The goal of this chapter is to discuss the importance of preclinical evaluation of potential therapies for neurological disorders in animal models that mimic the target human disorder as a prelude to the translation of these into clinical trials. The scope of neurological and/or neurosurgical disorders that could be considered herein includes both acute neurological insults as well as chronic conditions such as epilepsy, neuropathic pain and the neurodegenerative disorders, primarily Alzheimer’s disease, Parkinson’s disease and the motor neuron diseases, mainly amyotrophic lateral sclerosis. Animal models for all of these conditions have been devised and used to evaluate potential therapies. However, a single chapter cannot possibly do justice to this wide range of disorders and associated models. Thus, in order to keep the subject of neurological animal studies manageable, the focus of this chapter will be on models and basic principles of preclinical evaluation of therapies in the context of acute neurological injuries including stroke, cardiac arrest and cardiopulmonary resuscitation (CA/CPR), traumatic brain injury (TBI) and spinal cord injury (SCI). These acute insults represent 4 of the most catastrophic consequences that human beings can suffer. Furthermore, the discussion of how to test therapies in models of these conditions will be couched primarily in relation to pharmacological therapies. However, many, if not all principles that define a thorough preclinical evaluation of drugs in animal models are in fact equally applicable to gene and cellular transplant therapies.
There are approximately 750,000 strokes per year in the US, most, but certainly not all, affecting the elderly population. About 85% of strokes are ischemic in nature, involving a thromboembolic blockage of a brain artery; up to 15% of strokes are hemorrhagic. There are 2 types of hemorrhagic strokes: intracerebral hemorrhage (ICH) when blood is released into brain parenchyma producing brain damage by triggering brain edema (swelling) and mass effects, resulting in secondary ischemia within the brain tissue, and subarachnoid hemorrhage (SAH) when blood is released into the subarachnoid space from an aneurysm ballooning out from one of the major arteries, also causing a secondary ischemic insult from induction of delayed cerebral vasospasm peaking at 4–7 days after SAH. There are about 30,000 aneurysmal SAH per year in the US with a 2:1 female:male preponderance.
Cardiac arrest strikes about 600,000 people per year in the USA and leads to high mortality and poor neurological outcome. Many survivors of CA/CPR have moderate to severe neurological deficits many months following the event. Survival rates following CA/CPR have not changed for decades despite improvements in resuscitation techniques. The lack of effective treatment options to ameliorate reperfusion injury in the postresuscitation period likely accounts for the disappointing survival rates. Recently, however, induction of mild hypothermia in unresponsive cardiac arrest survivors showed improved neurological outcome and 6-month survival. This was the first demonstration in humans that development of brain injury after CA/CPR could be positively influenced by a postischemic intervention.
There are an estimated 1.5 million cases per annum of TBI in the US, ranging from mild to severe. Although most TBI cases are mild in severity, about 58,000 are severe (Glasgow Coma Score: 3–8) and 64,000 moderate (Glasgow Coma Score: 9–12) and such individuals often require intensive medical treatment and extended recovery periods. Further, there are about 11,000 new cases of SCI each year in the US with an overall prevalence of approximately 250,000. Although TBI and SCI affect active individuals of any age, most occur in young adults in the second and third decades of life. Moreover, the majority of stroke, TBI and SCI patients now survive their neurological insults due to improvements in emergency, neurological intensive care and surgical treatments. Nevertheless, the need for intensive rehabilitation and the reality of prolonged disability exacts a significant toll on the individual, his or her family and society. Effective ways of maintaining or recovering function could markedly improve the outlook for persons with these insults by enabling higher levels of independence and productivity.
Goals of Drug Therapies for Acute Neurological Disorders
Neuroprotection
The focus for pharmacological intervention to preserve neurological function after these acute central nervous system (CNS) injuries is based on the idea that most vascular and/or neurodegeneration that follows these injuries is not due to the primary ischemic, hemorrhagic or mechanical (that is, shearing of blood vessels and nerve cells) insults, but to secondary injury events set in motion by the primary injury. For example, most SCI cases do not involve actual physical transection of the cord, but the spinal cord is damaged as a result of a contusive, compressive or stretch injury. Thereby, usually some portions of the ascending sensory and descending motor tracts remain intact allowing for the possibility of neurological recovery. During the first minutes and hours following injury, a secondary degenerative process is initiated by the primary mechanical injury proportional to the magnitude of the initial insult. Nevertheless, the initial anatomical continuity of the injured spinal cord and our present knowledge of many factors involved in the secondary injury process have led to the hypothesis that pharmacological treatments which interrupt the secondary cascade, if applied early, could improve CNS tissue survival, and preserve the necessary anatomic substrates for functional recovery to take place. The goal of ameliorating the secondary injury is referred to as neuroprotection. Several reviews of poststroke, TBI or SCI secondary injury have been published [1–5].
In SCI, the secondary events occur initially in central gray matter and then spread to the surrounding white matter. The key issue in predicting recovery of function is the degree of preservation of the ascending and descending white matter tracts. However, many of the surviving white matter tracts do not conduct impulses due to posttraumatic demyelination or incomplete remyelination (that is, dysmyelination). Therefore, the goal of neuroprotective pharmacotherapy in SCI is to preserve as many of the white matter axons and as much of their investing myelin as possible. In TBI, a key determinant in neurological recovery is also the loss of axons. Based upon the often widespread loss of axons in injured brain, this phenomenon is referred to as diffuse axonal injury. However, it should be realized that a significant factor in influencing extent of neural injury both in TBI and SCI is a decrease in brain or spinal cord microvascular perfusion (that is, secondary ischemia). When this occurs, the result is an exacerbation of the injury process due to superimposed tissue ischemic hypoxia. Moreover, deficiencies in CNS hypoperfusion can be aggravated by systemic hypotension and/or hypoxia. Thus, it is important to note that secondary injury involves both parenchymal and microvascular events.
For focal ischemic stroke, the goal is to limit the extent of the infarction by preventing secondary injury in the partially perfused penumbral region surrounding the core of the infarct. For CA/CPR, which involves a transient global ischemic insult, the aim is to prevent the secondary degeneration of selectively vulnerable neuronal populations (for example, CA1 region of the hippocampus, layers 3, 5 and 6 of the cortex and intrinsic neurons of the caudate) that are caused by a combination of the ischemic episode plus the reperfusion of the brain after successful resuscitation (that is, reperfusion injury). In SAH, the main focus of attention has been on finding pharmacological ways to prevent the delayed vasospasm phenomenon that leads to secondary ischemic brain damage that might be focal or global in its extent. For ICH, the goal is to limit the deleterious effects of the hematoma on the surrounding tissue by preventing edema and ischemic damage.
Neurorestoration
Another approach to the treatment of acute neurological injuries involves the attempt to restore lost neurological function once the extent of the acute injury to the brain or spinal cord and associated neurological deficits has stabilized. Until a decade ago, it was firmly believed that once the brain or spinal cord was damaged by the secondary injury process, there was little, if any, capability for regeneration of axons and formation of new synapses to take place. However, over the last several years, it has been discovered that the CNS is indeed capable of significant structural and functional repair, plasticity and regeneration that might be pharmacologically or otherwise enhanced. Approaches for accomplishing this include reawakening the growth potential of the surviving neurons or antagonizing the multiple inhibitory factors that interfere with axonal growth and synaptogenesis. Alternatively, cellular replacement may be achievable in certain brain regions which possess nascent neural stem cells. It is increasingly apparent that these endogenous stem cell populations in brain and spinal cord might be pharmacologically stimulated to divide and differentiate into neuronal or oligodendroglial precursor cell types and ultimately neurons and remyelinating oligodendroglia, respectively. Indeed, the molecular mechanisms that control neurogenesis and gliogenesis can be targets for pharmacological intervention. Several pharmacological mechanisms can be targeted to enhance the function and/or structural plasticity of neuronal pathways that survive the ravages of postischemic or posttraumatic secondary injury [6–9].
Acute Neurological Injury Models
A listing of the in vivo models of acute neurological injury (that is, ischemia, hemorrhage, TBI and SCI) that have been or are currently being utilized for preclinical evaluation of neuroprotective or neurorestorative agents are provided in table 1.
Table 1.
In vivo models employed for discovery of neuroprotective and neurorestorative agents
| Stroke (focal ischemia) |
| Rat, mouse, cat or monkey temporary MCAO-microclip or intraluminal suture for 30 min to 2 h |
| Rat or mouse permanent MCAO-electrocoagulation or intraluminal suture |
| Cardiac arrest/resuscitation (transient global ischemia) |
| Rat 2-vessel (bilateral carotid) occlusion plus hypotension for 5–30 min |
| Rat 4-vessel occlusion for 5–30 min (permanent bilateral vertebral artery electocoagulation followed 24 h later with transient bilateral carotid occlusion) |
| Gerbil bilateral carotid occlusion for 5–15 min |
| Swine or canine cardiac arrest/resuscitation model with varying duration of arrest |
| Hemorrhagic stroke models (SAH or ICH) |
| Rabbit, cat or dog intra-cisterna magna injection of autologous blood |
| Rat intracranial injection of autologous blood via dorsolateral cranial burr hole |
| Monkey SAH via surgical placement of autologous blood clot around base of MCA |
| Rat striatal ICH |
| TBI |
| Diffuse |
| Rat or mouse fluid percussion – can be combined with hypotension or hypoxia |
| Rat impact acceleration – can be combined with hypotension or hypoxia |
| Mouse weight drop |
| Pig or primate rotational acceleration (nonimpact) |
| Focal |
| Rat or mouse controlled cortical impact |
| Axonal |
| Mouse optic nerve stretch |
| Subdural hematoma |
| Rat intracranial injection of autologous blood via dorsolateral cranial burr hole (same as SAH model) |
| SCI |
| Weight drop contusion |
| Wrathall device and model |
| Rat New York University (MASCIS) device and model |
| Rat Ohio State University (ESCID) device and model |
| Rat or mouse University of Kentucky (Infinite Horizons) device and model |
| Compression |
| Rat aneurysm clip compression (Fehlings and Tator model) |
| Cat weight compression (Anderson model) |
| Combination contusion and compression |
| Rat contusion followed by placement of Teflon wedges underneath vertebrae |
| Ischemic injury |
| Rabbit balloon in descending aorta inflated transiently above level of lumbar spinal arteries |
| Rat laser photoablation (Rose Bengal dye intravenously) |
| Excitotoxic injury |
| Kainic, quisqualic or ibotinic acid or dynorphin spinal cord microinjection |
| Regeneration models |
| Spinal cord transection, resection or hemisection |
| Dorsal rhizotomy |
Focal Ischemic Stroke Models
Various stroke models have been developed during the past 20 years [10]. However, the main ones in use today are the unilateral middle cerebral artery occlusion (MCAO) models used in rats and mice. Since these models were first developed in the 1980s, the MCAO has been variably induced by surgical ligation or cauterization via a small craniotomy over the middle cerebral artery (MCA), passage of an intraluminal nylon suture up into the ipsilateral cerebral circulation via the external carotid in the neck or via injection of a small autologous thrombus into the common carotid artery. The latter 2 are the most commonly employed today, and the thromboembolic paradigm is the most clinically relevant since the majority of human focal ischemic strokes involve a thromboembolic occlusion of the MCA. The MCAO models come in 2 varieties, temporary and permanent. The temporary MCAO involves removal of the vascular occlusion at varying times (30, 60, 90, 120 and 180 min) after the onset in order to allow reperfusion of ischemic tissue to take place. This is accomplished by surgical removal of the extraluminal or intraluminal occlusion device, and mimics either the instance where spontaneous thrombus dissolution may take place during the first 3 h after the beginning of the stroke due to activation of endogenous thrombolytic processes (believed to be a fairly rare occurrence), or the situation in which tissue plasminogen activator (tPA) is used for the purpose of dissolving the clot and restoring recirculation. Although removal of the vascular occlusion and reestablishment of the normal cerebral circulation is an obviously desirable therapeutic goal, it is well known that it can lead to reperfusion injury, caused by a burst of reactive oxygen species in the previously ischemic brain tissue. Thus, there is a need for neuroprotective agents to reduce the pathophysiological events from the initial ischemic insult and the subsequent deleterious side effects of recirculation. Accordingly, the temporary MCAO models are most useful for evaluating neuroprotective strategies that are used in conjunction with tPA and other treatments to reestablish blood flow. The effectiveness of pharmacologic thrombolysis decreases rapidly within 5 h of the onset of ischemia. Because of the narrow therapeutic window, currently only a small fraction of ischemic stroke patients receive treatment. Furthermore, MCAO animal studies have shown that reperfusion beyond the first 3 h does not lessen the extent of ischemic damage. Thus, the temporary MCAO models, although widely used in stroke research, actually have limited relevance to the majority of MCA territory strokes.
Another variety of focal ischemic stroke model, the permanent MCAO, where the occlusion is permanently left in place, may be a better model of the vast majority of strokes where recirculation has not been reestablished either spontaneously or pharmacologically during the critical first few hours after stroke onset. In this instance, the therapeutic goal is to reduce the expansion of the ischemic damage from the severely ischemic core area into the surrounding penumbral area. The ischemic penumbra is potentially salvageable for several hours due to its partial circulation from collateral blood vessels. While the permanent MCAO version may be the best option for preclinical evaluation of potential neuroprotective agents, testing of compounds in the temporary MCAO paradigm is also recommended. In either model, historically, investigators have used reductions in infarct size after short periods of observation (that is, 7 days) as the primary endpoint. This is not an ideal outcome measure since infarct size correlates poorly with functional outcome.
Most current stroke research with either the temporary or permanent MCAO models is carried out in mice or rats. The primary endpoints are generally behavioral and in motor or neurological function typically determined between 24 and 72 h after stroke onset, and sometimes after longer periods of time. However, MCAO models have been developed and are occasionally used in higher species including the cat, monkey and baboon; the use of these animal models for neuroprotective drug evaluation carries considerable expense. Some investigators believe that it is important to replicate pharmacological neuroprotective actions in these gyrencephalic species prior to movement of the compound into human clinical trials. In actuality, there is no solid comparative evidence that supports the notion that neuroprotective effects seen in rodent stroke models are not predictive of human efficacy. Furthermore, there are presently no firm data which support the commonly held idea that the therapeutic time window for a particular neuroprotective mechanism in a rat stroke model (for example 1 h) may be longer in nonhuman primates or humans (for example 6 h). On the contrary, the fact that various neuroprotective compounds which demonstrated a rather limited (1–2 h) therapeutic window for reduction of infarct size in rat MCAO models subsequently failed to improve outcome of stroke patients in clinical trials where the treatment initiation time varied from 6 to 24 h is consistent with the concept that the therapeutic window for neuroprotective effects may not be all that different between rodents and primates. At least no difference has been firmly demonstrated.
Cardiac Arrest/Resuscitation (Transient Global Ischemia) Models
Cardiac arrest produces immediate total body ischemia. Upon successful resuscitation, the previously ischemic organs, including the globally ischemic brain, are reperfused with blood and in the process suddenly flooded with oxygen. As noted before, this reperfusion/reoxygenation, while essential for maintenance of life, can nevertheless result in reperfusion injury. The combination of the ischemic insult plus the subsequent reperfusion injury phenomenon can damage selectively vulnerable neurons in proportion to the duration of blood flow interruption. The therapeutic goal is to mitigate this secondary neuronal injury which does not become fully manifest until between 24 and 48 h and perhaps a month after the insult. The most straightforward animal models involve the induction of a human-like cardiac arrest and resuscitation within the next several minutes. Most such studies have been performed in dogs, but rat, mouse and swine models are also utilized.
The vast majority of cardiac arrest/resuscitation neurologically focused studies utilize rodent models of transient global cerebral ischemia without stopping and restarting the heart. This general approach allows for cerebral ischemia like that occurring in cardiac arrest in humans to be studied in isolation. Of the 3 commonly used transient global cerebral ischemia models, the oldest is the gerbil bilateral carotid occlusion model. The gerbil brain has a high incidence of an incomplete Circle of Willis due to lack of the posterior communicating arteries that in other mammals connect the basilar to the carotid circulation. Therefore, 5 min of forebrain ischemia followed by reperfusion in the gerbil leads to a selective loss of hippocampal CA1 neurons that is apparent by 48 h; 10–15 min results in broader hippocampal damage as well as loss of cortical, striatal and nigrostriatal neurons [11, 12]. The gerbil model has fallen out of favor due to a high degree of interanimal variability based upon the fact that the circulatory anomaly is inconsistent with some gerbils having one or both posterior communicating arteries. Two other commonly used rat transient forebrain ischemia models are the 2-vessel occlusion plus hypotension paradigm developed by Siesjo and colleagues nearly 25 years ago [13, 14] and the 4-vessel occlusion model which involves prior surgical cauterization of the vertebral arteries followed by transient occlusion of both carotid arteries for 5–20 min [15, 16]. Brief episodes of forebrain ischemia (5–10 min) in either model produce selective hippocampal CA1 damage with longer episodes (10–20 min) producing additional damage in the cortex and striatum. Efficacy of neuroprotective compounds in the transient forebrain ischemia models suggests that these might be useful in cardiac arrest and resuscitation. However, confirmation of efficacy should be obtained in an actual cardiac arrest resuscitation model with improved survival, neurological recovery and a reduction in neuronal damage.
Hemorrhagic Stroke Models
As noted earlier, the 2 basic types of hemorrhagic strokes are ICH and SAH. For the former, the approach is simply to inject a volume of the animal’s own blood directly into brain parenchyma followed by an analysis of the volume of damage to the surrounding brain tissue. In the latter, most models involve injection of a volume of autologous blood, withdrawn immediately prior to SAH induction from the systemic circulation of the animal (for example, pig, rat, cat, rabbit and dog), into the subarachnoid space via injection into the cisterna magna or over one of the cerebral hemispheres via a small burr hole and puncture of the dura mater covering the brain. Common endpoints for drug evaluation include measurement of blood-brain barrier compromise or decreases in cerebral blood flow during the first several post-SAH hours or the assessment of cerebral vasospasm by histological or arteriographic methods between 2 and 7 days. A sophisticated SAH model involves the neurosurgical placement of an autologous blood clot around the base of the MCA in monkeys followed by arteriographic and histological ischemic damage measurements at 7 days. However, the cost of evaluating a single-dose level of a drug for its ability to inhibit delayed cerebral vasospasm in that model runs into the hundreds of thousands of dollars.
Subdural Hematoma Model
The rat lacks an arachnoid membrane, and thus the rat version of the SAH model involving injection of blood through the dura mater can also be thought of, and employed, as a subdural hematoma model. When autologous, nonheparinized blood is injected through the dorsal burr hole through the dura mater, it typically forms a clot over the dorsal surface of the brain mimicking a posttraumatic subdural hematoma, similar to the management of human subdural hematomas, the experimental protocol involves surgical removal of the clot at a specified time followed by histological measurement of ischemic damage caused by the hematoma [17].
TBI Models
In vivo TBI models include 3 basic types: diffuse, focal and axonal injury (table 1) [18]. Of the 3 diffuse injury models, the first is the rat fluid percussion TBI paradigm in which a transient hydraulic pressure pulse is applied to the exposed dura mater either over the midline of the brain or laterally over one of the hemispheres. The second is the rat impact-acceleration injury model in which a 0.5 or 1.0 kg weight is dropped onto a steel helmet cemented onto the exposed skull, and the third is the mouse weight-drop concussion paradigm. The pig or primate rotational acceleration models are useful for studying the phenomenon of diffuse axonal injury.
For the induction of focal TBIs, a controlled cortical impact model is widely used in either rats or mice and involves the infliction of a contusion injury through a small craniotomy. The magnitude of the injury is generally varied by the depth of the cortical indentation (usually 0.5–1.0 mm in mice and 1.0–2.0 mm in rats). The controlled cortical impact model mimics TBI-induced brain contusions, although a recent study has shown that the subsequent neurodegeneration is not as focal as generally thought [19]. A relatively new in vivo model utilizing a controlled stretch of the optic nerve in mice has been developed to examine the effects of stretch injury on axons.
SCI Models
Many SCI paradigms have been developed over the past 100 years. As shown in table 1, for evaluation of neuroprotective agents, the current rodent models use contusion, compression, ischemic and excitotoxic injury mechanisms. By far, the contusion models predominate in the experimental acute SCI field, and in particular the New York University [20] and University of Kentucky [21] controlled contusion devices dominate acute SCI research. For investigations of axonal regeneration in the injured spinal cord, either complete transaction or hemisection of the spinal cord or dorsal roots (rhizotomy) followed by histological assessments of axonal growth across the lesion site is used. Assessment of neurological recovery in rat SCI models most commonly employs the Basso/Beattie/Bresnahan locomotor recovery assessment tool (BBB Score) [22]. However, a variety of other motor recovery assessment tools are also often employed along with the BBB scoring system.
Pediatric Stroke and TBI Models
Ischemic brain injury and TBI occur in children and adults, and there is an alarming incidence of neonatal and pediatric stroke and TBI [23, 24]. Over the past several years, there has been an increasing realization that the response of the brain to ischemic brain injury or TBI in infants and children differs from the adult brain in regards to pathophysiology, secondary injury processes, cell death mechanisms (for example, necrotic vs. apoptotic), susceptibility of different brain regions and the capacity for plasticity and recovery [23–26]. This has prompted the development of stroke and TBI models in immature animals that are either uniquely designed for younger animals or are scaled-down versions of adult stroke or TBI models in either rodents or larger animals [23, 25–29]. Clearly, the development of therapies for acute neurological insults in the pediatric population should be preceded by studies of these in models employing immature animals.
What Have Previous Clinical Trials of Neuroprotective Agents Taught Us about the Needs for Preclinical Drug Evaluation in Animal Models?
In the early 1980s, pharmaceutical companies began developing neuroprotective drugs for the acute treatment of stroke and CNS injury. Eventually, many compounds made their way into large double-blind multicenter phase III clinical trials for stroke (ischemic and SAH), TBI and/or SCI. These efforts, which dominated neuroprotective clinical trials in the late 1980s and 1990s, were primarily directed at 3 general pharmacological mechanistic strategies to interrupt secondary injury processes: (1) inhibition of glutamate-mediated excitotoxicity [glutamate receptor antagonists and γ-amino-butyric acid (GABA) agonists], (2) reduction of intracellular calcium overload (L-type calcium channel blockers) and (3) interruption of reactive oxygen-mediated damage (free radical scavengers/antioxidants). Unfortunately, despite the multiple trials and literally hundreds of thousands of patients studied, little clinical benefit has resulted from these efforts as briefly reviewed below.
Glutamate Receptor Antagonists
Multiple glutamate receptor antagonists were taken into phase II and III trials, including the competitive NMDA receptor antagonists selfotel (CGS 19755) and aptiganel (CNS 1102) which block the binding of glutamate to its receptor complex recognition site, eliprodil which blocks the polyamine site and CP-101606 which blocks the NR2B subunit on the NMDA receptor complex. None of these produced a statistically significant improvement in neurological recovery in TBI or ischemic stroke trials [30, 31].
GABA Receptor Agonists
Another mechanism for countering glutamate excitotoxicity is to increase GABA-mediated inhibitory transmission with the administration of GABA receptor agonists. This approach resulted in the clinical evaluation of the GABA partial agonist compound chlomethiazole in a phase III stroke trial. However, no significant beneficial effect was demonstrated [30, 31].
Calcium Channel Blockers
Accumulation of intracellular calcium plays a major role in secondary injury after CNS injury or stroke. One mechanism for postinsult calcium overload involves depolarization-induced entry via voltage-dependent L-type channels. Accordingly, the first neuroprotective approach to be tested in phase III clinical trials in TBI or stroke was the competitive L-type calcium channel blocker, nimodipine, which was entered into the clinical trials in the late 1970s. In 2 different phase III multicenter TBI (moderate and severe) trials [32] and a single-stroke trial [31], no overall benefit was revealed with nimodipine treatment. However, retrospective analysis of the TBI trials has revealed that nimodipine may improve outcome in patients with traumatic SAH (tSAH) [32]. This is not an insignificant finding since about half of all patients with severe TBI, have tSAH as part of the pathophysiology. Furthermore, nimodipine has been shown to produce a slight, but significant increase in survival in aneurysmal SAH patients and have been approved in most countries for the treatment of that condition. Indeed, nimodipine represents the first agent to be approved for neuroprotective use even though much of its effect is probably mediated via protection of the microvasculature and vasodilation-mediated improvements in cerebral blood flow. Due to a manifestation of its microvascular vasodilation, the compound must be used with care, since it can lower arterial and cerebral perfusion pressures which can exacerbate posttraumatic, postischemic or post-SAH secondary brain injury.
Free Radical Scavengers
In order to interrupt reactive oxygen damage, the polyethylene conjugated form of the superoxide radical scavenger Cu/Zn superoxide dismutase (PEG-SOD) was evaluated in trials conducted in moderate and severe TBI patients. Although a positive trend was found in an initial small phase II trial [33], subsequent phase III trials failed to show any enhancement of neurological recovery [34].
A bigger development program was undertaken with the 21-aminosteroid lipid peroxidation inhibitor tirilazad. Tirilazad was extensively evaluated in animal models of SCI, TBI, ischemic stroke and SAH, and shown to exert a variety of neuroprotective and vasoprotective effects [35, 36]. Based upon these preclinical studies, clinical trials of tirilazad were conducted in TBI [34, 37], SAH [38], ischemic stroke [30, 31] and SCI [39]. In TBI, an initial North American trial of 1,100 patients comparing tirilazad treatment with placebo for 5 days, either initiated 4 h after injury, ended with such a confounding randomization imbalance that no meaningful efficacy analysis could be extracted. In contrast, a successfully completed European phase III trial failed to show an overall effect in moderately and severely injured patients. However, post hoc analysis revealed that the compound significantly improved survival in both moderately and severely injured male patients with tSAH [37]. This beneficial effect in the tSAH subgroup, which represents about half of severe TBIs, was not surprising, since the drug had previously been shown to improve recovery and survival in a phase III trial in aneurysmal SAH patients [38]. Interestingly, this effect in tSAH and aneurysmal SAH was mainly apparent in male patients. This gender difference was found to be partially due to a faster rate of metabolism of the drug in females. Nevertheless, subsequent female-only trials with higher tirilazad doses that were calculated to duplicate the exposure levels in males did not reveal the same level of efficacy as seen in male patients, although beneficial effects were apparent in the more severe SAH females [40, 41]. The issue of gender differences in neuroprotective drug responsiveness clouds the interpretation of tirilazad’s as well as other drugs’ neuroprotective efficacy.
Tirilazad was also extensively evaluated in 4 different phase III stroke trials [30, 31]. The first 2 (TESS I in Europe and RANTTAS I in the US) evaluated the effects of 6 mg/kg intravenously per day for 3 days with treatment beginning within 6 h after onset of the stroke. No effect was seen on 3- or 6-month outcome. Two subsequent higher dose trials (10 mg/kg per day in males; 15 mg/kg per day in females) were conducted. The first of these, the European TESS II which included patients enrolled within the first 6 h of the stroke, was stopped prematurely due to a significant increase in morbidity and mortality in the high-dose tirilazad group. Prudence dictated the simultaneous cessation of the parallel US high-dose RANTTAS II trial. However, subsequent analysis of the 3-month recovery scores of the approximately 100 patients who had already been enrolled in RANTTAS II revealed a nearly significant improvement in neurological recovery. The only difference between the 2 trials was that in TESS II, the enrollment window was 6 h, whereas in RANTTAS II, treatment began within 4 h. The contrasting results of TESS II and RANTTAS II indicate that tirilazad may be effective in stroke patients if given in the first 4 h, but may in fact be harmful if delayed until 6 h. Another issue besides the therapeutic window is the issue of how long to maintain treatment. The decision to treat stroke patients in all tirilazad trials for 72 h was based on the limits of safety rather than on a demonstration of the benefits of such lengthy treatment in preclinical stroke models [35]. The toxicity of the drug in TESS II indicates that it is possible to overtreat with the drug. Thus, the possibility exists that a shorter treatment duration may have yielded more positive results. The fact that neither the optimum therapeutic window nor the optimal treatment duration were ever determined for tirilazad or any other neuroprotective drug prior to their being advanced into clinical trials for TBI or stroke may have played a role in the failures of NMDA antagonists, the calcium channel blocker nimodipine and the antioxidants PEG-SOD and tirilazad in achieving an overall beneficial effect.
Most recently, the nitrone-based free radical scavenger NXY-059, which had been more thoroughly tested in stroke models than any previous stroke-directed neuroprotective compound [42], was evaluated in phase III trials in ischemic stroke. Although an initial trial showed an apparent benefit [43], a subsequent larger trial failed to confirm the efficacy of the drug [44].
This brief history of neuroprotective drug discovery and development over the past 20–25 years could be fairly characterized as a series of often high profile and expensive failures. Although these have largely dampened the enthusiasm of the pharmaceutical industry for this therapeutic area, much has been learned from them that could, and should, serve as a roadmap for future efforts aimed at pharmacological neuroprotection and improved neurological recovery after stroke, TBI and SCI. Postmortem analyses of mistakes made in stroke [30, 31] and TBI [32] drug development have been published and a careful reading of them reveals a host of shortcomings in past preclinical testing of candidate neuroprotective agents and in clinical trial design and conduct that need to be addressed in the future. A summary is provided in table 2.
Table 2.
Reasons for past failures in neuroprotective drug discovery and development
| Inadequate understanding of secondary injury mechanisms |
| Lack of definition of time course of glutamate receptor functional changes |
| Lack of definition of the sources and spatial and temporal characteristics of reactive oxygen generation → inability to rationally determine therapeutic window and optimum treatment duration |
| Lack of understanding of the interrelationship of secondary injury mechanisms |
| Focus on secondary injury mechanisms with short therapeutic windows → need to identify and target injury mechanisms with longer therapeutic windows |
| Lack of understanding of the relative therapeutic windows in animal models and humans; is the time course of secondary injury in mice, rats and men similar? |
| Inadequate preclinical testing |
| Lack of testing in multiple models |
| Failure to compare efficacy in male and female animals |
| Incomplete dose response and definition of therapeutic plasma levels |
| Incomplete definition of therapeutic window |
| Lack of definition of pharmacokinetics, timing of needed maintenance dosing and optimum treatment duration |
| Poor clinical trial design |
| Gross mismatch between preclinical and clinical testing |
| Imprecise outcome scales (Glasgow Coma Scale; Glasgow Outcome Scale; ASIA scale, NIH stroke scale) |
| Lumping of all kinds of ischemic strokes or moderate and severe TBIs |
| Lack of identification and a priori plan to analyze subgroups (tSAH; MCA territory strokes) |
| Lack of biomarker to follow the progression of the pathophysiology and monitor mechanistic drug effects |
| Lack of standardization of neurorehabilitation protocols |
First of all, the discovery of the first generation of neuroprotective agents including glutamate receptor antagonists, calcium channel blockers and antioxidants occurred prior to the elucidation of an adequate understanding of the intricacies of the targeted secondary injury mechanisms. In each case, there was an inadequate knowledge of the time course and interrelationships of these events, their therapeutic windows for effective treatment intervention and how these were either similar or different between species, injury models, genders as well as between animals and humans. In the case of reactive oxygen mechanisms, our knowledge of the key reactive oxygen species as well as their sources and cellular targets was inadequate to guide the design of optimum antioxidant neuroprotective compounds. Secondly, the preclinical efficacy testing of compounds was often woefully inadequate and even naïve. From this experience, we can derive several lessons that need to be considered in preclinical evaluations of neuroprotective agents so that the chance of translational success in clinical trials is maximized.
Issues that Need to Be Addressed in Preclinical Neuroprotective or Neurorestorative Drug Evaluation
Neuroprotective Drug Evaluation
The following issues/questions need to be addressed in preclinical evaluation of drugs for acute neuroprotection.
A thorough demonstration of the time course of the target pathophysiological mechanism in relevant animal models is necessary to determine when treatment needs to begin and how long it must be maintained. This must be done in both male and female animals based upon several studies showing that the magnitude and duration of post-ischemic and posttraumatic pathophysiology may differ greatly between genders in certain models [45–50].
A rigorous dose-response analysis in regards to effects on the target mechanism, ability to reduce posttraumatic neurodegeneration and improve behavioral and neurological recovery is necessary.
A correlation of neuroprotective action with plasma and CNS tissue pharmacokinetics; that is, a definition of the effective neuroprotective concentration and a dosing protocol that is adequate to maintain the therapeutic concentration for as long as the target secondary injury mechanism is active is needed.
A comparison of single- versus multiple-dose regimens in order to establish optimum treatment regimen (intravenous bolus plus infusion make the most sense) should be undertaken.
A determination of the therapeutic window in order to know how early treatment must begin is necessary. It has been argued that even if a particular agent only has a 1-hour window in a rat stroke, TBI or SCI model, the window in humans with the corresponding condition is likely to be much longer. However, there is little evidence to support this assumption. Consequently, clinical trial design should take the preclinical therapeutic window definition for a particular agent seriously in regards to how soon the compound may need to be given to patients. With this in mind, a failure to demonstrate a clinically practical therapeutic window for a particular agent in an animal model may mean that this agent and its corresponding secondary injury mechanism may be too short to be effectively addressed in real world therapeutics.
The above-mentioned parameters dose response, optimum treatment duration and therapeutic window are most likely to vary between TBI, ischemic stroke, cardiac arrest/resuscitation, SAH and SCI models.
A comparison of the neuroprotective pharmacology (dose response, optimum treatment duration and therapeutic window) in multiple injury models (focal versus diffuse TBI) in order to determine whether the agent in question only works in certain types of injuries is needed.
A comparison of the neuroprotective pharmacology in male versus female animals is necessary.
A determination of pharmacodynamic and pharmacokinetic interactions with other commonly used ancillary treatments (anticonvulsants, minor and major tranquilizers) should be undertaken.
Health characteristics of animals must be taken into consideration. While much has been learned from preclinical ischemia studies concerning mechanisms of injury and neuroprotection, it must be considered that the animal models of ischemia do not closely mimic the human disease. In most cases, animals that are studied are usually young, normal, healthy animals, whereas humans suffering the diseases/disorders mentioned often have other existing morbidities, such as age, hypertension, diabetes, myocardial infarction, arrhythmias or other ongoing disease processes. These ongoing disease processes likely alter how and when therapies may be effective.
For a further discussion of the ideal characterization of an acute neuroprotective agent, the reader is referred to the chapter by del Zoppo et al. [this vol., pp. 34–38] which discusses the STAIR criteria for preclinical evaluation of therapies for acute stroke.
Neurorestorative Drug Evaluation
The issues/questions discussed above for testing of neuroprotective drugs are equally relevant to neurorestorative drug evaluation:
A definition of the time course of endogenous repair mechanisms (trophic and growth factor expression, growth-associated protein expression) is necessary.
A rigorous dose-response analysis in regards to effects on the target mechanism and ability to improve behavioral and neurological recovery is required.
A correlation of neurorestorative action with plasma and CNS tissue pharmacokinetics is needed; that is, a definition of the effective neurorestorative concentration, be it cellular or pharmacologic, and a dosing protocol that is adequate to maintain the therapeutic concentration for as long as it is needed to maximize the behavioral recovery improvement.
Optimum treatment regimen should be established (Is chronic short-term treatment all that is required?).
A determination of the therapeutic window is necessary in order to know how early treatment must begin.
A comparison of the neurorestorative pharmacology and cellular therapy (dose-response, optimum treatment duration and therapeutic window) in multiple injury models (focal vs. diffuse TBI) is required in order to determine whether the agent in question only works in certain types of injuries.
A comparison of the neurorestorative approach in male versus female animals is needed.
A determination of pharmacodynamic and pharmacokinetic interactions with other commonly used ancillary treatments (anticonvulsants, minor and major tranquilizers) should be undertaken.
Gene and Cellular Therapies
The principles and needed therapeutic definitions outlined above for neuroprotective or neurorestorative drugs are equally applicable to the preclinical evaluation of gene or cellular therapies for either acute neurological injuries or chronic neurodegenerative conditions. What is the ideal number of gene copies or cells needed to achieve the best effect (that is, dose)? What is the ideal timing for gene vector administration or cellular transplant (that is, therapeutic window)? Is a single administration all that is required or is repeated administration or transplant needed in order to maximize efficacy (that is, optimum duration of treatment)? Do gender-based hormonal differences or ancillary drug treatments make a difference in the response to gene administration or transplant survival and proliferation?
Utility of Transgenic and Gene Knockout Models for Preclinical Therapeutic Evaluation
The development of transgenic and genetic knockout (KO) technologies in mice (and to some extent in rats) has provided important tools that have helped to identify and validate the importance of certain secondary injury mechanisms as well as genes that control neuronal repair, plasticity and axonal regeneration. These have been employed extensively over the past decade in neurological research. For example, the importance of oxidative damage mechanisms in acute neurological injury models has been confirmed by the demonstration that if one increases the expression of certain antioxidant genes (such as Cu,Zn superoxide dismutase) by incorporation of multiple copies of that gene into the mouse genome, this results in a decreased sensitivity to ischemic or traumatic insults [51]. Alternatively, genetic knockout of the same antioxidant gene increased vulnerability of the CNS to the same injuries [51]. However, certain caveats are often cited in regards to the use and interpretation of genetically modified mice. One of the main ones is that the overexpression or KO of a gene does not necessarily occur in isolation. Changes in one gene may lead to upstream or downstream changes in the expression of other genes which play a role in the phenotype of the model.
An increasingly employed contemporary strategy for controlling genetic overexpression or KO is through the use of gene constructs that include a switch for regulation of the temporal expression of the gene in question via activation or inhibition of the promoter region of the gene. This strategy, referred to as conditional overexpression or more commonly conditional KO, typically involves a genetic response element (switch) that can be triggered to turn off gene expression upon administration of a drug. The most common one is the TET-OFF switch which shuts off the expression of the target gene upon administration of the tetracycline compound doxycycline via the drinking water. Cessation of doxycycline administration usually allows the gene to come back on. This technology is being increasingly applied in neurological research. For instance, conditional KO mice are being used to explore the role of certain matrix proteins in TBI models [52] and the effects of altered neurofilament expression in neuronal structure and function [53], just to name 2 examples. This approach lessens the problem seen in nonconditional transgenic and KO mice in which the chronic change in one gene may cause changes in other genes such that the phenotype is not specifically related to the changes in expression of the target gene. Moreover, the conditional on-off approach allows for the manipulation of genes, whereas if they were knocked out permanently in the mouse, the result would be embryonic or early postnatal lethality.
In addition to the control of the temporal expression of a gene, it is now possible to specifically manipulate the expression of a particular gene in specific brain regions. This region-specific KO is accomplished by use of the Cre recombinase (also known as Cre-Lox) technology in which mice are first generated with an inducible tissue-specific promoter for expression of Cre. These mice are crossed with a second mouse line in which the gene of interest can be knocked in by flanked Cre recognition sequences known as Lox-P sites. The target gene in the resulting double transgenic mouse is then induced through administration of a drug [54]. One recent application of this technology involved an examination of the role of vascular endothelial growth factor in regulating brain angiogenesis and neuronal apoptotic cell death [55]. Lastly, it is now possible to develop conditional KO mice in which a gene of interest can be knocked out in a particular cell type such as in astrocytes [56] or endothelial cells [57].
Although there may be some applications of genetically modified mice in drug testing, these technologies are mainly useful for identifying the physiological or pathophysiological role of certain candidate genes and validation of potential neuroprotective or neurorestorative therapeutic targets.
Outcome Measures in Preclinical Models
A multitude of physiological, neurophysiological, neurochemical, histological, imaging and behavioral outcome measures have been employed in animal models of acute neurological injury. The choice of endpoints depends upon the species, the particular acute insult, the main pathophysiological elements, whether the therapeutic approach is neuroprotective or neurorestorative and whether the target mechanism is known and measurable. In general, the preclinical evaluation of potential therapies should include multiple endpoints. Table 3 lists the main endpoints/outcome measures and their timing range that have been employed for therapeutic efficacy evaluation in models of the different types of acute neurological insults.
Table 3.
Endpoints and outcome measures commonly employed in in vivo models for discovery of neuroprotective and neurorestorative agents
| Indication | Endpoints/outcome measures | Timing |
|---|---|---|
| Stroke (focal ischemia) | Metabolic imaging (PET, MRS) | minute → hours |
| Neurochemical or immunohistochemical mechanistic markers |
minute → hours | |
| Physiological – CBF, edema | minute → hours | |
| Infarct size – histological or by MRI | 24 h → 7 days | |
| Behavioral recovery – multiple scales | 24 h → 7 days | |
| Cardiac arrest/resuscitation (transient global ischemia) |
Metabolic imaging (PET, MRS) | minute → hours |
| Neurochemical or immunohistochemical mechanistic markers |
minute → hours | |
| Physiological – CBF | minute → hours | |
| Neurophysiological – evoked potential, EEG |
minute → hours | |
| Histological assessment of neuronal loss | 3 h → 7 days (sometimes longer | |
| Survival and behavioral recovery | 24 h → 7 days (sometimes longer) | |
| Hemorrhagic stroke models (SAH or ICH) |
Physiological – CBF, BBB opening, edema | minute → hours |
| Neurochemical or immunohistochemical mechanistic markers |
minute → hours | |
| Biochemical measurements in SAH clot | 1 → 7 days | |
| Cerebral vasospasm – angiography | 48 h → 7 days | |
| Histological assessment of ischemic damage |
48 h → 7 days | |
| TBI | Metabolic imaging (PET, MRS) | minute → hours |
| Neurochemical or immunohistochemical mechanistic markers |
minute → hours | |
| Physiological – CBF, BBB opening, edema | minute → hours | |
| Histological assessment of neuronal/ axonal loss |
24 h → 7 days | |
| Behavioral recovery – motor and cognitive scales |
48 h → 28 days | |
| Subdural hematoma | Metabolic imaging (PET, MRS) | minute → hours |
| Neurochemical or immunohistochemical mechanistic markers |
minute → hours | |
| Physiological – CBF, BBB opening, edema | minute → hours | |
| Histological assessment of neuronal/ axonal loss |
24 h → 7 days | |
| SCI | Neurochemical or immunohistochemical mechanistic markers |
minute → hours |
| Physiological – SCBF | minute → hours | |
| Neurophysiological – sensory or motor evoked potentials |
minute → hours → days 42 days |
|
| Histological assessment of neuronal/ axonal loss |
42 days | |
| Locomotor recovery | ||
Restrictions and Ethical Considerations in Animal Studies
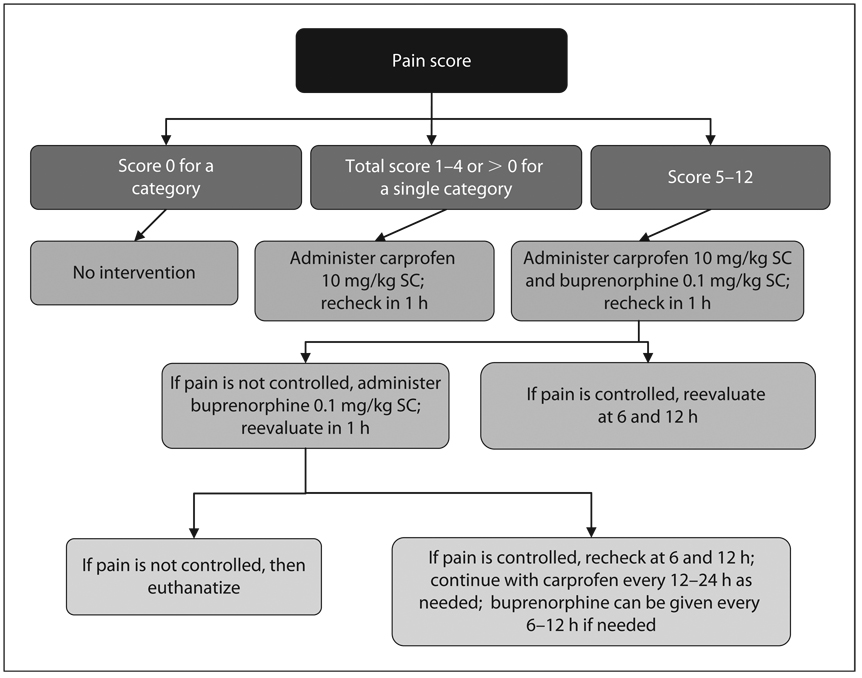
Animal modeling in the acute neurological injury arena is associated with a higher degree of relevance for therapeutic evaluation due to the fact that the current stable of models are able to reliably replicate human traumatic, ischemic or hemorrhagic neurological injuries. In other words, the animal models are closer to the human condition. This has been achieved largely as a result of our fairly well-established understanding of the pathophysiology and neuropathology of human stroke, TBI and SCI, epilepsy, Alzheimer’s disease and Parkinson’s disease. Thus, the rationale for the use of animal models in the discovery of therapeutic approaches for these diseases is strong even though there are lingering questions about the similarity in the pathophysiological time courses in mice and rats versus humans. In sharp contrast, animal modeling of many of the psychiatric disorders currently involves guesswork and assumptions. Psychiatric disease models can at best emulate a particular aspect, but not the complete symptom complex seen for example in schizophrenia, depression or anxiety. However, despite the arguably greater validity of acute CNS injury models, because they each involve surgical preparation and inflicting damage to the brain or spinal cord, there is the possibility of pain and distress which must be considered and minimized by appropriate use of analgesics and other veterinary care. As with all types of animal models, approval for the use of CNS injury paradigms requires careful review by veterinary staff and an Institutional Animal Care and Use Committee to insure that the models are being used by competent investigators and that that the methods have been refined to minimize distress, alleviate pain and reduce the number of animals necessary for the conduct of good scientific evaluation. In regards to pain assessment and minimization, table 4 provides a pain assessment scale for use in mouse or rat CNS injury paradigms. Figure 1 shows an algorithm that can be used for pain management, should the pain assessment indicate that analgesic intervention is needed; however, it is exceedingly rare that analgesia is required. Moreover, although neither of the analgesics listed – carprofen, a nonsteroidal anti-inflammatory agent and buprenorphine, a κ-opoid receptor agonist – have been specifically examined in acute neurological injury models, other NSAIDS and κ-agonists have been shown to produce neuroprotective effects. Thus, the use of these analgesic agents might potentially complicate acute neuroprotection studies. However, current National Research Council Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (2003) dictate that pain assessments and pharmacological analgesia be available should particular animals require it. The guidelines also call for the responsible minimization of the number of animals used. Concerning the responsible reduction of the number of mice, rats or other animals used in preclinical evaluation of therapies for acute neurological injuries, all models covered in this chapter have a long record of published use employing a variety of short-term and longer-term endpoints. Consultation of this literature can provide a clear idea of the variability and required sample sizes that are needed in the hands of experienced investigators.
Table 4.
Pain scale for rodents after cranial surgery
| Criteria/score | 0 | 1 | 2 | 3 | Total |
|---|---|---|---|---|---|
| Locomotion | Moving normally around cage, not hugging the sides of the cage |
Stumbling, falling or hugging the sides of the cage |
Writhing, stumbling and/ or falling; OR movement only when stimulated |
No movement | |
| Pain on palpation of surgery site |
None | Mild (occasional vocalization or pulls head back, or kicks at evaluator) |
Moderate (frequent vocalization and pulls head back, or kicks at evaluator) |
Severe (vociferous vocalization, withdraws head, bites, struggles) |
|
| Behavior | Normal cage exploration, normal food and water consumption, animal calm in cage; previously social animal still social |
Minimal exploration, increased or decreased food and/or water consumption; previously social animal has become withdrawn or aggressive |
No cage exploration, hunched posture, anorexic for 24 h |
No cage exploration, hunched posture, piloerection, anorexic, increased respiratory rate or labored breathing |
|
| Appearance of incision |
Clean, no scratching at incision, no redness, no swelling |
Mild scratching at incision, redness, suture intact; mild swelling |
Severe scratching, incision open; obvious swelling |
Incision infected (redness, swelling, purulent drainage) |
|
| Total pain score: ______ | |||||
| Initials: ______________________ | |||||
| Date: ________________________ | |||||
| Time: ________________________ | |||||
| Analgesic administered based on total pain score and flow chart: ___________________________ | |||||
Fig. 1.
Analgesic flow chart for rodents after cranial surgery.
Summary
This chapter on the role of animal studies in preclinical therapeutic evaluation has been set within the context of acute ischemic, hemorrhagic and traumatic injuries. Although there has been a long list of translational failures in regards to neuroprotective drugs for ischemic stroke and TBI, this experience has provided us with several valuable lessons in regards to what we did wrong in past efforts and what we need to do better to achieve translational success in the future. Among these lessons is the knowledge that preclinical evaluation of drugs, gene therapies and cellular transplantation in animal models needs to be thorough and define the optimal treatment parameters, that is, dose, timing, duration, gender differences in responsiveness and how other pharmacological treatments may positively or negatively impact neuroprotective or neurorestorative efficacy. Subsequent to a thorough preclinical evaluation, clinical trial design needs to carefully consider and take full advantage of the therapeutic parameters derived from animal studies. Although preclinical evaluation in animal models needs to be thorough and statistically rigorous, careful consideration of animal welfare and minimization of sample sizes should not be ignored in the process.
References
- 1.Kofler J, Hattori K, Sawada M, DeVries C, Martin LJ, Hurn PD, Traystman R. Histopathological and behavioral characterization of a novel model of cardiac arrest and cardiopulmonary resuscitation in mice. J Neurosci Methods. 2004;136:33–44. doi: 10.1016/j.jneumeth.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 2.Nishikawa T, Kirsch JR, Koehler RC, Miyabe M, Traystman RJ. Competitive N-methyl-d-aspartate receptor blockade reduces brain injury following transient focal ischemia in cats. Stroke. 1994;25:2258–2264. doi: 10.1161/01.str.25.11.2258. [DOI] [PubMed] [Google Scholar]
- 3.Nishikawa T, Kirsch JR, Koehler RC, Miyabe M, Traystman RJ. Nitric oxide synthase inhibition reduces caudate injury following transient focal ischemia in cats. Stroke. 1994;25:877–885. doi: 10.1161/01.str.25.4.877. [DOI] [PubMed] [Google Scholar]
- 4.Noppens RR, Kofler J, Hurn PD, Traystman RJ. Dose-dependent neuroprotection by 17β-estradiol after cardiac arrest and cardiopulmonary resuscitation. Crit Care Med. 2005;33 doi: 10.1097/01.ccm.0000169884.81769.f7. 15951-602. [DOI] [PubMed] [Google Scholar]
- 5.Traystman RJ, Kirsch JR, Koehler RC. Oxygen radical mechanisms of brain injury following ischemia and reperfusion. J Appl Physiol. 1991;71:1185–1195. doi: 10.1152/jappl.1991.71.4.1185. [DOI] [PubMed] [Google Scholar]
- 6.Fournier AE, Strittmatter SM. Repulsive factors and axon regeneration in the CNS. Curr Opin Neurobiol. 2001;11:89–94. doi: 10.1016/s0959-4388(00)00178-1. [DOI] [PubMed] [Google Scholar]
- 7.Gallo G, Letourneau PC. Regulation of growth cone actin filaments by guidance cues. J Neurobiol. 2004;58:92–102. doi: 10.1002/neu.10282. [DOI] [PubMed] [Google Scholar]
- 8.McKerracher L. Spinal cord repair: strategies to promote axon regeneration. Neurobiol Dis. 2001;8:11–18. doi: 10.1006/nbdi.2000.0359. [DOI] [PubMed] [Google Scholar]
- 9.Ren JM, Finklestein SP. Growth factor treatment of stroke. Curr Drug Targets CNS Neurol Disord. 2005;4:121–125. doi: 10.2174/1568007053544101. [DOI] [PubMed] [Google Scholar]
- 10.Ginsberg MD, Busto R. Small animal models of global and focal cerebral ischemia. In: Ginsberg MD, Bogousslavsky J, editors. Cerebrovascular Disease: Pathophysiology, Diagnosis and Management. Malden: Blackwell Science; 1998. pp. 14–35. [Google Scholar]
- 11.Andrus PK, Fleck TJ, Oostveen JA, Hall ED. Neuroprotective effects of the novel brain-penetrating pyrrolopyrimidine antioxidants U-101033E and U-104067F against post-ischemic degeneration of nigrostriatal neurons. J Neurosci Res. 1997;47:650–654. doi: 10.1002/(sici)1097-4547(19970315)47:6<650::aid-jnr11>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 12.Hall ED, Andrus PK, Oostveen JA, Althaus JS, Von Voigtlander PF. Neuroprotective effects of the dopamine D2/D3 agonist pramipixole against post-ischemic or methamphetamine-induced degeneration of nigrostriatal neurons. Brain Res. 1996;742:80–88. doi: 10.1016/s0006-8993(96)00968-7. [DOI] [PubMed] [Google Scholar]
- 13.Kagstrom E, Smith ML, Siesjo BK. Local cerebral blood flow in the recovery period following complete cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1983;3:170–182. doi: 10.1038/jcbfm.1983.24. [DOI] [PubMed] [Google Scholar]
- 14.Smith ML, Bendek G, Dahlgren N, Rosen I, Wieloch T, Siesjo BK. Models for studying long-term recovery following forebrain ischemia in the rat. 2. A 2-vessel occlusion model. Acta Neurol Scan. 1984;69:385–401. doi: 10.1111/j.1600-0404.1984.tb07822.x. [DOI] [PubMed] [Google Scholar]
- 15.Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10:267–272. doi: 10.1161/01.str.10.3.267. [DOI] [PubMed] [Google Scholar]
- 16.Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- 17.Miller JD, Bullock R, Graham DI, Chen MH, Teasdale GM. Ischemic brain damage in a model of acute subdural hematoma. Neurosurg. 1990;27:433–439. doi: 10.1097/00006123-199009000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Kline A, Dixon C. Contemporary in vivo models of brain trauma and a comparison of injury responses. In: Miller L, Hayes R, editors. Head Trauma: Basic, Preclinical and Clinical Directions. New York: Wiley-Liss; 2001. pp. 65–84. [Google Scholar]
- 19.Hall ED, Sullivan PG, Gibson TR, Pavel KM, Thompson BM, Scheff SW. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J Neurotrauma. 2005;22:252–265. doi: 10.1089/neu.2005.22.252. [DOI] [PubMed] [Google Scholar]
- 20.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 21.Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE., Jr Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- 22.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 23.Prins ML, Hovda DA. Developing experimental models to address traumatic brain injury in children. J Neurotrauma. 2003;20:123–137. doi: 10.1089/08977150360547053. [DOI] [PubMed] [Google Scholar]
- 24.Schneier AJ, Shields BJ, Hostetler SG, Xiang H, Smith GA. Incidence of pediatric traumatic brain injury and associated hospital resource utilization in the United States. Pediatrics. 2006;118:483–492. doi: 10.1542/peds.2005-2588. [DOI] [PubMed] [Google Scholar]
- 25.Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116–123. doi: 10.1542/peds.109.1.116. [DOI] [PubMed] [Google Scholar]
- 26.Bittigau P, Sifringer M, Felderhoff-Mueser U, Hansen HH, Ikonomidou C. Neuropathological and biochemical features of traumatic injury in the developing brain. Neurotox Res. 2003;5:475–490. doi: 10.1007/BF03033158. [DOI] [PubMed] [Google Scholar]
- 27.Duhaime AC. Large animal models of traumatic injury to the immature brain. Dev Neurosci. 2006;28:380–387. doi: 10.1159/000094164. [DOI] [PubMed] [Google Scholar]
- 28.Smith SL, Hall ED. Tirilazad widens the therapeutic window for riluzole-induced attenuation of progressive cortical degeneration in an infant rat model of the shaken baby syndrome. J Neurotrauma. 1998;15:707–719. doi: 10.1089/neu.1998.15.707. [DOI] [PubMed] [Google Scholar]
- 29.Smith SL, Andrus PK, Gleason DD, Hall ED. Infant rat model of the shaken baby syndrome: preliminary characterization and evidence for the role of free radicals in cortical hemorrhaging and progressive neuronal degeneration. J Neurotrauma. 1998;15:693–705. doi: 10.1089/neu.1998.15.693. [DOI] [PubMed] [Google Scholar]
- 30.Cheng YD, Al-Khoury L, Zivin JA. Neuroprotection for ischemic stroke: Two decades of success and failure. NeuroRx. 2004;1:36–45. doi: 10.1602/neurorx.1.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labiche LA, Grotta JC. Clinical trials for cytoprotection in stroke. NeuroRx. 2004;1:46–70. doi: 10.1602/neurorx.1.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray GD, Teasdale GM, Schmitz H. Nimodipine in traumatic subarachnoid haemorrhage: A re-analysis of the HIT I and HIT II trials. Acta Neurochir (Wien) 1996;138:1163–1167. doi: 10.1007/BF01809745. [DOI] [PubMed] [Google Scholar]
- 33.Muizelaar JP, Kupiec JW, Rapp LA. PEG-SOD after head injury. J Neurosurg. 1995;83:942. doi: 10.3171/jns.1995.83.5.0942. [DOI] [PubMed] [Google Scholar]
- 34.Narayan RK, Michel ME, Ansell B. Clinical trials in head injury. J Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall ED, McCall JM, Means ED. Therapeutic potential of the lazaroids (21-aminosteroids) in acute central nervous system trauma, ischemia and subarachnoid hemorrhage. Adv Pharmacol. 1994;28:221–268. doi: 10.1016/s1054-3589(08)60497-4. [DOI] [PubMed] [Google Scholar]
- 36.Hall ED. Lazaroid: Mechanisms of Action and Implications for Disorders of the CNS. The Neuroscientist. 1997;3:42–51. [Google Scholar]
- 37.Marshall LF, Maas AI, Marshall SB, Bricolo A, Fearnside M, Iannotti F, Klauber MR, Lagarrigue J, Lobato M, Persson L, Pickard J, Piek J, Servadei F, Wellis GN, Morris GF, Means ED, Musch B. A multicenter trial on the efficacy of using tirilazad mesylate in cases of head injury. J Neurosurg. 1998;89:519–525. doi: 10.3171/jns.1998.89.4.0519. [DOI] [PubMed] [Google Scholar]
- 38.Kassell NF, Haley EC, Jr, Apperson-Hansen C, Alves WM. Randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: a cooperative study in Europe, Australia, and New Zealand. J Neurosurg. 1996;84:221–228. doi: 10.3171/jns.1996.84.2.0221. [DOI] [PubMed] [Google Scholar]
- 39.Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, Fehlings M, Herr DL, Hitchon PW, Marshall LF, Nockels RP, Pascale V, Perot PL, Jr, Piepmeier J, Sonntag VK, Wagner F, Wilberger JE, Winn HR, Young W. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597–1604. [PubMed] [Google Scholar]
- 40.Lanzino G, Kassell NF. Double-blind, randomized, vehicle-controlled study of high-dose tirilazad mesylate in women with aneurysmal subarachnoid hemorrhage. Part II. A cooperative study in North America. J Neurosurg. 1999;90:1018–1024. doi: 10.3171/jns.1999.90.6.1018. [DOI] [PubMed] [Google Scholar]
- 41.Lanzino G, Kassell NF, Dorsch NW, Pasqualin A, Brandt L, Schmiedek P, Truskowski LL, Alves WM. Double-blind, randomized, vehicle-controlled study of high-dose tirilazad mesylate in women with aneurysmal subarachnoid hemorrhage. Part I. A cooperative study in Europe, Australia, New Zealand, and South Africa. J Neurosurg. 1999;90:1011–1017. doi: 10.3171/jns.1999.90.6.1011. [DOI] [PubMed] [Google Scholar]
- 42.Green AR, Shuaib A. Therapeutic strategies for the treatment of stroke. Drug Discov Today. 2006;11:681–693. doi: 10.1016/j.drudis.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Lees KR, Zivin JA, Ashwood T, Davalos A, Davis S, Deiner HC, Grotta J, Lyden P, Shuaib A, Hardemark HG, Wasiewski W. NXY-059 for acute ischemic stroke. N Engl J Med. 2006;354:588–600. doi: 10.1056/NEJMoa052980. [DOI] [PubMed] [Google Scholar]
- 44.Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis S, Diener HC, Ashwood T, Wasiewski W, Emeribe U. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 45.Hall ED, Pazara KE, Linseman KL. Sex differences in postischemic neuronal necrosis in gerbils. J Cereb Blood Flow Metab. 1991;11:292–298. doi: 10.1038/jcbfm.1991.61. [DOI] [PubMed] [Google Scholar]
- 46.Roof RL, Hall ED. Estrogen-related gender difference in survival rate and cortical blood flow after impact-acceleration head injury in rats. J Neurotrauma. 2000;17:1155–1169. doi: 10.1089/neu.2000.17.1155. [DOI] [PubMed] [Google Scholar]
- 47.Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke. 1998;29:1666–1670. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- 48.Hurn PD, Littleton-Kearney MT, Kirsch JR, Dharmarajan AM, Traystman RJ. Postischemic cerebral blood flow recovery in the female: Effect of 17β-estradiol. J Cereb Blood Flow Metab. 1995;15:666–672. doi: 10.1038/jcbfm.1995.82. [DOI] [PubMed] [Google Scholar]
- 49.Wise PM, Dubal DB, Wilson ME, Rau SW, Bottner M. Minireview: neuroprotective effects of estrogen-new insights into mechanisms of action. Endocrinology. 2001;142:969–973. doi: 10.1210/endo.142.3.8033. [DOI] [PubMed] [Google Scholar]
- 50.Wise PM, Dubal DB, Wilson ME, Rau SW, Liu Y. Estrogens: trophic and protective factors in the adult brain. Front Neuroendocrinol. 2001;22:33–66. doi: 10.1006/frne.2000.0207. [DOI] [PubMed] [Google Scholar]
- 51.Chan PH, Epstein CJ, Li Y. Transgenic mice and knockout mutants in the study of oxidative stress in brain injury. J Neurotrauma. 1995;12:815–824. doi: 10.1089/neu.1995.12.815. [DOI] [PubMed] [Google Scholar]
- 52.Tate CC, Garcia AJ, LaPlaca MC. Plasma fibronectin is neuroprotective following traumatic brain injury. Exp Neurol. 2007;207:13–22. doi: 10.1016/j.expneurol.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 53.Millecamps S, Gowing G, Corti O, Mallet J, Julien JP. Conditional NF-L transgene expression in mice for in vivo analysis of turnover and transport rate of neurofilaments. J Neurosci. 2007;27:4947–4956. doi: 10.1523/JNEUROSCI.5299-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Neal KR, Agah R. Conditional targeting: inducible deletion by Cre recombinase. Methods Mol Biol. 2007;366:309–320. doi: 10.1007/978-1-59745-030-0_17. [DOI] [PubMed] [Google Scholar]
- 55.Raab S, Beck H, Gaumann A. Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb Haemost. 2004;91:595–605. doi: 10.1160/TH03-09-0582. [DOI] [PubMed] [Google Scholar]
- 56.Casper KB, Jones K, McCarthy KD. Characterization of astrocyte-specific conditional knockouts. Genesis. 2007;45:292–299. doi: 10.1002/dvg.20287. [DOI] [PubMed] [Google Scholar]
- 57.Licht AH, Raab S, Hofmann U, Breier G. Endothelium-specific Cre recombinase activity in flk-1-Cre transgenic mice. Dev Dyn. 2004;229:312–318. doi: 10.1002/dvdy.10416. [DOI] [PubMed] [Google Scholar]