R and any of the sleep measures derived from either PSG or PSQ. This was true both for all 24 subject-sessions considered together and also when only the control bedtime condition was considered separately (p > 0.10 all cases). Thus, it would appear the phase angle between Tmin and rise-time was a much less potent predictor of sleep than was the phase angle between bedtime and Tmin.
DISCUSSION
The primary hypotheses to be tested by the study were that TIB and TST would be longer in the earlier bedtime condition and shorter in the later bedtime condition, as compared to control. These hypotheses were confirmed. However, by additionally considering sleep efficiency and WASO, we were able to determine that these improvements that occurred under the earlier condition were achieved at a certain cost. Although by putting a senior to bed 2 h earlier than normal one might allow them almost 0.5 h more TST, this increase might be won at the cost of an almost 5% decrease in SE, and more than a 30 min increase in WASO. Moreover, the results of the analyses on the PSQ variables (see Table 1) indicated that it was the later, rather than the earlier, bedtime condition that resulted in superior post-sleep subjective ratings by the subject. This is important, because some experts in geriatric sleep (e.g., Hoch et al., 2001) believe that it is preserving higher levels of sleep efficiency that should be the goal of a senior seeking to preserve good sleep and health into later life. In such a view, the sleep efficiency goal is more important than the actual duration goal (i.e., number of minutes of sleep obtained). Such an approach ties into behavioral treatments of insomnia that often specifically seek to increase SE by reducing TIB. Sleep restriction is a major component of Cognitive Behavioral Treatment for Insomnia (CBT-I) (see Hoelscher & Edinger, 1988; Morin et al., 1993). Sleep restriction requires patients to limit the time spent in bed while awake and thus favors sleep consolidation (Spielman et al., 1987). Thus, according to this approach, the later bedtime condition with its associated average sleep efficiency of 83.9% would be preferable to the earlier bedtime condition with its associated average sleep efficiency of 74.0%, even though almost 50 min less actual sleep was then being obtained. Supportive evidence for sleep efficiency being preferable to more minutes of sleep came from the PSQ ratings by the subjects themselves, for whom the later bedtime condition produced the highest VAS ratings of slept as much as needed? and slept soundly?.
Higher sleep efficiency may also be important for the continued well-being of the individual. In a study of 185 healthy older adults, Dew and colleagues (2003) have shown that after controlling for age, sex, and baseline medical burden, individuals with SE < 80% were at 1.93 greater risk of death during a 4–19 yr follow-up compared to better sleepers.
In contrast to the above discussion, an alternative approach would be more concerned about seniors getting enough sleep per se, and would thus concentrate on the total minutes of sleep obtained each night. This approach would favor the earlier over the later bedtime condition, as the earlier condition gives the subject more than 6 h of sleep, which some have posited as the minimum core amount of sleep needed for full functioning (Horne, 1988). However, it should be noted that there was apparently no increase in daytime alertness or performance consequent upon the increased sleep obtained in the earlier condition (see Table 2).
A second finding of interest with regard to the PSG monitoring studies was the comparatively short durations of sleep obtained the last three nights of the control bedtime condition. Under this condition, subjects went to bed at their habitual bedtime and were free to sleep for as long as they liked. The present protocol was unusual in that subjects were in temporal isolation without knowledge of clock time, and were entirely free to determine, on a night-by-night basis, their own rise-times during the 72 h in question. Arguably, under this condition, they could be regarded as essentially “sleep satiated.” Even so, an average of only 5.9 h of sleep was obtained. Moreover, even under the earlier bedtime condition, when more sleep was obtained, the duration of sleep only increased to 6.25 h. Thus, non-pharmacological manipulations that seek to increase the sleep duration of seniors to the 7.5–8 h range may be doomed to failure. Even under optimal circadian conditions, healthy seniors over the age of 70 yrs may be physically unable to attain the amount of sleep seen in younger adulthood.
A third finding of interest with regard to the PSG sleep monitoring studies was the absence of any worsening of SL under the earlier bedtime condition (earlier: 29 min vs. control: 27 min), when subjects were put to bed at almost exactly during the “forbidden zone” for sleep. Historically, a major consistent finding with regard to the interaction between the circadian pacemaker and sleep period onset is that sleep is very difficult to obtain about 2–3 h before the individual’s habitual bedtime. This has been referred to as a “forbidden zone” for sleep (Dijk & Czeisler, 1994; Lavie & Scherson, 1981; Richardson et al., 1982). Thus, for example, in studies involving ultra-short daylengths (90 or 20 min), very little sleep is usually obtained in the ultra-short “night,” coinciding with the time span of about 2–3 h before habitual bedtime. Thus, in the present study, one might expect rather long SL when bedtime is moved earlier by 2 h. The absence of any such effect may be attributed to the age of our subjects. In an earlier “90 minute day” (60 min awake, 30 min for sleep) study involving seniors (Buysse et al., 2005), we showed an age-related decrease in the amplitude of the circadian rhythm in sleep propensity relative to that of younger adults. Monk and Kupfer (2000) have posited that age-related attenuation of circadian rhythms may occur in rhythms, such as subjective alertness and sleep propensity, which are “downstream” from the circadian pacemaker.
Because no bright light levels were experienced, and the change in bedtime was only 2 h, we expected the circadian pacemaker to remain largely unchanged under the three bedtime conditions (earlier, control, and later). However, there were statistically reliable, albeit <2 h, differences observed in circadian phase when we calculated the phase of the final three circadian cycles of rectal temperature. It should be remembered, though, that such estimates also included the masking effects of sleep on temperature, the timing of which was delayed or advanced by 2 h. Thus, in terms of the phase of the circadian pacemaker itself, it may well be that both phase changes were overestimated in the cosinor analysis of the temperature rhythm. Only by keeping subjects awake for at least 24 h in an “unmasking protocol” could circadian phase be accurately determined from the temperature rhythm—a procedure clearly ruled out in the current experiment. Thus, it should be recognized that all of the present discussions of circadian phase, including phase angles, are from an inexact measure, because of the known nonlinear interaction between sleep-dependent and circadian-dependent thermoregulatory mechanisms that cannot be unmasked using mathematical procedures. Although very recent research has thus favored melatonin as the “gold standard” circadian marker (hence its attempted application in this study), it is noteworthy that much of the advance in our knowledge of human circadian rhythms over the last half-century has derived from studying circadian rectal temperature rhythms in subjects living on nychthemeral routines. Circadian phase and phase angle results are thus presented here with the caveat that they may be inexact. The notion that circadian phase differences were overestimated by the temperature rhythm analysis was to some extent borne out by the DLMO findings. These findings, although very incomplete, found no clear evidence for a phase advance in the earlier bedtime condition, or phase delay in the later bedtime condition. Estimating pacemaker phase by salivary DLMO avoids the effects of masking, but it suffers from the absence of sample collection during sleep. Our DLMO results were often inconclusive because of sampling limitations. Clearly, future experiments should plan to have a much longer evening saliva collection interval, or, preferably, 24 h blood collections, and collect right up until bedtime.
The analysis of phase angle differences between bedtime and Tmin (PA-B) and rise-time and Tmin (PA-R) revealed some insights into the mechanism by which gains in TIB and TST, and losses in SE, might have occurred by moving bedtime 2 h earlier. It would appear that subjects chose to wake at approximately the same phase angle between Tmin and rise-time, whatever the bedtime condition, thus allowing extra TIB and TST under the earlier condition when more time became available for sleep. Under the later bedtime condition, the relatively constant phase angle with rise-time, combined with a 2 h delay in bedtime, led to a compression of the sleep episode, with a consequent reduction in WASO and increase in SE. Thus, the present effects would appear to represent a combination of circadian and homeostatic influences. Further research is needed to determine whether equivalent effects occur in younger adults, and if they are the same in men and women (Tonetti et al., 2008), and whether these results can be verified in a larger sample of seniors. The issue of sleep quality and quantity, especially in seniors, has attracted even greater attention because of the increasing proportion today of elderly persons, well beyond the age of 65 yrs, who in many countries continue to remain in the workforce and to adhere to various diverse work schedules (see, e.g., Bohle et al., 2008; Costa & Di Milia, 2008; Folkard, 2008).
CONCLUSION
Advancing the bedtime of seniors by 2 h can increase the amount of sleep obtained but will decrease their SE. Delaying the bedtime of seniors by 2 h can increase their SE and improve subjective sleep quality, but will result in less overall sleep being obtained. Even under optimal circadian and/or behavioral conditions, healthy seniors appear to be only able to achieve about 6.25 h of actual, PSG-documented, sleep.
ACKNOWLEDGMENTS
Special thanks are owed to Melissa Clark, Joette Zarotney, and Gail Oterson, for subject recruitment and to Margaret Rosenberg for data analysis. Gratitude is also owed to our subjects for their gracious cooperation in a challenging protocol. Support for this work was provided by National Institute on Aging grants AG-13396 and AG-020677, NASA grant NNJ04HF76G, and CTSA grant RR-024153.
The views expressed are not necessarily those of the University of Pittsburgh or the National Institute on Aging.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- Bohle P, Di Milia L, Fletcher A, Rajaratnam S. Introduction: Aging and the multifaceted influences on adaptation to working time. Chronobiol. Int. 2008;25:155–164. doi: 10.1080/07420520802074058. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Browman KE, Monk TH, Reynolds CF, Fasiczka AL, Kupfer DJ. Napping and 24-hour sleep/wake patterns in healthy elderly and young adults. J. Am. Geriatr. Soc. 1992;40:779–786. doi: 10.1111/j.1532-5415.1992.tb01849.x. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Monk TH, Carrier J, Begley A. Circadian patterns of sleep, sleepiness, and performance in older and younger adults. Sleep. 2005;28:1365–1376. doi: 10.1093/sleep/28.11.1365. [DOI] [PubMed] [Google Scholar]
- Campbell SS, Terman M, Lewy AJ, Dijk DJ, Eastman CI, Boulos Z. Light treatment for sleep disorders: Consensus report V. Age related disturbances. J. Biol. Rhythms. 1995;10:151–154. doi: 10.1177/074873049501000207. [DOI] [PubMed] [Google Scholar]
- Costa G, Di Milia L. Aging and shift work: A complex problem to face. Chronobiol. Int. 2008;25:165–182. doi: 10.1080/07420520802103410. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Allan JS, Strogatz SH, Ronda JM, Sanchez R, Rios CD, Freitag WO, Richardson GS, Kronauer RE. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233:667–670. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Dumont M, Duffy JF, Steinberg JD, Richardson GS, Brown EN, Sanchez R, Rios CD, Ronda JM. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet. 1992;340:933–936. doi: 10.1016/0140-6736(92)92817-y. [DOI] [PubMed] [Google Scholar]
- Dardente H, Cermakian N. Molecular circadian rhythms in central and peripheral clocks in mammals. Chronobiol. Int. 2007;24:195–213. doi: 10.1080/07420520701283693. [DOI] [PubMed] [Google Scholar]
- Dew MA, Hoch CC, Buysse DJ, Monk TH, Begley AE, Houck PR, Hall M, Kupfer DJ, Reynolds CF. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom. Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci. Lett. 1994;166:63–68. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Zeitzer JM, Rimmer DW, Klerman EB, Dijk DJ, Czeisler CA. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. Am. J. Physiol. Endocrinol. Metab. 2002;282:E297–E303. doi: 10.1152/ajpendo.00268.2001. [DOI] [PubMed] [Google Scholar]
- Folkard S. Shift work, safety, and aging. Chronobiol. Int. 2008;25:183–196. doi: 10.1080/07420520802106694. [DOI] [PubMed] [Google Scholar]
- Gooneratne NS, Metlay JP, Guo W, Pack FM, Kapoor S, Pack AI. The validity and feasibility of saliva melatonin assessment in the elderly. J. Pineal Res. 2003;34:88–94. doi: 10.1034/j.1600-079x.2003.02945.x. [DOI] [PubMed] [Google Scholar]
- Hoch CC, Reynolds CF, Buysse DJ, Monk TH, Nowell P, Begley AE, Hall F, Dew MA. Protecting sleep quality in later life: A pilot study of bed restriction and sleep hygiene. J. Gerontol.: Psychol. Sci. 2001;56B doi: 10.1093/geronb/56.1.p52. [DOI] [PubMed] [Google Scholar]
- Hoelscher TJ, Edinger JD. Treatment of sleep-maintenance insomnia in older adults: sleep period reduction, sleep education, and modified stimulus control. Psychol. Aging. 1988;3:258–263. doi: 10.1037//0882-7974.3.3.258. [DOI] [PubMed] [Google Scholar]
- Horne J. Why we sleep: The functions of sleep in humans and other mammals. New York: Oxford University Press; 1988. p. 319. [Google Scholar]
- Horne JA, Östberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Lavie P, Scherson A. Ultrashort sleep-waking schedule, I. Evidence of ultradian rhythmicity in ‘sleepability’. Electroencephalogr. Clin. Neurophysiol. 1981;52:163–174. doi: 10.1016/0013-4694(81)90164-4. [DOI] [PubMed] [Google Scholar]
- Monk TH. Aging human circadian rhythms: Conventional wisdom may not always be right. J. Biol. Rhythms. 2005;20:366–374. doi: 10.1177/0748730405277378. [DOI] [PubMed] [Google Scholar]
- Monk TH, Kupfer DJ. Circadian rhythms in healthy aging: Effects downstream from the pacemaker. Chronobiol. Int. 2000;17:355–368. doi: 10.1081/cbi-100101051. [DOI] [PubMed] [Google Scholar]
- Monk TH, Reynolds CF, Buysse DJ, Hoch CC, Jarrett DB, Jennings JR, Kupfer DJ. Circadian characteristics of healthy 80-year-olds and their relationship to objectively recorded sleep. J. Gerontol.: Med. Sci. 1991;46:M171–M175. doi: 10.1093/geronj/46.5.m171. [DOI] [PubMed] [Google Scholar]
- Monk TH, Reynolds CF, Kupfer DJ, Buysse DJ, Coble PA, Hayes AJ, Machen MA, Petrie SR, Ritenour AM. The Pittsburgh Sleep Diary. J. Sleep Res. 1994;3:111–120. [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Reynolds CF, Kupfer DJ, Houck PR. Circadian temperature rhythms of older people. Exp. Gerontol. 1995;30:455–474. doi: 10.1016/0531-5565(95)00007-4. [DOI] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Reynolds CF, Berga SL, Jarrett DB, Begley AE, Kupfer DJ. Circadian rhythms in human performance and mood under constant conditions. J. Sleep Res. 1997;6:9–18. doi: 10.1046/j.1365-2869.1997.00023.x. [DOI] [PubMed] [Google Scholar]
- Monk TH, Thompson WK, Buysse DJ, Hall M, Nofzinger EA, Reynolds CF. Sleep in healthy seniors: A diary study of the relation between bedtime and the amount of sleep obtained. J. Sleep Res. 2006;15:256–260. doi: 10.1111/j.1365-2869.2006.00534.x. [DOI] [PubMed] [Google Scholar]
- Monk TH, Begley AE, Billy BD, Fletcher ME, Germain A, Mazumdar S, Moul DE, Shear MK, Thompson WK, Zarotney JR. Sleep and circadian rhythms of spousally bereaved seniors. Chronobiol. Int. 2008;25:83–98. doi: 10.1080/07420520801909320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin CM, Kowatch RA, Barry T, Walton E. Cognitive-behavior therapy for late-life insomnia. J. Consult. Clin. Psychol. 1993;61:137–146. doi: 10.1037//0022-006x.61.1.137. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Touitou Y, Smolensky MH. Ethical and methodological standards for laboratory and medical biological rhythm research. Chronobiol. Int. 2008;25:999–1016. doi: 10.1080/07420520802544530. [DOI] [PubMed] [Google Scholar]
- Prinz PN. Age impairments in sleep, metabolic and immune functions. Exp. Gerontol. 2004;39:1739–1743. doi: 10.1016/j.exger.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. Washington DC: NIH Publication 204. U.S. Government Printing Office Department of Health Education and Welfare; A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. 1968
- Richardson GS, Carskadon MA, Orav EJ, Dement WC. Circadian variation of sleep tendency in elderly and young adult subjects. Sleep. 1982;5:S82–S94. doi: 10.1093/sleep/5.s2.s82. [DOI] [PubMed] [Google Scholar]
- Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP, Vitiello MV, Zhdanova IV. Circadian rhythm sleep disorders: Part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. Sleep. 2007;30:1484–1501. doi: 10.1093/sleep/30.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10:45–56. [PubMed] [Google Scholar]
- Suhner AG, Murphy PJ, Campbell SS. Failure of timed bright light exposure to alleviate age-related sleep maintenance insomnia. J. Am. Geriatr. Soc. 2002;50:617–623. doi: 10.1046/j.1532-5415.2002.50154.x. [DOI] [PubMed] [Google Scholar]
- Tonetti L, Fabbri M, Natale V. Sex differences in sleep-time preferences and sleep need: A cross-sectional survey among Italian pre-adolescents, adolescents, and adults. Chronobiol. Int. 2008;25:745–759. doi: 10.1080/07420520802394191. [DOI] [PubMed] [Google Scholar]
- Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: Validation and comparison to plasma melatonin. J. Biol. Rhythms. 1997;12:457–466. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]